Emotional Functioning in Long-Term Breast Cancer Survivors: A Cross-Sectional Study on Its Influence and Key Predictors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Procedures
2.2. Measures
2.2.1. Emotional Functioning
2.2.2. Sociodemographic and Clinical Data Collection
2.2.3. Health-Related Quality of Life
2.2.4. Mood State
2.2.5. Self-Perceived Physical Fitness
2.2.6. Physical Activity Level
2.2.7. Pain Measures
2.2.8. Cancer-Related Fatigue
2.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Health-Related Quality of Life
3.3. Mood State
3.4. Self-Perceived Physical Fitness
3.5. Physical Activity Level
3.6. Pain
3.7. Cancer-Related Fatigue
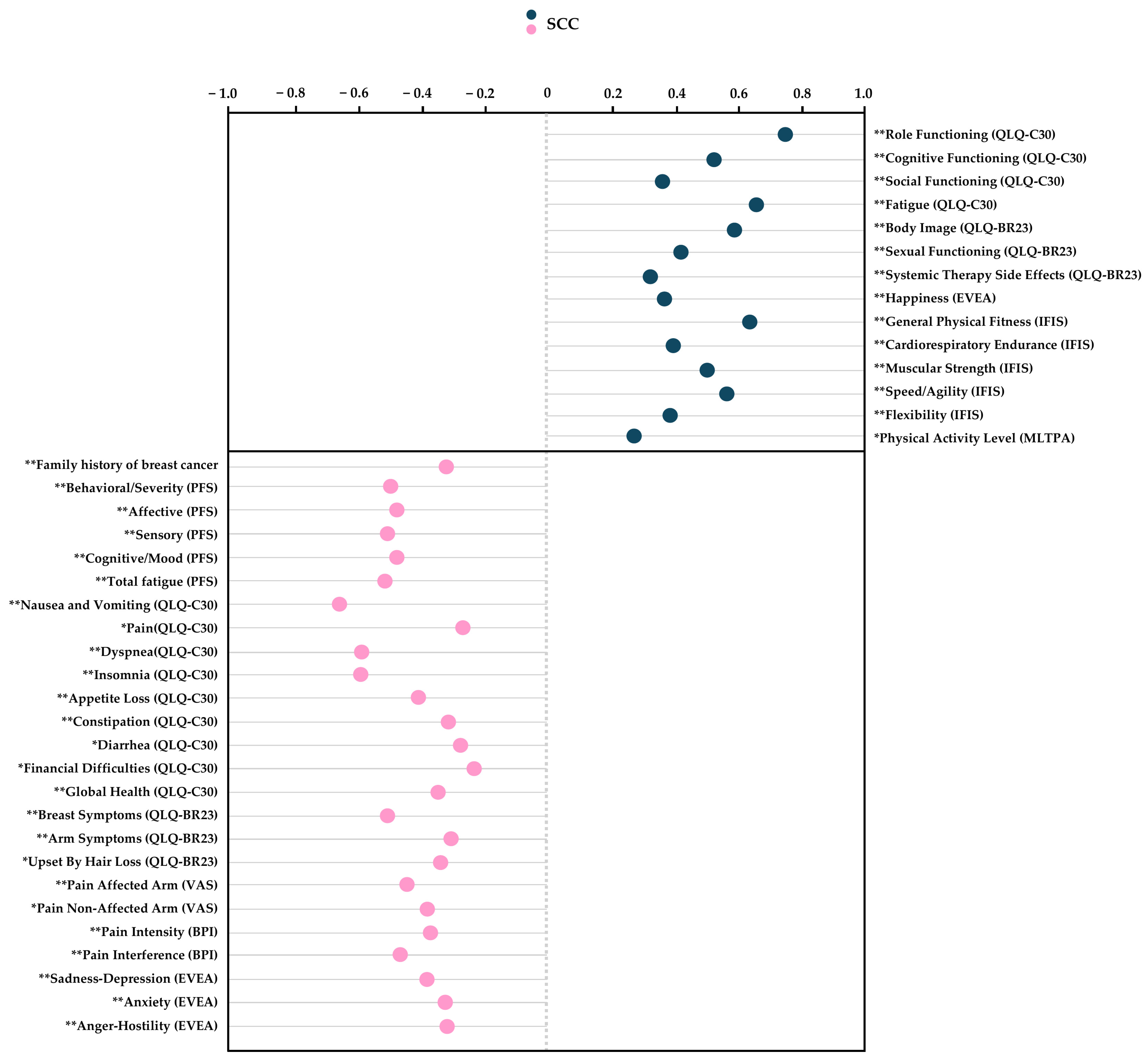
3.8. Correlation Analysis
3.9. Multiple Linear Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPI | Brief Pain Inventory |
| BC | Breast cancer |
| CI | Confidence interval |
| CRF | Cancer-related fatigue |
| EVEA | Scale for Mood Assessment |
| HRQoL | Health-related quality of life |
| ICC | Intraclass correlation coefficient |
| IFIS | International Fitness Scale |
| LTBCSs | Long-term breast cancer survivors |
| MET | Metabolic equivalent task |
| MLTPA | Minnesota Leisure Time Physical Activity |
| PA | Physical activity |
| PFS | Piper Fatigue Scale |
| QLQ-C30 | EORTC Core Quality of Life Quality of Life Questionnaire |
| QLQ-BR23 | The Breast Cancer-Specific Module |
| SCC | Spearman’s correlation coefficient |
| SD | Standard deviation |
| VAS | Visual Analogue Scale |
References
- Milojevich, H.M.; Lindquist, K.A.; Sheridan, M.A. Adversity and Emotional Functioning. Affect. Sci. 2021, 2, 324–344. [Google Scholar] [CrossRef] [PubMed]
- Ebbestad, F.E.; Ammitzbøll, G.; Horsbøll, T.A.; Andersen, I.; Johansen, C.; Zehran, B.; Dalton, S.O. The Long-Term Burden of a Symptom Cluster and Association with Longitudinal Physical and Emotional Functioning in Breast Cancer Survivors. Acta Oncol. 2023, 62, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kamata, A.; Hino, K.; Kamiyama, K.; Takasaka, Y. Very Late Recurrence in Breast Cancer: Is Breast Cancer a Chronic Disease? Cureus 2022, 14, e22804. [Google Scholar] [CrossRef] [PubMed]
- Breidenbach, C.; Heidkamp, P.; Hiltrop, K.; Pfaff, H.; Enders, A.; Ernstmann, N.; Kowalski, C. Prevalence and Determinants of Anxiety and Depression in Long-Term Breast Cancer Survivors. BMC Psychiatry 2022, 22, 101. [Google Scholar] [CrossRef]
- Zhu, Y.; Jha, S.C.; Shutta, K.H.; Huang, T.; Balasubramanian, R.; Clish, C.B.; Hankinson, S.E.; Kubzansky, L.D. Psychological Distress and Metabolomic Markers: A Systematic Review of Posttraumatic Stress Disorder, Anxiety, and Subclinical Distress. Neurosci. Biobehav. Rev. 2022, 143, 104954. [Google Scholar] [CrossRef]
- Tang, W.-Z.; Mangantig, E.; Iskandar, Y.H.P.; Cheng, S.-L.; Yusuf, A.; Jia, K. Prevalence and Associated Factors of Psychological Distress among Patients with Breast Cancer: A Systematic Review and Meta-Analysis. BMJ Open 2024, 14, e077067. [Google Scholar] [CrossRef]
- Durosini, I.; Triberti, S.; Savioni, L.; Sebri, V.; Pravettoni, G. The Role of Emotion-Related Abilities in the Quality of Life of Breast Cancer Survivors: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 12704. [Google Scholar] [CrossRef]
- Götze, H.; Friedrich, M.; Taubenheim, S.; Dietz, A.; Lordick, F.; Mehnert, A. Depression and Anxiety in Long-Term Survivors 5 and 10 Years after Cancer Diagnosis. Support. Care Cancer 2020, 28, 211–220. [Google Scholar] [CrossRef]
- Ren, Y.; Maselko, J.; Tan, X.; Olshan, A.F.; Stover, A.M.; Bennett, A.V.; Reeder-Hayes, K.E.; Edwards, J.K.; Reeve, B.B.; Troester, M.A.; et al. Emotional and Functional Well-Being in Long-Term Breast Cancer Survivorship. Cancer Causes Control 2024, 35, 1191–1200. [Google Scholar] [CrossRef]
- Aitken, L.-A.; Hossan, S.Z. The Psychological Distress and Quality of Life of Breast Cancer Survivors in Sydney, Australia. Healthcare 2022, 10, 2017. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-Luque, A.; Victoria Cerezo, M.; López, E.; Sibole, J.V. Emotional distress among long-term breast cancer survivors: The role of insomnia and worry. Behav. Psychol. 2020, 28, 533–549. [Google Scholar]
- Rodriguez-Gonzalez, A.; Hernández, R.; Cruz-Castellanos, P.; Fernández-Montes, A.; Castillo-Trujillo, O.; Muñoz, M.M.; Cano-Cano, J.M.; Corral, M.J.; Esteban, E.; Jiménez-Fonseca, P.; et al. Using the Emotional Functioning in Clinical Practice to Detect Psychological Distress in Patients with Advanced Thoracic and Colorectal Cancer. Health Qual. Life Outcomes 2023, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Oort, Q.; Zwinkels, H.; Koekkoek, J.A.F.; Vos, M.J.; Reijneveld, J.C.; Taphoorn, M.J.B.; Dirven, L. Is the EORTC QLQ-C30 Emotional Functioning Scale Appropriate as an Initial Screening Measure to Identify Brain Tumour Patients Who May Possibly Have a Mood Disorder? Psychooncology 2022, 31, 995–1002. [Google Scholar] [CrossRef]
- Tavoli, A.; Tavoli, Z.; Montazeri, A. The relationship between emotional functioning of the EORTC QLQ-C30 and A measure of anxiety and depression (HADS) in cancer patients. Int. J. Cancer Manag. 2019, 12, e94568. [Google Scholar] [CrossRef]
- Snyder, C.F.; Blackford, A.L.; Okuyama, T.; Akechi, T.; Yamashita, H.; Toyama, T.; Carducci, M.A.; Wu, A.W. Using the EORTC-QLQ-C30 in Clinical Practice for Patient Management: Identifying Scores Requiring a Clinician’s Attention. Qual. Life Res. 2013, 22, 2685–2691. [Google Scholar] [CrossRef]
- Lidington, E.; Giesinger, J.M.; Janssen, S.H.M.; Tang, S.; Beardsworth, S.; Darlington, A.-S.; Starling, N.; Szucs, Z.; Gonzalez, M.; Sharma, A.; et al. Identifying Health-Related Quality of Life Cut-off Scores That Indicate the Need for Supportive Care in Young Adults with Cancer. Qual. Life Res. 2022, 31, 2717–2727. [Google Scholar] [CrossRef]
- Tran, T.X.M.; Jung, S.-Y.; Lee, E.-G.; Cho, H.; Kim, N.Y.; Shim, S.; Kim, H.Y.; Kang, D.; Cho, J.; Lee, E.; et al. Fear of Cancer Recurrence and Its Negative Impact on Health-Related Quality of Life in Long-Term Breast Cancer Survivors. Cancer Res. Treat. 2022, 54, 1065–1073. [Google Scholar] [CrossRef]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on Medical Research Involving Human Subjects: A Review of Seventh Revision. J. Nepal Health Res. Counc. 2020, 17, 548–552. [Google Scholar] [CrossRef]
- Cocks, K.; Wells, J.R.; Johnson, C.; Schmidt, H.; Koller, M.; Oerlemans, S.; Velikova, G.; Pinto, M.; Tomaszewski, K.A.; Aaronson, N.K.; et al. Content Validity of the EORTC Quality of Life Questionnaire QLQ-C30 for Use in Cancer. Eur. J. Cancer 2023, 178, 128–138. [Google Scholar] [CrossRef]
- Calderon, C.; Ferrando, P.J.; Lorenzo-Seva, U.; Ferreira, E.; Lee, E.M.; Oporto-Alonso, M.; Obispo-Portero, B.M.; Mihic-Góngora, L.; Rodríguez-González, A.; Jiménez-Fonseca, P. Psychometric Properties of the Spanish Version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30). Qual. Life Res. 2022, 31, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; de Rooij, B.H.; Kieffer, J.; Oerlemans, S.; Mols, F.; Aaronson, N.K.; van der Graaf, W.T.A.; van de Poll-Franse, L.V. The EORTC QLQ-C30 Summary Score as Prognostic Factor for Survival of Patients with Cancer in the “Real-World”: Results from the Population-Based PROFILES Registry. Oncologist 2019, 25, e722–e732. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Gutiérrez, S.; García-Vera, M.P. Psychometric properties of the Scale for Mood Assessment (EVEA): A review. Ansiedad Y Estrés 2014, 20, 27–49. [Google Scholar]
- Álvarez-Gallardo, I.C.; Soriano-Maldonado, A.; Segura-Jiménez, V.; Carbonell-Baeza, A.; Estévez-López, F.; McVeigh, J.G.; Delgado-Fernández, M.; Ortega, F.B. International FItness Scale (IFIS): Construct Validity and Reliability in Women with Fibromyalgia: The al-Ándalus Project. Arch. Phys. Med. Rehabil. 2016, 97, 395–404. [Google Scholar] [CrossRef]
- Aandstad, A. Self-Perceived and Self-Tested Endurance: Associations with Objective Measures. Percept. Mot. Skills 2022, 129, 1492–1503. [Google Scholar] [CrossRef]
- Henström, M.; Leppänen, M.H.; Henriksson, P.; Söderström, E.; Sandborg, J.; Ortega, F.B.; Löf, M. Self-Reported (IFIS) versus Measured Physical Fitness, and Their Associations to Cardiometabolic Risk Factors in Early Pregnancy. Sci. Rep. 2021, 11, 22719. [Google Scholar] [CrossRef]
- Ruiz Comellas, A.; Pera, G.; Baena Díez, J.M.; Mundet Tudurí, X.; Alzamora Sas, T.; Elosua, R.; Torán Monserrat, P.; Heras, A.; Forés Raurell, R.; Fusté Gamisans, M.; et al. Validation of a Spanish short version of the Minnesota leisure time Physical Activity Questionnaire (VREM). Rev. Esp. Salud Publica 2012, 86, 495–508. [Google Scholar]
- Herrmann, S.D.; Willis, E.A.; Ainsworth, B.E.; Barreira, T.V.; Hastert, M.; Kracht, C.L.; Schuna, J.M., Jr.; Cai, Z.; Quan, M.; Tudor-Locke, C.; et al. 2024 Adult Compendium of Physical Activities: A Third Update of the Energy Costs of Human Activities. J. Sport Health Sci. 2024, 13, 6–12. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Mendes, M.d.A.; da Silva, I.; Ramires, V.; Reichert, F.; Martins, R.; Ferreira, R.; Tomasi, E. Metabolic Equivalent of Task (METs) Thresholds as an Indicator of Physical Activity Intensity. PLoS ONE 2018, 13, e0200701. [Google Scholar] [CrossRef]
- Matthews, C.E.; Moore, S.C.; Arem, H.; Cook, M.B.; Trabert, B.; Håkansson, N.; Larsson, S.C.; Wolk, A.; Gapstur, S.M.; Lynch, B.M.; et al. Amount and Intensity of Leisure-Time Physical Activity and Lower Cancer Risk. J. Clin. Oncol. 2020, 38, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Reneman, M.F.; Posthumus, J.B.; Stewart, R.E. Reliability and Validity of the Visual Analogue Scale for Disability in Patients with Chronic Musculoskeletal Pain. Int. J. Rehabil. Res. 2008, 31, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Badia, X.; Muriel, C.; Gracia, A.; Manuel Núñez-Olarte, J.; Perulero, N.; Gálvez, R.; Carulla, J.; Cleeland, C.S. Validación española del cuestionario Brief Pain Inventory en pacientes con dolor de causa neoplásica. Med. Clin. 2003, 120, 52–59. [Google Scholar] [CrossRef]
- Piper, B.F.; Dibble, S.L.; Dodd, M.J.; Weiss, M.C.; Slaughter, R.E.; Paul, S.M. The Revised Piper Fatigue Scale: Psychometric Evaluation in Women with Breast Cancer. Oncol. Nurs. Forum 1998, 25, 677–684. [Google Scholar] [PubMed]
- Cantarero-Villanueva, I.; Fernández-Lao, C.; Díaz-Rodríguez, L.; Cuesta-Vargas, A.I.; Fernández-de-las-Peñas, C.; Piper, B.F.; Arroyo-Morales, M. The Piper Fatigue Scale-Revised: Translation and Psychometric Evaluation in Spanish-Speaking Breast Cancer Survivors. Qual. Life Res. 2014, 23, 271–276. [Google Scholar] [CrossRef]
- Stover, A.M.; Reeve, B.B.; Piper, B.F.; Alfano, C.M.; Smith, A.W.; Mitchell, S.A.; Bernstein, L.; Baumgartner, K.B.; McTiernan, A.; Ballard-Barbash, R. Deriving Clinically Meaningful Cut-Scores for Fatigue in a Cohort of Breast Cancer Survivors: A Health, Eating, Activity, and Lifestyle (HEAL) Study. Qual. Life Res. 2013, 22, 2279–2292. [Google Scholar] [CrossRef]
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.S.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P.; et al. Cancer-Related Fatigue, Version 2.2015. J. Natl. Compr. Canc. Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef]
- Fabi, A.; Bhargava, R.; Fatigoni, S.; Guglielmo, M.; Horneber, M.; Roila, F.; Weis, J.; Jordan, K.; Ripamonti, C.I. Cancer-Related Fatigue: ESMO Clinical Practice Guidelines for Diagnosis and Treatment. Ann. Oncol. 2020, 31, 713–723. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Academic: New York, NY, USA, 1977. [Google Scholar]
- Vatcheva, K.P.; Lee, M.; McCormick, J.B.; Rahbar, M.H. Multicollinearity in Regression Analyses Conducted in Epidemiologic Studies. Epidemiology 2016, 6, 227–235. [Google Scholar] [CrossRef]
- Phoosuwan, N.; Lundberg, P.C. Psychological Distress and Health-Related Quality of Life among Women with Breast Cancer: A Descriptive Cross-Sectional Study. Support. Care Cancer 2022, 30, 3177–3186. [Google Scholar] [CrossRef]
- Maass, S.W.M.C.; Boerman, L.M.; Verhaak, P.F.M.; Du, J.; de Bock, G.H.; Berendsen, A.J. Long-Term Psychological Distress in Breast Cancer Survivors and Their Matched Controls: A Cross-Sectional Study. Maturitas 2019, 130, 6–12. [Google Scholar] [CrossRef]
- Abdelhadi, O. The impact of psychological distress on quality of care and access to mental health services in cancer survivors. Front. Health Serv. 2023, 3, 1111677. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, N.; Zhong, L.; Wang, S.; Zheng, Y.; Yang, B.; Zhang, J.; Lin, Y.; Wang, Z. Prognostic Value of Depression and Anxiety on Breast Cancer Recurrence and Mortality: A Systematic Review and Meta-Analysis of 282,203 Patients. Mol. Psychiatry 2020, 25, 3186–3197. [Google Scholar] [CrossRef] [PubMed]
- Baziliansky, S.; Cohen, M. Emotion Regulation and Psychological Distress in Cancer Survivors: A Systematic Review and Meta-analysis. Stress Health 2021, 37, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Fresno-Alba, S.; Leyton-Román, M.; Mesquita da Silva, S.; Jiménez-Castuera, R. Predicting Quality of Life in Women with Breast Cancer Who Engage in Physical Exercise: The Role of Psychological Variables. Healthcare 2023, 11, 2088. [Google Scholar] [CrossRef]
- Baceviciene, M.; Jankauskiene, R.; Emeljanovas, A. Self-Perception of Physical Activity and Fitness Is Related to Lower Psychosomatic Health Symptoms in Adolescents with Unhealthy Lifestyles. BMC Public Health 2019, 19, 980–991. [Google Scholar] [CrossRef]
- Sun, M.; Liu, C.; Lu, Y.; Zhu, F.; Li, H.; Lu, Q. Effects of Physical Activity on Quality of Life, Anxiety and Depression in Breast Cancer Survivors: A Systematic Review and Meta-Analysis. Asian Nurs. Res. 2023, 17, 276–285. [Google Scholar] [CrossRef]
- Patsou, E.D.; Alexias, G.D.; Anagnostopoulos, F.G.; Karamouzis, M.V. Effects of Physical Activity on Depressive Symptoms during Breast Cancer Survivorship: A Meta-Analysis of Randomised Control Trials. ESMO Open 2017, 2, e000271. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Zhang, S.; Zhou, Y.; Lv, Y.; Feng, L.; Yu, L. Effects of Exercise on Depression and Anxiety in Breast Cancer Survivors: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Cancer Med. 2025, 14, e70671. [Google Scholar] [CrossRef]
- Bjerkeset, E.; Röhrl, K.; Schou-Bredal, I. Symptom Cluster of Pain, Fatigue, and Psychological Distress in Breast Cancer Survivors: Prevalence and Characteristics. Breast Cancer Res. Treat. 2020, 180, 63–71. [Google Scholar] [CrossRef]
- Lobefaro, R.; Rota, S.; Porcu, L.; Brunelli, C.; Alfieri, S.; Zito, E.; Taglialatela, I.; Ambrosini, M.; Spagnoletti, A.; Zimatore, M.; et al. Cancer-Related Fatigue and Depression: A Monocentric, Prospective, Cross-Sectional Study in Advanced Solid Tumors. ESMO Open 2022, 7, 100457. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-P.; Von Ah, D.; Chen, M.-K.; Saligan, L.N. Relationship of Cancer-Related Fatigue with Psychoneurophysiological (PNP) Symptoms in Breast Cancer Survivors. Eur. J. Oncol. Nurs. 2024, 68, 102469. [Google Scholar] [CrossRef]
- Cipriani, G.E.; Bartoli, M.; Amanzio, M. Are Sleep Problems Related to Psychological Distress in Healthy Aging during the COVID-19 Pandemic? A Review. Int. J. Environ. Res. Public Health 2021, 18, 10676. [Google Scholar] [CrossRef] [PubMed]
- Karamouzis, M.V.; Patsou, E.; Alexias, G.; Anagnostopoulos, F. Association between Physical Activity, Psychological Mood Profile and Self-Esteem among Breast Cancer Survivors. Ann. Oncol. 2016, 27, vi95. [Google Scholar] [CrossRef]
- Halbach, S.M.; Midding, E.; Ernstmann, N.; Würstlein, R.; Weber, R.; Christmann, S.; Kowalski, C. Male Breast Cancer Patients’ Perspectives on Their Health Care Situation: A Mixed-Methods Study. Breast Care 2020, 15, 22–29. [Google Scholar] [CrossRef]
- Arraras, J.I.; Nolte, S.; Liegl, G.; Rose, M.; Manterola, A.; Illarramendi, J.J.; Zarandona, U.; Rico, M.; Teiejria, L.; Asin, G.; et al. General Spanish Population Normative Data Analysis for the EORTC QLQ-C30 by Sex, Age, and Health Condition. Health Qual. Life Outcomes 2021, 19, 208–219. [Google Scholar] [CrossRef]
| Characteristics | LTBCSs Emotional Functioning | ||
|---|---|---|---|
| Psychological Distress | Satisfactory Psychological Well-Being | p/X2 | |
| ≤90 (QLQ-C30) (n = 38) | ≥91 (QLQ-C30) (n = 42) | ||
| Mean age ± SD, years | 49.76 ± 7.20 | 49.04 ± 8.83 | 0.57 a |
| Mean time since diagnosis ± SD, months | 90.97 ± 28.56 | 90.01 ± 30.15 | 0.64 a |
| Mean time since the first surgery ± SD, months | 87.89 ± 29.04 | 87.34 ± 30.55 | 0.69 a |
| Marital Status, n (%) | |||
| Not married | 6 (15.8) | 7 (16.7) | |
| Married | 24 (63.2) | 31 (73.8) | |
| Divorced or separated | 6 (15.8) | 2 (4.8) | |
| Widowed | 2 (5.3) | 2 (4.8) | 0.42 b |
| Educational level, n (%) | |||
| Primary school | 18 (47.4) | 16 (38.1) | |
| Secondary school | 10 (26.3) | 8 (19.0) | |
| University | 10 (26.3) | 18 (42.9) | 0.29 b |
| Employment Status, n (%) | |||
| Housewife | 13 (34.2) | 12 (28.6) | |
| Currently working | 5 (13.2) | 12 (28.6) | |
| Sick leave | 15 (39.5) | 15 (35.7) | |
| Retired | 5 (13.2) | 3 (7.1) | 0.35 b |
| Tobacco consumption, n (%) | |||
| Non-consumption | 18 (47.4) | 22 (52.4) | |
| Smoker | 11 (28.9) | 8 (19.0) | |
| Ex-smoker | 9 (23.7) | 12 (28.6) | 0.57 b |
| Alcohol consumption, n (%) | |||
| Non-consumption | 14 (36.8) | 16 (38.1) | |
| Monthly | 12 (31.6) | 7 (16.7) | |
| Weekly | 9 (23.7) | 18 (42.9) | |
| Daily | 3 (7.9) | 1 (2.4) | 0.15 b |
| Family history of breast cancer, n (%) | |||
| No | 12 (31.6) | 27 (64.3) | |
| Yes | 26 (68.4) | 15 (35.7) | 0.06 b |
| Menopause, n (%) | |||
| No | 4 (10.5) | 7 (16.7) | |
| Yes | 34 (89.5) | 35 (83.3) | 0.42 b |
| Tumor stage, n (%) | |||
| I | 7 (18.4) | 16 (38.1) | |
| II | 26 (68.4) | 20 (47.6) | |
| III a | 5 (13.2) | 6 (14.3) | 0.12 b |
| Type of treatment, n (%) | |||
| None | 0 (0) | 0 (0) | |
| Radiotherapy | 3 (7.9) | 0 (0) | |
| Chemotherapy | 3 (7.9) | 3 (7.1) | |
| Radiotherapy and chemotherapy | 32 (84.2) | 39 (92.9) | 0.17 b |
| Surgery, n (%) | |||
| Lumpectomy | 6 (15.8) | 5 (23.8) | |
| Quadrantectomy | 17 (44.7) | 17 (52.4) | |
| Unilateral mastectomy | 12 (31.6) | 11 (21.4) | |
| Bilateral mastectomy | 3 (7.9) | 3 (2.4) | 0.41 b |
| Type of medication, n (%) | |||
| None | 8 (21.1) | 11 (26.2) | |
| Tamoxifen | 14 (36.8) | 17 (40.5) | |
| Other types | 16 (42.1) | 14 (33.3) | 0.70 b |
| Metastasis, n (%) | |||
| No | 30 (78.9) | 36 (85.7) | |
| Yes | 8 (21.1) | 6 (14.3) | 0.42 b |
| Recurrence, n (%) | |||
| No | 32 (84.2) | 35 (83.3) | |
| Yes | 6 (15.8) | 7 (16.7) | 0.91 b |
| Currently seeing a psychologist or in the last three months, n (%) | |||
| No | 17 (44.7) | 16 (38.1) | |
| Yes | 21 (55.3) | 26 (61.9) | 0.54 b |
| Currently seeing a physiotherapist or in the last three months, n (%) | |||
| No | 13 (34.2) | 16 (38.1) | |
| Yes | 25 (65.8) | 26 (61.9) | 0.71 b |
| Variables | LTBCSs Emotional Functioning | p Values | Cohen’s d | |
|---|---|---|---|---|
| Psychological Distress | Satisfactory Psychological Well-Being | |||
| ≤90 (QLQ-C30) (n = 38) | ≥91 (QLQ-C30) (n = 42) | |||
| Functioning Scales QLQ-C30, mean ± SD, median; IQR, and (95% CI) | ||||
| Physical Functioning | 35.08 ± 21.84 33.33; 0.00 (27.90–42.26) | 29.75 ± 16.26 33.33; 0.00 (24.68–34.82) | 0.21 | 0.28 |
| Role Functioning | 69.18 ± 21.33 73.33; 26.67 (62.17–76.19) | 92.83 ± 10.85 93.33; 12.58 (89.44–96.21) | <0.01 ** | >1.20 |
| Cognitive Functioning | 50.65 ± 30.90 50.00; 45.83 (40.50–60.81) | 77.97 ± 24.47 79.16; 33.33 (70.34–85.60) | <0.01 ** | 0.98 |
| Social Functioning | 53.06 ± 32.61 50.00; 50.00 (42.34–63.79) | 69.84 ± 27.35 83.33; 37.50 (61.31–78.36) | 0.01 * | 0.56 |
| Symptom Scales QLQ-C30, mean ± SD, median; IQR, and (95% CI) | ||||
| Fatigue | 88.88 ± 21.03 100.00; 16.67 (82.33–95.44) | 54.38 ± 32.34 58.33; 37.51 (43.75–65.01) | <0.01 ** | >1.20 |
| Nausea and Vomiting | 54.96 ± 28.05 44.44; 55.55 (45.74–64.19) | 19.84 ± 23.98 11.11; 33.33 (12.36–27.31) | <0.01 ** | >1.20 |
| Pain | 10.96 ± 21.32 0.00; 16.67 (3.95–17.97) | 4.36 ± 14.28 0.00; 0.00 (−0.08–8.81) | 0.03 * | 0.36 |
| Single Items QLQ-C30, mean ± SD, median; IQR, and (95% CI) | ||||
| Dyspnea | 55.70 ± 30.82 50.00; 50.00 (45.56–65.82) | 23.41 ± 25.25 16.67; 33.33 (15.54–31.28) | <0.01 ** | 1.15 |
| Insomnia | 38.59 ± 33.35 33.33; 0.52 (27.63–49.56) | 9.52 ± 21.19 0.00; 0.00 (2.91–16.12) | <0.01 ** | 1.04 |
| Appetite Loss | 62.28 ± 30.18 66.67; 66.67 (52.35–72.20) | 39.28 ± 34.10 33.33; 66.67 (28.65–49.91) | <0.01 ** | 0.71 |
| Constipation | 16.66 ± 29.76 0.00; 33.33 (6.88–26.44) | 7.14 ± 20.20 0.00; 0.00 (0.84–13.44) | 0.06 | 0.37 |
| Diarrhea | 32.45 ± 36.75 33.33; 66.67 (20.37–44.53) | 18.65 ± 25.55 0.00; 33.33 (10.68–26.61) | 0.11 | 0.44 |
| Financial Difficulties | 18.42 ± 28.68 0.00; 33.33 (8.99–27.84) | 6.34 ± 15.15 0.00; 0.00 (1.62–11.07) | 0.03 | 0.53 |
| Global Health Status QLQ-C30, mean ± SD, median; IQR, and (95% CI) | ||||
| Global Health Status | 14.28 ± 28.64 0.00; 33.33 (5.35–23.21) | 32.54 ± 36.67 0.00; 0.00 (20.48–44.59) | <0.01 ** | 0.56 |
| Summary Score QLQ-C30, mean ± SD, median; IQR, and (95% CI) | ||||
| Summary Score | 56.81 ± 15.32 59.10; 20.07 (51.77–61.85) | 73.79 ± 10.59 76.02; 12.10 (70.48–77.09) | <0.01 ** | >1.20 |
| Functional Scales QLQ-BR23, mean ± SD (95% CI) | ||||
| Body Image | 51.53 ± 18.96 50.00; 33.34 (45.29–57.77) | 74.60 ± 19.90 75.00; 25.00 (68.40–80.80) | <0.01 ** | 1.19 |
| Sexual Functioning | 66.22 ± 31.17 75.00; 50.00 (55.98–76.47) | 87.10 ± 20.59 100; 16.67 (80.68–93.52) | <0.01 ** | 0.79 |
| Sexual Enjoyment | 19.73 ± 23.84 16.67; 33.33 (11.90–27.57) | 23.80 ± 18.45 33.33; 33.33 (18.05–29.55) | 0.16 | 0.19 |
| Future Perspective | 30.70 ± 26.14 33.33; 33.33 (22.10–39.29) | 33.33 ± 24.41 33.33; 0.00 (25.72–40.94) | 0.55 | 0.10 |
| Symptom Scales QLQ-BR23, mean ± SD, median; IQR, and (95% CI) | ||||
| Systemic Therapy Side Effects | 65.08 ± 39.61 66.67; 66.67 (52.73–77.42) | 42.98 ± 33.69 33.33; 66.67 (31.90–54.05) | <0.01 ** | 0.60 |
| Breast Symptoms | 37.84 ± 20.71 33.33; 28.57 (31.03–44.65) | 20.26 ± 20.84 14.29; 23.81 (13.76–20.76) | <0.01 ** | 0.85 |
| Arm Symptoms | 34.21 ± 30.55 25.00; 47.75 (24.16–44.25) | 18.84 ± 22.09 12.50; 25.00 (11.96–25.73) | 0.01 * | 0.58 |
| Upset By Hair Loss | 41.81 ± 33.12 38.88; 66.67 (30.92–52.70) | 22.75 ± 25.84 22.22; 33.33 (14.69–30.80) | <0.01 ** | 0.64 |
| Variables | LTBCSs Emotional Functioning | p/X2 | Cohen’s d | ||
|---|---|---|---|---|---|
| Psychological Distress | Satisfactory Psychological Well-Being | ||||
| ≤90 (QLQ-C30) (n = 38) | ≥91 (QLQ-C30) (n = 42) | ||||
| EVEA, mean ± SD, median; IQR, and (95% CI) a | |||||
| Sadness–depression | 3.86 ± 2.83 4.12; 5.06 (2.93–4.79) | 2.06 ± 2.29 1.12; 3.56 (1.34–2.78) | <0.01 ** | 0.70 | |
| Anxiety | 3.95 ± 2.72 4.25; 4.63 (3.05–4.85) | 2.24 ± 2.23 1.50; 2.75 (1.54–2.94) | <0.01 ** | 0.69 | |
| Anger–hostility | 3.12 ± 2.67 2.62; 4.50 (2.24–4.00) | 1.45 ± 2.04 0.50; 2.00 (0.82–2.09) | <0.01 ** | 0.70 | |
| Happiness | 5.79 ± 8.44 4.12; 2.50 (3.01–8.57) | 6.17 ± 2.41 6.62; 3.50 (5.42–6.92) | <0.01 ** | 0.06 | |
| IFIS, mean ± SD, median; IQR, and (95% CI) a | |||||
| General physical fitness | 2.68 ± 0.77 3.00; 1.00 (2.42–2.93) | 3.83 ± 0.85 4.00; 2.00 (3.56–4.09) | <0.01 ** | >1.20 | |
| Cardiorespiratory endurance | 2.44 ± 1.00 2.50; 1.00 (2.11–2.77) | 3.28 ± 1.04 3.00; 1.25 (2.96–3.61) | <0.01 ** | 0.82 | |
| Muscular strength | 2.42 ± 0.82 2.00; 1.00 (2.14–2.69) | 3.28 ± 1.01 3.00; 1.25 (2.96–3.60) | <0.01 ** | 0.93 | |
| Speed/agility | 2.39 ± 0.71 2.00; 1.00 (2.15–2.63) | 3.45 ± 0.88 3.00; 1.00 (3.17–3.72) | <0.01 ** | >1.20 | |
| Flexibility | 2.47 ± 0.89 2.50; 1.00 (2.18–2.76) | 3.23 ± 1.03 3.00; 1.00 (2.91–3.55) | <0.01 ** | 0.79 | |
| MLTPA, n (%) b | |||||
| Inactive: ≤3 (MET hour/week) | 12 (31.6) | 9 (21.4) | <0.01 ** | ||
| Low active: 3.1–7.4 (MET hour/week) | 17 (44.7) | 15 (35.7) | |||
| Active: ≥7.5 (MET hour/week) | 9 (23.7) | 18 (42.9) | - | ||
| VAS (cm), mean ± SD, median; IQR, and (95% CI) a | |||||
| Dominant arm | 3.39 ± 2.79 4.00; 5.25 (2.47–4.31) | 1.33 ± 1.94 0.00; 2.25 (0.72–1.93) | <0.01 ** | 0.86 | |
| Non-dominant arm | 2.13 ± 2.94 0.50; 3.50 (1.16–3.09) | 0.85 ± 2.29 0.00; 0.00 (0.14–1.57) | <0.01 ** | 0.49 | |
| BPI, mean ± SD, median; IQR, and (95% CI) a | |||||
| Intensity | 3.06 ± 2.73 3.33; 5.42 (2.16–3.96) | 1.57 ± 2.04 0.66; 2.84 (0.93–2.20) | 0.01 * | 0.62 | |
| Interference | 3.14 ± 2.90 3.07; 5.33 (2.18–4.09) | 1.02 ± 1.95 0.00; 1.07 (0.41–1.63) | <0.01 ** | 0.86 | |
| PFS (domains), mean ± SD, median; IQR, and (95% CI) a | |||||
| Behavioral/severity | 4.17 ± 2.84 3.91; 5.04 (3.23–5.10) | 1.92 ± 2.46 0.53; 3.43 (1.15–2.69) | <0.01 ** | 0.85 | |
| Affective | 4.68 ± 2.86 4.80; 3.90 (3.73–5.62) | 2.09 ± 2.94 0.50; 3.50 (1.17–3.00) | <0.01 ** | 0.89 | |
| Sensory | 4.98 ± 2.74 5.50; 3.65 (4.08–5.88) | 2.10 ± 2.73 0.90; 3.25 (1.25–2.96) | <0.01 ** | 1.05 | |
| Cognitive/mood | 4.57 ± 2.64 5.08; 3.75 (3.70–5.43) | 1.96 ± 2.68 1.00; 2.37 (1.13–2.80) | <0.01 ** | 0.98 | |
| Total fatigue | 4.61 ± 2.64 4.84; 2.85 (3.79–5.43) | 2.01 ± 2.53 0.79; 2.85 (1.22–2.80) | <0.01 ** | 1.00 | |
| PFS (Cut-score type A), n (%) b | |||||
| No fatigue | 0 | 4 (10.5) | 22 (52.4) | ||
| Mild | 1–3 | 9 (23.7) | 12 (28.6) | ||
| Moderate | 4–6 | 20 (52.6) | 4 (9.5) | ||
| Severe | 7–10 | 5 (13.2) | 4 (9.5) | <0.01 ** | - |
| PFS (Cut-score type B), n (%) b | |||||
| No fatigue | 0 | 4 (10.5) | 22 (52.4) | ||
| Mild | 1–2 | 5 (13.2) | 11 (26.2) | ||
| Moderate | 3–5 | 19 (50) | 5 (11.9) | ||
| Severe | 6–10 | 10 (26.3) | 4 (9.5) | <0.01 ** | - |
| Model | Variables/ Predictors | β | 95% CI | t | F | p-Values | Regression Equation Y = a + bX |
|---|---|---|---|---|---|---|---|
| Model 1 (r2 = 0.573) | Role Functioning (QLQ-C30) | 0.75 | 0.87 ± 1.30 | 10.23 | 104.65 | <0.01 ** | Emotional Functioning = −9.47 + (1.08 Role Functioning) |
| Model 2 (r2 = 0.601) | Role Functioning (QLQ-C30) | 0.65 | 0.06 ± 1.18 | 7.68 | 57.88 | <0.01 ** | Emotional Functioning = −9.41 + (0.93 Role Functioning) + (0.18 Cognitive Functioning) |
| Cognitive Functioning (QLQ-C30) | 0.19 | 0.02 ± 0.34 | 2.30 | 0.02 * | |||
| Model 3 (r2 = 0.622) | Role Functioning (QLQ-C30) | 0.54 | 0.50 ± 1.06 | 5.54 | 41.69 | <0.01 ** | Emotional Functioning = −13.90 + (0.78 Role Functioning) + (−0.17 Cognitive Functioning) + (5.52 Self-Perceived General Physical Fitness) |
| Cognitive Functioning (QLQ-C30) | 0.18 | 0.01 ± 0.33 | 2.16 | 0.03 * | |||
| Self-Perceived General Physical Fitness (IFIS) | 0.18 | 0.22 ± 10.81 | 2.07 | 0.04 * | |||
| Model 4 (r2 = 0.642) | Role Functioning (QLQ-C30) | 0.59 | 0.56 ± 1.13 | 5.97 | 33.57 | <0.01 ** | Emotional Functioning = −32.10 + (0.84 Role Functioning) + (0.24 Cognitive Functioning) + (6.15 Self-Perceived General Physical Fitness) + (2.02 Sadness–Depression) |
| Cognitive Functioning (QLQ-C30) | 0.26 | 0.07 ± 0.42 | 2.86 | <0.01 ** | |||
| Self-Perceived General Physical Fitness (IFIS) | 0.20 | 0.92 ± 11.37 | 2.34 | 0.02 * | |||
| Sadness–Depression (EVEA) | 0.18 | 0.03 ± 4.00 | 2.02 | 0.04 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Salvago, F.; Atienzar-Aroca, S.; Pujol-Fuentes, C.; Figueroa-Mayordomo, M.; Molina-García, C.; Gutiérrez-García, P.; Medina-Luque, J. Emotional Functioning in Long-Term Breast Cancer Survivors: A Cross-Sectional Study on Its Influence and Key Predictors. Cancers 2025, 17, 1574. https://doi.org/10.3390/cancers17091574
Álvarez-Salvago F, Atienzar-Aroca S, Pujol-Fuentes C, Figueroa-Mayordomo M, Molina-García C, Gutiérrez-García P, Medina-Luque J. Emotional Functioning in Long-Term Breast Cancer Survivors: A Cross-Sectional Study on Its Influence and Key Predictors. Cancers. 2025; 17(9):1574. https://doi.org/10.3390/cancers17091574
Chicago/Turabian StyleÁlvarez-Salvago, Francisco, Sandra Atienzar-Aroca, Clara Pujol-Fuentes, Maria Figueroa-Mayordomo, Cristina Molina-García, Palmira Gutiérrez-García, and Jose Medina-Luque. 2025. "Emotional Functioning in Long-Term Breast Cancer Survivors: A Cross-Sectional Study on Its Influence and Key Predictors" Cancers 17, no. 9: 1574. https://doi.org/10.3390/cancers17091574
APA StyleÁlvarez-Salvago, F., Atienzar-Aroca, S., Pujol-Fuentes, C., Figueroa-Mayordomo, M., Molina-García, C., Gutiérrez-García, P., & Medina-Luque, J. (2025). Emotional Functioning in Long-Term Breast Cancer Survivors: A Cross-Sectional Study on Its Influence and Key Predictors. Cancers, 17(9), 1574. https://doi.org/10.3390/cancers17091574









