Definition of Microscopic Tumor Clearance (R0) in Pancreatic Cancer Resections
Abstract
:1. Introduction
| Study | Year | Study period | Number of patients | R1/R2 rates |
|---|---|---|---|---|
| Willet et al. [8] | 1993 | 1978–1991 | 72 | 51% |
| Yeo et al. [43] | 1997 | 1990–1996 | 282 | 29% |
| Richter et al. [6] | 2003 | 1972–1998 | 194 | 37% |
| Wagner et al. [7] | 2004 | 1993–2001 | 165 | 23.6% |
| Cameron et al. [44] | 2006 | 1969–2003 | 405 | 36% |
| Kuhlmann et al. [45] | 2006 | 1992–2001 | 160 | 50% |
| Verbeke et al. [17] | 2006 | 1995–2003 | 26 | 85% |
| Winter et al. [46] | 2006 | 1970–2006 | 1175 | 42% |
| Raut et al. [9] | 2007 | 1990–2004 | 360 | 17% |
| Esposito et al. [16] | 2008 | 2005–2006 | 111 | 76% |
| Campbell et al. [18] | 2009 | 1997–2007 | 163 | 79% |
| Jamieson et al. [4] | 2010 | 1996–2007 | 148 | 74% |
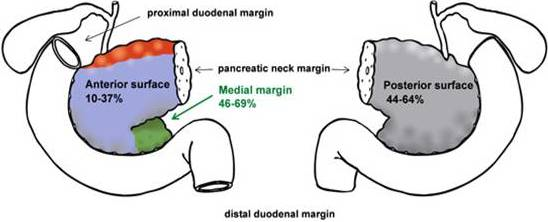
2. Definition of the Resection Margins
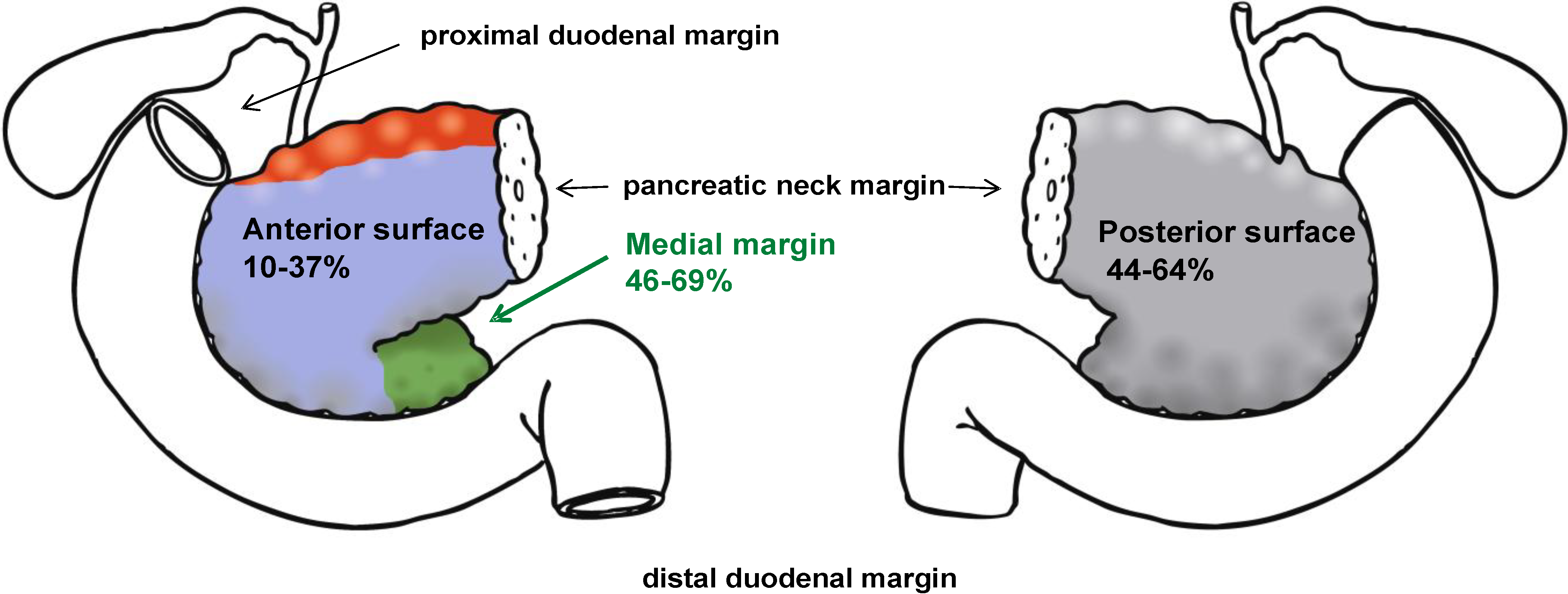
| Parameter | Esposito et al. [16] | Verbeke et al. [17] | Jamieson et al. [4] | Campbell et al. [18] |
|---|---|---|---|---|
| Protocol | RCPath guidelines | RCPath guidelines | RCPath guidelines | RCPath guidelines |
| Cases | 111 | 26 | 148 | 163 |
| Study period | 2005-2006 | 1995-2003 | 1996-2007 | 1997-2007 |
| Margin definition | </= 1 mm | </= 1 mm | </= 1 mm | </= 1 mm |
| R-classification | ||||
| R0 | 24% | 15% | 26% | 21% |
| R1 (1 mm rule) | 76% | 85% | 74% | 79% |
| R1 (0 mm rule) | No data | No data | 55% | 45% |
| Margin involvement | ||||
| Posterior | 47% | 64% | 44% | 54% |
| Medial | 69% | 55% | 46% | 50% |
| Anterior surface | 10% | 18% | 37% | * |
| Pancreatic duct | 4% | 9% | ||
| Bile duct | 5% | 0 | 3% | 3% |
| Stomach/Duodenum | 4% | 0 | 2% | 5% |
| Transection | 30% | |||
| Only one single margin involved | 68% | 55% | 58% | 65% |
| Two or more margins involved (multifocal) | 32% | 45% | 42% | 35% |
Standardization of Pathological Investigation
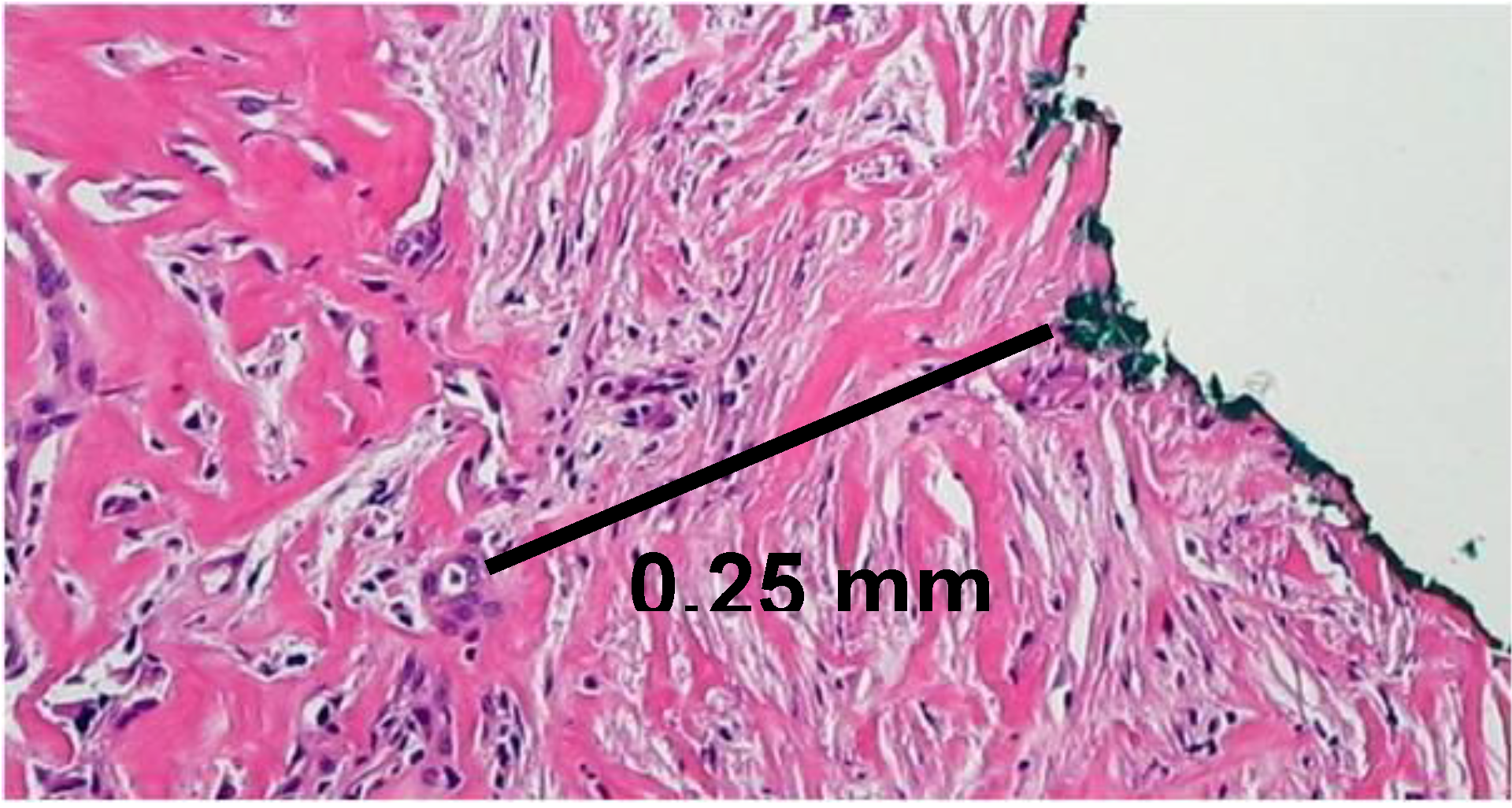
3. Margin Clearance
| Study | Year | R1 RCPath (1 mm rule) | R1 UICC (0 mm rule) | Ratio RCPath/ UICC |
|---|---|---|---|---|
| Jamieson et al. [4] | 2009 | 74% | 55% | 1.4 |
| Campbell et al. [18] | 2009 | 79% | 45% | 1.8 |
| Gaedcke et al. [22] | 2009 | 82.6% (R1/R2) | 63% (R1/R2) | 1.3 |
4. Conclusions and Future Perspectives
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Bentrem, D.J.; Ko, C.Y.; Stewart, A.K.; Winchester, D.P.; Talamonti, M.S. National failure to operate on early stage pancreatic cancer. Ann. Surg. 2007, 246, 173–180. [Google Scholar] [CrossRef]
- Jamieson, N.B.; Foulis, A.K.; Oien, K.A.; Going, J.J.; Glen, P.; Dickson, E.J.; Imrie, C.W.; McKay, C.J.; Carter, R. Positive mobilization margins alone do not influence survival following pancreatico-duodenectomy for pancreatic ductal adenocarcinoma. Ann. Surg. 2010, 251, 1003–1010. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Stocken, D.D.; Dunn, J.A.; Almond, J.; Beger, H.G.; Pederzoli, P.; Bassi, C.; Dervenis, C.; Fernandez-Cruz, L.; Lacaine, F.; Buckels, J.; Deakin, M.; Adab, F.A.; Sutton, R.; Imrie, C.; Ihse, I.; Tihanyi, T.; Olah, A.; Pedrazzoli, S.; Spooner, D.; Kerr, D.J.; Friess, H.; Büchler, M.W.; European Study Group for Pancreatic Cancer. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann. Surg. 2001, 234, 758–768. [Google Scholar] [CrossRef]
- Richter, A.; Niedergethmann, M.; Sturm, J.W.; Lorenz, D.; Post, S.; Trede, M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J. Surg. 2003, 27, 324–329. [Google Scholar] [CrossRef]
- Wagner, M.; Redaelli, C.; Lietz, M.; Seiler, C.A.; Friess, H.; Büchler, M.W. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br. J. Surg. 2004, 91, 586–594. [Google Scholar] [CrossRef]
- Willett, C.G.; Lewandrowski, K.; Warshaw, A.L.; Efird, J.; Compton, C.C. Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Ann. Surg. 1993, 217, 144–148. [Google Scholar] [CrossRef]
- Raut, C.P.; Tseng, J.F.; Sun, C.C.; Wang, H.; Wolff, R.A.; Crane, C.H.; Hwang, R.; Vauthey, J.N.; Abdalla, E.K.; Lee, J.E.; Pisters, P.W.; Evans, D.B. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann. Surg. 2007, 246, 52–60. [Google Scholar] [CrossRef]
- Van den Broeck, A.; Sergeant, G.; Ectors, N.; Van Steenbergen, W.; Aerts, R.; Topal, B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur. J. Surg. Oncol. 2009, 35, 600–604. [Google Scholar] [CrossRef]
- Smeenk, H.G.; Tran, T.C.; Erdmann, J.; van Eijck, C.H.; Jeekel, J. Survival after surgical management of pancreatic adenocarcinoma: Does curative and radical surgery truly exist? Langenbecks Arch. Surg. 2005, 390, 94–103. [Google Scholar] [CrossRef]
- Kleeff, J.; Reiser, C.; Hinz, U.; Bachmann, J.; Debus, J.; Jaeger, D.; Friess, H.; Büchler, M.W. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann. Surg. 2007, 245, 566–572. [Google Scholar] [CrossRef]
- Verbeke, C.S. Resection margins and R1 rates in pancreatic cancer--are we there yet? Histopathology. 2008, 52, 787–796. [Google Scholar] [CrossRef]
- Nagakawa, T.; Nagamori, M.; Futakami, F.; Tsukioka, Y.; Kayahara, M.; Ohta, T.; Ueno, K.; Miyazaki, I. Results of extensive surgery for pancreatic carcinoma. Cancer 1996, 77, 640–645. [Google Scholar]
- Tsuchiya, R.; Noda, T.; Harada, N.; Miyamoto, T.; Tomioka, T.; Yamamoto, K.; Yamaguchi, T.; Izawa, K.; Tsunoda, T.; Yoshino, R.; et al. Collective review of small carcinomas of the pancreas. Ann. Surg. 1986, 203, 77–81. [Google Scholar] [CrossRef]
- Esposito, I.; Kleeff, J.; Bergmann, F.; Reiser, C.; Herpel, E.; Friess, H.; Schirmacher, P.; Büchler, M.W. Most pancreatic cancer resections are R1 resections. Ann. Surg. Oncol. 2008, 15, 1651–1660. [Google Scholar] [CrossRef]
- Verbeke, C.S.; Leitch, D.; Menon, K.V.; McMahon, M.J.; Guillou, P.J.; Anthoney, A. Redefining the R1 resection in pancreatic cancer. Br. J. Surg. 2006, 93, 1232–1237. [Google Scholar] [CrossRef]
- Campbell, F.; Smith, R.A.; Whelan, P.; Sutton, R.; Raraty, M.; Neoptolemos, J.P.; Ghaneh, P. Classification of R1 resections for pancreatic cancer: The prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology 2009, 55, 277–283. [Google Scholar] [CrossRef]
- Liszka, Ł.; Pajak, J.; Zielińska-Pajak, E.; Gołka, D.; Mrowiec, S.; Lampe, P. Different approaches to assessment of lymph nodes and surgical margin status in patients with ductal adenocarcinoma of the pancreas treated with pancreaticoduodenectomy. Pathology 2010, 42, 138–146. [Google Scholar] [CrossRef]
- Sobin, L.H.; Gaspodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumors, 7th ed.; Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Hruban, R.H.; Pitman, M.B.; Klimstra, D. Tumors of the pancreas; Armed Forces Institute of Pathology: Washington, DC, USA, 2007. [Google Scholar]
- Gaedcke, J.; Gunawan, B.; Grade, M.; Szöke, R.; Liersch, T.; Becker, H.; Ghadimi, B.M. The mesopancreas is the primary site for R1 resection in pancreatic head cancer: Relevance for clinical trials. Langenbecks Arch. Surg. 2010, 395, 451–458. [Google Scholar] [CrossRef]
- Kim, J.; Reber, H.A.; Dry, S.M.; Elashoff, D.; Chen, S.L.; Umetani, N.; Kitago, M.; Hines, O.J.; Kazanjian, K.K.; Hiramatsu, S.; Bilchik, A.J.; Yong, S.; Shoup, M.; Hoon, D.S. Unfavourable prognosis associated with K-ras gene mutation in pancreatic cancer surgical margins. Gut 2006, 55, 1598–1605. [Google Scholar] [CrossRef]
- Chang, D.K.; Johns, A.L.; Merrett, N.D.; Gill, A.J.; Colvin, E.K.; Scarlett, C.J.; Nguyen, N.Q.; Leong, R.W.; Cosman, P.H.; Kelly, M.I.; Sutherland, R.L.; Henshall, S.M.; Kench, J.G.; Biankin, A.V. Margin clearance and outcome in resected pancreatic cancer. J. Clin. Oncol. 2009, 27, 2855–2862. [Google Scholar] [CrossRef]
- Wittekind, C.; Compton, C.; Quirke, P.; Nagtegaal, I.; Merkel, S.; Hermanek, P.; Sobin, L.H. A uniform residual tumor (R) classification: Integration of the R classification and the circumferential margin status. Cancer 2009, 115, 3483–3488. [Google Scholar] [CrossRef]
- Pultrum, B.B.; Honing, J.; Smit, J.K.; van Dullemen, H.M.; van Dam, G.M.; Groen, H.; Hollema, H.; Plukker, J.T. A critical appraisal of circumferential resection margins in esophageal carcinoma. Ann. Surg. Oncol. 2010, 17, 812–820. [Google Scholar] [CrossRef]
- Dexter, S.P.; Sue-Ling, H.; McMahon, M.J.; Quirke, P.; Mapstone, N.; Martin, I.G. Circumferential resection margin involvement: An independent predictor of survival following surgery for oesophageal cancer. Gut 2001, 48, 667–670. [Google Scholar] [CrossRef]
- Sagar, P.M.; Johnston, D.; McMahon, M.J.; Dixon, M.F.; Quirke, P. Significance of circumferential resection margin involvement after oesophagectomy for cancer. Br. J. Surg. 1993, 80, 1386–1388. [Google Scholar] [CrossRef]
- Shaib, Y.; El-Serag, H.B. The epidemiology of cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 115–125. [Google Scholar] [CrossRef]
- Hayashi, S.; Miyazaki, M.; Kondo, Y.; Nakajima, N. Invasive growth patterns of hepatic hilar ductal carcinoma. A histologic analysis of 18 surgical cases. Cancer 1994, 73, 2922–2929. [Google Scholar] [CrossRef]
- Shimada, H.; Niimoto, S.; Matsuba, A.; Nakagawara, G.; Kobayashi, M.; Tsuchiya, S. The infiltration of bile duct carcinoma along the bile duct wall. Int. Surg. 1988, 73, 87–90. [Google Scholar]
- Sakamoto, E.; Nimura, Y.; Hayakawa, N.; Kamiya, J.; Kondo, S.; Nagino, M.; Kanai, M.; Miyachi, M.; Uesaka, K. The pattern of infiltration at the proximal border of hilar bile duct carcinoma: A histologic analysis of 62 resected cases. Ann. Surg. 1998, 227, 405–411. [Google Scholar] [CrossRef]
- Aljiffry, M.; Walsh, M.J.; Molinari, M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990-2009. World J. Gastroenterol. 2009, 15, 4240–4262. [Google Scholar] [CrossRef]
- Seyama, Y.; Kubota, K.; Sano, K.; Noie, T.; Takayama, T.; Kosuge, T.; Makuuchi, M. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann. Surg. 2003, 238, 73–83. [Google Scholar]
- Otani, K.; Chijiiwa, K.; Kai, M.; Ohuchida, J.; Nagano, M.; Tsuchiya, K.; Kondo, K. Outcome of surgical treatment of hilar cholangiocarcinoma. J. Gastrointest. Surg. 2008, 12, 1033–1040. [Google Scholar] [CrossRef]
- Kloek, J.J.; Ten Kate, F.J.; Busch, O.R.; Gouma, D.J.; van Gulik, T.M. Surgery for extrahepatic cholangiocarcinoma: Predictors of survival. HPB (Oxford) 2008, 10, 190–195. [Google Scholar] [CrossRef]
- Kobayashi, A.; Miwa, S.; Nakata, T.; Miyagawa, S. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br. J. Surg. 2010, 97, 56–64. [Google Scholar] [CrossRef]
- Japanese Society of Biliary Surgery, General Rules for Surgical and Pathological Studies on Cancer of Biliary Tract, 2d ed.; Tokyo: Kanehara, Japan, 1989; pp. 53–56.
- Sasaki, R.; Takeda, Y.; Funato, O.; Nitta, H.; Kawamura, H.; Uesugi, N.; Sugai, T.; Wakabayashi, G.; Ohkohchi, N. Significance of ductal margin status in patients undergoing surgical resection for extrahepatic cholangiocarcinoma. World J. Surg. 2007, 31, 1788–1796. [Google Scholar] [CrossRef]
- Endo, I.; House, M.G.; Klimstra, D.S.; Gönen, M.; D'Angelica, M.; Dematteo, R.P.; Fong, Y.; Blumgart, L.H.; Jarnagin, W.R. Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann. Surg. Oncol. 2008, 15, 2104–2112. [Google Scholar] [CrossRef]
- Wakai, T.; Shirai, Y.; Moroda, T.; Yokoyama, N.; Hatakeyama, K. Impact of ductal resection margin status on long-term survival in patients undergoing resection for extrahepatic cholangiocarcinoma. Cancer 2005, 103, 1210–1216. [Google Scholar] [CrossRef]
- Autschbach, F. The pathological assessment of total mesorectal excision: What are the relevant resection margins? Recent Results Cancer Res. 2005, 165, 30–39. [Google Scholar] [CrossRef]
- Yeo, C.J.; Cameron, J.L.; Sohn, T.A.; Lillemoe, K.D.; Pitt, H.A.; Talamini, M.A.; Hruban, R.H.; Ord, S.E.; Sauter, P.K.; Coleman, J.; Zahurak, M.L.; Grochow, L.B.; Abrams, R.A. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: Pathology, complications, and outcomes. Ann. Surg. 1997, 226, 248–257. [Google Scholar] [CrossRef]
- Cameron, J.L.; Riall, T.S.; Coleman, J.; Belcher, K.A. One thousand consecutive pancreaticoduodenectomies. Ann. Surg. 2006, 244, 10–15. [Google Scholar] [CrossRef]
- Kuhlmann, K.; de Castro, S.; van Heek, T.; Busch, O.; van Gulik, T.; Obertop, H.; Gouma, D. Microscopically incomplete resection offers acceptable palliation in pancreatic cancer. Surgery 2006, 139, 188–196. [Google Scholar] [CrossRef]
- Winter, J.M.; Cameron, J.L.; Campbell, K.A.; Arnold, M.A.; Chang, D.C.; Coleman, J.; Hodgin, M.B.; Sauter, P.K.; Hruban, R.H.; Riall, T.S.; Schulick, R.D.; Choti, M.A.; Lillemoe, K.D.; Yeo, C.J. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J. Gastrointest. Surg. 2006, 10, 1199-1210; discussion 1210-1211. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schlitter, A.M.; Esposito, I. Definition of Microscopic Tumor Clearance (R0) in Pancreatic Cancer Resections. Cancers 2010, 2, 2001-2010. https://doi.org/10.3390/cancers2042001
Schlitter AM, Esposito I. Definition of Microscopic Tumor Clearance (R0) in Pancreatic Cancer Resections. Cancers. 2010; 2(4):2001-2010. https://doi.org/10.3390/cancers2042001
Chicago/Turabian StyleSchlitter, Anna Melissa, and Irene Esposito. 2010. "Definition of Microscopic Tumor Clearance (R0) in Pancreatic Cancer Resections" Cancers 2, no. 4: 2001-2010. https://doi.org/10.3390/cancers2042001
APA StyleSchlitter, A. M., & Esposito, I. (2010). Definition of Microscopic Tumor Clearance (R0) in Pancreatic Cancer Resections. Cancers, 2(4), 2001-2010. https://doi.org/10.3390/cancers2042001