Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update
Abstract
:1. Introduction
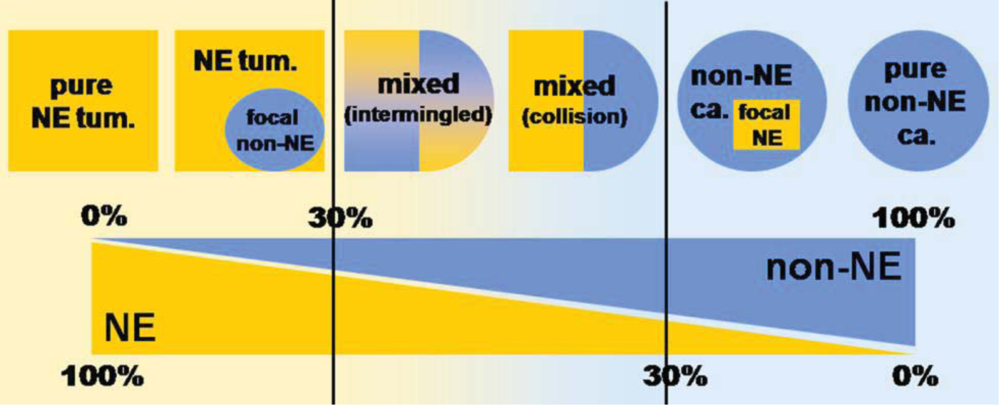
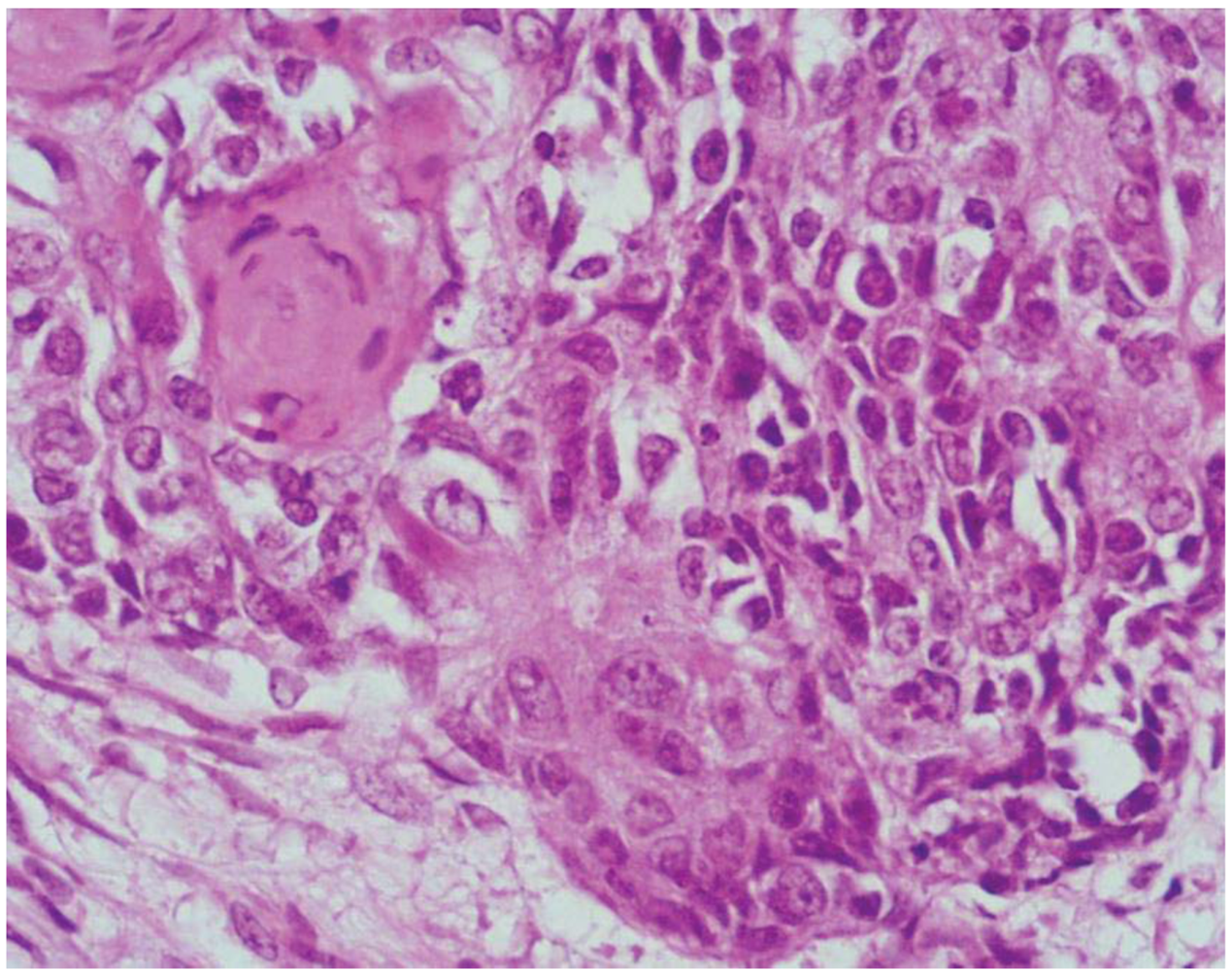
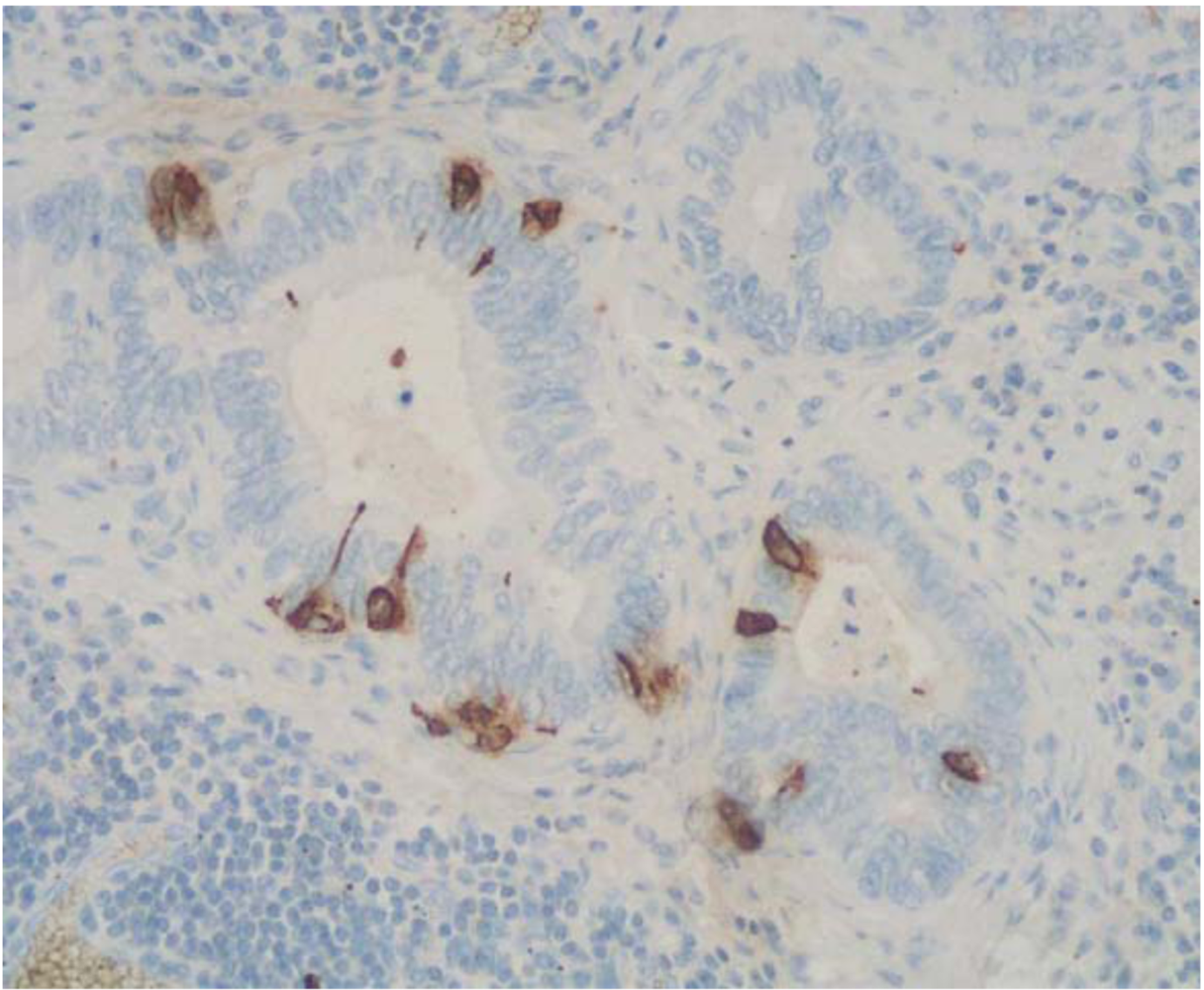
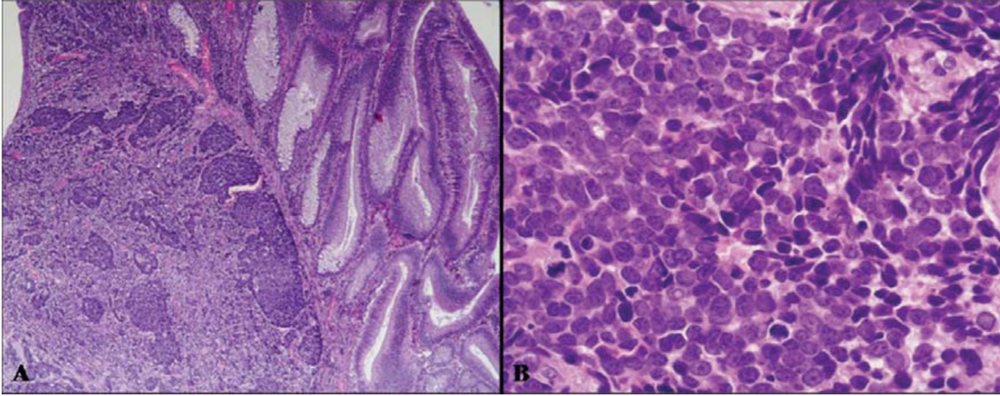
2. Mixed Adenoneuroendocrine Carcinomas (MANECs)
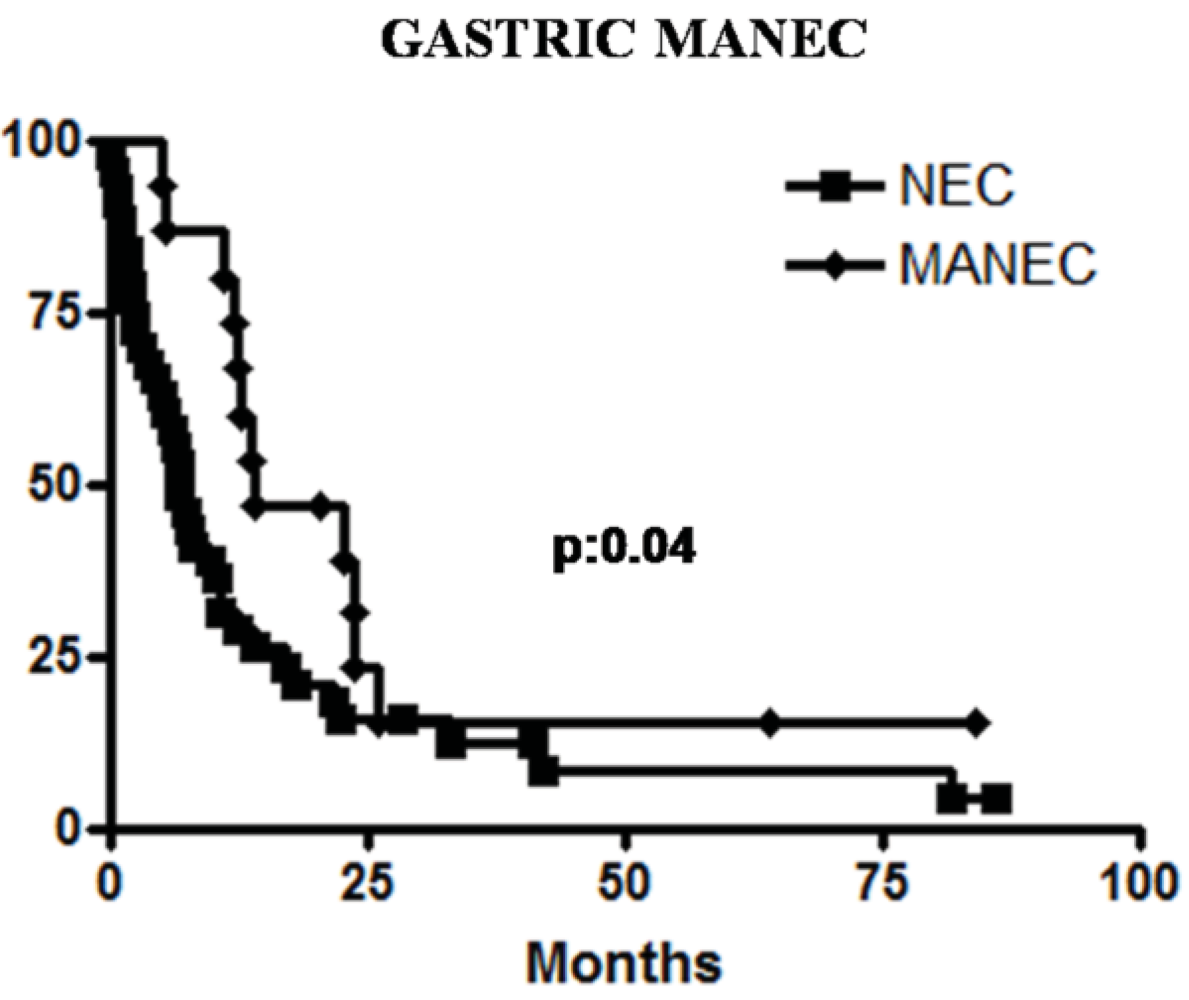
2.1. High Grade Malignant MANEC
| Mixed Adenoneuroendocrine Carcinoma (MANEC) |
| High grade malignant |
| Mixed adenoma/adenocarcinoma-NEC |
| Intermediate grade malignant |
| Mixed adenocarcinoma-G1/G2 NET * |
| Amphicrine carcinoma |
| Mixed Adenoneuroendocrine Tumor (MANET)—Provisional category |
| Low grade malignant |
| Adenoma-NET |
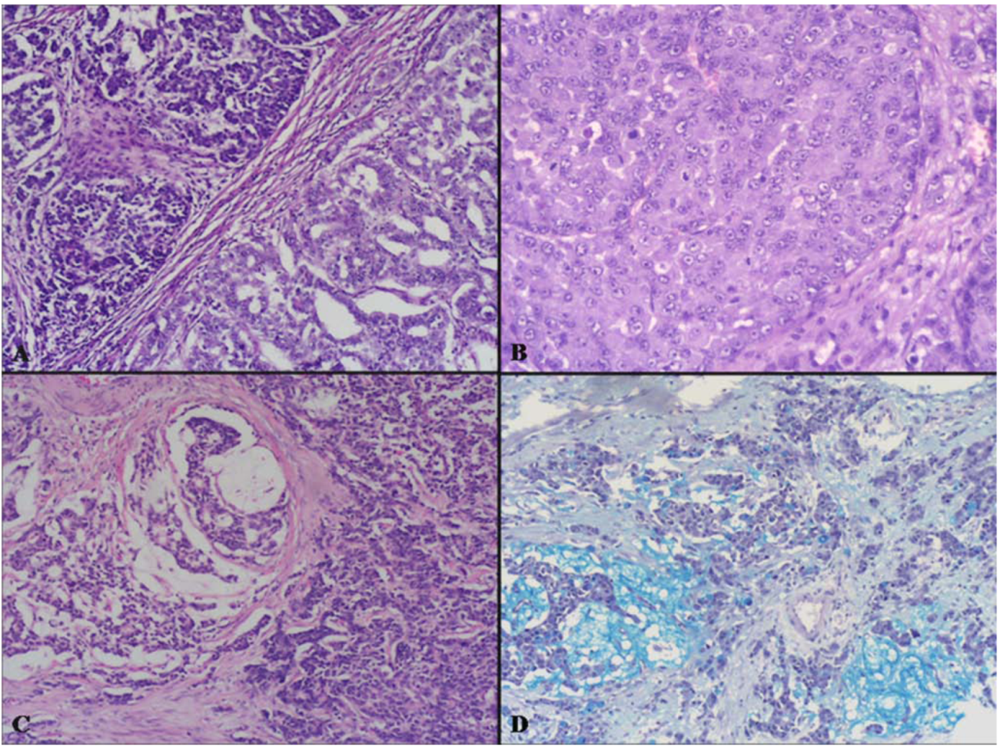
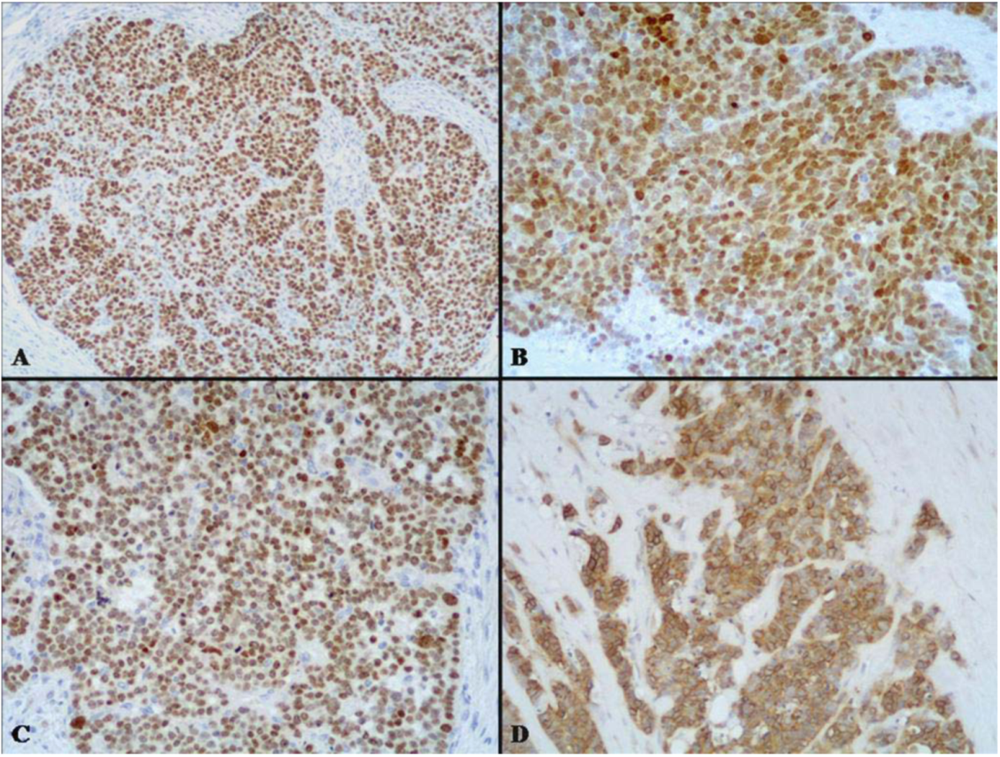
2.2. Intermediate Grade Malignant MANEC
2.2.1. Mixed Adenocarcinoma-Neuroendocrine Tumor (NET)
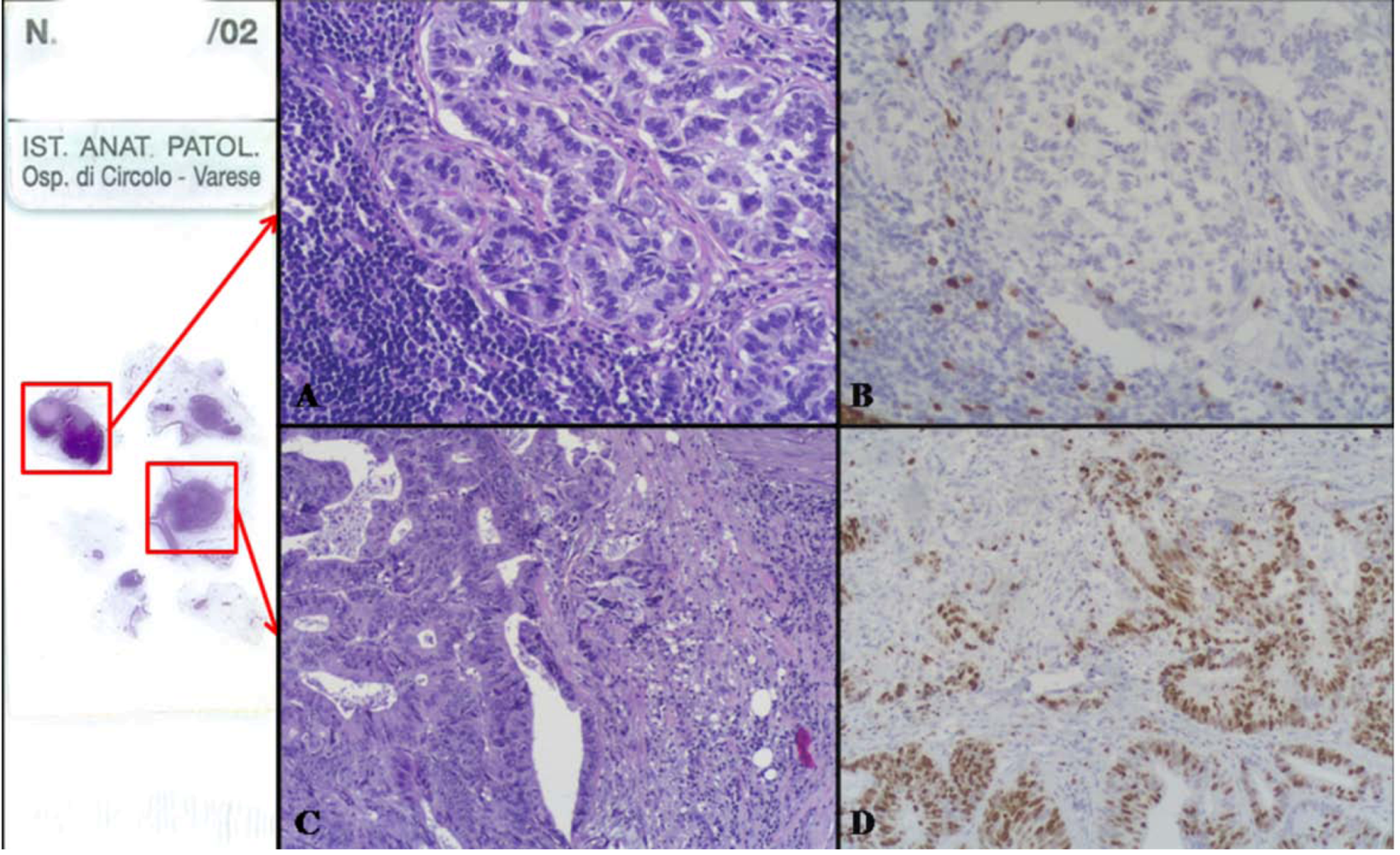
| Parameter | Caruso et al. [48] | Pasquinelli et al. [65] | Adhikari et al. [68] | Ronellenfitsch et al. [69] | Nugent et al. [70] |
|---|---|---|---|---|---|
| Age (year) | 53 | 79 | 52 | 53 | 64 |
| Sex | F | F | M | M | F |
| Site in the stomach | Body | Body | Body | Body | Body |
| Satellite NE micronodules | Present | Present | Present | Present | Present |
| Carcinoma type | Intestinal | Signet ring cell | Intestinal | Intestinal | Signet ring cell |
| Chronic atrophic gastritis | Present | Present | Present | Present | Present |
| Metastasis | Absent | Liver, Bone | Liver, LN | Absent | LN |
| Prognosis | Alive | DOD | Alive | Alive | Alive |
| Age (yeras) | Sex | Site | Size (cm) | Associated non acinar component | LN metastasis | Distant metastasis | Follow-up (months) | |
|---|---|---|---|---|---|---|---|---|
| Fukunaga et al. [72] | 77 | F | Fu | 1.2 | Solid, glandular | No | No | AFD (7) |
| Sun and Wasserman [73] | 86 | F | A | 5 | Solid, signet ring | No | No | nk |
| Jain et al. [71] | 41 | F | B | 1.5 | Solid, glandular | Present | No | AFD (24) |
| 61 | M | Fu | 3.3 | Solid, glandular | Present | Liver | Lost | |
| 72 | M | B | nk | Solid, glandular | No | No | DOC (4) | |
| Ambrosini-Spaltro et al. [74] | 52 | M | A | 4 | Trabecular | No | No | nk |
| Kusafuka et al. [75] | 59 | M | B | 6 | Solid | Present | Liver peritoneum | DOD (3) |
| Capella et al. [76] | 67 | F | A/B | 6 | Solid | Present | No | POD |
| 49 | M | B/Fu | 15 | Diffuse, glandular | Present | Liver | DOD (14) | |
| 55 | M | A | 8 | Diffuse, signet ring | Present | No | DOD (9) | |
| 91 | M | B | 3,7 | Glandular | Present | Liver | DOD (2) | |
| 58 | M | A | 5,5 | Diffuse, signet ring | No | No | NED (9) |
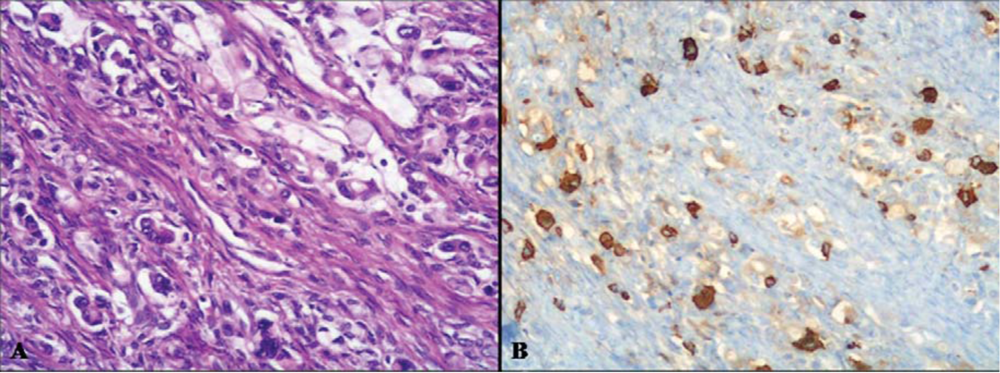
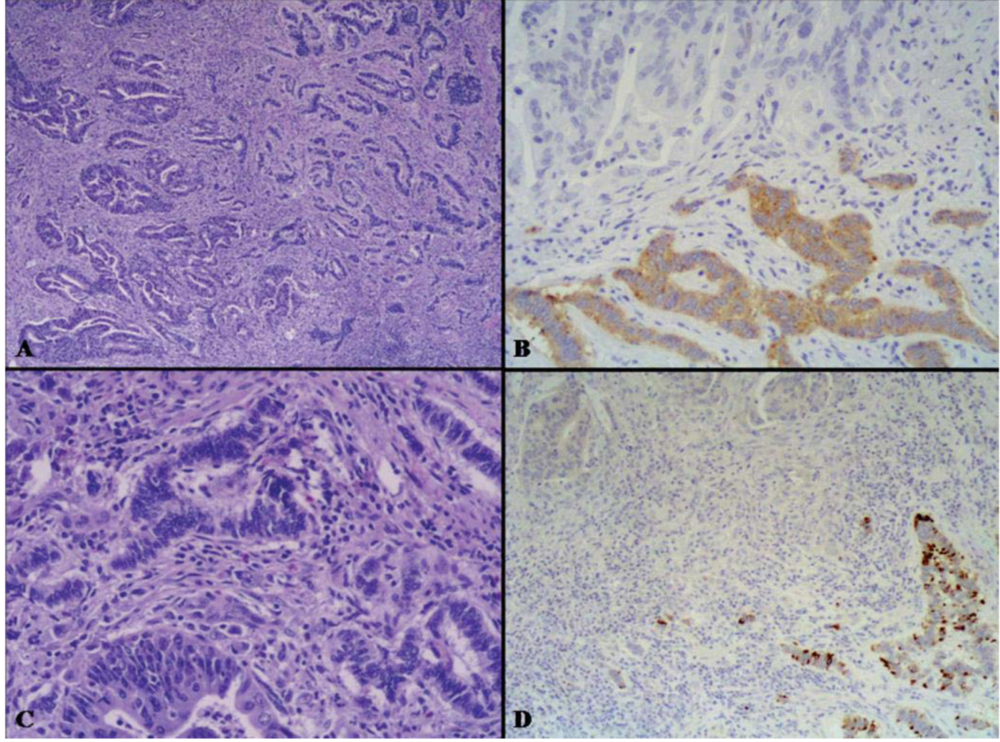
2.2.2. Amphicrine Carcinoma
3. Mixed Adenoneuroendocrine Tumors (MANETs)
3.1. Adenoma-Neuroendocrine Tumor (NET)
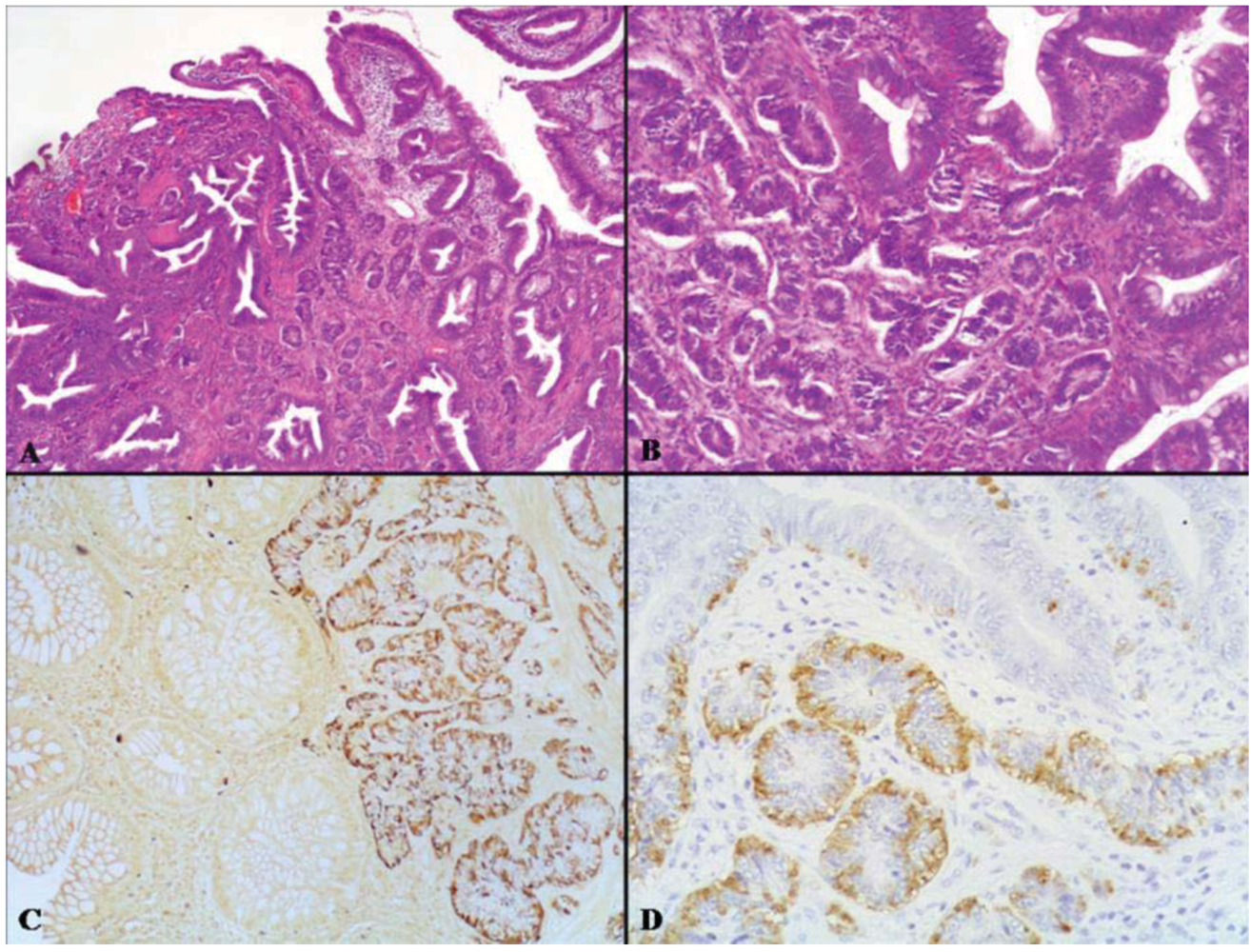
| Varghese et al [91] | Moyana et al. [9] | Lyda et al. [10] | |||
|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 1 | Case 2 | ||
| Age (year) | 76 | 68 | 75 | 37 | 80 |
| Sex | M | F | F | M | M |
| Site | Terminal ileum | Cecum | Rectum | Rectum | Ascending colon |
| Size (cm) | 4 | 1.5 | 2 | 1 | 3 |
| Pathology | TA-NET | VA-NET | Adenoma-NET | VA-NET | Adenoma-NET |
| Follow-up | Nk | Alive | Alive | Alive | DOC |
4. Pathogenesis and Molecular Findings
5. Management of MANEC
6. Conclusions
Conflict of interest
References
- Cordier, R. Les cellules argentaffines dans les tumeurs intestinales. Arch. Int. Med. Exp. 1924, 1, 5. [Google Scholar]
- Lewin, K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am. J. Surg. Pathol. 1987, 11, S71–S86. [Google Scholar] [CrossRef]
- Solcia, E.; Klöppel, G.; Sobin, L.H. Histological Typing of Endocrine Tumours (WHO International Histological Classification of Tumours), 2nd ed; Springer: Berlin, Germany, 2000. [Google Scholar]
- Rindi, G.; Arnold, R.; Bosman, F.T.; Capella, C.; Kilmstra, D.S.; Kloppel, G.; Komminoth, P.; Solcia, E. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In WHO Classification of Tumours of the Digestive System, 4th; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; IARC Press: Lyon, France, 2010; pp. 13–14. [Google Scholar]
- Capella, C.; La Rosa, S.; Uccella, S.; Billo, P.; Cornaggia, M. Mixed endocrine-exocrine tumors of the gastrointestinal tract. Semin. Diagn. Pathol. 2000, 17, 91–103. [Google Scholar]
- Volante, M.; Rindi, G.; Papotti, M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: A comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006, 449, 499–506. [Google Scholar] [CrossRef]
- Komminoth, P.; Arnold, R.; Capella, C.; Klimstra, D.S.; Klöppel, G.; Solcia, E.; Rindi, G. Neuroendocrine neoplasms of the appendix. In WHO Classification of Tumours of the Digestive System, 4th; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; IARC Press: Lyon, France, 2010; pp. 126–128. [Google Scholar]
- Carr, N.J.; Sobin, L.H. Adenocarcinoma of the appendix. In WHO Classification of Tumours of the Digestive System, 4th; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; IARC Press: Lyon, France, 2010; pp. 122–125. [Google Scholar]
- Moyana, T.N.; Qizilbash, A.H.; Murphy, F. Composite glandular-carcinoid tumors of the colon and rectum. Report of two cases. Am. J. Surg. Pathol. 1988, 12, 607–611. [Google Scholar] [CrossRef]
- Lyda, M.; Fenoglio-Preiser, C.M. Adenoma-carcinoid tumors of the colon. Arch. Pathol. Lab. Med. 1998, 122, 262–265. [Google Scholar]
- Briggs, J.C.; Ibrahim, N.B.N. Oat cell carcinoma of the oesophagus: A clinico-pathological study of 23 cases. Histopathology 1983, 7, 261–277. [Google Scholar] [CrossRef]
- Tanabe, T.; Nishimaki, T.; Kanda, T.; Nakagawa, S.; Ohashi, M.; Hatakeyama, K. Esophageal composite carcinoma with tripartite differentiation: Clinicopathological analysis of three cases. Esophagus 2005, 2, 91–96. [Google Scholar] [CrossRef]
- Yun, J.P.; Zhang, M.F.; Hou, J.H.; Tian, Q.H.; Fu, J.; Liang, X.M.; Wu, Q.L.; Rong, T.H. Primary small cell carcinoma of the esophagus: Clinicopathological and immunohistochemical features of 21 cases. BMC Cancer 2007, 7, 38–45. [Google Scholar]
- Maru, D.M.; Khurana, H.; Rashid, A.; Correa, A.M.; Anandasabapathy, S.; Krishnan, S.; Komaki, R.; Ajani, J.A.; Swisher, S.G.; Hofstetter, W.L. Retrospective study of clinicopathologic features and prognosis of high-grade neuroendocrine carcinoma of the esophagus. Am. J. Surg. Pathol. 2008, 32, 1404–1411. [Google Scholar] [CrossRef]
- Shia, J.; Tang, L.H.; Weiser, M.R.; Brenner, B.; Adsay, N.V.; Stelow, E.B.; Saltz, L.B.; Qin, J.; Landmann, R.; Leonard, G.D.; et al. Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? Am. J. Surg. Pathol. 2008, 32, 719–731. [Google Scholar] [CrossRef]
- Matsui, K.; Jin, X.M.; Kitagawa, M.; Miwa, A. Clinicopathologic features of neuroendocrine carcinomas of the stomach: Appraisal of small cell and large cell variants. Arch. Pathol. Lab. Med. 1998, 122, 1010–1017. [Google Scholar]
- Kim, K.M.; Kim, M.J.; Cho, B.K.; Choi, S.W.; Rhyu, M.G. Genetic evidence for the multi-step progression of mixed glandular-neuroendocrine gastric carcinomas. Virchows Arch. 2002, 440, 85–93. [Google Scholar] [CrossRef]
- Lee, E.J.; Park, S.M.; Maeng, L.; Lee, A.; Kim, K.M. Composite glandular-endocrine cell carcinomas of the stomach: Clinicopathologic and methylation study. APMIS 2005, 113, 569–576. [Google Scholar] [CrossRef]
- Rayhan, N.; Sano, T.; Qian, Z.R.; Obari, A.K.; Hirokawa, M. Histological and immunohistochemical study of composite neuroendocrine-exocrine carcinomas of the stomach. J. Med. Invest. 2005, 52, 191–202. [Google Scholar] [CrossRef]
- Li, A.F.; Li, A.C.; Hsu, C.Y.; Li, W.Y.; Hsu, H.S.; Chen, J.Y. Small cell carcinomas in gastrointestinal tract: Immunohistochemical and clinicopathological features. J. Clin. Pathol. 2010, 63, 620–625. [Google Scholar] [CrossRef]
- La Rosa, S.; Inzani, F.; Vanoli, A.; Klersy, C.; Dainese, L.; Rindi, G.; Capella, C.; Bordi, C.; Solcia, E. Histologic characterization and improved prognostic evaluation of 209 gastric neuroendocrine neoplasms. Hum. Pathol. 2011, 42, 1373–1384. [Google Scholar] [CrossRef]
- Nassar, H.; Albores-Saavedra, J.; Klimstra, D.S. High-grade neuroendocrine carcinoma of the ampulla of Vater: A clinicopathologic and immunohistochemical analysis of 14 cases. Am. J. Surg. Pathol. 2005, 29, 588–594. [Google Scholar] [CrossRef]
- Mills, S.E.; Allen, M.S., Jr.; Cohen, A.R. Small-cell undifferentiated carcinoma of the colon. Am. J. Surg. Pathol. 1983, 7, 643–651. [Google Scholar] [CrossRef]
- Gaffey, M.J.; Mills, S.E.; Lack, E.E. Neuroendocrine carcinoma of the colon and rectum. A clinicopathologic, ultrastructural and immunohistochemical study of 24 cases. Am. J. Surg. Pathol. 1990, 14, 1010–1023. [Google Scholar] [CrossRef]
- Sarsfield, P.; Anthony, P.P. Small cell undifferentiated (“neuroendocrine”) carcinoma. Histopathology 1990, 16, 357–363. [Google Scholar] [CrossRef]
- Vortmeyer, A.O.; Lubensky, I.A.; Merino, J.A.; Wang, C.Y.; Pham, T.; Furth, E.E.; Zhuang, Z. Concordance of genetic alterations in poorly differentiated colorectal neuroendocrine carcinomas and associated adenocarcinomas. J. Natl. Cancer Inst. 1997, 89, 1448–1453. [Google Scholar] [CrossRef]
- Makino, A.; Serra, S.; Chetty, R. Composite adenocarcinoma and large cell neuroendocrine carcinoma of the rectum. Virchows Arch. 2006, 448, 644–647. [Google Scholar] [CrossRef]
- Nair, M.S.; Fafemi, O.; Borgestein, R.; Rees, J. Composite carcinoma of the colon: A case report with literature review. Int. J. Oncol. 2008, 5, 2. [Google Scholar]
- Duffy, A.; Shia, J.; Klimstra, D.; Temple, L.; O'Reilly, E.M. Collision tumor of the large bowel in the context of advanced pregnancy and ulcerative colitis. Clin. Colorectal Cancer 2008, 7, 402–405. [Google Scholar] [CrossRef]
- Li, Y.; Yau, A.; Schaeffer, D.; Magliocco, A.; Gui, X.; Urbanski, S.; Waghray, R.; Owen, D.; Gao, Z.H. Colorectal glandular-neuroendocrine mixed tumors: Pathologic spectrum and clinical implications. Am. J. Surg. Pathol. 2011, 35, 413–425. [Google Scholar]
- La Rosa, S.; Marando, A.; Furlan, D.; Sahnane, N.; Capella, C. Colorectal Poorly Differentiated neuroendocrine carcinomas (NECs) and mixed adenoneuroendocrine carcinomas (MANECs): Insights into the diagnostic immunophenotype, assessment of methylation profile and search for prognostic markers. Am. J. Surg. Pathol. 2012, in press. [Google Scholar]
- Sterling, R.K. Ectopic ACTH syndrome associated with anorectal carcinoma. Report of a case and review of the literature. Dig. Dis. Sci. 1993, 38, 955–959. [Google Scholar] [CrossRef]
- Balachandra, B.; Marcus, V.; Jass, J.R. Poorly differentiated tumours of the anal canal: A diagnostic strategy for the surgical pathologist. Histopathology 2007, 50, 163–174. [Google Scholar] [CrossRef]
- Pantvaidya, G.; Pramesh, C.S.; Deshpande, M.S.; Jambhekar, N.A.; Sharma, S.; Deshpande, R.K. Small cell carcinoma of the esophagus: The Tata Memorial Hospital experience. Ann. Thorac. Surg. 2002, 74, 1924–1927. [Google Scholar] [CrossRef]
- Matsui, K.; Kitagawa, M.; Miwa, A.; Kuroda, Y.; Tsuji, M. Small cell carcinoma of the stomach: A clinicopathologic study of 17 cases. Am. J. Gastroenterol. 1991, 86, 1167–1175. [Google Scholar]
- Rindi, G.; Bordi, C.; Rappel, S.; La Rosa, S.; Stolte, M.; Solcia, E. Gastric carcinoids and neuroendocrine carcinomas: Pathogenesis, pathology, and behavior. World J. Surg. 1996, 20, 168–172. [Google Scholar] [CrossRef]
- Bernick, P.E.; Klimstra, D.S.; Shia, J.; Minsky, B.; Saltz, L.; Shi, W.; Thaler, H.; Guillem, J.; Paty, P.; Cohen, A.M.; et al. Neuroendocrine carcinomas of the colon and rectum. Dis. Colon Rectum 2004, 47, 163–169. [Google Scholar]
- Klimstra, D.S.; Modlin, I.R.; Adsay, N.V.; Chetty, R.; Deshpande, V.; Gönen, M.; Jensen, R.T.; Kidd, M.; Kulke, M.H.; Lloyd, R.V.; et al. Pathology reporting of neuroendocrine tumors: Application of the Delphic consensus process to the development of a minimum pathology data set. Am. J. Surg. Pathol. 2010, 34, 300–313. [Google Scholar] [CrossRef]
- Onishi, R.; Sano, T.; Nakamura, Y.; Namiuchi, S.; Sawada, S.; Ihara, C.; Shimatsu, A. Ectopic adrenocorticotropin syndrome associated with undifferentiated carcinoma of the colon showing multidirectional neuroendocrine, exocrine and squamous differentiation. Virchows Arch. 1996, 427, 537–541. [Google Scholar]
- Tanoue, S.; Shimoda, T.; Suzuki, M.; Ikegami, M.; Ishikawa, E.; Sano, T. Anaplastic carcinoma of the esophagus. Acta Pathol. Jpn. 1983, 33, 831–841. [Google Scholar]
- Watson, K.J.; Shulkes, A.; Smallwood, R.A.; Douglas, M.C.; Hurley, R.; Kalnins, R.; Moran, L. Watery diarrhea-hypokalemia-achlorydria syndrome and carcinoma of the esophagus. Gastroenterology 1988, 88, 798–803. [Google Scholar]
- La Rosa, S.; Chiaravalli, A.M.; Placidi, C.; Papanikolaou, N.; Cerati, M.; Capella, C. TTF1 expression in normal lung neuroendocrine cells and related tumors. Immunohistochemical study comparing two different monoclonal antibodies. Virchows Arch. 2010, 457, 497–507. [Google Scholar] [CrossRef]
- Rapa, I.; Ceppi, P.; Bollito, E.; Rosas, R.; Cappia, S.; Bacillo, E.; Porpiglia, F.; Berutti, A.; Papotti, M.; Volante, M. Human ASH1 expression in prostate cancer with neuroendocrine differentiation. Mod. Pathol. 2008, 21, 700–707. [Google Scholar] [CrossRef]
- Capella, C.; Hage, E.; Solcia, E.; Usellini, L. Ultrastructural similarity of endocrine-like cells of the human lung and some related cells of the gut. Cell Tissue Res. 1978, 186, 25–37. [Google Scholar]
- Damjanov, I.; Amenta, P.S.; Bosman, F.T. Undifferentiated carcinoma of the colon containing exocrine, neuroendocrine and squamous cells. Virchows Arch. 1983, 401, 57–66. [Google Scholar]
- Klöppel, G.; Rindi, G.; Anlauf, M.; Perren, A.; Komminoth, P. Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Arch. 2007, 451, S9–S27. [Google Scholar] [CrossRef]
- Chong, F.K.; Graham, J.H.; Madoff, I.M. Mucin producing carcinoid (“composite tumors”) of upper third of esophagus: A variant of carcinoid tumor. Cancer 1979, 44, 1853–1859. [Google Scholar] [CrossRef]
- Caruso, M.L.; Pilato, F.P.; D'Adda, T.; Baggi, M.T.; Fucci, L.; Valentini, A.M.; Lacatena, M.; Bordi, C. Composite carcinoid-adenocarcinoma of the stomach associated with multiple gastric carcinoids and nonantral gastric atrophy. Cancer 1989, 64, 1534–1539. [Google Scholar] [CrossRef]
- Shah, I.; Schlageter, M.O.; Bohem, N. Composite carcinoid-adenocarcinoma of ampulla of Vater. Hum. Pathol. 1990, 21, 1188–1190. [Google Scholar] [CrossRef]
- Bates, H.R., Jr.; Belter, L.F. Composite carcinoid tumor (argentaffinoma-adenocarcinoma) of the colon: Report of 2 cases. Dis. Colon Rectum 1967, 10, 467–470. [Google Scholar]
- Auber, F.; Gambiez, L.; Desreumaux, P.; Mudry, J.; Lecomte-Houcke, M.; Cortot, A.; Quandalle, P.; Colombel, J.F. Mixed adenocarcinoid tumor and Crohn’s disease. J. Clin. Gastroenterol. 1998, 26, 353–354. [Google Scholar]
- Cary, N.R.; Barron, D.J.; McGoldrick, J.P.; Wells, F.C. Combined oesophageal adenocarcinoma and carcinoid in Barrett’ oesophagitis: Potential role of enterochromaffin-like cells in oesophageal malignancy. Thorax 1993, 48, 404–405. [Google Scholar] [CrossRef]
- Tahara, E.; Ito, H.; Nakagami, K.; Shimamoto, F.; Yamamoto, M.; Sumii, K. Scirrhous argyrophil cell carcinoma of the stomach with multiple production of polypeptide hormones, amine, CEA, lysozyme and HCG. Cancer 1982, 49, 1904–1915. [Google Scholar] [CrossRef]
- Yang, G.C.; Rotterdam, H. Mixed (composite) glandular-endocrine cell carcinoma of the stomach. Report of a case and review of the literature. Am. J. Surg. Pathol. 1991, 15, 592–598. [Google Scholar] [CrossRef]
- Fujiyoshi, Y.; Kuhara, H.; Eimoto, T. Composite glandular-endocrine cell carcinoma of the stomach. Report of two cases with goblet cell carcinoid component. Pathol. Res. Pract. 2005, 200, 823–829. [Google Scholar] [CrossRef]
- Musialik, J.A.; Kohut, M.J.; Marek, T.; Wodołazski, A.; Hartleb, M. Composite neuroendocrine and adenomatous carcinoma of the papilla of Vater. World J. Gastroenterol. 2009, 15, 4199–4200. [Google Scholar] [CrossRef]
- Klappenbach, R.S.; Kurman, R.J.; Sinclair, C.F.; James, L.P. Composite carcinoma-carcinoid tumors of the gastrointestinal tract. A morphologic, histochemical and immunocytochemical study. Am. J. Clin. Pathol. 1985, 84, 137–143. [Google Scholar]
- Hernandez, F.J.; Fernandez, B.B. Mucus-secreting colonic carcinoid tumors. Light and electron-microscopic study of three cases. Dis. Colon Rectum 1974, 17, 387–396. [Google Scholar] [CrossRef]
- Peonim, V.; Thakerngpol, K.; Pacharee, P.; Stitnimankarn, T. Adenosquamous carcinoma and carcinoidal differentiation of the colon. Report of a case. Cancer 1983, 52, 1122–1125. [Google Scholar] [CrossRef]
- Hock, Y.L.; Scott, K.W.; Grace, R.H. Mixed adenocarcinoma/carcinoid tumour of large bowel in a patient with Crohn’s disease. J. Clin. Pathol. 1993, 46, 183–185. [Google Scholar] [CrossRef]
- Lew, A.; Lewin, K.J.; Zarchy, T.; Pisegna, J.R. Adenocarcinoma of the colon with neuroendocrine features and secretory diarrhea. Am. J. Gastroenterol. 1999, 94, 1690–1694. [Google Scholar]
- Soga, J.; Tazawa, K.; Aizawa, O.; Wada, K.; Tuto, T. Argentaffin cell adenocarcinoma of the stomach: An atypical carcinoid? Cancer 1971, 28, 991–1003. [Google Scholar]
- Yamashina, M.; Flinner, R.A. Concurrent occurrence of adenocarcinoma and carcinoid tumor in the stomach: A composite tumor or collision tumors? Am. J. Clin. Pathol. 1985, 83, 233–236. [Google Scholar]
- Murayama, H.; Imai, T.; Kikuchi, M. Solid carcinomas of the stomach. A combined histochemical, light and electron microscopic study. Cancer, 1983; 51, 1673–1681. [Google Scholar]
- Pasquinelli, G.; Santini, D.; Preda, P.; Cariani, G.; Bonora, G.; Martinelli, G.N. Composite gastric carcinoma and precursor lesions with amphicrine features in chronic gastritis. Ultrastruct. Pathol. 1993, 17, 9–24. [Google Scholar] [CrossRef]
- Jiao, Y.F.; Nakamura, S.; Arai, T.; Sugai, T.; Uesugi, N.; Habano, W.; Suzuki, M.; Tazawa, H.; Goukon, Y. Adenoma, adenocarcinoma and mixed carcinoid-adenocarcinoma arising in a small lesion of the colon. Pathol. Int. 2003, 53, 457–462. [Google Scholar] [CrossRef]
- Ulich, T.R.; Kollin, M.; Lewin, K.J. Composite gastric carcinoma. Report of a tumor of the carcinoma-carcinoid spectrum. Arch. Pathol. Lab. Med. 1988, 112, 91–93. [Google Scholar]
- Adhikari, D.; Conte, C.; Eskreis, D.; Urmacher, C.; Ellen, K. Combined adenocarcinoma and carcinoid tumor in atrophic gastritis. Ann. Clin. Lab. Sci. 2002, 32, 422–427. [Google Scholar]
- Ronellenfitsch, U.; Ströbel, P.; Schwarzbach, M.H.; Staiger, W.I.; Gragert, D.; Kähler, G. A composite adenoendocrine carcinoma of the stomach arising from a neuroendocrine tumor. J. Gastrointest. Surg. 2007, 11, 1573–1575. [Google Scholar] [CrossRef]
- Nugent, S.L.; Cunningham, S.C.; Alexiev, B.A.; Bellavance, E.; Papadimitriou, J.C.; Hanna, N. Composite signet-ring cell/neuroendocrine carcinoma of the stomach with a metastatic neuroendocrine carcinoma component: A better prognosis entity. Diagn. Pathol. 2007, 2, 43–50. [Google Scholar] [CrossRef]
- Jain, D.; Eslami-Varzaneh, F.; Takano, A.M.; Ayer, U.; Umashankar, R.; Muller, R.; Klimstra, D.S. Composite glandular and endocrine tumors of the stomach with pancreatic acinar differentiation. Am. J. Surg. Pathol. 2005, 29, 1524–1529. [Google Scholar] [CrossRef]
- Fukunaga, M. Gastric carcinoma resembling pancreatic mixed acinar-endocrine carcinoma. Hum. Pathol. 2002, 33, 569–573. [Google Scholar] [CrossRef]
- Sun, Y.; Wasserman, P.G. Acinar cell carcinoma in the stomach: A case report with literature review. Hum. Pathol. 2004, 35, 263–265. [Google Scholar] [CrossRef]
- Ambrosini-Spaltro, A.; Poti, O.; de Palma, M.; Filotico, M. Pancreatic-type acinar cell carcinoma of the stomach beneath a focus of pancreatic metaplasia of the gastric mucosa. Hum. Pathol. 2009, 40, 746–749. [Google Scholar] [CrossRef]
- Kusafuka, K.; Bando, E.; Muramatsu, K.; Ito, H.; Tanizawa, Y.; Kawamura, T.; Mochizuki, T.; Terashima, M.; Nakajima, T. Pancreatic-type mixed acinar-endocrine carcinoma with alpha-fetoprotein production arising from the stomach: A report of an extremely rare case. Med. Mol. Morphol. 2009, 42, 167–174. [Google Scholar] [CrossRef]
- Capella, C.; La Rosa, S.; Chiaravalli, A.M. Department of Surgical and Morphological Sciences, Varese, Italy. Unpublished work 2012.
- Moncur, J.T.; Lacy, B.E.; Longnecker, D.S. Mixed acinar-endocrine carcinoma arising in the ampulla of Vater. Hum. Pathol. 2002, 33, 449–451. [Google Scholar] [CrossRef]
- Knight, B.K.; Hayes, M.M. Mixed adenocarcinoma and carcinoid tumour of the colon. A report of 4 cases with postulates on histogenesis. S. Afr. Med. J. 1987, 72, 708–710. [Google Scholar]
- Tahara, E.; Ito, H.; Nakagami, K.; Shimamoto, F.; Yamamoto, M.; Sumii, K. Scirrhous argyrophil cell carcinoma of the stomach with multiple production of polypeptide hormones, amine, CEA, lysozyme and HCG. Cancer 1982, 49, 1904–1915. [Google Scholar] [CrossRef]
- Papotti, M.; Galliano, D.; Monga, G. Signet-ring cell carcinoid of the gallbladder. Histopathology 1990, 17, 255–259. [Google Scholar] [CrossRef]
- Tang, L.H.; Shia, J.; Soslow, R.A.; Dhall, D.; Wong, W.D.; O'Reilly, E.; Paty, P.; Weiser, M.R.; Guillem, J.; Temple, L.; et al. Pathologic classification and clinical behavior of the spectrum of goblet cell carcinoid tumors of the appendix. Am. J. Surg. Pathol. 2008, 32, 1429–1443. [Google Scholar] [CrossRef]
- Burke, A.; Lee, Y.K. Adenocarcinoid (goblet cell carcinoid) of the duodenum presenting as gastric outlet obstruction. Hum. Pathol. 1990, 21, 238–239. [Google Scholar] [CrossRef]
- Jones, M.A.; Griffith, L.M.; West, A.B. Adenocarcinoid tumor of the periampullary region: A novel duodenal neoplasm presenting as biliary tract obstruction. Hum. Pathol. 1989, 20, 198–200. [Google Scholar] [CrossRef]
- Burke, A.P.; Sobin, L.H.; Federspiel, B.H.; Shekitka, K.M.; Helwig, E.B. Goblet cell carcinoids and related tumors of the vermiform appendix. Am. J. Clin. Pathol. 1990, 94, 27–35. [Google Scholar]
- Lyss, A.P.; Thompson, J.J.; Gluck, J.H. Adenocarcinoid tumor of the colon arising in preexisting ulcerative colitis. Cancer 1981, 48, 833–839. [Google Scholar] [CrossRef]
- Feyrter, F. Uber Diffuse Endokrine Epitheliale Organe (in German); Johann Ambrosius Barth: Leipzig, Germany, 1938. [Google Scholar]
- Ratzenhofer, M.; Leb, D. Uber die feinstruktur der argentaffinen und der anderen escheinungsformen der “Hellen Zellen” Feyrter’s im kaninchen magen. Z. Zellforsch 1965, 67, 113–150. [Google Scholar] [CrossRef]
- Ratzenhofer, M.; Aubock, I.; Becker, H. Elektronen und fluoreszenzmikroskopische untersuchungen der appendicite neurogene. Verh. Dtsch. Ges. Pathol. 1969, 53, 218–224. [Google Scholar]
- Chejfec, G.; Capella, C.; Solcia, E.; Jao, W.; Gould, V.E. Amphicrine cells, dysplasias, and neoplasia. Cancer 1985, 56, 2683–2690. [Google Scholar] [CrossRef]
- Reis-Filho, J.S.; Schmitt, F.C. Amphicrine gastric carcinoma. Arch. Pathol. Lab. Med. 2001, 125, 1513–1514. [Google Scholar]
- Varghese, N.M.; Zaitoun, A.M.; Thomas, S.M.; Senapati, A.; Theodossi, A. Composite glandular-carcinoid tumour of the terminal ileum. J. Clin. Pathol. 1994, 47, 427–429. [Google Scholar] [CrossRef]
- Cheng, H.; Leblond, C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. Unitarian theory of the origin of the four epithelial cell types. Am. J. Anat. 1974, 141, 537–561. [Google Scholar] [CrossRef]
- Furlan, D.; Cerutti, R.; Uccella, S.; La Rosa, S.; Rigoli, E.; Genasetti, A.; Capella, C. Different molecular profiles characterize well-differentiated endocrine tumors and poorly differentiated endocrine carcinomas of the gastroenteropancreatic tract. Clin. Cancer Res. 2004, 10, 947–57. [Google Scholar]
- Hervieu, V.; Scoazec, J.Y. Mixed endocrine tumors. Ann. Pathol. 2005, 25, 511–528. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
La Rosa, S.; Marando, A.; Sessa, F.; Capella, C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers 2012, 4, 11-30. https://doi.org/10.3390/cancers4010011
La Rosa S, Marando A, Sessa F, Capella C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers. 2012; 4(1):11-30. https://doi.org/10.3390/cancers4010011
Chicago/Turabian StyleLa Rosa, Stefano, Alessandro Marando, Fausto Sessa, and Carlo Capella. 2012. "Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update" Cancers 4, no. 1: 11-30. https://doi.org/10.3390/cancers4010011
APA StyleLa Rosa, S., Marando, A., Sessa, F., & Capella, C. (2012). Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers, 4(1), 11-30. https://doi.org/10.3390/cancers4010011




