Different Serotonergic Expression in Nevomelanocytic Tumors
Abstract
:1. Introduction
2. Materials and methods
2.1. Tissue Specimens
2.2. Immunohistochemical Procedure
2.3. Double Staining
2.4. Microscopy
2.5. Statistics
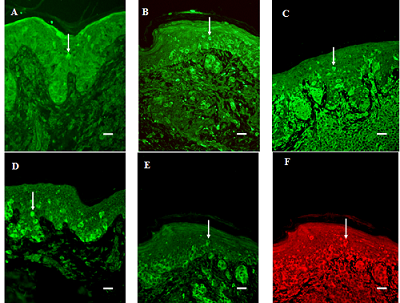
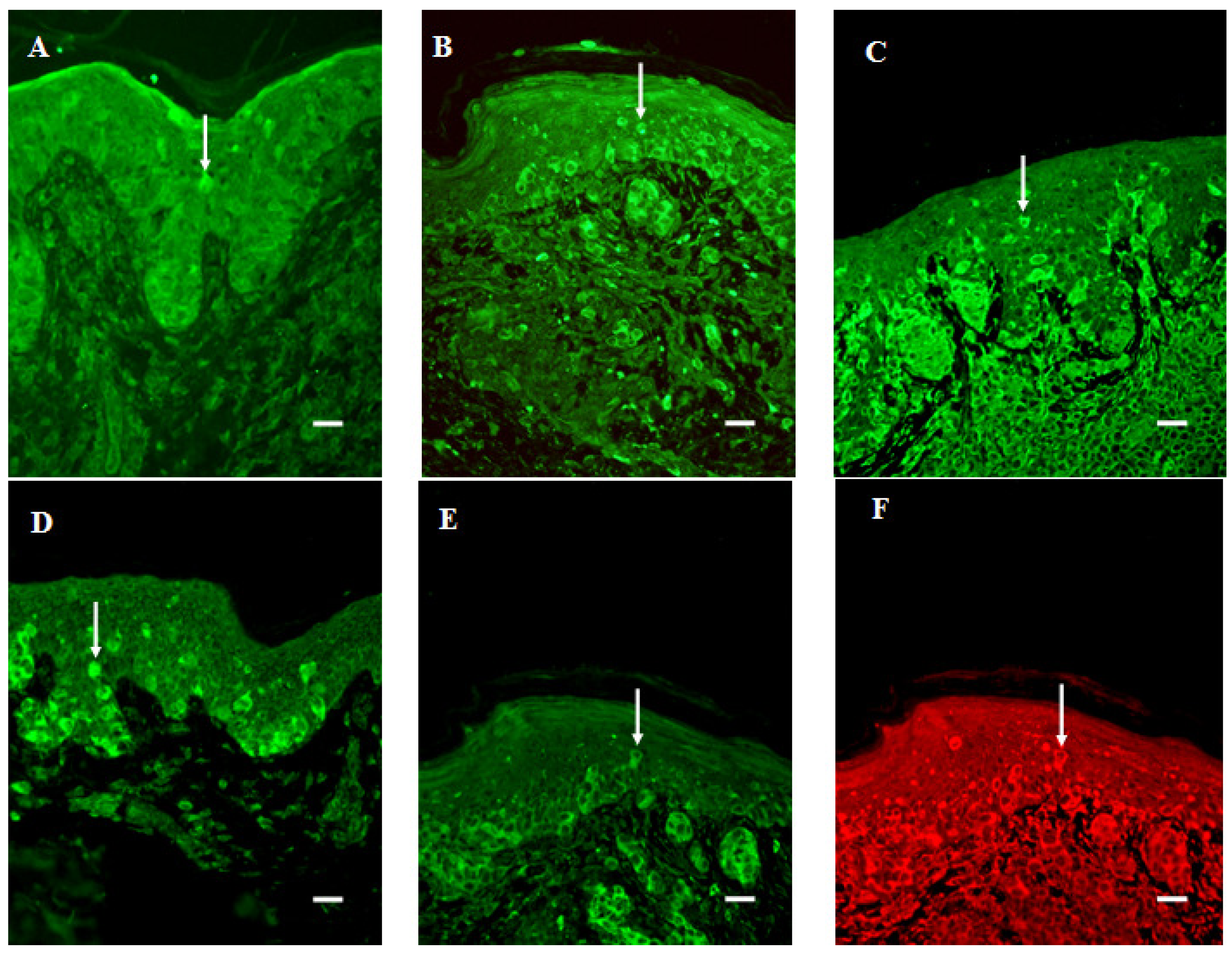
3. Results
3.1. General Findings
3.2. Migration of Cells into the Suprabasal Epidermis
3.3. Expression in the Junction Area between Epidermis and Dermis
3.4. The Strength of the Signal from Tumor Cells in the Dermis
3.5. Vessel Immunoreactivity

| Cells in the suprabasal epidermis | Junction area | Dermis Signal strength in tumour cells | Vessels | i SERT | i 5-HT1A | ||
|---|---|---|---|---|---|---|---|
| BCN | 5-HT | 0 | 1.0 ± 0.7 | 1.0 ± 0 | 0 ± 0 | ||
| DN | 5-HT | 0.6 ± 0.7 | 0.8 ± 0.8 | 1.1 ± 0.3 | 0.1 ±0.3 | ||
| SSM | 5-HT | 1.5 ± 0.5 | 0.5 ± 0.5 | 1.3 ± 0.6 | 0.2 ± 0.4 |
3.6. Infiltration of SERT- and 5-HT1AR-Immunoreactive Cells into the Dermal Region of the Tumor


4. Discussion
5. Conclusion
Acknowledgements
References
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Azmitia, E.C. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res. Bull. 2001, 56, 413–424. [Google Scholar] [CrossRef]
- Bockaert, J.; Claeysen, S; Bécamel, C.; Dumuis, A.; Marin, P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006, 326, 553–572. [Google Scholar] [CrossRef]
- Siddiqui, E.J. The effect of serotonin and serotonin antagonists on bladder cancer cell proliferation. BJU Int. 2006, 97, 634–639. [Google Scholar] [CrossRef]
- Siddiqui, E.J.; Thompson, C.S.; Mikhailidis, D.P.; Mumtaz, F.H. The role of serotonin in tumor growth(review). Oncol. Rep. 2005, 14, 1593–1597. [Google Scholar]
- Sonier, B.; Arseneault, M.; Lavigne, C.; Ouellette, R.J.; Vaillancourt, C. The 5-HT2A serotonergic receptor is expressed in the MCF-7 human breast cancer cell line and reveals a mitogenic effect of serotonin. Biochem. Biophys. Res. Commun. 2006, 343, 1053–1059. [Google Scholar] [CrossRef]
- Xu, W.; Tamim, H.; Shapiro, S.; Stang, M.R.; Collet, J.P. Use of antidepressants and risk of colorectal cancer: a nested control-case study. Lancet Oncol. 2006, 7, 301–308. [Google Scholar] [CrossRef]
- Bahl, S. Use of antidepressant medications and the possible association with breast cancer risk. Psychother. Psychosom. 2003, 72, 185–194. [Google Scholar] [CrossRef]
- Moorman, P.G.; Grubber, J.M.; Millikan, R.C.; Newman, B. Antidepressant medications and their association with invasive breast cancer and carcinoma in situ of the breast. Epidemiology 2003, 14, 307–314. [Google Scholar]
- Johansson, O.; Liu, P.Y.; Bondesson, L.; Nordlind, K.; Olsson, M..J; Löntz, W.; Verhofstad, A.; Liang, Y.; Gangi, S. A serotonin-like immunoreactivity is present in human cutaneous melanocytes. J. Invest. Dermatol. 1998, 111, 1010–1014. [Google Scholar] [CrossRef]
- Slominski, A.; Semak, I.; Pisarchik, A.; Sweatman, T.; Szczesniewski, A.; Wortsman, J. Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002, 511, 102–106. [Google Scholar] [CrossRef]
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J.; Szczesniewski, A.; Slugocki, G.; McNulty, J.; Kauser, S.; Tobin, D.J.; Jing, C.; Johansson, O. Serotonergic and melatonergic system are fully expressed in human skin. FASEB J. 2002, 16, 896–898. [Google Scholar]
- Slominski, A.; Pisarchik, A.; Johansson, O.; Jing, C.; Semak, I.; Slugocki, G.; Wortsman, J. Tryptophan hydroxylase expression in human skin cells. Biochim. Biophys. Acta 2003, 1639, 80–86. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Tobin, D.J. The cutaneous serotonergic/melatonergic system: securing a place under the sun. FASEB J. 2005, 19, 176–194. [Google Scholar] [CrossRef]
- Lundeberg, L.; El-Nour, H.; Mohabbati, S.; Morales, M.; Azmitia, E.; Nordlind, K. Expression of serotonin receptors in allergic contact eczematous human skin. Arch. Dermatol. Res. 2002, 294, 393–398. [Google Scholar]
- Slominski, A.; Pisarchik, A.; Zbytek, B.; Tobin, D.J.; Kauser, S.; Wortsman, J. Functional activity of serotonergic and melatonergic systems expressed in the skin. J. Cell Physiol. 2003, 196, 144–153. [Google Scholar] [CrossRef]
- McEwan, M.; Parsons, P.G. Inhibition of melanization in human melanoma cells by a serotonin uptake inhibitor. J. Invest. Dermatol. 1987, 89, 82–86. [Google Scholar]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Azmitia, E.C.; Yu, I.; Akbari, H.M.; Kheck, N.; Whitaker-Azmitia, P.M.; Marshak, D.R. Antipeptide antibodies against the 5-HT1A receptor. J. Chem. Neuroanat. 1992, 15, 289–298. [Google Scholar]
- DeFelipe, J.; Arellano, J.I.; Gómez, A.; Azmitia, E.C.; Munoz, A. Pyramidal cell axons show a local specialization for GABA and 5-HT inputs in monkey and human cerebral cortex. J. Compar. Neurol. 2001, 433, 148–155. [Google Scholar] [CrossRef]
- Wu, C.; Yoder, E.J.; Shih, J.; Chen, K.; Dias, P.; Shi, L.; Ji, X.D.; Wei, J.; Conner, J.M.; Kumar, S.; Ellisman, M.H.; Singh, S.K. Development and characterization of monoclonal antibodies specific to the serotonin 5-HT2 receptor. J. Histochem. Cytochem. 1998, 46, 811–824. [Google Scholar] [CrossRef]
- Wu, C.; Singh, S.K., Dias; Kumar, S.; Mann, D.M. Activated astrocytes display increased 5-HT2a receptor expression in pathological states. Exp. Neurol. 1999, 158, 529–533. [Google Scholar] [CrossRef]
- Eastwood, S.L.; Burnet, P.W.; Gittins, R.; Baker, K.; Harrison, P.J. Expression of serotonin 5-HT2A receptors in the human cerebellum and alterations in schizophrenia. Synapse 2001, 42, 104–114. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Wayne, W.D. Biostatistics: A Foundation for Analysis in the Health Sciences, 6th ed.; John Wiley & Sons: New York, NY, USA, 1995. [Google Scholar]
- Lüttgen, M.; Ögren, S.O.; Meister, B. 5-HT1A receptor mRNA and immunoreactivity in the rat medial septum/diagonal band of Broca-relationships to GABAergic and cholinergic neurons. J. Chem. Neuroanat. 2005, 29, 93–111. [Google Scholar] [CrossRef]
- Martin-Ruiz, R.; Puig, M.V.; Celada, P.; Shapiro, D.A.; Roth, B.L.; Mengod, G.; Artigas, F. Control of serotonergic function in median prefrontal cortex by serotonin-2A receptors though a glutamate-dependent mechanism. J. Neurosci. 2001, 21, 9856–9866. [Google Scholar]
- Zhang, Y.; Gray, T.S.; D´Souza, D.N.; Carrasco, G.A.; Damjanoska, K.J., Dudas; Zainelli, G.M.; Sullivan Hanley, N.R.; Battaglia, G.; Muma, N.A.; Van de Kar, L.D. Desensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons in vivo. J. Pharmacol. Exper. Therapeut. 2004, 310, 59–66. [Google Scholar] [CrossRef]
- Berendsen, H.H. Interactions between 5-hydroxytryptamine receptor subtypes: is a disturbed receptor balance contributing to the symptomatology of depression in humans? Pharmacol. Ther. 1995, 66, 17–37. [Google Scholar] [CrossRef]
- Reddy, M.V.; Storer, R.D.; Laws, G.M.; Armstrong, M.J.; Barnum, J.E.; Gara, J.P.; McKnight, C.G.; Skopek, T.R.; Sina, J.F.; DeLuca, J.G.; Galloway, S.M. Genotoxicity of naturally occurring indole compounds: correlation between covalent DNA binding and other genotoxicity tests. Environ. Mol. Mutagen. 2002, 40, 1–17. [Google Scholar] [CrossRef]
- Zilkha-Falb, R.; Ziv, I.; Nardi, N.; Offen, D., Melamed; Barzilai, A. Monoamine-induced apoptotic neuronal cell death. Cell Mol. Neurobiol. 1997, 17, 101–118. [Google Scholar] [CrossRef]
- Serafeim, A.; Grafton, G.; Chamba, A.; Gregory, C.D.; Blakely, R.D.; Bowery, N.G.; Barnes, N.M.; Gordon, J. 5-Hydroxytryptamine drives apoptosis in biopsylike Burkitt lymphoma cells: reversal by selective serotonin reuptake inhibitors. Blood 2002, 99, 2545–2553. [Google Scholar] [CrossRef]
- Serafeim, A.; Holder, M.J.; Grafton, G.; Chamba, A.; Drayson, M.T.; Luong, Q.T.; Bunce, C.M.; Gregory, C.D.; Barnes, N.M.; Gordon, J. Selective serotonin reuptake inhibitors directly signal for apoptosis in biopsy-like Burkitt lymphoma cells. Blood 2003, 101, 3212–3219. [Google Scholar] [CrossRef]
- Levkovitz, Y.; Gil-Ad, I.; Zeldich, E.; Dayag, M.; Weizman, A. Differential induction of apoptosis by antidepressants in glioma and neuroblastoma cell lines. J. Molec. Neurosci. 2005, 27, 29–42. [Google Scholar] [CrossRef]
- Brandes, L.J.; Arron, R.J.; Bogdanovic, R.P.; Tong, J.; Zaborniak, C.L.; Hogg, G.R.; Warrington, R.C.; Fang, W.; LaBella, F.S. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res. 1992, 52, 3796–3800. [Google Scholar]
- Meredith, E.J.; Holder, M.J.; Chamba, A.; Challa, A.; Drake-Lee, A.; Bunce, C.M.; Drayson, M.T.; Pilkington, G.; Blakely, R.D.; Dyer, M.J.; Barnes, N.M.; Gordon, J. The serotonin transporter (SLC6A4) is present in B-cell clones of diverse malignant origin: probing a potential antitumor target for psychotropics. FASEB J. 2005, 19, 1187–1189. [Google Scholar]
- Passaretti, T.V.; Wilcox, B.D.; Jeffrey, J.J. Serotonin regulation of gene expression in uterine extracellular matrix: reciprocal effects on collagens and collagenase. Mol. Cell. Endocrinol. 1996, 120, 125–132. [Google Scholar] [CrossRef]
- Hayashi, T.; Sumi, D.; Matsui-Hirai, H.; Fukatsu, A.; Arockia Rani, P.J.; Kano, H.; Tsunekawa, T.; Iguchi, A. Sarpogrelate HCl, a selective 5-HT2A antagonist, retards the progression of atherosclerosis through a novel mechanism. Atherosclerosis 2003, 168, 23–31. [Google Scholar] [CrossRef]
- Laemmel, E.; Stücker, O.; Darmon, P.L.; Vicaut, E. Characterization of the specific response to serotonin of mouse tumor-feeding arterioles. Int. J. Radiat. Biol. 1998, 74, 379–386. [Google Scholar] [CrossRef]
- Stucker, O.; Laemmel, E.; Teisseire, B.; Vicaut, E. Specific response of mouse tumor-feeding arterioles to stimulation by 5-HT1A agonists. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 1125–1131. [Google Scholar] [CrossRef]
- Ribatti, D.; Vacca, A.; Ria, R.; Marzullo, A.; Nico, B.; Filotico, R.; Roncali, L.; Dammacco, F. Neovascularization, expression of fibroblast growth factor-2, and mast cells with tryptase activity increase simultaneously with pathological progression in human malignant melanoma. Eur. J. Cancer 2003, 39, 666–674. [Google Scholar] [CrossRef]
- Kervinen, H.; Kaartinen, M.; Mäkynen, H.; Palosuo, T.; Mänttäri, M.; Kovanen, P.T. Serum tryptase levels in acute coronary syndromes. Int. J. Cardiol. 2005, 104, 138–143. [Google Scholar] [CrossRef]
- Ch´ng, S.; Wallis, R.A.; Yuan, L.; Davis, P.F.; Tan, S.T. Mast cells and cutaneous malignancies. Modern Pathol. 2006, 19, 149–159. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Naimi-Akbar, C.; Ritter, M.; Demel, S.; El-Nour, H.; Hedblad, M.-A.; Azmitia, E.C.; Nordlind, K. Different Serotonergic Expression in Nevomelanocytic Tumors. Cancers 2010, 2, 1166-1177. https://doi.org/10.3390/cancers2021166
Naimi-Akbar C, Ritter M, Demel S, El-Nour H, Hedblad M-A, Azmitia EC, Nordlind K. Different Serotonergic Expression in Nevomelanocytic Tumors. Cancers. 2010; 2(2):1166-1177. https://doi.org/10.3390/cancers2021166
Chicago/Turabian StyleNaimi-Akbar, Clara, Markus Ritter, Sasika Demel, Husameldin El-Nour, Mari-Anne Hedblad, Efrain C. Azmitia, and Klas Nordlind. 2010. "Different Serotonergic Expression in Nevomelanocytic Tumors" Cancers 2, no. 2: 1166-1177. https://doi.org/10.3390/cancers2021166