Proton Radiobiology
Abstract
:1. Introduction
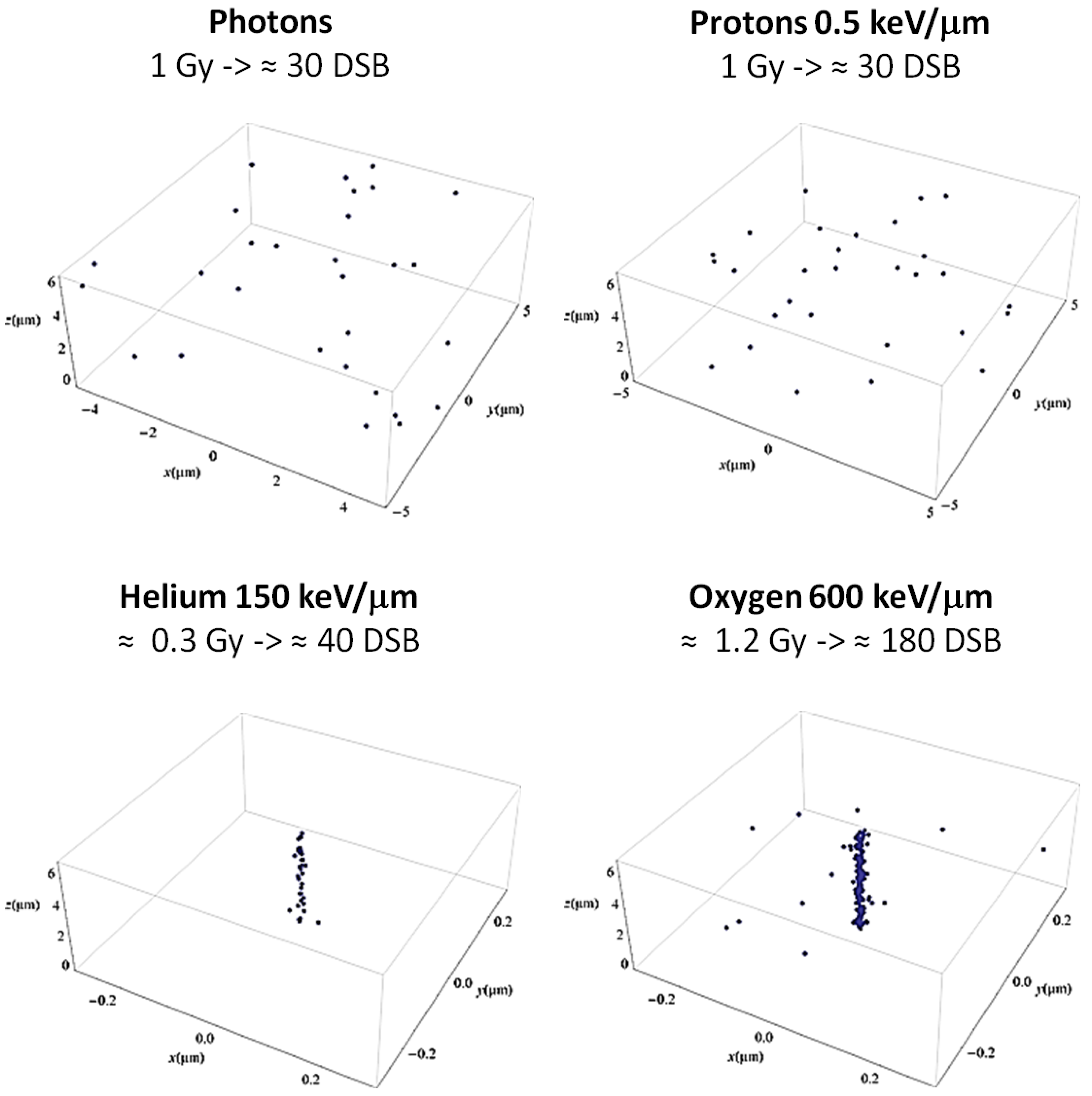
2. Radiation Quality-Dependent Cell Response
2.1. Sub-Cellular Level: DNA and Non-DNA Targets
2.2. Cellular Level
2.3. Tissue Level
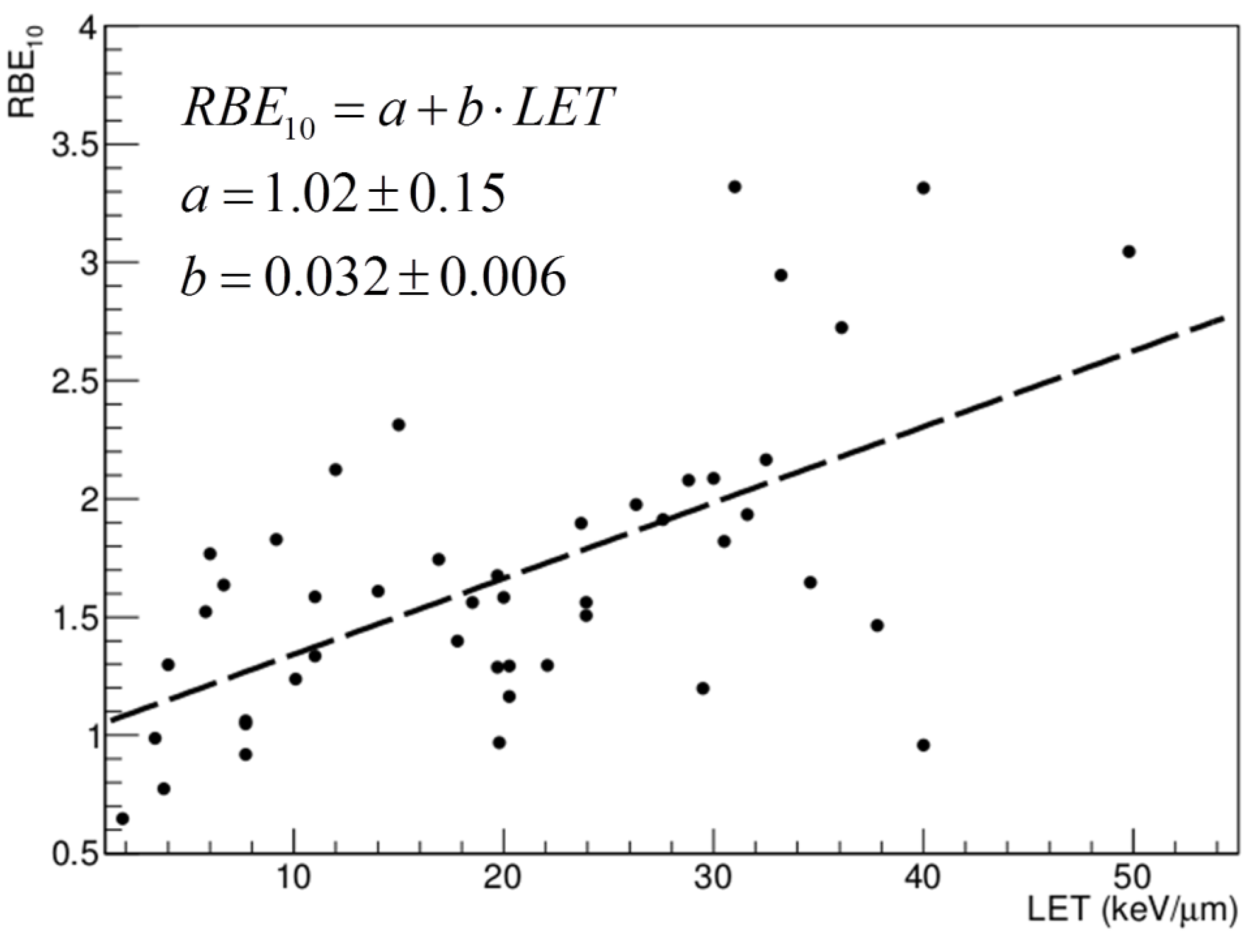
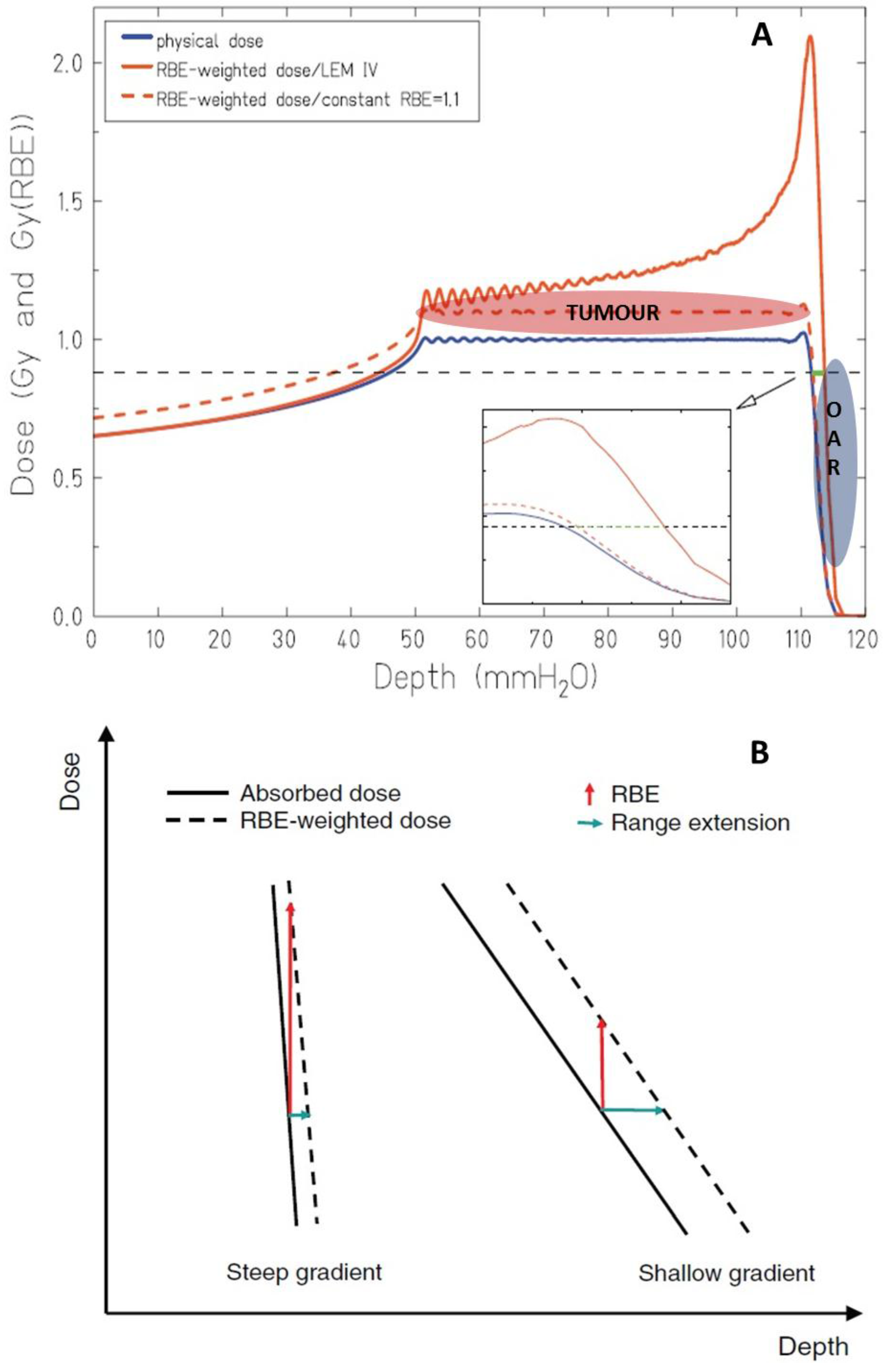
3. Range Uncertainties: Biological Range Extension and the Role of RBE
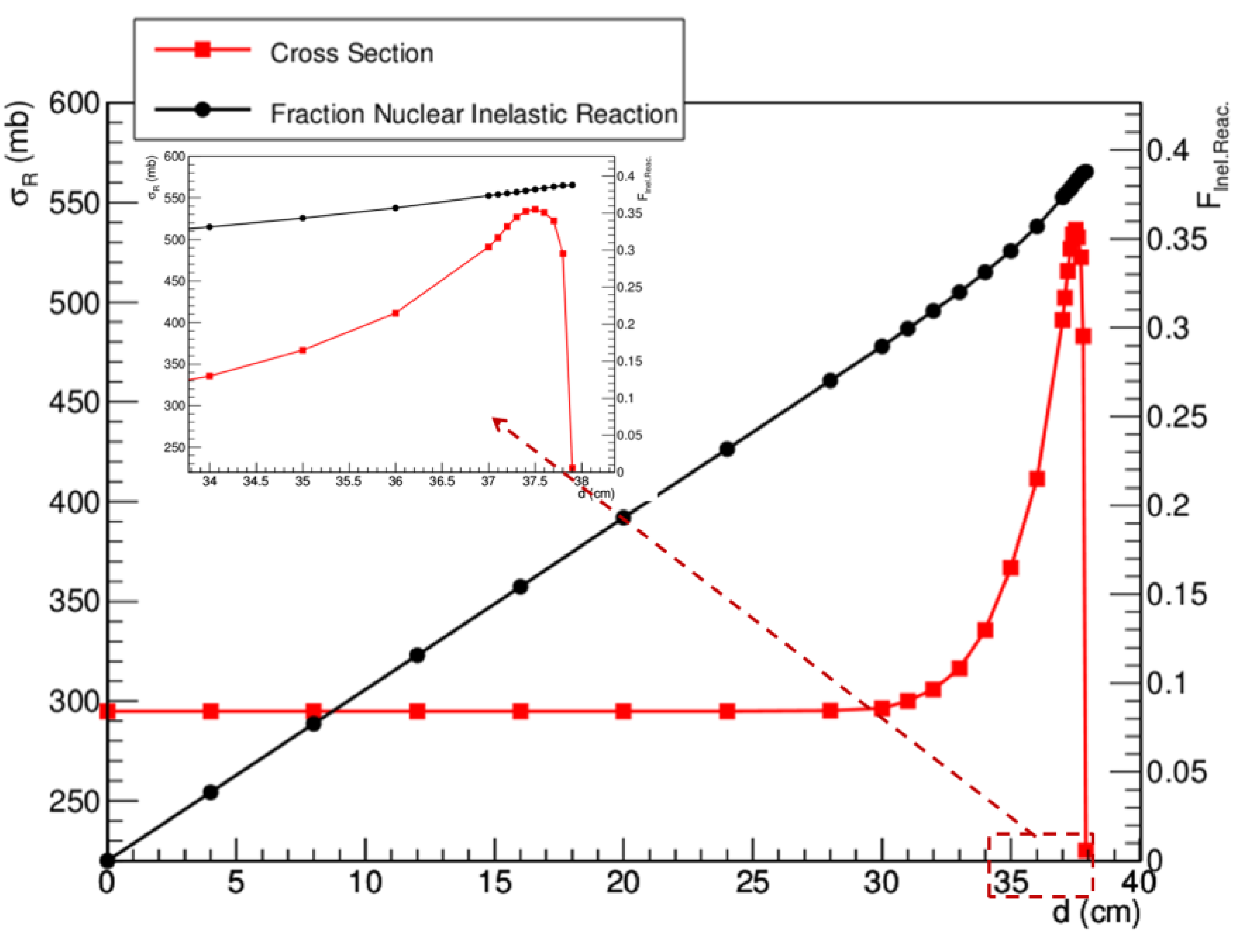
4. Nuclear Interactions
| Fragment | E (MeV) | LET (keV/μm) | Range (μm) |
|---|---|---|---|
| 15O | 1.0 | 983 | 2.3 |
| 15N | 1.0 | 925 | 2.5 |
| 14N | 2.0 | 1137 | 3.6 |
| 13C | 3.0 | 951 | 5.4 |
| 12C | 3.8 | 912 | 6.2 |
| 11C | 4.6 | 878 | 7.0 |
| 10B | 5.4 | 643 | 9.9 |
| 8Be | 6.4 | 400 | 15.7 |
| 6Li | 6.8 | 215 | 26.7 |
| 4He | 6.0 | 77 | 48.5 |
| 3He | 4.7 | 89 | 38.8 |
| 2H | 2.5 | 14 | 68.9 |

4.1. Transport Codes
4.2. Target Fragments and RBE
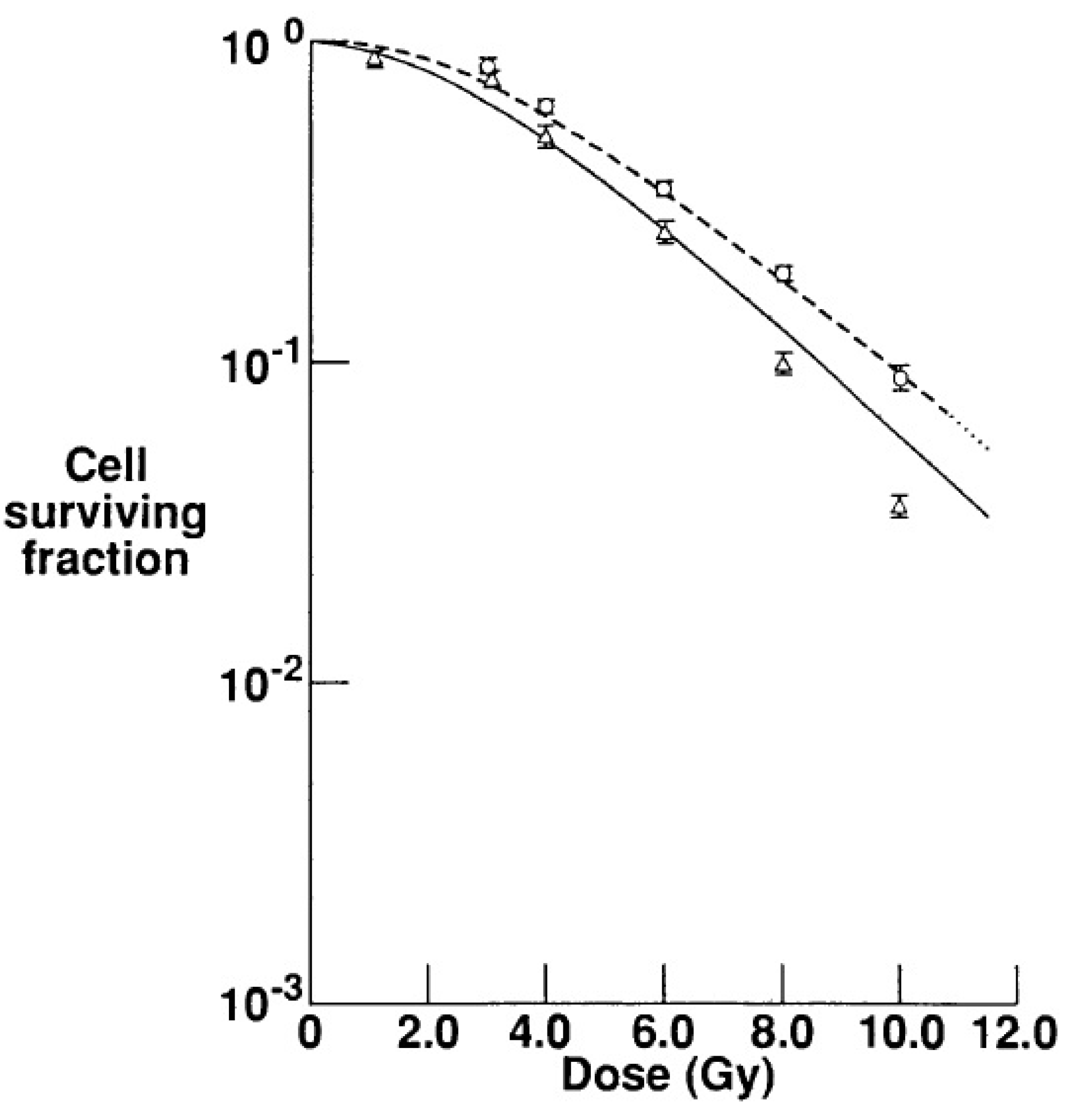

5. Conclusions: The Times They Are a-Changin
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jermann, M. Particle therapy statistics in 2013. Int. J. Part. Ther. 2014, 1, 40–43. [Google Scholar] [CrossRef]
- Loeffler, J.S.; Durante, M. Charged particle therapy--optimization, challenges and future directions. Nat. Rev. Clin. Oncol. 2013, 10, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Lomax, A.J.; Bortfeld, T.; Goitein, G.; Debus, J.; Dykstra, C.; Tercier, P.A.; Coucke, P.A.; Mirimanoff, R.O. A treatment planning inter-comparison of proton and intensity modulated photon radiotherapy. Radiother. Oncol. 1999, 51, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H.; Niemierko, A.; Ancukiewicz, M.; Gerweck, L.E.; Goitein, M.; Loeffler, J.S.; Suit, H.D. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Goodhead, D.T. Energy deposition stochastics and track structure: What about the target? Radiat. Prot. Dosimetry. 2006, 122, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar]
- Friedrich, T.; Scholz, U.; Elsässer, T.; Durante, M.; Scholz, M. Systematic analysis of RBE and related quantities using a database of cell survival experiments with ion beam irradiation. J. Radiat. Res. 2012. [Google Scholar] [CrossRef]
- Sinclair, W.K. The shape of radiation survival curves of mammalian cells cultured in vitro. In Biophysical Aspects of Radiation Quality; Technical Reports Series No. 58; IAEA: Vienna, Austria, 1966. [Google Scholar]
- Paganetti, H.; van Luijk, P. Biological considerations when comparing proton therapy with photon therapy. Semin. Radiat. Oncol. 2013, 23, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Prise, K.M.; O’Sullivan, J.M. Radiation-induced bystander signalling in cancer therapy. Nat. Rev. Cancer 2009, 9, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Girdhani, S.; Lamont, C.; Hahnfeldt, P.; Abdollahi, A.; Hlatky, L. Proton irradiation suppresses angiogenic genes and impairs cell invasion and tumor growth. Radiat. Res. 2012, 178, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Girdhani, S.; Sachs, R.; Hlatky, L. Biological effects of proton radiation: What we know and don’t know. Radiat. Res. 2013, 179, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.H.; Park, S.; Peyton, M.; Girard, L.; Xie, Y.; Minna, J.D.; Story, M.D. Distinct transcriptome profiles identified in normal human bronchial epithelial cells after exposure to gamma-rays and different elemental particles of high Z and energy. BMC. Genomics 2013, 14, 372. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Kellerer, A.M.; Kraft-Weyrather, W.; Kraft, G. Computation of cell survival in heavy ion beams for therapy. The model and its approximation. Radiat. Environ. Biophys. 1997, 36, 59–66. [Google Scholar] [CrossRef]
- Elsässer, T.; Weyrather, W.K.; Friedrich, T.; Durante, M.; Iancu, G.; Krämer, M.; Kragl, G.; Brons, S.; Winter, M.; Weber, K.J.; et al. Quantification of the relative biological effectiveness for ion beam radiotherapy: Direct experimental comparison of proton and carbon ion beams and a novel approach for treatment planning. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1177–1183. [Google Scholar]
- Dasu, A.; Toma-Dasu, I. Impact of variable RBE on proton fractionation. Med. Phys. 2013, 40, 011705. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Marshall, T.I.; Perozziello, F.M.; Manti, L.; Currell, F.J.; Hanton, F.; McMahon, S.J.; Kavanagh, J.N.; Cirrone, G.A.; Romano, F.; et al. Relative biological effectiveness variation along monoenergetic and modulated Bragg peaks of a 62-MeV therapeutic proton beam: A preclinical assessment. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 27–35. [Google Scholar]
- Grassberger, C.; Paganetti, H. Elevated LET components in clinical proton beams. Phys. Med. Biol. 2011, 56, 6677–6691. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; Cherubini, R.; Dalla, V.M.; Dini, V.; Esposito, G.; Moschini, G.; Sapora, O.; Simone, G.; Tabocchini, M.A. DNA fragmentation in V79 cells irradiated with light ions as measured by pulsed-field gel electrophoresis. I. Experimental results. Int. J. Radiat. Biol. 2002, 78, 475–482. [Google Scholar] [CrossRef]
- Calugaru, V.; Nauraye, C.; Noel, G.; Giocanti, N.; Favaudon, V.; Megnin-Chanet, F. Radiobiological characterization of two therapeutic proton beams with different initial energy spectra used at the Institut Curie Proton Therapy Center in Orsay. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Sutherland, B.M. Spectrum of complex DNA damages depends on the incident radiation. Radiat. Res. 2006, 165, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Leloup, C.; Garty, G.; Assaf, G.; Cristovao, A.; Breskin, A.; Chechik, R.; Shchemelinin, S.; Paz-Elizur, T.; Livneh, Z.; Schulte, R.W.; et al. Evaluation of lesion clustering in irradiated plasmid DNA. Int. J. Radiat. Biol. 2005, 81, 41–54. [Google Scholar]
- Grosse, N.; Fontana, A.O.; Hug, E.B.; Lomax, A.; Coray, A.; Augsburger, M.; Paganetti, H.; Sartori, A.A.; Pruschy, M. Deficiency in homologous recombination renders Mammalian cells more sensitive to proton vs. photon irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 175–181. [Google Scholar] [CrossRef]
- Finnberg, N.; Wambi, C.; Ware, J.H.; Kennedy, A.R.; El-Deiry, W.S. Gamma-radiation (GR) triggers a unique gene expression profile associated with cell death compared to proton radiation (PR) in mice in vivo. Cancer Biol. Ther. 2008, 7, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Gerelchuluun, A.; Hong, Z.; Sun, L.; Suzuki, K.; Terunuma, T.; Yasuoka, K.; Sakae, T.; Moritake, T.; Tsuboi, K. Induction of in situ DNA double-strand breaks and apoptosis by 200 MeV protons and 10 MV X-rays in human tumour cell lines. Int. J. Radiat. Biol. 2011, 87, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Green, L.M.; Murray, D.K.; Bant, A.M.; Kazarians, G.; Moyers, M.F.; Nelson, G.A.; Tran, D.T. Response of thyroid follicular cells to gamma irradiation compared to proton irradiation. I. Initial characterization of DNA damage, micronucleus formation, apoptosis, cell survival, and cell cycle phase redistribution. Radiat. Res. 2001, 155, 32–42. [Google Scholar]
- Costes, S.V.; Boissiere, A.; Ravani, S.; Romano, R.; Parvin, B.; Barcellos-Hoff, M.H. Imaging features that discriminate between foci induced by high- and low-LET radiation in human fibroblasts. Radiat. Res. 2006, 165, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Davis, E.; O’Neill, P.; Durante, M.; Cucinotta, F.A.; Wu, H. Immunofluorescence detection of clustered gamma-H2AX foci induced by HZE-particle radiation. Radiat. Res. 2005, 164, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Leatherbarrow, E.L.; Harper, J.V.; Cucinotta, F.A.; O’Neill, P. Induction and quantification of gamma-H2AX foci following low and high LET-irradiation. Int. J. Radiat. Biol. 2006, 82, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Goetz, W.; Morgan, M.N.; Baulch, J.E. The effect of radiation quality on genomic DNA methylation profiles in irradiated human cell lines. Radiat. Res. 2011, 175, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet. 2007, 8, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, C.; Piro, S.; Tabbi, G.; Ragusa, M.; di Pietro, V.; Zimmitti, V.; Cuda, F.; Anello, M.; Consoli, U.; Salinaro, E.T.; et al. Cellular and molecular effects of protons: Apoptosis induction and potential implications for cancer therapy. Apoptosis 2006, 11, 57–66. [Google Scholar]
- Lee, K.B.; Lee, J.S.; Park, J.W.; Huh, T.L.; Lee, Y.M. Low energy proton beam induces tumor cell apoptosis through reactive oxygen species and activation of caspases. Exp. Mol. Med. 2008, 40, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Giedzinski, E.; Rola, R.; Fike, J.R.; Limoli, C.L. Efficient production of reactive oxygen species in neural precursor cells after exposure to 250 MeV protons. Radiat. Res. 2005, 164, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, S.H.; Fang, B.; Gillin, M.; Mohan, R.; Chang, J.Y. Therapy-resistant cancer stem cells have differing sensitivity to photon vs. proton beam radiation. J. Thorac. Oncol. 2013, 8, 1484–1491. [Google Scholar] [CrossRef]
- Mishra, K.P. Cell membrane oxidative damage induced by gamma-radiation and apoptotic sensitivity. J. Environ. Pathol. Toxicol. Oncol. 2004, 23, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Kale, R.K. Radiation induced peroxidative damage: Mechanism and significance. Indian J. Exp. Biol. 2001, 39, 291–309. [Google Scholar] [PubMed]
- Somosy, Z. Radiation response of cell organelles. Micron 2000, 31, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Benderitter, M.; Vincent-Genod, L.; Pouget, J.P.; Voisin, P. The cell membrane as a biosensor of oxidative stress induced by radiation exposure: A multiparameter investigation. Radiat. Res. 2003, 159, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Baluchamy, S.; Ravichandran, P.; Periyakaruppan, A.; Ramesh, V.; Hall, J.C.; Zhang, Y.; Jejelowo, O.; Gridley, D.S.; Wu, H.; Ramesh, G.T. Induction of cell death through alteration of oxidants and antioxidants in lung epithelial cells exposed to high energy protons. J. Biol. Chem. 2010, 285, 24769–24774. [Google Scholar] [CrossRef] [PubMed]
- Luotola, J. Membrane damage caused by free radicals—Radiation perspective. Med. Biol. 1984, 62, 115–118. [Google Scholar] [PubMed]
- Durante, M. New challenges in high-energy particle radiobiology. Br. J. Radiol. 2014, 87, 20130626. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Bell, M.H.; Nirodi, C.S.; Story, M.D.; Minna, J.D. Radiogenomics predicting tumor responses to radiotherapy in lung cancer. Semin. Radiat. Oncol. 2010, 20, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Raju, M.R. Proton radiobiology, radiosurgery and radiotherapy. Int. J. Radiat. Biol. 1995, 67, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Gerweck, L.E.; Kozin, S.V. Relative biological effectiveness of proton beams in clinical therapy. Radiother. Oncol. 1999, 50, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; Bettega, D.; Calzolari, P.; Cera, F.; Cherubini, R.; Dalla, V.M.; Durante, M.; Favaretto, S.; Gialanella, G.; Grossi, G.; et al. Inactivation of human normal and tumour cells irradiated with low energy protons. Int. J. Radiat. Biol. 2000, 76, 831–839. [Google Scholar]
- Bettega, D.; Calzolari, P.; Chauvel, P.; Courdi, A.; Herault, J.; Iborra, N.; Marchesini, R.; Massariello, P.; Poli, G.L.; Tallone, L. Radiobiological studies on the 65 MeV therapeutic proton beam at Nice using human tumour cells. Int. J. Radiat. Biol. 2000, 76, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.T.; Inoue, T.; Inoue, T.; Yamazaki, H.; Fukushima, S.; Fournier-Bidoz, N.; Koizumi, M.; Ozeki, S.; Hatanaka, K. Comparison of radiobiological effective depths in 65-MeV modulated proton beams. Br. J. Cancer 1997, 76, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Brahme, A. Development of radiation therapy optimization. Acta Oncol. 2000, 39, 579–595. [Google Scholar] [CrossRef] [PubMed]
- Wedenberg, M.; Lind, B.K.; Hardemark, B. A model for the relative biological effectiveness of protons: The tissue specific parameter alpha/beta of photons is a predictor for the sensitivity to LET changes. Acta Oncol. 2013, 52, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ahmad, S. Empirical model estimation of relative biological effectiveness for proton beam therapy. Radiat. Prot. Dosim. 2012, 149, 116–123. [Google Scholar] [CrossRef]
- Frese, M.C.; Wilkens, J.J.; Huber, P.E.; Jensen, A.D.; Oelfke, U.; Taheri-Kadkhoda, Z. Application of constant vs. variable relative biological effectiveness in treatment planning of intensity-modulated proton therapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 80–88. [Google Scholar]
- Frese, M.C.; Yu, V.K.; Stewart, R.D.; Carlson, D.J. A mechanism-based approach to predict the relative biological effectiveness of protons and carbon ions in radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.B. A microdosimetric-kinetic theory of the dependence of the RBE for cell death on LET. Med. Phys. 1998, 25, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Carabe, A.; Moteabbed, M.; Depauw, N.; Schuemann, J.; Paganetti, H. Range uncertainty in proton therapy due to variable biological effectiveness. Phys. Med. Biol. 2012, 57, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Tilly, N.; Johansson, J.; Isacsson, U.; Medin, J.; Blomquist, E.; Grusell, E.; Glimelius, B. The influence of RBE variations in a clinical proton treatment plan for a hypopharynx cancer. Phys. Med. Biol. 2005, 50, 2765–2777. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Attardi, L.D. The role of apoptosis in cancer development and treatment response. Nat. Rev. Cancer 2005, 5, 231–237. [Google Scholar] [PubMed]
- Green, L.M.; Tran, D.T.; Murray, D.K.; Rightnar, S.S.; Todd, S.; Nelson, G.A. Response of thyroid follicular cells to gamma irradiation compared to proton irradiation: II. The role of connexin 32. Radiat. Res. 2002, 158, 475–485. [Google Scholar] [CrossRef]
- Ristic-Fira, A.M.; Todorovic, D.V.; Koricanac, L.B.; Petrovic, I.M.; Valastro, L.M.; Cirrone, P.G.; Raffaele, L.; Cuttone, G. Response of a human melanoma cell line to low and high ionizing radiation. Ann. NY Acad. Sci. 2007, 1095, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Narang, H.; Bhat, N.; Gupta, S.K.; Santra, S.; Choudhary, R.K.; Kailash, S.; Krishna, M. Differential activation of mitogen-activated protein kinases following high and low LET radiation in murine macrophage cell line. Mol. Cell Biochem. 2009, 324, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Moertel, H.; Georgi, J.C.; Distel, L.; Eyrich, W.; Fritsch, M.; Grabenbauer, G.; Sauer, R. Effects of low energy protons on clonogenic survival, DSB repair and cell cycle in human glioblastoma cells and B14 fibroblasts. Radiother. Oncol. 2004, 73, S115–S118. [Google Scholar] [CrossRef] [PubMed]
- Antoccia, A.; Sgura, A.; Berardinelli, F.; Cavinato, M.; Cherubini, R.; Gerardi, S.; Tanzarella, C. Cell cycle perturbations and genotoxic effects in human primary fibroblasts induced by low-energy protons and X/gamma-rays. J. Radiat. Res. 2009, 50, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Significance and implementation of RBE variations in proton beam therapy. Technol. Cancer Res. Treat. 2003, 2, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.J.; Cao, Y.; Li, C.Y.; Dewhirst, M.W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004, 5, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Sonveaux, P.; Brouet, A.; Havaux, X.; Gregoire, V.; Dessy, C.; Balligand, J.L.; Feron, O. Irradiation-induced angiogenesis through the up-regulation of the nitric oxide pathway: Implications for tumor radiotherapy. Cancer Res. 2003, 63, 1012–1019. [Google Scholar] [PubMed]
- Park, C.M.; Park, M.J.; Kwak, H.J.; Lee, H.C.; Kim, M.S.; Lee, S.H.; Park, I.C.; Rhee, C.H.; Hong, S.I. Ionizing radiation enhances matrix metalloproteinase-2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res. 2006, 66, 8511–8519. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Teshima, T.; Kagawa, K.; Hishikawa, Y.; Takahashi, Y.; Kawaguchi, A.; Suzumoto, Y.; Nojima, K.; Furusawa, Y.; Matsuura, N. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res. 2005, 65, 113–120. [Google Scholar] [PubMed]
- Bernier, J.; Bentzen, S.M.; Vermorken, J.B. Molecular therapy in head and neck oncology. Nat. Rev. Clin. Oncol. 2009, 6, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Teshima, T.; Kawaguchi, N.; Hamada, Y.; Mori, S.; Madachi, A.; Ikeda, S.; Mizuno, H.; Ogata, T.; Nojima, K.; et al. Heavy ion irradiation inhibits in vitro angiogenesis even at sublethal dose. Cancer Res. 2003, 63, 4253–4257. [Google Scholar]
- Jang, G.H.; Ha, J.H.; Huh, T.L.; Lee, Y.M. Effect of proton beam on blood vessel formation in early developing zebrafish (Danio rerio) embryos. Arch. Pharm. Res. 2008, 31, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Gridley, D.S.; Bonnet, R.B.; Bush, D.A.; Franke, C.; Cheek, G.A.; Slater, J.D.; Slater, J.M. Time course of serum cytokines in patients receiving proton or combined photon/proton beam radiation for resectable but medically inoperable non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Kajioka, E.H.; Andres, M.L.; Mao, X.W.; Moyers, M.F.; Nelson, G.A.; Gridley, D.S. Hematological and TGF-beta variations after whole-body proton irradiation. Vivo 2000, 14, 703–708. [Google Scholar]
- Rithidech, K.N.; Reungpatthanaphong, P.; Honikel, L.; Rusek, A.; Simon, S.R. Dose-rate effects of protons on in vivo activation of nuclear factor-kappa B and cytokines in mouse bone marrow cells. Radiat. Environ. Biophys. 2010, 49, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Bjornstad, K.A.; Rosen, C.J.; Lin, S.; Blakely, E.A. Particle radiation alters expression of matrix metalloproteases resulting in ECM remodeling in human lens cells. Radiat. Environ. Biophys. 2007, 46, 187–194. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.P.; Bjornstad, K.A.; Chang, P.Y.; Chou, W.; Lockett, S.J.; Blakely, E.A. Modulation of lens cell adhesion molecules by particle beams. Phys. Med. 2001, 17, S247–S248. [Google Scholar]
- Tian, J.; Pecaut, M.J.; Coutrakon, G.B.; Slater, J.M.; Gridley, D.S. Response of extracellular matrix regulators in mouse lung after exposure to photons, protons and simulated solar particle event protons. Radiat. Res. 2009, 172, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Hei, T.K.; Zhou, H.; Ivanov, V.N.; Hong, M.; Lieberman, H.B.; Brenner, D.J.; Amundson, S.A.; Geard, C.R. Mechanism of radiation-induced bystander effects: A unifying model. J. Pharm. Pharmacol. 2008, 60, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Lorimore, S.A.; Chrystal, J.A.; Robinson, J.I.; Coates, P.J.; Wright, E.G. Chromosomal instability in unirradiated hemaopoietic cells induced by macrophages exposed in vivo to ionizing radiation. Cancer Res. 2008, 68, 8122–8126. [Google Scholar] [CrossRef] [PubMed]
- Lorimore, S.A.; Mukherjee, D.; Robinson, J.I.; Chrystal, J.A.; Wright, E.G. Long-lived inflammatory signaling in irradiated bone marrow is genome dependent. Cancer Res. 2011, 71, 6485–6491. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Ishiwata, Y.; Nakagawa, K.; Yokochi, S.; Taruki, C.; Akuta, T.; Ohtomo, K.; Matsushima, K.; Tamatani, T.; Kanegasaki, S. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1alpha. Clin. Cancer Res. 2008, 14, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Hiniker, S.M.; Chen, D.S.; Knox, S.J. Abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 2035–2036. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar]
- Durante, M.; Reppingen, N.; Held, K.D. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol. Med. 2013, 19, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Gragoudas, E.S.; Lane, A.M.; Munzenrider, J.; Egan, K.M.; Li, W. Long-term risk of local failure after proton therapy for choroidal/ciliary body melanoma. Trans. Am. Ophthalmol. Soc. 2002, 100, 43–48. [Google Scholar] [PubMed]
- Munzenrider, J.E.; Verhey, L.J.; Gragoudas, E.S.; Seddon, J.M.; Urie, M.; Gentry, R.; Birnbaum, S.; Ruotolo, D.M.; Crowell, C.; McManus, P.; et al. Conservative treatment of uveal melanoma: Local recurrence after proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 493–498. [Google Scholar]
- Marucci, L.; Lane, A.; Li, W.; Egan, K.; Gragoudas, E.; Adams, J.; Collier, J.; Munzenrider, J.E. Conservation treatment of the eye: Conformal proton reirradiation for recurrent uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Sethi, R.V.; Giantsoudi, D.; Raiford, M.; Malhi, I.; Niemierko, A.; Rapalino, O.; Caruso, P.; Yock, T.I.; Tarbell, N.J.; Paganetti, H.; et al. Patterns of failure after proton therapy in medulloblastoma; linear energy transfer distributions and relative biological effectiveness associations for relapses. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 655–663. [Google Scholar]
- Schneider, U.; Lomax, A.; Timmermann, B. Second cancers in children treated with modern radiotherapy techniques. Radiother. Oncol. 2008, 89, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Sethi, R.V.; Shih, H.A.; Yeap, B.Y.; Mouw, K.W.; Petersen, R.; Kim, D.Y.; Munzenrider, J.E.; Grabowski, E.; Rodriguez-Galindo, C.; Yock, T.I.; et al. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer 2014, 120, 126–133. [Google Scholar]
- Chung, C.S.; Yock, T.I.; Nelson, K.; Xu, Y.; Keating, N.L.; Tarbell, N.J. Incidence of second malignancies among patients treated with proton vs. photon radiation. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 46–52. [Google Scholar] [CrossRef]
- Newhauser, W.D.; Durante, M. Assessing the risk of second malignancies after modern radiotherapy. Nat. Rev. Cancer 2011, 11, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Moyers, M.F.; Miller, D.W.; Bush, D.A.; Slater, J.D. Methodologies and tools for proton beam design for lung tumors. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Goitein, M. Calculation of the uncertainty in the dose delivered during radiation therapy. Med. Phys. 1985, 12, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys. Med. Biol. 2012, 57, R99–R117. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Both, S.; Bentefour, H.; Paly, J.J.; Tochner, Z.; Efstathiou, J.; Lu, H.M. Improvement of prostate treatment by anterior proton fields. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Jakel, O.; Reiss, P. The influence of metal artefacts on the range of ion beams. Phys. Med. Biol. 2007, 52, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Newhauser, W.; Fontenot, J.; Koch, N.; Dong, L.; Lee, A.; Zheng, Y.; Waters, L.; Mohan, R. Monte Carlo simulations of the dosimetric impact of radiopaque fiducial markers for proton radiotherapy of the prostate. Phys. Med. Biol. 2007, 52, 2937–2952. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Pedroni, E.; Lomax, A. The calibration of CT Hounsfield units for radiotherapy treatment planning. Phys. Med. Biol. 1996, 41, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.; Bortfeld, T.; Schlegel, W. Correlation between CT numbers and tissue parameters needed for Monte Carlo simulations of clinical dose distributions. Phys. Med. Biol. 2000, 45, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Albertini, F.; Bolsi, A.; Lomax, A.J.; Rutz, H.P.; Timmerman, B.; Goitein, G. Sensitivity of intensity modulated proton therapy plans to changes in patient weight. Radiother. Oncol. 2008, 86, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Knopf, A.C.; Lomax, A. In vivo proton range verification: A review. Phys. Med. Biol. 2013, 58, R131–R160. [Google Scholar] [CrossRef] [PubMed]
- Grün, R.; Friedrich, T.; Krämer, M.; Zink, K.; Durante, M.; Engenhart-Cabillic, R.; Scholz, M. Biologically effective proton range analysis. Med. Phys. 2013, 40, 1–10. [Google Scholar] [CrossRef]
- Johansson, J. Comparative Treatment Planning in Radiotherapy and Clinical Impact of Proton Relative Biological Effectiveness; Acta Universitatis Upsaliensis: Uppsala, Sweden, 2006; p. 62. [Google Scholar]
- Gensheimer, M.F.; Yock, T.I.; Liebsch, N.J.; Sharp, G.C.; Paganetti, H.; Madan, N.; Grant, P.E.; Bortfeld, T. In vivo proton beam range verification using spine MRI changes. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.V. Microscopic abrasion-ablation approximation to projectile fragmentation. Phys. Rev. C. Nucl. Phys. 1995, 51, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.V.; Mastroleo, R.C.; Hussein, M.S. Fragment production in heavy-ion reactions. Phys. Rev. C. 1992, 46, R30–R33. [Google Scholar] [CrossRef]
- Bradt, H.L.; Peters, B. The heavy nuclei of the primary cosmic radiation. Phys. Rev. 1950, 77, 54–76. [Google Scholar] [CrossRef]
- Sihver, L.; Tsao, C.H.; Silberberg, R.; Kanai, T.; Barghouty, A.F. Total reaction and partial cross section calculations in proton-nucleus (Zt ≤ 26) and nucleus-nucleus reactions (Zp and Zt ≤ 26). Phys. Rev. C 1993, 47, 1225–1236. [Google Scholar] [CrossRef]
- Durante, M.; Cucinotta, F.A. Physical basis of radiation protection in space travel. Rev. Mod. Phys. 2011, 83, 1245–1281. [Google Scholar] [CrossRef]
- Bichsel, H. Stochastics of energy loss and biological effects of heavy ions in radiation therapy. Adv. Quantum Chem. 2013, 65, 1–38. [Google Scholar]
- Goldhaber, A.S. Statistical models of fragmentation processes. Phys. Lett. 1974, 53B, 306–308. [Google Scholar] [CrossRef]
- Giacomelli, M.; Sihver, L.; Skvarc, J.; Yasuda, N.; Ilic, R. Projectile like fragment emission angles in fragmentation reactions of light heavy ions in the energy region <200 MeV/nucleon: Modeling and simulations. Phys. Rev. C 2004, 69, 064601. [Google Scholar]
- Paganetti, H. Nuclear interactions in proton therapy: Dose and relative biological effect distributions originating from primary and secondary particles. Phys. Med. Biol. 2002, 47, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Krämer, M.; Durante, M. Ion beam transport calculations and treatment plans in particle therapy. Eur. Phys. J. D 2010, 60, 195–202. [Google Scholar] [CrossRef]
- Krämer, M.; Scifoni, E.; Schmitz, F.; Sokol, O.; Durante, M. Overview of recent advances in treatment planning for ion beam radiotherapy. Eur. Phys. J. D 2014, 68, 306–311. [Google Scholar] [CrossRef]
- Sihver, L.; Lantz, M.; Böhlen, T.T.; Mairani, A.; Cerutti, A.F.; Ferrari, A. A comparison of total reaction cross section models used in FLUKA, GEANT4 and PHITS. In Proceedings of the IEEE Aerospace Conference, Big Sky, MT, USA, 3–10 March 2012; pp. 1–10.
- Böhlen, T.T.; Cerutti, F.; Dosanjh, M.; Ferrari, A.; Gudowska, I.; Mairani, A.; Quesada, J.M. Benchmarking nuclear models of FLUKA and GEANT4 for carbon ion therapy. Phys. Med. Biol. 2010, 55, 5833–5847. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, C.A.; Carlsson, G.A. Proton dosimetry with 185 MeV protons. Dose buildup from secondary protons and recoil electrons. Health Phys. 1977, 33, 481–484. [Google Scholar]
- Cucinotta, F.A.; Katz, R.; Wilson, J.W.; Townsend, L.W.; Shinn, J.; Hajnal, F. Biological effectiveness of high-energy protons: Target fragmentation. Radiat. Res. 1991, 127, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Butts, J.J.; Katz, R. Theory of RBE for heavy ion bombardment of dry enzymes and viruses. Radiat. Res. 1967, 30, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Katz, R.; Sharma, S.C. Heavy particles in therapy: An application of track theory. Phys. Med. Biol. 1974, 19, 413–435. [Google Scholar] [CrossRef] [PubMed]
- Gueulette, J.; Bohm, L.; de Coster, B.M.; Vynckier, S.; Octave-Prignot, M.; Schreuder, A.N.; Symons, J.E.; Jones, D.T.; Wambersie, A.; Scalliet, P. RBE variation as a function of depth in the 200-MeV proton beam produced at the National Accelerator Centre in Faure (South Africa). Radiother. Oncol. 1997, 42, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Koike, S.; Uzawa, A.; Takai, N.; Fukawa, T.; Furusawa, Y.; Aoki, M.; Miyato, Y. Biological gain of carbon-ion radiotherapy for the early response of tumor growth delay and against early response of skin reaction in mice. J. Radiat. Res. 2005, 46, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Karger, C.P.; Peschke, P.; Sanchez-Brandelik, R.; Scholz, M.; Debus, J. Radiation tolerance of the rat spinal cord after 6 and 18 fractions of photons and carbon ions: Experimental results and clinical implications. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Wedenberg, M.; Toma-Dasu, I. Disregarding RBE variation in treatment plan comparison may lead to bias in favor of proton plans. Med. Phys. 2014, 41, 091706. [Google Scholar] [CrossRef] [PubMed]
- Niemierko, A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med. Phys. 1997, 24, 103–110. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tommasino, F.; Durante, M. Proton Radiobiology. Cancers 2015, 7, 353-381. https://doi.org/10.3390/cancers7010353
Tommasino F, Durante M. Proton Radiobiology. Cancers. 2015; 7(1):353-381. https://doi.org/10.3390/cancers7010353
Chicago/Turabian StyleTommasino, Francesco, and Marco Durante. 2015. "Proton Radiobiology" Cancers 7, no. 1: 353-381. https://doi.org/10.3390/cancers7010353
APA StyleTommasino, F., & Durante, M. (2015). Proton Radiobiology. Cancers, 7(1), 353-381. https://doi.org/10.3390/cancers7010353







