Evaluation of Circulating Tumor Cells and Related Events as Prognostic Factors and Surrogate Biomarkers in Advanced NSCLC Patients Receiving First-Line Systemic Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Patient Demographics
| Factors | Subgroup | n | % |
|---|---|---|---|
| Average age at baseline | 62.7 (40–83) | ||
| Sex | Male | 37 | 86 |
| Female | 6 | 14 | |
| Smoker | Current smoker | 19 | 44.2 |
| Prior smoker | 15 | 34.9 | |
| Never smoker | 7 | 16.3 | |
| Unknown | 2 | 4.6 | |
| ECOG PS | 0–1 | 30 | 69.8 |
| 2 | 13 | 30.2 | |
| Histology | Adenocarcinoma | 31 | 72.1 |
| Squamous-cell | 12 | 27.9 | |
| Tumour stage | IIIB | 4 | 9.3 |
| IV | 39 | 90.7 | |
| Metastasis location | Bone | 11 | 25.6 |
| Liver | 5 | 11.6 | |
| Supra-adrenal | 9 | 20.9 | |
| Pleural | 16 | 37.2 | |
| Others | 5 | 10.6 | |
| QT treatment | CDDP-PEM | 15 | 34.9 |
| CDDP-/CBDCA- | 22 | 51.2 | |
| GEM-VNB | 3 | 7 | |
| The best suportive care | 3 | 7 | |
2.2. CTC and CTC Related Objects at Baseline
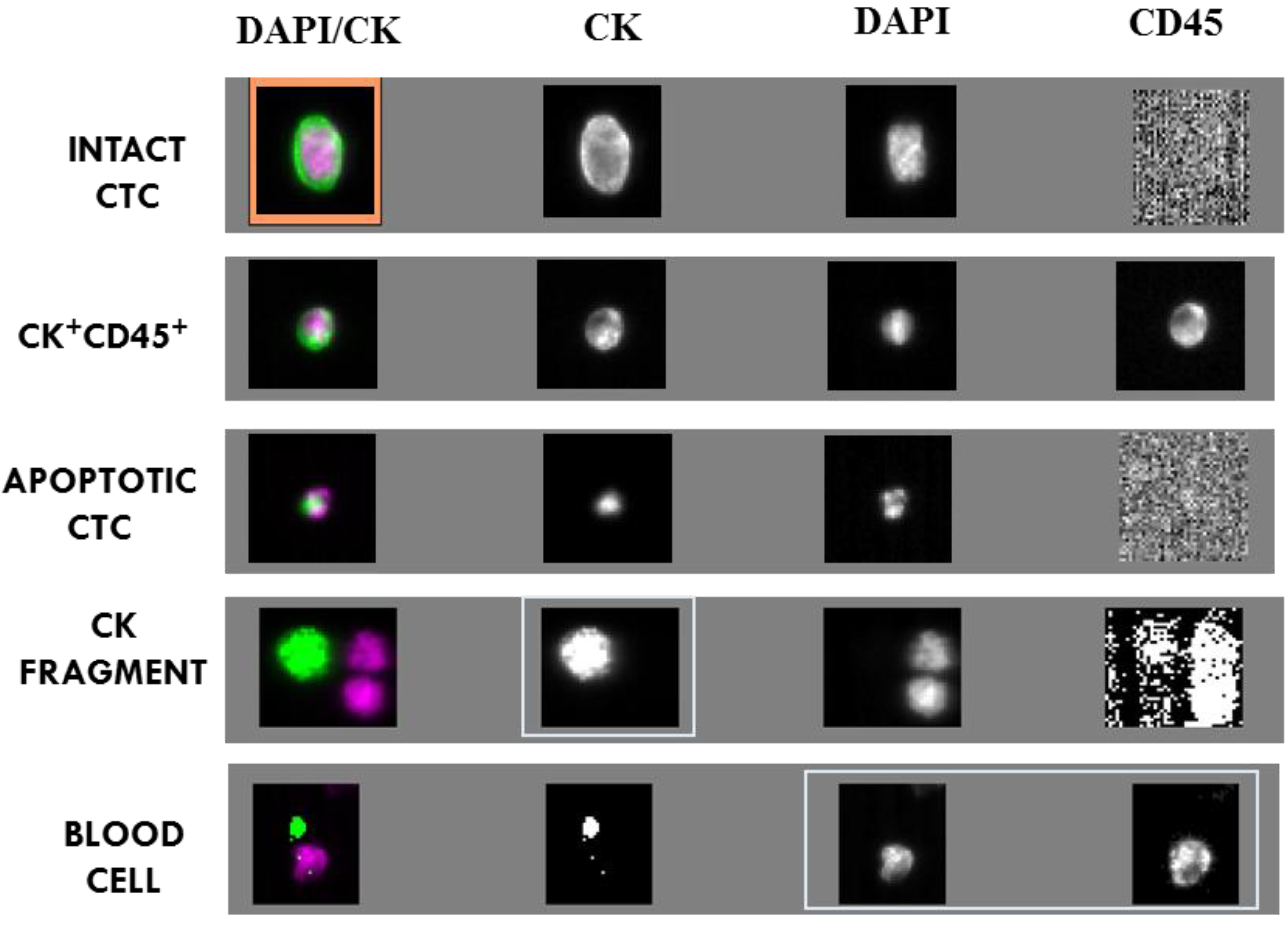
| Type of Event | Baseline | 2nd C | 5th C | |||
|---|---|---|---|---|---|---|
| Mean ± SEM | Positive patients | Mean ± SEM | Positive patients | Mean ± SEM | Positive patients | |
| INTACT CTC | 18.9 ± 14.8 | 18 (41.9%) | 0.87 ± 0.22 | 9 (31%) | 0.77 ± 0.42 | 6 (28.6%) |
| CK+ CD45+ CELLS | 2.6 ± 1.1 | 16 (37.2%) | ND | ND | ND | ND |
| APOPTOTIC CTC | 8.4 ± 6.8 | 14 (32.6%) | 10.57 ± 6.4 | 5 (17.2%) | 3 ± 3 | 3 (14.3%) |
| CK FRAGMENTS | 19.2 ± 9.6 | 32 (74.4%) | 1.85 ± 0.61 | 12 (41.4%) | 3.1 ± 1.35 | 14 (66.6%) |
| BLOOD CELLS | 55 ± 20.48 | 43 (100%) | ND | ND | ND | ND |
2.3. Correlation between CTC/CTC-Related Objects at Baseline and Clinico-Pathologic Features
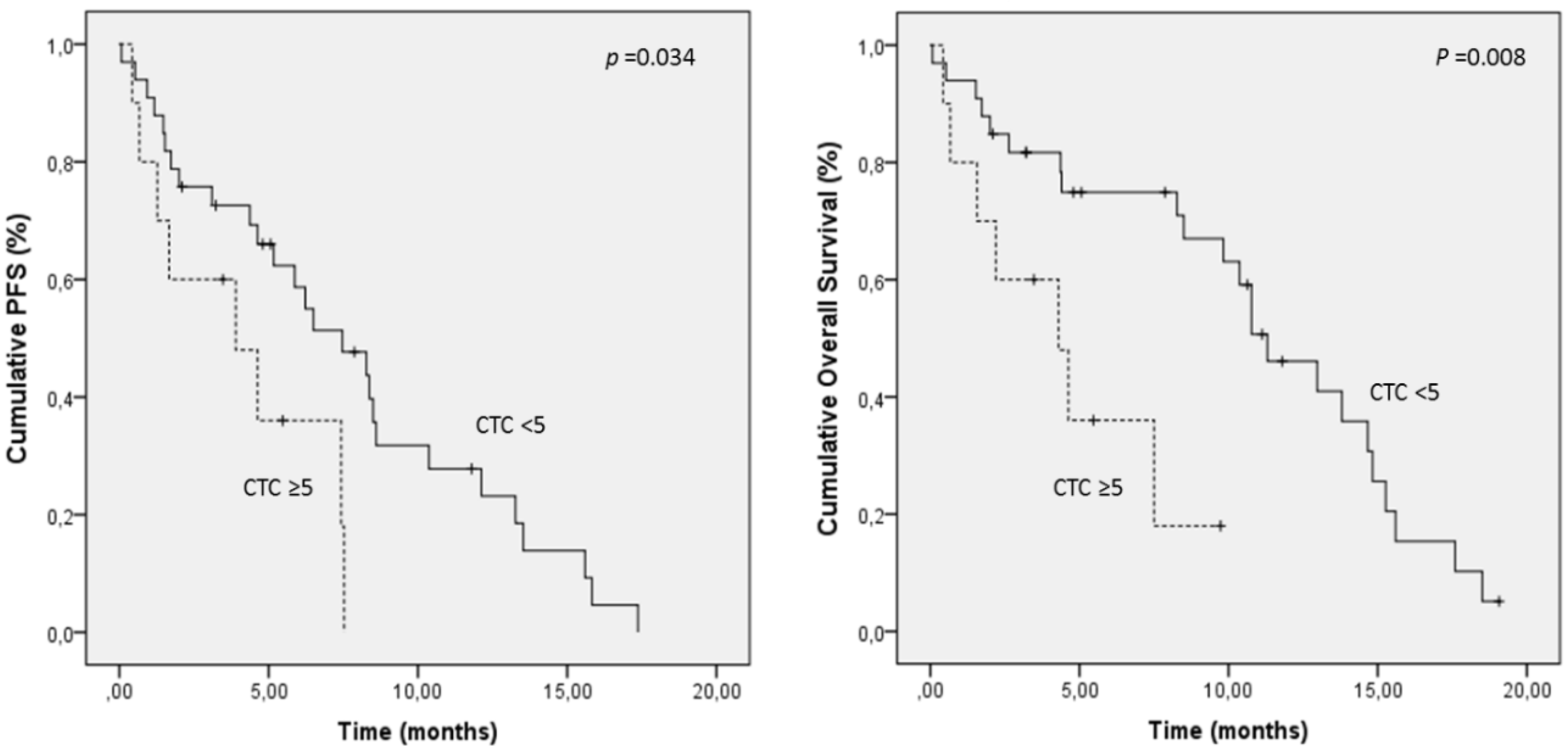
2.4. Prognostic Significance of CTC/CTC-Related Objects at Baseline
| Factor | Cut-Off | Median PFS | p | Median OS | p |
|---|---|---|---|---|---|
| INTACT CTC | 5 | 4.1 vs. 7.6 | 0.034 * | 4.6 vs. 10.7 | 0.008 * |
| APOPTOTIC CTC | 2 | 3.4 vs. 7.6 | 0.017 * | 3.6 vs. 10.5 | 0.001 * |
| CK FRAGMENTS | 2 | 3.4 vs. 8.7 | 0.035 * | 5.4 vs. 11.2 | 0.10 |
| CK+ CD45+ | 2 | 6.5 vs. 6.8 | 0.59 | 7.3 vs. 9.6 | 0.86 |
| BLOOD CELLS | 17 | 6.7 vs. 7.1 | 0.60 | 9.1 vs. 9.6 | 0.97 |
| INTACT/APOPTOTIC CTC | 2 | 4.3 vs. 8.4 | 0.006 * | 5.7 vs. 10.9 | 0.014 * |
| INTACT/APOPTOTIC CTC + CK+ FRAGMENTS | 5 | 4.1 vs. 8.5 | 0.003 * | 6.5 vs. 10.9 | 0.026 * |
| Factor | PFS | Factor | OS | ||
|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | ||
| Bone mets (no vs. yes) | 2 (0.84–5) | 0.11 | Bone mets. (no vs. yes) | 2.6 (1–6.5) | 0.038 * |
| Intact CTC (<5 vs. ≥5) | 4.3 (1.3–14.4) | 0.016 | ECOG PS (≤1 vs. 2) | 2.1 (0.9–4.6) | 0.071 |
| Apoptotic CTC (<2 vs. ≥2) | 1.3 (1.1–6.2) | 0.16 | Intact CTC (<5 vs. ≥5) | 2.9 (0.7–11.4) | 0.11 |
| CK+ fragments (<2 vs. ≥2) | 1.9 (1.2–4.8) | 0.15 | Apoptotic CTC (<2 vs. 2) | 1.68 (1.13–4.4) | 0.064 |
| Bone mets. (no vs. yes) | 2.4 (1.1–5.5) | 0.028 * | Bone mets. (no vs. yes) | 2.5 (1.2–5.6) | 0.029 * |
| Intact/apoptotic CTC (<2 vs. ≥2) | 1.4 (0.69–2.9) | 0.33 | ECOG PS (≤1 vs. 2) | 2.5 (1.2–5.5) | 0.016 * |
| Intact/apoptotic CTC (<2 vs. ≥2) | 1.3 (0.6–3) | 0.45 | |||
| Bone mets. (no vs. yes) | 2.2 (0.9–4.9) | 0.052 | |||
| Intact/apoptotic CTC+ CK+ fragments (<5 vs. ≥5) | 3 (1.4–6.5) | 0.005 * | Bone mets. (no vs. yes) | 3 (0.96–5.5) | 0.61 |
| ECOG PS (≤1 vs. 2) | 2.3 (1–5) | 0.029 * | |||
| Intact/apoptotic CTC+ CK+ fragments (<5 vs. ≥5) | 2.3 (0.9–5.7) | 0.07 | |||
2.5. Changes of CTC/CTC-Related Objects within the Treatment
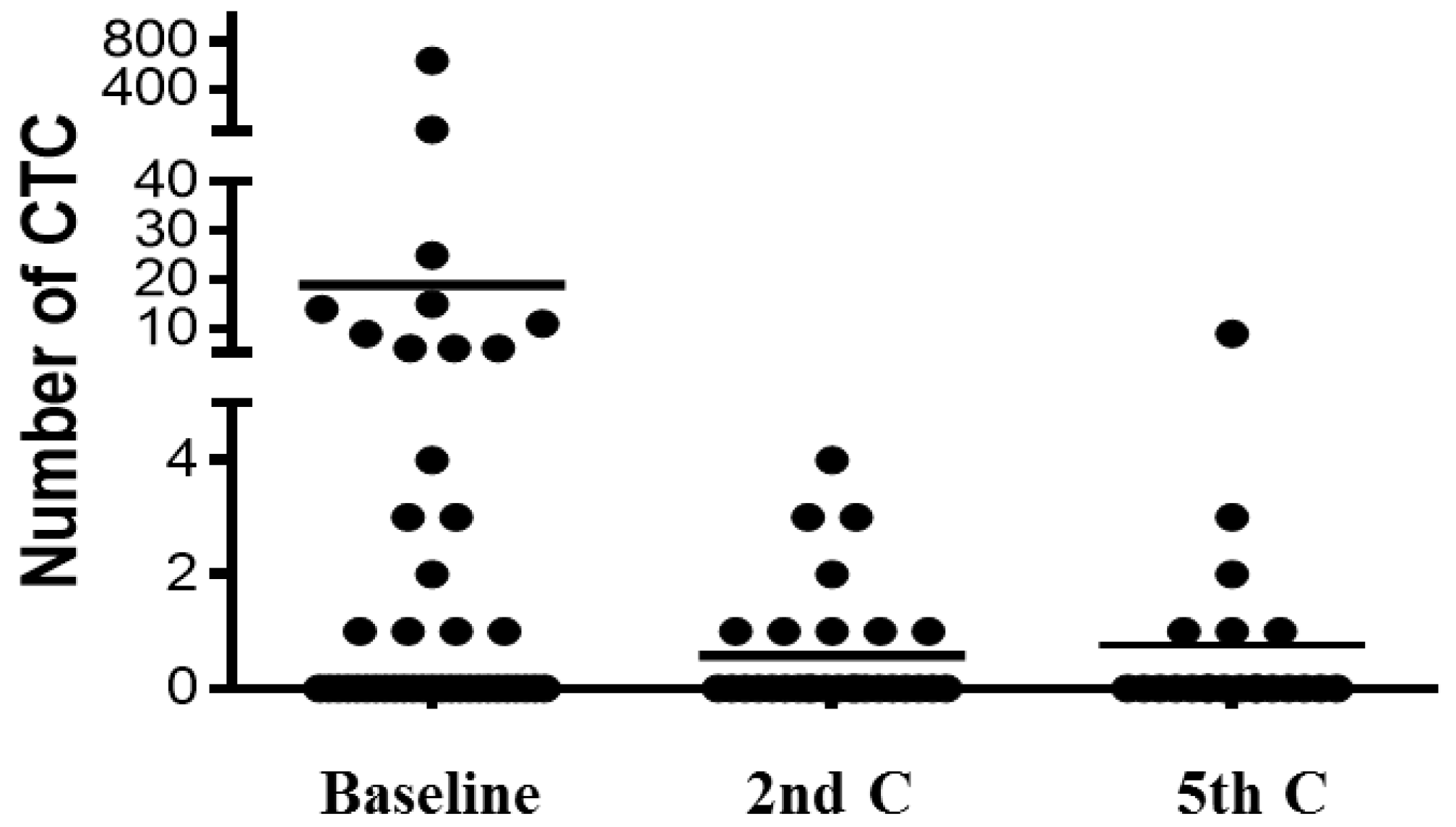
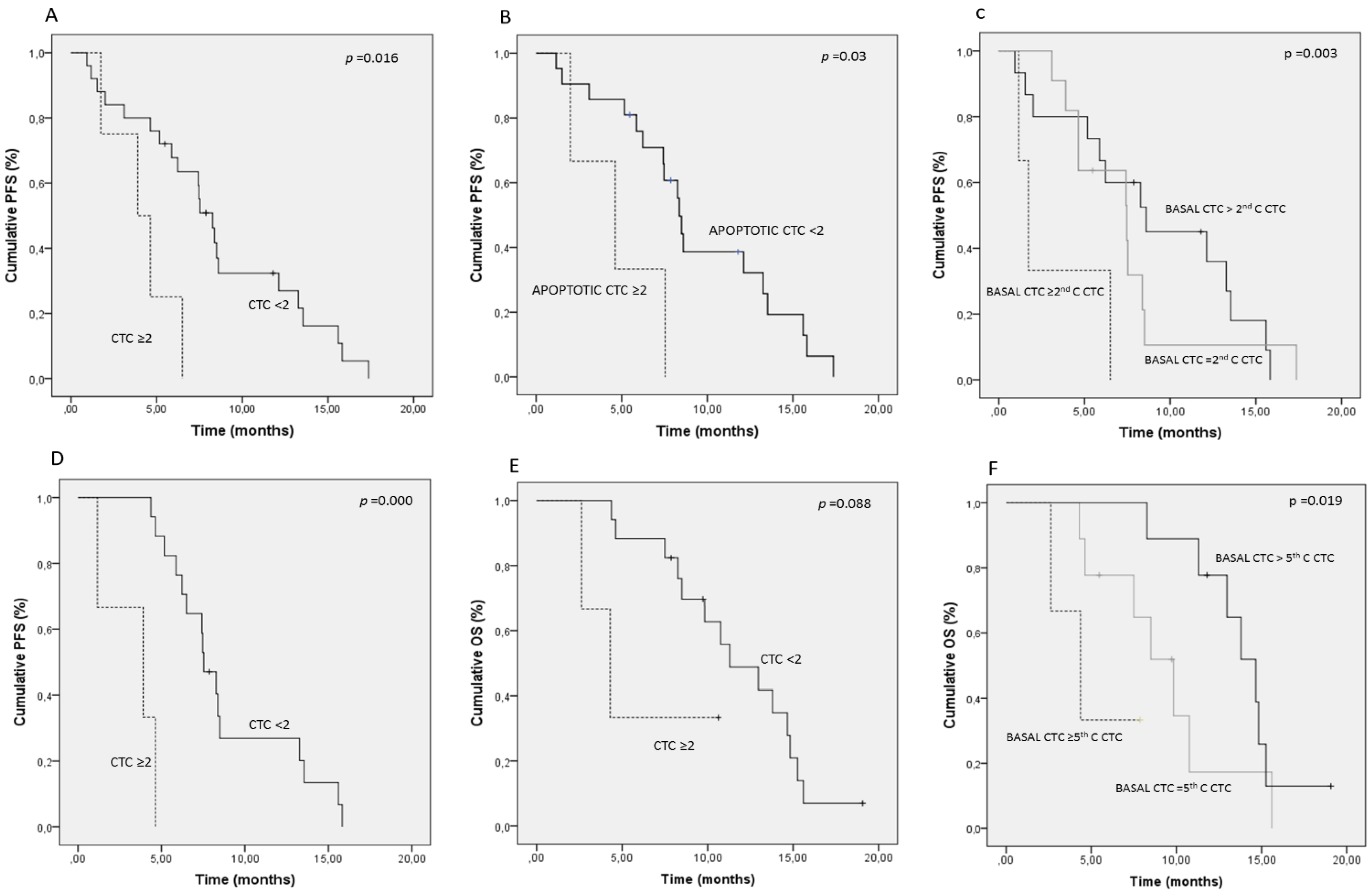
3. Experimental
3.1. Study Design
3.2. CTC Analysis
3.3. Statistical Analysis
4. Conclusions
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef]
- Shepherd, F.A.; Pereira, J.R.; Ciuleanu, T.; Tan, E.H.; Hirsh, V.; Thongprasert, S.; Campos, D.; Maoleekoonpiroj, S.; Smylie, M.; Martins, R.; et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 2005, 353, 123–132. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, D.W.; Nakagawa, K.; Seto, T.; Crino, L.; Ahn, M.J.; de Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Pantel, K. Circulating tumour cells: Liquid biopsy of cancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef]
- Alunni-Fabbroni, M.; Sandri, M.T. Circulating tumour cells in clinical practice: Methods of detection and possible characterization. Methods 2010, 50, 289–297. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumour cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- De Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumour cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef]
- Cohen, S.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.A.; Mitchell, E.; Miller, M.C.; et al. Prognostic significance of circulating tumour cells in patients with metastatic colorectal cancer. Ann. Oncol. 2009, 20, 1223–1229. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Went, P.T.; Lugli, A.; Meier, S.; Bundi, M.; Mirlacher, M.; Sauter, G.; Dirnhofer, S. Frequent EpCam protein expression in human carcinomas. Hum. Pathol. 2004, 35, 122–128. [Google Scholar] [CrossRef]
- Mikolajczyk, S.D.; Millar, L.S.; Tsinberg, P.; Coutts, S.M.; Zomorrodi, M.; Pham, T.; Bischoff, F.Z.; Pircher, T.J. Detection of EpCAM-negative and cytokeratin-negative circulating tumour cells in peripheral blood. J. Oncol. 2011, 2011. [Google Scholar] [CrossRef]
- Lecharpentier, A.; Vielh, P.; Perez-Moreno, P.; Planchard, D.; Soria, J.C.; Farace, F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br. J. Cancer 2011, 105, 1338–1341. [Google Scholar] [CrossRef]
- Krebs, M.G.; Sloane, R.; Priest, L.; Lancashire, L.; Hou, J.M.; Greystoke, A.; Ward, T.H.; Ferraldeschi, R.; Hughes, A.; Clack, G.; et al. Evaluation and prognostic significance of circulating tumour cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 2011, 29, 1556–1563. [Google Scholar] [CrossRef]
- Punnoose, E.A.; Atwal, S.; Liu, W.; Raja, R.; Fine, B.M.; Hughes, B.G.; Hicks, R.J.; Hampton, G.M.; Amler, L.C.; Pirzkall, A.; et al. Evaluation of circulating tumour cells and circulating tumour DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin. Cancer Res. 2012, 18, 2391–2401. [Google Scholar] [CrossRef]
- Fehm, T.; Solomayer, E.F.; Meng, S.; Tucker, T.; Lane, N.; Wang, J.; Gebauer, G. Methods for isolating circulating epithelial cells and criteria for their classification as carcinoma cells. Cytotherapy 2005, 7, 171–185. [Google Scholar] [CrossRef]
- Hofman, V.; Bonnetaud, C.; Ilie, M.I.; Vielh, P.; Vignaud, J.M.; Flejou, J.F.; Lantuejoul, S.; Piaton, E.; Mourad, N.; Butori, C.; et al. Preoperative circulating tumour cell detection using the isolation by size of epithelial tumour cell method for patients with lung cancer is a new prognostic biomarker. Clin. Cancer Res. 2011, 17, 827–835. [Google Scholar] [CrossRef]
- Krebs, M.G.; Hou, J.M.; Sloane, R.; Lancashire, L.; Priest, L.; Nonaka, D.; Ward, T.H.; Backen, A.; Clack, G.; Hughes, A.; et al. Analysis of circulating tumour cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J. Thorac. Oncol. 2012, 7, 306–315. [Google Scholar] [CrossRef]
- Hou, J.M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical significance and molecular characteristics of circulating tumour cells and circulating tumour microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef]
- Riethdorf, S.; Fritsche, H.; Muller, V.; Rau, T.; Schindlbeck, C.; Rack, B.; Janni, W.; Coith, C.; Beck, K.; Janicke, F.; et al. Detection of circulating tumour cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch system. Clin. Cancer Res. 2007, 13, 920–928. [Google Scholar] [CrossRef]
- Coumans, F.A.; Doggen, C.J.; Attard, G.; de Bono, J.S.; Terstappen, L.W. All circulating EpCAM+CK+CD45− objects predict overall survival in castration-resistant prostate cancer. Ann. Oncol. 2010, 21, 1851–1857. [Google Scholar] [CrossRef]
- Gradilone, A.; Iacovelli, R.; Cortesi, E.; Raimondi, C.; Gianni, W.; Nicolazzo, C.; Petracca, A.; Palazzo, A.; Longo, F.; Frati, L.; et al. Circulating tumour cells and “suspicious objects” evaluated through CellSearch in metastatic renal cell carcinoma. Anticancer Res. 2011, 31, 4219–4221. [Google Scholar]
- Wendel, M.; Bazhenova, L.; Boshuizen, R.; Kolatkar, A.; Honnatti, M.; Cho, E.H.; Marrinucci, D.; Sandhu, A.; Perricone, A.; Thistlethwaite, P.; et al. Fluid biopsy for circulating tumour cell identification in patients with early-and late-stage non-small cell lung cancer: A glimpse into lung cancer biology. Phys. Biol. 2012, 9. [Google Scholar] [CrossRef]
- Mandrekar, S.J.; Qi, Y.; Hillman, S.L.; Ziegler, K.L.A.; Reuter, N.F.; Rowland, K.M.; Kuross, S.A.; Marks, R.S.; Schild, S.E.; Adjei, A.A. Endpoints in phase II trials for advanced non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 3–9. [Google Scholar] [CrossRef]
Appendix
| Factor | PFS | OS | ||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| Age (<65 vs. ≥65 years) | 1.36 (0.6–2.7) | 0.38 | 2 (0.93–4.4) | 0.07 |
| Sex (male vs. female) | 2.62 (0.8–8.7) | 0.11 | 3.2 (0.7–13.6) | 0.11 |
| ECOG PS (≤1 vs. 2) | 1.9 (0.94–3.9) | 0.07 | 2.4 (1.1–5.2) | 0.022 * |
| Histology (adeno. vs. squamous) | 0.7 (0.36–1.7) | 0.55 | 0.8 (0.34–1.9) | 0.62 |
| Stage (IIIB vs. IV) | 2.5 (0.7–8.3) | 0.13 | 4 (0.9–17.4) | 0.057 |
| N° mets. (≤1 vs. ≥2) | 1.4 (0.7–2.9) | 0.29 | 1.58 (0.7–3.3) | 0.22 |
| Bone mets. (no vs.yes) | 2.4 (1.1–5.3) | 0.038 * | 2.6 (1.1–6.1) | 0.026 * |
| Liver mets. (no vs. yes) | 1 (0.3–3.5) | 0.94 | 1.6 (0.47–5.8) | 0.40 |
| Pleural mets. (no vs. yes) | 0.5 (0.25–1.2) | 0.13 | 0.7 (0.36–1.6) | 0.51 |
| Supra-adrenal mets. (no vs. yes) | 1.8 (0.8–4.1) | 0.16 | 1.4 (0.6–1.4) | 0.42 |
| Baseline intact CTC (<5 vs. ≥5) | 2.4 (1–5.7) | 0.043 * | 3.1 (1.2–8.2) | 0.016 * |
| Baseline CK+ CD45+ (<2 vs. ≥2) | 0.6 (0.25–1.6) | 0.35 | 0.6 (0.3–1.5) | 0.31 |
| Baseline apoptotic CTC (<2 vs. ≥2) | 1.8 (1.2–5.5) | 0.025 * | 2.6 (1.2–8.3) | 0.009 * |
| Baseline CK+ fragments (<2 vs. ≥2) | 2 (1.3–4.4) | 0.036 * | 1.7 (0.8–3.9) | 0.15 |
| Baseline Blood cells (<17 vs. ≥17) | 0.8 (0.39–1.6) | 0.51 | 1.1 (0.5–2.4) | 0.78 |
| Baseline intact/apoptotic CTC (<2 vs. ≥2) | 1.9 (0.9–2.9) | 0.016 * | 2.3 (0.6–2.8) | 0.021 * |
| Baseline intact/apoptotic CTC+ CK+ fragments (<5 vs. ≥5) | 3.1 (1.5–6.8) | 0.003 * | 2.7 (1.1–6.6) | 0.025 * |
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Muinelo-Romay, L.; Vieito, M.; Abalo, A.; Nocelo, M.A.; Barón, F.; Anido, U.; Brozos, E.; Vázquez, F.; Aguín, S.; Abal, M.; et al. Evaluation of Circulating Tumor Cells and Related Events as Prognostic Factors and Surrogate Biomarkers in Advanced NSCLC Patients Receiving First-Line Systemic Treatment. Cancers 2014, 6, 153-165. https://doi.org/10.3390/cancers6010153
Muinelo-Romay L, Vieito M, Abalo A, Nocelo MA, Barón F, Anido U, Brozos E, Vázquez F, Aguín S, Abal M, et al. Evaluation of Circulating Tumor Cells and Related Events as Prognostic Factors and Surrogate Biomarkers in Advanced NSCLC Patients Receiving First-Line Systemic Treatment. Cancers. 2014; 6(1):153-165. https://doi.org/10.3390/cancers6010153
Chicago/Turabian StyleMuinelo-Romay, Laura, Maria Vieito, Alicia Abalo, Marta Alonso Nocelo, Francisco Barón, Urbano Anido, Elena Brozos, Francisca Vázquez, Santiago Aguín, Miguel Abal, and et al. 2014. "Evaluation of Circulating Tumor Cells and Related Events as Prognostic Factors and Surrogate Biomarkers in Advanced NSCLC Patients Receiving First-Line Systemic Treatment" Cancers 6, no. 1: 153-165. https://doi.org/10.3390/cancers6010153
APA StyleMuinelo-Romay, L., Vieito, M., Abalo, A., Nocelo, M. A., Barón, F., Anido, U., Brozos, E., Vázquez, F., Aguín, S., Abal, M., & López, R. L. (2014). Evaluation of Circulating Tumor Cells and Related Events as Prognostic Factors and Surrogate Biomarkers in Advanced NSCLC Patients Receiving First-Line Systemic Treatment. Cancers, 6(1), 153-165. https://doi.org/10.3390/cancers6010153




