May Patients Receiving GLP-1 Agonists Be at Lower Risk of Prostate Cancer Aggressiveness and Progression?
Simple Summary
Abstract
1. Introduction
2. Incretin Hormones
3. Incretin Hormone Receptor Agonists
4. Prostate Cancer, Obesity, and Diabetes
4.1. Prostate Cancer and Obesity
4.2. Prostate Cancer and Diabetes
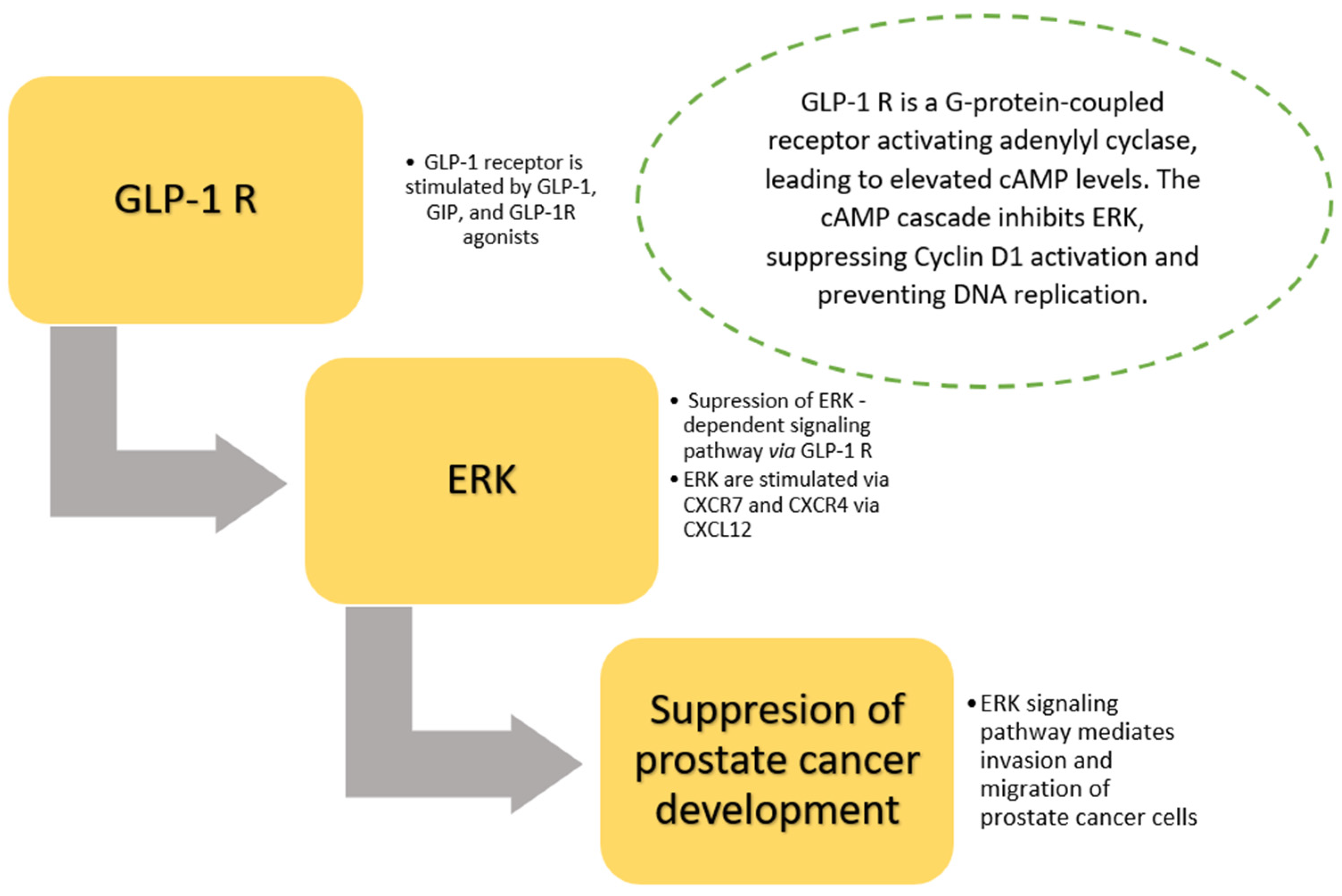
5. Prostate Cancer and Incretin Hormones—Dependent Pathway
6. Prostate Cancer and GLP-1 Receptor Agonists
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| Akt | protein kinase B |
| AMPK | 5′AMP-activated protein kinase |
| BAX | BCL2-associated X protein (apoptosis regulator) |
| BMI | body mass index |
| cAMP | cyclic adenosine monophosphate |
| CRPC | castration-resistant prostate cancer |
| CXCL | ligand of chemokine receptor |
| CXCR | chemokine receptor CXCR |
| DDP-4 | dipeptidyl peptidase 4 |
| DHT | dihydrotestosterone |
| EPAC | exchange protein directly activated by cAMP |
| ERK | extracellular signal-regulated kinase |
| FDA | Food and Drug Administration |
| GIP | glucose-dependent insulinotropic polypeptide |
| GLP-1 | glucagon-like peptide 1 |
| GLP-1 RAs | glucagon-like peptide 1 receptor agonists |
| GPCR | G protein–coupled receptor |
| GSIS | glucose-stimulated insulin secretion |
| HRR | homologous recombination repair |
| HUVECs | human umbilical vein endothelial cells |
| IL-1 | interleukin 1 |
| IL-6 | interleukin 6 |
| IR | irradiation |
| JAK | Janus tyrosine kinase family |
| LNCaP | androgen-sensitive human prostate cells |
| MAPK | mitogen-activated protein kinase |
| mHSPC | metastatic hormone sensitive prostate cancer |
| mTOR | mechanistic target of rapamycin |
| PI3K | phosphoinositide 3-kinase |
| PKC | protein kinase C |
| PPAT | periprostatic adipose tissue |
| SGLT2 | sodium–glucose cotransporter 2 |
| SKP2 | S-phase kinase-associated protein 2 |
| Src | Src family of protein tyrosine kinases |
| STAT | signal transducer and activator of transcription |
| TNF-β | tumor necrosis factor β |
| VGCCs | voltage-gated calcium channels |
References
- Alfaris, N.; Waldrop, S.; Johnson, V.; Boaventura, B.; Kendrick, K.; Stanford, F.C. GLP-1 single, dual, and triple receptor agonists for treating type 2 diabetes and obesity: A narrative review. eClinical Med. 2024, 75, 102782. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wei, J.; He, X.; Lian, J.; Han, D.; An, P.; Zhou, T.; Liu, S.; Wang, F.; Min, J. Quantitative association between body mass index and the risk of cancer: A global Meta-analysis of prospective cohort studies. Int. J. Cancer 2018, 143, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Siegel, R.L.; Torre, L.A.; Pearson-Stuttard, J.; Islami, F.; Fedewa, S.A.; Goding Sauer, A.; Shuval, K.; Gapstur, S.M.; Jacobs, E.J.; et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer. J. Clin. 2019, 69, 88–112. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramadani, F.G.; Perdana, N.R.; Ringoringo, D.R.L. Body mass index, obesity and risk of prostate cancer: A systematic review and meta-analysis. Cent. Eur. J. Urol. 2024, 77, 176–188. [Google Scholar]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kim, C.H. Differential Risk of Cancer Associated with Glucagon-like Peptide-1 Receptor Agonists: Analysis of Real-world Databases. Endocr. Res. 2022, 47, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, Y.; Yang, S.; He, G.; Jiang, Z.; Gang, X.; Wang, G. Antidiabetic medications and the risk of prostate cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Pharmacol. Res. 2022, 177, 106094. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yin, H.; Yu, O.H.Y.; Azoulay, L. Incretin-Based Drugs and the Incidence of Prostate Cancer Among Patients With Type 2 Diabetes. Epidemiology 2022, 33, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. S1), 5–21. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Philippe, J.; Mojsov, S.; Chick, W.L.; Habener, J.F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl. Acad. Sci. USA 1987, 84, 3434–3438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, N.K.; Hackett, T.A.; Galli, A.; Flynn, C.R. GLP-1: Molecular mechanisms and outcomes of a complex signaling system. Neurochem. Int. 2019, 128, 94–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, G.; Chepurny, O.G.; Malester, B.; Rindler, M.J.; Rehmann, H.; Bos, J.L.; Schwede, F.; Coetzee, W.A.; Holz, G.G. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic beta cells and rat INS-1 cells. J. Physiol. 2006, 573 Pt 3, 595–609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Britsch, S.; Krippeit-Drews, P.; Lang, F.; Gregor, M.; Drews, G. Glucagon-like peptide-1 modulates Ca2+ current but not K+ATP current in intact mouse pancreatic B-cells. Biochem. Biophys. Res. Commun. 1995, 207, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Leech, C.A.; Habener, J.F. Insulinotropic glucagon-like peptide-1-mediated activation of non-selective cation currents in insulinoma cells is mimicked by maitotoxin. J. Biol. Chem. 1997, 272, 17987–17993. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.; Parker, H.E.; Adriaenssens, A.E.; Hodgson, J.M.; Cork, S.C.; Trapp, S.; Gribble, F.M.; Reimann, F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 2014, 63, 1224–1233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef] [PubMed]
- van Bloemendaal, L.; Veltman, D.J.; Ten Kulve, J.S.; Groot, P.F.; Ruhé, H.G.; Barkhof, F.; Sloan, J.H.; Diamant, M.; Ijzerman, R.G. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes Obes. Metab. 2015, 17, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Calanna, S.; Christensen, M.; Holst, J.J.; Laferrère, B.; Gluud, L.L.; Vilsbøll, T.; Knop, F.K. Secretion of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes: Systematic review and meta-analysis of clinical studies. Diabetes Care. 2013, 36, 3346–3352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barrera, J.G.; Sandoval, D.A.; D’Alessio, D.A.; Seeley, R.J. GLP-1 and energy balance: An integrated model of short-term and long-term control. Nat. Rev. Endocrinol. 2011, 7, 507–516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hammoud, R.; Drucker, D.J. Beyond the pancreas: Contrasting cardiometabolic actions of GIP and GLP1. Nat. Rev. Endocrinol. 2023, 19, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Knura, M.; Garczorz, W.; Borek, A.; Drzymała, F.; Rachwał, K.; George, K.; Francuz, T. The Influence of Anti-Diabetic Drugs on Prostate Cancer. Cancers 2021, 13, 1827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holst, J.J.; Vilsbøll, T.; Deacon, C.F. The incretin system and its role in type 2 diabetes mellitus. Mol. Cell Endocrinol. 2009, 297, 127–136. [Google Scholar] [CrossRef]
- Liu, Q.K. Mechanisms of action and therapeutic applications of GLP-1 and dual GIP/GLP-1 receptor agonists. Front. Endocrinol. 2024, 15, 1431292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Choi, T.; Al-Aly, Z. Mapping the effectiveness and risks of GLP-1 receptor agonists. Nat. Med. 2025, 31, 951–962, Erratum in Nat Med. 2025, 31, 1038. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. GLP-1 agonists: US sees 700% increase over four years in number of patients without diabetes starting treatment. BMJ 2024, 386, q1645. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Attia, A.; Elshazli, R.M.; Abdelmaksoud, A.; Tatum, D.; Aiash, H.; Toraih, E.A. Differential Effects of GLP-1 Receptor Agonists on Cancer Risk in Obesity: A Nationwide Analysis of 1.1 Million Patients. Cancers 2024, 17, 78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allott, E.H.; Masko, E.M.; Freedland, S.J. Obesity and prostate cancer: Weighing the evidence. Eur. Urol. 2013, 63, 800–809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacInnis, R.J.; English, D.R. Body size and composition and prostate cancer risk: Systematic review and meta-regression analysis. Cancer Causes Control 2006, 17, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Aronson, W.J. Examining the relationship between obesity and prostate cancer. Rev. Urol. 2004, 6, 73–81. [Google Scholar] [PubMed] [PubMed Central]
- Hong, H.; Koch, M.O.; Foster, R.S.; Bihrle, R.; Gardner, T.A.; Fyffe, J.; Ulbright, T.M.; Eble, J.N.; Cheng, L. Anatomic distribution of periprostatic adipose tissue: A mapping study of 100 radical prostatectomy specimens. Cancer 2003, 97, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Artime, A.; García-Soler, B.; Sainz, R.M.; Mayo, J.C. Emerging Roles for Browning of White Adipose Tissue in Prostate Cancer Malignant Behaviour. Int. J. Mol. Sci. 2021, 22, 5560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drewa, J.; Lazar-Juszczak, K.; Adamowicz, J.; Juszczak, K. Periprostatic Adipose Tissue as a Contributor to Prostate Cancer Pathogenesis: A Narrative Review. Cancers 2025, 17, 372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, J.; Euhus, D.M.; Scherer, P.E. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr. Rev. 2011, 32, 550–570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hurwitz, A.A.; Foster, B.A.; Allison, J.P.; Greenberg, N.M.; Kwon, E.D. The TRAMP mouse as a model for prostate cancer. Curr. Protoc. Immunol. 2001, 45, 20.5.1–20.5.23. [Google Scholar] [CrossRef] [PubMed]
- Brestoff, J.R.; Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 2015, 161, 146–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Howe, L.R.; Subbaramaiah, K.; Hudis, C.A.; Dannenberg, A.J. Molecular pathways: Adipose inflammation as a mediator of obesity-associated cancer. Clin. Cancer. Res. 2013, 9, 6074–6083. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsushita, M.; Fujita, K.; Nonomura, N. Influence of diet and nutrition on prostate cancer. Int. J. Mol. Sci. 2020, 21, 1447. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Song, M.; Preston, M.A.; Ma, W.; Hu, Y.; Pernar, C.H.; Stopsack, K.H.; Ebot, E.M.; Fu, B.C.; Zhang, Y.; et al. The association of diabetes with risk of prostate cancer defined by clinical and molecular features. Br. J. Cancer. 2020, 123, 657–665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.; Giovannucci, E.; Jeon, J.Y. Diabetes and mortality in patients with prostate cancer: A meta-analysis. Springerplus 2016, 5, 1548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faris, J.E.; Smith, M.R. Metabolic sequelae associated with androgen deprivation therapy for prostate cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 240–246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karzai, F.H.; Madan, R.A.; Dahut, W.L. Metabolic syndrome in prostate cancer: Impact on risk and outcomes. Future Oncol. 2016, 12, 1947–1955. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cannarella, R.; Condorelli, R.A.; Barbagallo, F.; La Vignera, S.; Calogero, A.E. Endocrinology of the Aging Prostate: Current Concepts. Front. Endocrinol. 2021, 12, 554078. [Google Scholar] [CrossRef]
- Guo, C.; Huang, T.; Chen, A.; Chen, X.; Wang, L.; Shen, F.; Gu, X. Glucagon-like peptide 1 improves insulin resistance in vitro through anti-inflammation of macrophages. Braz. J. Med. Biol. Res. 2016, 49, e5826. [Google Scholar] [CrossRef]
- Kim, S.; Keku, T.O.; Martin, C.; Galanko, J.; Woosley, J.T.; Schroeder, J.C.; Satia, J.A.; Halabi, S.; Sandler, R.S. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer. Res. 2008, 68, 323–328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Juge-Aubry, C.E.; Somm, E.; Giusti, V.; Pernin, A.; Chicheportiche, R.; Verdumo, C.; Rohner-Jeanrenaud, F.; Burger, D.; Dayer, J.M.; Meier, C.A. Adipose tissue is a major source of interleukin-1 receptor antagonist: Upregulation in obesity and inflammation. Diabetes 2003, 52, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Bahmad, H.F.; Jalloul, M.; Azar, J.; Moubarak, M.M.; Samad, T.A.; Mukherji, D.; Al-Sayegh, M.; Abou-Kheir, W. Tumor Microenvironment in Prostate Cancer: Toward Identification of Novel Molecular Biomarkers for Diagnosis, Prognosis, and Therapy Development. Front. Genet. 2021, 12, 652747. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Abdul, M.; Hoosein, N. Modulation of neuroendocrine differentiation in prostate cancer by interleukin-1 and -2. Prostate. Suppl. 1998, 8, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.P.; Li, J.; Tewari, A.K. Inflammation and prostate cancer: The role of interleukin 6 (IL-6). BJU Int. 2014, 113, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Gribble, F.M.; Reimann, F. Metabolic Messengers: Glucagon-like peptide 1. Nat. Metab. 2021, 3, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785, Erratum in: Lancet Diabetes Endocrinol. 2020, 8, e2. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.S.; Kalff, V.; Williams, S.G.; Murphy, D.G.; Colman, P.G.; Hofman, M.S. The GLP-1 receptor is expressed in vivo by human metastatic prostate cancer. Endocr. Oncol. 2024, 4, e230015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.proteinatlas.org/ENSG00000112164-GLP1R/cell+line#prostate_cancer (accessed on 1 February 2025).
- Skriver, C.; Friis, S.; Knudsen, L.B.; Catarig, A.M.; Clark, A.J.; Dehlendorff, C.; Mørch, L.S. Potential preventive properties of GLP-1 receptor agonists against prostate cancer: A nationwide cohort study. Diabetologia 2023, 66, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Bu, H.M.; Ma, X.H.; Lu, S.; Zhao, S.; Cui, Y.L.; Sun, J. Glucagon-like Peptide-1 Analogues Inhibit Proliferation and Increase Apoptosis of Human Prostate Cancer Cells in vitro. Exp. Clin. Endocrinol. Diabetes 2017, 125, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y.; Kimama, T.; Kawanami, T.; Hamaguchi, Y.; Terawaki, Y.; Tanaka, T.; Murase, K.; Motonaga, R.; Tanabe, M.; Yanase, T. Combined Treatment with Exendin-4 and Metformin Attenuates Prostate Cancer Growth. PLoS ONE 2015, 10, e0139709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nomiyama, T.; Kawanami, T.; Irie, S.; Hamaguchi, Y.; Terawaki, Y.; Murase, K.; Tsutsumi, Y.; Nagaishi, R.; Tanabe, M.; Morinaga, H.; et al. Exendin-4, a GLP-1 receptor agonist, attenuates prostate cancer growth. Diabetes 2014, 63, 3891–3905. [Google Scholar] [CrossRef] [PubMed]
- Sfanos, K.S.; Yegnasubramanian, S.; Nelson, W.G.; De Marzo, A.M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 2018, 15, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, T.; Nomiyama, T.; Kawanami, T.; Hamaguchi, Y.; Horikawa, T.; Tanaka, T.; Irie, S.; Motonaga, R.; Hamanoue, N.; Tanabe, M.; et al. Activation of overexpressed glucagon-like peptide-1 receptor attenuates prostate cancer growth by inhibiting cell cycle progression. J. Diabetes Investig. 2020, 11, 1137–1149. [Google Scholar] [CrossRef]
- He, W.; Li, J. Exendin-4 enhances radiation response of prostate cancer. Prostate 2018, 78, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, S.; Montazeri, H.; Tarighi, P. Synergistic anti-tumor effects of Liraglutide, a glucagon-like peptide-1 receptor agonist, along with Docetaxel on LNCaP prostate cancer cell line. Eur. J. Pharmacol. 2020, 878, 173102. [Google Scholar] [CrossRef]
- Wenjing, H.; Shao, Y.; Yu, Y.; Huang, W.; Feng, G.; Li, J. Exendin-4 enhances the sensitivity of prostate cancer to enzalutamide by targeting Akt activation. Prostate 2020, 80, 367–375. [Google Scholar] [CrossRef]
- Aronis, K.N.; Chamberland, J.P.; Mantzoros, C.S. GLP-1 promotes angiogenesis in human endothelial cells in a dose-dependent manner, through the Akt, Src and PKC pathways. Metabolism 2013, 62, 1279–1286. [Google Scholar] [CrossRef]
- Alhajahjeh, A.; Al-Faouri, R.; Bahmad, H.F.; Bader, T.; Dobbs, R.W.; Abdulelah, A.A.; Abou-Kheir, W.; Davicioni, E.; Lee, D.I.; Shahait, M. From Diabetes to Oncology: Glucagon-like Peptide-1 (GLP-1) Receptor Agonist’s Dual Role in Prostate Cancer. Cancers 2024, 16, 1538. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macri, E.; Loda, M. Role of p27 in prostate carcinogenesis. Cancer Metastasis Rev. 1998, 17, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.H.; Ou, L.; Gay, A.; Besson, A.; Kriwacki, R.W. Mapping Interactions between p27 and RhoA that Stimulate Cell Migration. J. Mol. Biol. 2018, 430, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Garrett-Mayer, E.; de Wit, R.; Tannock, I.; Eisenberger, M. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin. Cancer. Res. 2010, 16, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Conduit, C.; Inderjeeth, A.J.; Allen, R.; Martin, A.J.; Parulekar, W.; Mulroe, E.; McJannett, M.; Zielinski, R.R.; Thomson, A.; Tan, T.H.; et al. Enzalutamide with standard first-line therapy for metastatic hormone-sensitive prostate cancer: A plain language summary of the ENZAMET trial (ANZUP 1304). Future Oncol. 2025, 21, 627–636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Navarro, G.; Xu, W.; Jacobson, D.A.; Wicksteed, B.; Allard, C.; Zhang, G.; De Gendt, K.; Kim, S.H.; Wu, H.; Zhang, H.; et al. Extranuclear Actions of the Androgen Receptor Enhance Glucose-Stimulated Insulin Secretion in the Male. Cell Metab. 2016, 23, 837–851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Handgraaf, S.; Philippe, J. The Role of Sexual Hormones on the Enteroinsular Axis. Endocr. Rev. 2019, 40, 1152–1162. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drewa, J.; Lazar-Juszczak, K.; Adamowicz, J.; Juszczak, K. May Patients Receiving GLP-1 Agonists Be at Lower Risk of Prostate Cancer Aggressiveness and Progression? Cancers 2025, 17, 1576. https://doi.org/10.3390/cancers17091576
Drewa J, Lazar-Juszczak K, Adamowicz J, Juszczak K. May Patients Receiving GLP-1 Agonists Be at Lower Risk of Prostate Cancer Aggressiveness and Progression? Cancers. 2025; 17(9):1576. https://doi.org/10.3390/cancers17091576
Chicago/Turabian StyleDrewa, Julia, Katarzyna Lazar-Juszczak, Jan Adamowicz, and Kajetan Juszczak. 2025. "May Patients Receiving GLP-1 Agonists Be at Lower Risk of Prostate Cancer Aggressiveness and Progression?" Cancers 17, no. 9: 1576. https://doi.org/10.3390/cancers17091576
APA StyleDrewa, J., Lazar-Juszczak, K., Adamowicz, J., & Juszczak, K. (2025). May Patients Receiving GLP-1 Agonists Be at Lower Risk of Prostate Cancer Aggressiveness and Progression? Cancers, 17(9), 1576. https://doi.org/10.3390/cancers17091576




