Complex of MUC1, CIN85 and Cbl in Colon Cancer Progression and Metastasis
Abstract
:1. Introduction
2. Results
2.1. Detection of CIN85 and Abnormal MUC1 in Human Colon Cancer
| Characteristics | No. | MUC1 * | P-Value | CIN85 | P-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neg | Weak | Moderate | High | Neg | Weak | Moderate | High | ||||
| Normal | 4 | 3 | 1 | 0 | 0 | 0.048 | 2 | 2 | 0 | 0 | 0.082 |
| Benign | 16 | 5 | 6 | 5 | 0 | 3 | 3 | 8 | 3 | ||
| Cancer | 55 | 9 | 15 | 14 | 14 | 5 | 12 | 19 | 19 | ||
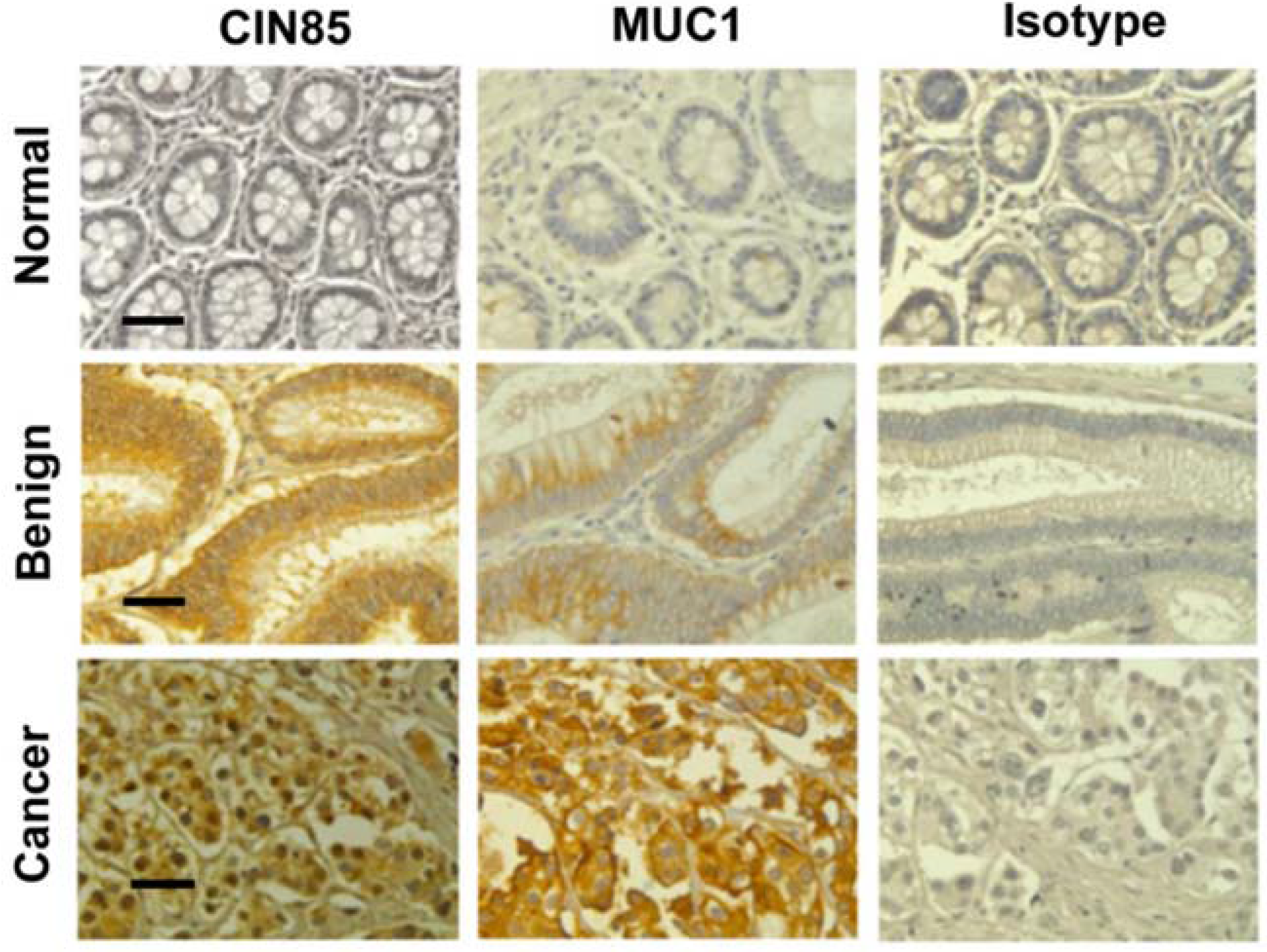
| Group | No. | MUC1 * Mod/HIgh CIN85 Neg/Low | MUC1 * Neg/Low CIN85 Mod/High | MUC1 */CIN85 (Mod/High) | P-Value | |
|---|---|---|---|---|---|---|
| Co-Expression | No Co-Expression | |||||
| LN Positive | 31 | 2 | 6 | 17 | 6 | 0.043 |
| LN Negative | 24 | 6 | 9 | 6 | 3 | |
| Metastasis Positive | 6 | 0 | 0 | 6 | 0 | 0.024 |
| Metastasis Negative | 49 | 8 | 15 | 17 | 9 | |
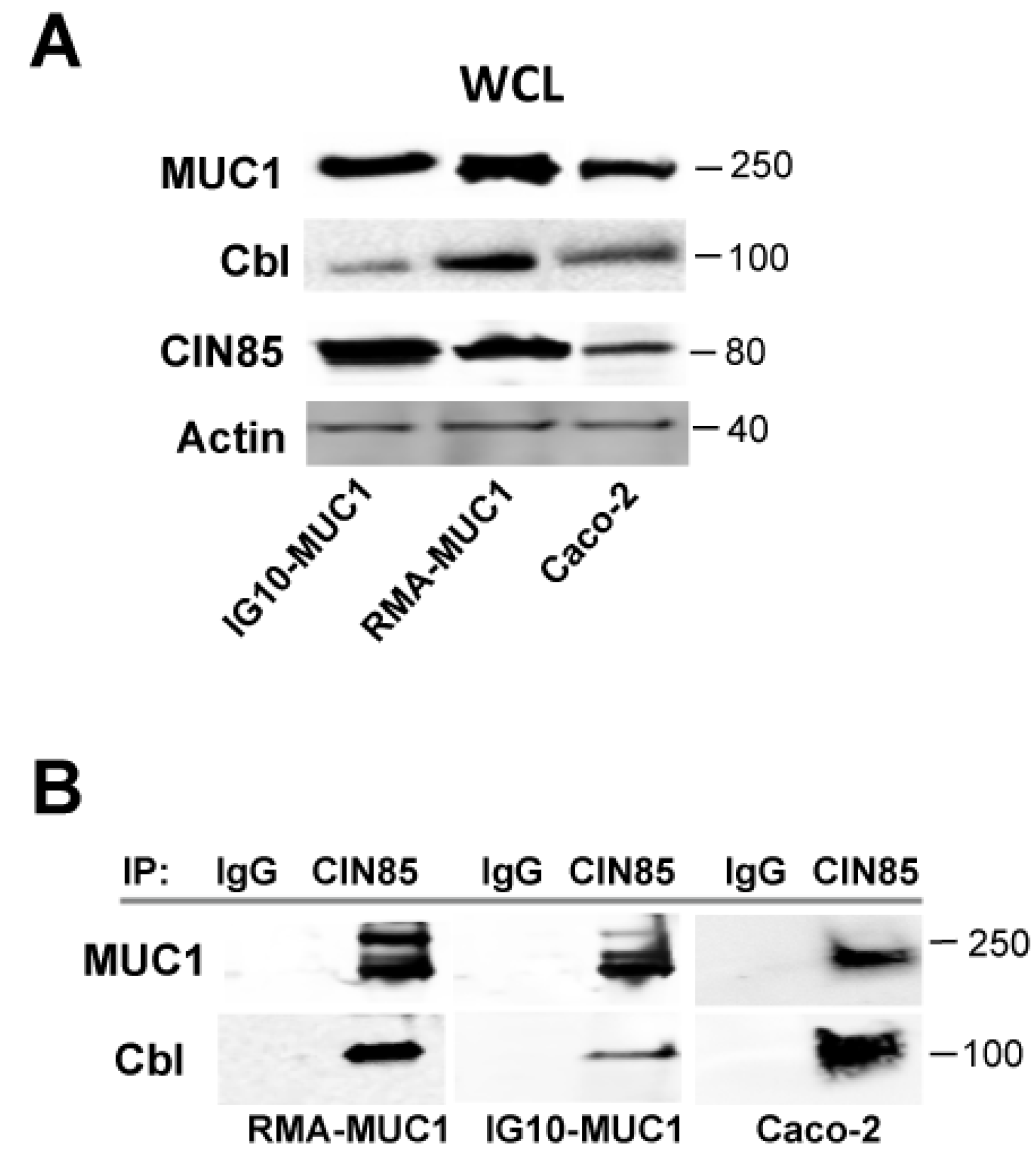
2.2. Novel Association of Cbl with CIN85 and MUC1
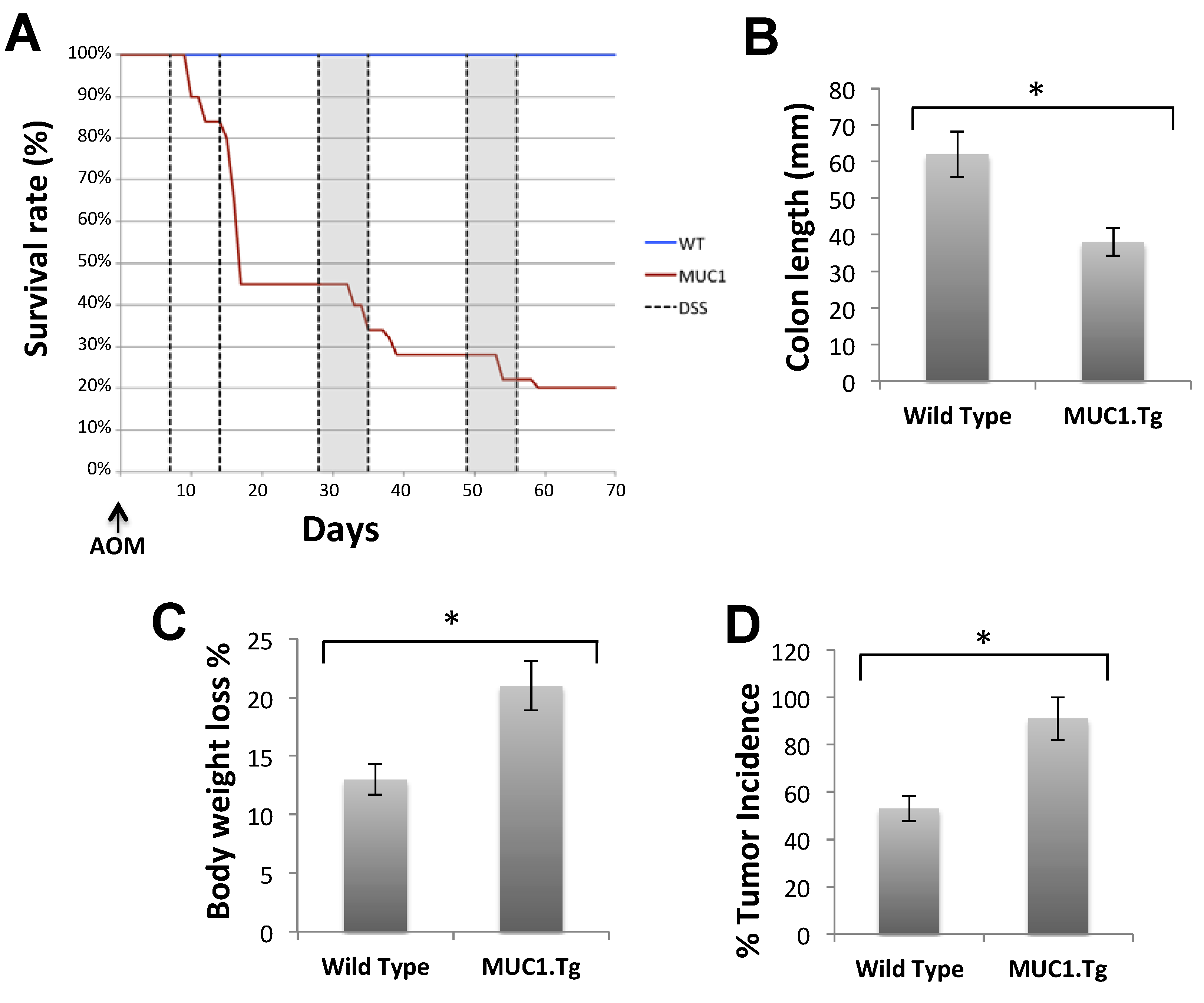
2.3. MUC1, CIN85 and Cbl in AOM/DSS-Induced Mouse Colon Carcinogenesis
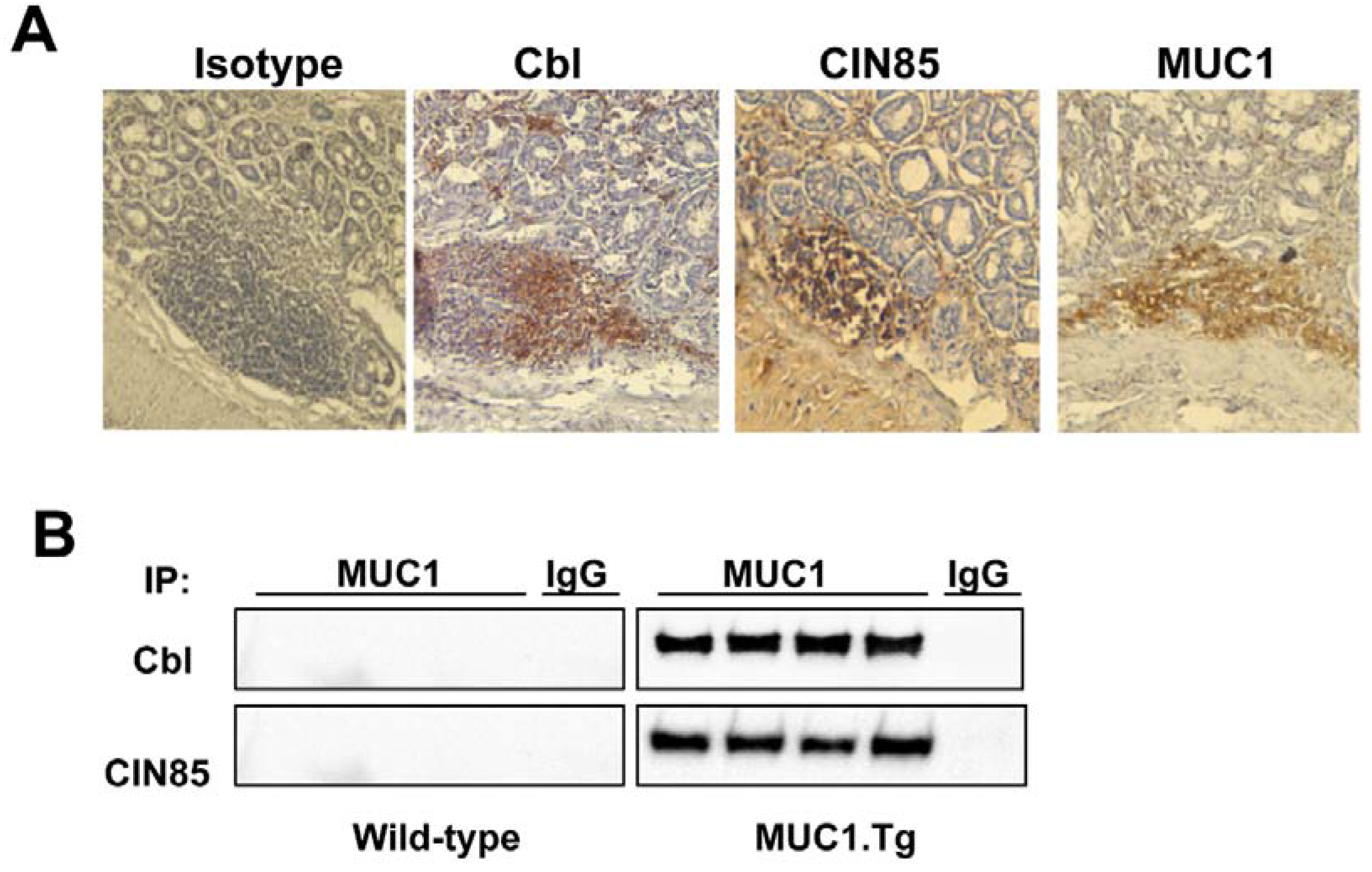
3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. Plasmid and Transfection
4.3. Animals and tumor induction
4.4. Western Blotting and Immunoprecipitation
4.5. Immunohistochemistry
4.6. Scoring of Immunoreactivity
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Shoda, J.; Kawamoto, T.; Shinozaki, E.; Miyahara, N.; Hotta, S.; Iizuka, Y.; Nakahara, A.; Tanaka, N.; Yanaka, A.; et al. Expression of muc1 recognized by monoclonal antibody my.1e12 is a useful biomarker for tumor aggressiveness of advanced colon carcinoma. Clin. Exp. Metastasis 2004, 21, 321–329. [Google Scholar]
- Cascio, S.; Farkas, A.M.; Hughey, R.P.; Finn, O.J. Altered glycosylation of muc1 influences its association with cin85: The role of this novel complex in cancer cell invasion and migration. Oncotarget 2013, 4, 1686–1697. [Google Scholar] [PubMed]
- Ciborowski, P.; Finn, O.J. Non-glycosylated tandem repeats of MUC1 facilitate attachment of breast tumor cells to normal human lung tissue and immobilized extracellular matrix proteins (ECM) in vitro: Potential role in metastasis. Clin. Exp. Metastasis 2002, 19, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Nakamori, S.; Ota, D.M.; Cleary, K.R.; Shirotani, K.; Irimura, T. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology 1994, 106, 353–361. [Google Scholar] [PubMed]
- Suzuki, Y.; Sutoh, M.; Hatakeyama, S.; Mori, K.; Yamamoto, H.; Koie, T.; Saitoh, H.; Yamaya, K.; Funyu, T.; Habuchi, T.; et al. MUC1 carrying core 2 O-glycans functions as a molecular shield against NK cell attack, promoting bladder tumor metastasis. Int. J. Oncol. 2012, 40, 1831–1838. [Google Scholar]
- Hattrup, C.L.; Gendler, S.J. Structure and function of the cell surface (tethered) mucins. Ann. Rev. Physiol. 2008, 70, 431–457. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Cascio, S.; Zhang, L.; Finn, O.J. MUC1 protein expression in tumor cells regulates transcription of proinflammatory cytokines by forming a complex with nuclear factor-kappab p65 and binding to cytokine promoters: Importance of extracellular domain. J. Biol. Chem. 2011, 286, 42248–42256. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I. CIN85/CMS family of adaptor molecules. FEBS Lett. 2002, 529, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Havrylov, S.; Redowicz, M.J.; Buchman, V.L. Emerging roles of Ruk/CIN85 in vesicle-mediated transport, adhesion, migration and malignancy. Traffic 2010, 11, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Niiro, H.; Jabbarzadeh-Tabrizi, S.; Kikushige, Y.; Shima, T.; Noda, K.; Ota, S.; Tsuzuki, H.; Inoue, Y.; Arinobu, Y.; Iwasaki, H.; et al. CIN85 is required for Cbl-mediated regulation of antigen receptor signaling in human B cells. Blood 2012, 119, 2263–2273. [Google Scholar]
- Kim, J.; Kang, D.; Sun, B.K.; Kim, J.H.; Song, J.J. TRAIL/MEKK4/p38/HSP27/Akt survival network is biphasically modulated by the Src/CIN85/c-Cbl complex. Cell. Signal. 2013, 25, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.M.; Onodera, Y.; Mazaki, Y.; Miyoshi, H.; Hashimoto, S.; Sabe, H. CIN85, a Cbl-interacting protein, is a component of amap1-mediated breast cancer invasion machinery. EMBO J. 2007, 26, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, A.; Gilestro, G.F.; Lanzardo, S.; Comoglio, P.M.; Migone, N.; Giordano, S. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature 2002, 416, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Soubeyran, P.; Kowanetz, K.; Szymkiewicz, I.; Langdon, W.Y.; Dikic, I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 2002, 416, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Szymkiewicz, I.; Kowanetz, K.; Soubeyran, P.; Dinarina, A.; Lipkowitz, S.; Dikic, I. CIN85 participates in Cbl-b-mediated down-regulation of receptor tyrosine kinases. J. Biol. Chem. 2002, 277, 39666–39672. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Finn, O.J. MUC1 immunotherapy is here to stay. Expert Opin. Biol. Ther. 2013, 13, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Tanaka, S.; Haruma, K.; Sumii, K.; Kajiyama, G.; Shimamoto, F.; Kohno, N. Clinical significance of MUC1 and E-cadherin expression, cellular proliferation, and angiogenesis at the deepest invasive portion of colorectal cancer. Int. J. Oncol. 2000, 16, 55–64. [Google Scholar] [PubMed]
- Roy, L.D.; Sahraei, M.; Subramani, D.B.; Besmer, D.; Nath, S.; Tinder, T.L.; Bajaj, E.; Shanmugam, K.; Lee, Y.Y.; Hwang, S.I.; et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 2011, 30, 1449–1459. [Google Scholar]
- Tamura, Y.; Higashi, M.; Kitamoto, S.; Yokoyama, S.; Osako, M.; Horinouchi, M.; Shimizu, T.; Tabata, M.; Batra, S.K.; Goto, M.; et al. MUC4 and MUC1 expression in adenocarcinoma of the stomach correlates with vessel invasion and lymph node metastasis: An immunohistochemical study of early gastric cancer. PLOS ONE 2012, 7, e49251. [Google Scholar]
- Lau, S.K.; Shields, D.J.; Murphy, E.A.; Desgrosellier, J.S.; Anand, S.; Huang, M.; Kato, S.; Lim, S.T.; Weis, S.M.; Stupack, D.G.; et al. EGFR-mediated carcinoma cell metastasis mediated by integrin alphavbeta5 depends on activation of c-Src and cleavage of muc1. PLOS ONE 2012, 7, e36753. [Google Scholar]
- Schroeder, J.A.; Adriance, M.C.; Thompson, M.C.; Camenisch, T.D.; Gendler, S.J. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene 2003, 22, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascio, S.; Finn, O.J. Complex of MUC1, CIN85 and Cbl in Colon Cancer Progression and Metastasis. Cancers 2015, 7, 342-352. https://doi.org/10.3390/cancers7010342
Cascio S, Finn OJ. Complex of MUC1, CIN85 and Cbl in Colon Cancer Progression and Metastasis. Cancers. 2015; 7(1):342-352. https://doi.org/10.3390/cancers7010342
Chicago/Turabian StyleCascio, Sandra, and Olivera J. Finn. 2015. "Complex of MUC1, CIN85 and Cbl in Colon Cancer Progression and Metastasis" Cancers 7, no. 1: 342-352. https://doi.org/10.3390/cancers7010342
APA StyleCascio, S., & Finn, O. J. (2015). Complex of MUC1, CIN85 and Cbl in Colon Cancer Progression and Metastasis. Cancers, 7(1), 342-352. https://doi.org/10.3390/cancers7010342





