Patterns of Recurrence After Postoperative Stereotactic Radiotherapy for Brain Metastases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Radiological Characterization
2.3. Treatment Outcomes
2.4. Statistical Analyses
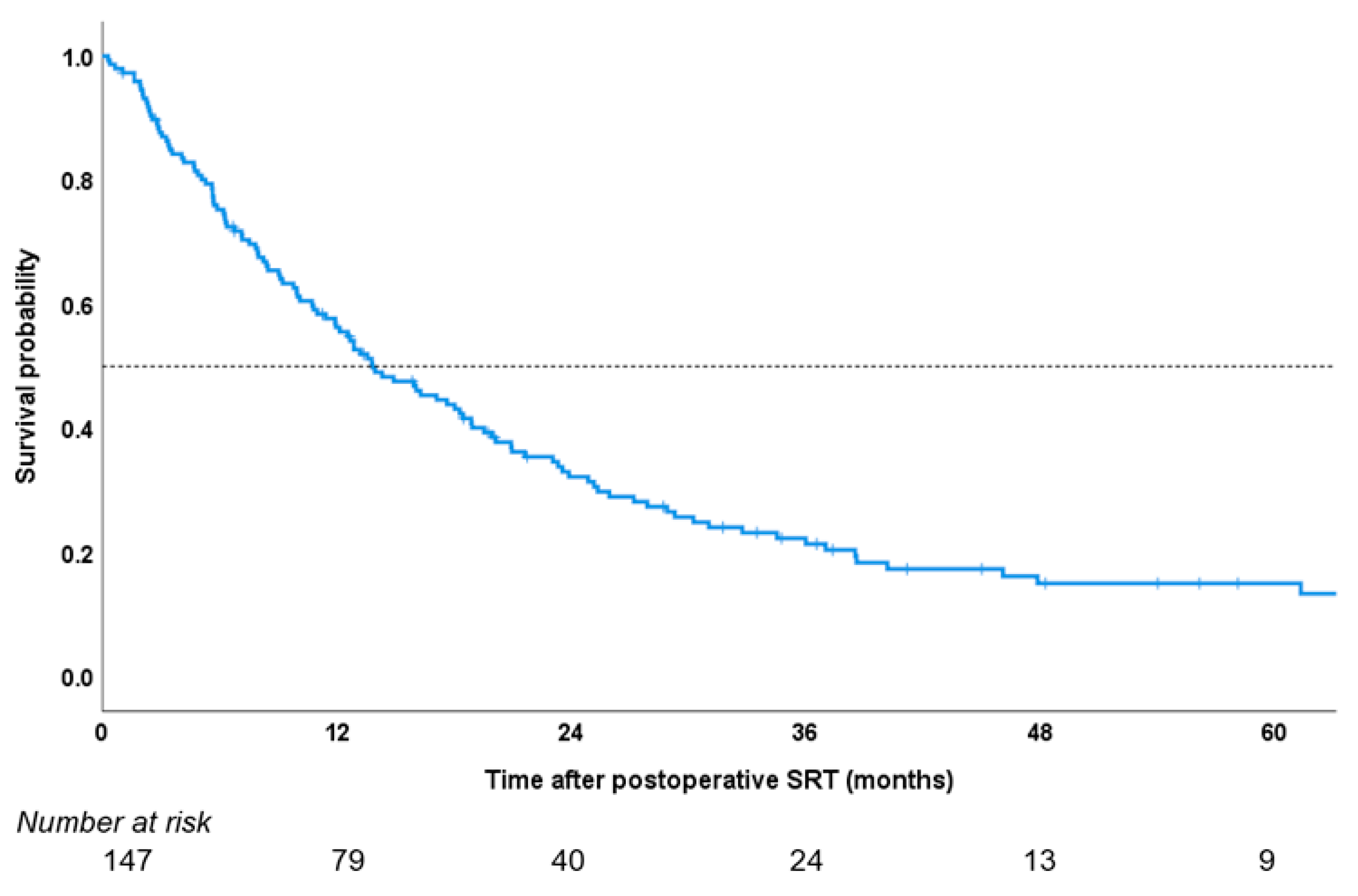
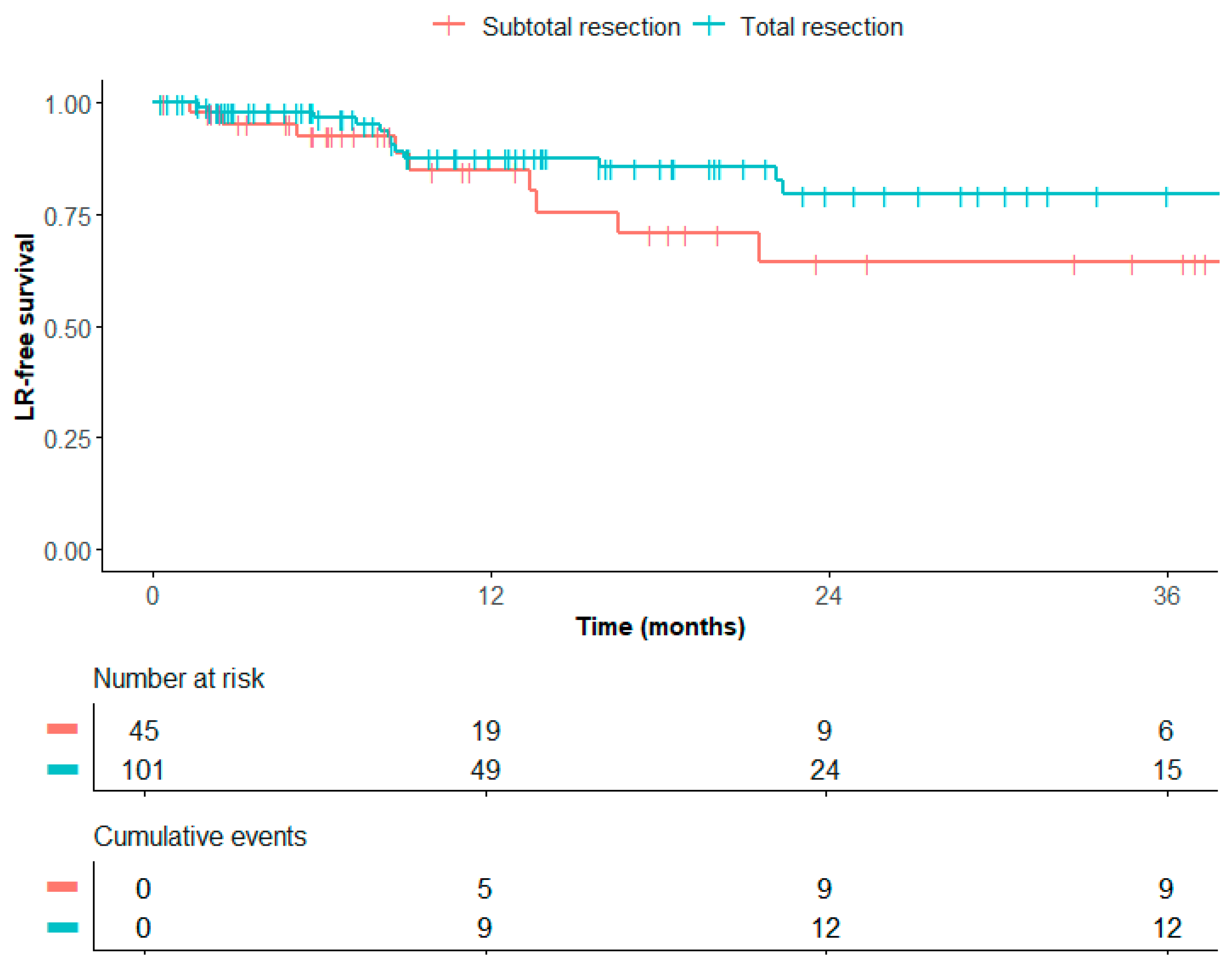
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BM(s) | Brain metastasis/metastases |
| CSF | Cerebrospinal fluid |
| CTV | Clinical target volume |
| DSC | Dynamic susceptibility contrast |
| IQR | Interquartile range |
| LMD | Leptomeningeal disease |
| LR | Local recurrence |
| MRI | Magnetic resonance imaging |
| NSCLC | Non-small-cell lung cancer |
| PTV | Planning target volume |
| QA | Quality assurance |
| SRT | Stereotactic radiotherapy |
| VIBE | Volumetric-interpolated breath-hold examination |
Appendix A
References
- Le Rhun, E.; Guckenberger, M.; Smits, M.; Dummer, R.; Bachelot, T.; Sahm, F.; Galldiks, N.; de Azambuja, E.; Berghoff, A.S.; Metellus, P.; et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann. Oncol. 2021, 32, 1332–1347. [Google Scholar] [CrossRef] [PubMed]
- Hilkens, N.A.; Enting, R.H.; Hendriks, L.E.L.; Lagerwaard, F.J.; de Vos, F.Y.F.L.; Gijtenbeek, J.M.M. Herziene richtlijn ‘Hersenmetastasen’. Ned. Tijdschr. Voor Geneeskd. 2020, 164, 1–4. [Google Scholar]
- Mahajan, A.; Ahmed, S.; McAleer, M.F.; Weinberg, J.S.; Li, J.; Brown, P.; Settle, S.; Prabhu, S.S.; Lang, F.F.; Levine, N.; et al. Prospective Randomized Trial of Post-operative Stereotactic Radiosurgery versus Observation for Completely Resected Brain Metastases. Lancet Oncol. 2017, 18, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Bilger, A.; Bretzinger, E.; Fennell, J.; Nieder, C.; Lorenz, H.; Oehlke, O.; Grosu, A.L.; Specht, H.M.; Combs, S.E. Local control and possibility of tailored salvage after hypofractionated stereotactic radiotherapy of the cavity after brain metastases resection. Cancer Med. 2018, 7, 2350–2359. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Bilger, A.; Diehl, C.; Bretzinger, E.; Lorenz, H.; Oehlke, O.; Specht, H.M.; Kirstein, A.; Grosu, A.L. Multicenter analysis of stereotactic radiotherapy of the resection cavity in patients with brain metastases. Cancer Med. 2018, 7, 2319–2327. [Google Scholar] [CrossRef]
- Foreman, P.M.; Jackson, B.E.; Singh, K.P.; Romeo, A.K.; Guthrie, B.L.; Fisher, W.S.; Riley, K.O.; Markert, J.M.; Willey, C.D.; Bredel, M.; et al. Postoperative radiosurgery for the treatment of metastatic brain tumor: Evaluation of local failure and leptomeningeal disease. J. Clin. Neurosci. 2018, 49, 48–55. [Google Scholar] [CrossRef]
- Johnson, M.D.; Avkshtol, V.; Baschnagel, A.M.; Meyer, K.; Ye, H.; Grills, I.S.; Chen, P.Y.; Maitz, A.; Olson, R.E.; Pieper, D.R.; et al. Surgical Resection of Brain Metastases and the Risk of Leptomeningeal Recurrence in Patients Treated with Stereotactic Radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 537–543. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Sahgal, A.; Detsky, J.; Atenafu, E.G.; Myrehaug, S.; Tseng, C.L.; Husain, Z.; Heyn, C.; Maralani, P.; Ruschin, M.; et al. Predictors of leptomeningeal disease following hypofractionated stereotactic radiotherapy for intact and resected brain metastases. Neuro-Oncology 2020, 22, 84–93. [Google Scholar] [CrossRef]
- Routman, D.M.; Yan, E.; Vora, S.; Peterson, J.; Mahajan, A.; Chaichana, K.L.; Laack, N.; Brown, P.D.; Parney, I.F.; Burns, T.C.; et al. Preoperative stereotactic radiosurgery for brain metastases. Front. Neurol. 2018, 9, 959. [Google Scholar] [CrossRef]
- Prabhu, R.S.; Patel, K.R.; Press, R.H.; Soltys, S.G.; Brown, P.D.; Mehta, M.P.; Asher, A.L.; Burri, S.H. Preoperative vs postoperative radiosurgery for resected brain metastases: A review. Neurosurgery 2019, 84, 19–29. [Google Scholar] [CrossRef]
- Cheng, H.; Perez-Soler, R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018, 19, e43–e55. [Google Scholar] [CrossRef]
- Franzoi, M.A.; Hortobagyi, G.N. Leptomeningeal carcinomatosis in patients with breast cancer. Crit. Rev. Oncol./Hematol. 2019, 135, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Steininger, J.; Gellrich, F.F.; Engellandt, K.; Meinhardt, M.; Westphal, D.; Beissert, S.; Meier, F.; Oliva, G.C.I. Leptomeningeal Metastases in Melanoma Patients: An Update on and Future Perspectives for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 11443. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Le Rhun, E.; Besse, B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev. 2017, 53, 128–137. [Google Scholar] [CrossRef]
- Puri, S.; Chaudhry, A.; Bayable, A.; Ganesh, A.; Daher, A.; Gadi, V.K.; Maraka, S. Systemic Treatment for Brain Metastasis and Leptomeningeal Disease in Breast Cancer Patients. Curr. Oncol. Rep. 2023, 25, 1419–1430. [Google Scholar] [CrossRef]
- Sherman, W.J.; Romiti, E.; Michaelides, L.; Moniz-Garcia, D.; Chaichana, K.L.; Quinones-Hinojosa, A.; Porter, A.B. Systemic Therapy for Melanoma Brain and Leptomeningeal Metastases. Curr. Treat. Options Oncol. 2023, 24, 1962–1977. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, R.; Jerzak, K.J.; Larrouquere, L.; Muller, V.; Le Rhun, E. Pharmacotherapy for leptomeningeal disease in breast cancer. Cancer Treat Rev. 2024, 122, 102653. [Google Scholar] [CrossRef]
- Le Rhun, E.; Weller, M.; van den Bent, M.; Brandsma, D.; Furtner, J.; Ruda, R.; Schadendorf, D.; Seoane, J.; Tonn, J.C.; Wesseling, P.; et al. Leptomeningeal metastasis from solid tumours: EANO-ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. ESMO Open 2023, 8, 101624. [Google Scholar] [CrossRef]
- Ahn, J.H.; Lee, S.H.; Kim, S.; Joo, J.; Yoo, H.; Lee, S.H.; Shin, S.H.; Gwak, H.S. Risk for leptomeningeal seeding after resection for brain metastases: Implication of tumor location with mode of resection: Clinical article. J. Neurosurg. 2012, 116, 984–993. [Google Scholar] [CrossRef]
- Kumar, A.M.S.; Miller, J.; Hoffer, S.A.; Mansur, D.B.; Coffey, M.; Lo, S.S.; Sloan, A.E.; Machtay, M. Postoperative hypofractionated stereotactic brain radiation (HSRT) for resected brain metastases: Improved local control with higher BED(10). J. Neurooncol. 2018, 139, 449–454. [Google Scholar] [CrossRef]
- Crouzen, J.A.; Petoukhova, A.L.; Broekman, M.L.D.; Fiocco, M.; Fisscher, U.J.; Franssen, J.H.; Gadellaa-van Hooijdonk, C.G.M.; Kerkhof, M.; Kiderlen, M.; Mast, M.E.; et al. SAFESTEREO: Phase II randomized trial to compare stereotactic radiosurgery with fractionated stereotactic radiosurgery for brain metastases. BMC Cancer 2023, 23, 273. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Niyazi, M.; Andratschke, N.; Guckenberger, M.; Palmer, J.D.; Shih, H.A.; Lo, S.S.; Soltys, S.; Russo, I.; Brown, P.D.; et al. Current status and recent advances in resection cavity irradiation of brain metastases. Radiat. Oncol. 2021, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Atalar, B.; Modlin, L.A.; Choi, C.Y.H.; Adler, J.R.; Gibbs, I.C.; Chang, S.D.; Harsh, I.V.G.R.; Li, G.; Nagpal, S.; Hanlon, A.; et al. Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 713–718. [Google Scholar] [CrossRef]
- Keller, A.; Doré, M.; Cebula, H.; Thillays, F.; Proust, F.; Darié, I.; Martin, S.A.; Delpon, G.; Lefebvre, F.; Noël, G.; et al. Hypofractionated Stereotactic Radiation Therapy to the Resection Bed for Intracranial Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Ferris, M.J.; Switchenko, J.; Press, R.H.; Buchwald, Z.; Olson, J.J.; Eaton, B.R.; Curran, W.J.; Shu, H.K.G.; Crocker, I.R.; et al. Postoperative stereotactic radiosurgery for resected brain metastases: A comparison of outcomes for large resection cavities. Pract. Radiat. Oncol. 2017, 7, e419–e425. [Google Scholar] [CrossRef]
- Shi, S.; Sandhu, N.; Jin, M.C.; Wang, E.; Jaoude, J.A.; Schofield, K.; Zhang, C.; Liu, E.; Gibbs, I.C.; Hancock, S.L.; et al. Stereotactic Radiosurgery for Resected Brain Metastases: Single-Institutional Experience of Over 500 Cavities. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 764–771. [Google Scholar] [CrossRef]
- Akanda, Z.Z.; Hong, W.; Nahavandi, S.; Haghighi, N.; Phillips, C.; Kok, D.L. Post-operative stereotactic radiosurgery following excision of brain metastases: A systematic review and meta-analysis. Radiother. Oncol. 2020, 142, 27–35. [Google Scholar] [CrossRef]
- Ojerholm, E.; Lee, J.Y.K.; Thawani, J.P.; Miller, D.; O’Rourke, D.M.; Dorsey, J.F.; Geiger, G.A.; Nagda, S.; Kolker, J.D.; Lustig, R.A.; et al. Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J. Neurosurg. 2014, 121, 75–83. [Google Scholar] [CrossRef]
- Lowe, S.R.; Wang, C.P.; Brisco, A.; Whiting, J.; Arrington, J.; Ahmed, K.; Yu, M.; Robinson, T.; Oliver, D.; Etame, A.; et al. Surgical and anatomic factors predict development of leptomeningeal disease in patients with melanoma brain metastases. Neuro-Oncology 2022, 24, 1307–1317. [Google Scholar] [CrossRef]
- Puri, A.; Mylavarapu, C.; Xu, J.; Patel, T.A.; Teh, B.S.; Tremont-Lukats, I.; Chang, J.C.; Niravath, P. Clinical factors and association with treatment modalities in patients with breast cancer and brain metastases who develop leptomeningeal metastases. Breast Cancer Res. Treat. 2022, 193, 613–623. [Google Scholar] [CrossRef]
- Iwai, Y.; Yamanaka, K.; Yasui, T. Boost radiosurgery for treatment of brain metastases after surgical resections. Surg. Neurol. 2008, 69, 181–186; discussion 6. [Google Scholar] [CrossRef] [PubMed]
- Morshed, R.A.; Saggi, S.; Cummins, D.D.; Molinaro, A.M.; Young, J.S.; Viner, J.A.; Villanueva-Meyer, J.E.; Goldschmidt, E.; Boreta, L.; Braunstein, S.E.; et al. Identification of risk factors associated with leptomeningeal disease after resection of brain metastases. J. Neurosurg. 2023, 139, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Tewarie, I.A.; Jessurun, C.A.C.; Hulsbergen, A.F.C.; Smith, T.R.; Mekary, R.A.; Broekman, M.L.D. Leptomeningeal disease in neurosurgical brain metastases patients: A systematic review and meta-analysis. Neuro-Oncol. Adv. 2021, 3, vdab162. [Google Scholar] [CrossRef] [PubMed]
- Suki, D.; Hatiboglu, M.A.; Patel, A.J.; Weinberg, J.S.; Groves, M.D.; Mahajan, A.; Sawaya, R. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery 2009, 64, 664–676. [Google Scholar] [CrossRef]
- DePaoli, B.; Gozal, Y.M.; Pater, L.E.; Breneman, J.C.; Warnick, R.E.; Elson, J.; Struve, T.D. Ventricular violation increases the risk of leptomeningeal disease in cavity-directed radiosurgery treated patients. J. Radiat. Oncol. 2018, 8, 23–29. [Google Scholar] [CrossRef]
- Jung, J.-M.; Kim, S.; Joo, J.; Shin, K.H.; Gwak, H.-S.; Lee, S.H. Incidence and Risk Factors for Leptomeningeal Carcinomatosis in Breast Cancer Patients with Parenchymal Brain Metastases. J. Korean Neurosurg. Soc. 2012, 52, 193. [Google Scholar] [CrossRef]
- Jo, K.-I.; Lim, D.-H.; Kim, S.-T.; Im, Y.-S.; Kong, D.S.; Seol, H.J.; Nam, D.-H.; Lee, J.-I. Leptomeningeal seeding in patients with brain metastases treated by gamma knife radiosurgery. J. Neuro-Oncol. 2012, 109, 293–299. [Google Scholar] [CrossRef]
- Huang, A.J.; Huang, K.E.; Page, B.R.; Ayala-Peacock, D.N.; Lucas, J.T.; Lesser, G.J.; Laxton, A.W.; Tatter, S.B.; Chan, M.D. Risk factors for leptomeningeal carcinomatosis in patients with brain metastases who have previously undergone stereotactic radiosurgery. J. Neuro-Oncol. 2014, 120, 163–169. [Google Scholar] [CrossRef]
- Ma, R.; Levy, M.; Gui, B.; Lu, S.E.; Narra, V.; Goyal, S.; Danish, S.; Hanft, S.; Khan, A.J.; Malhotra, J.; et al. Risk of leptomeningeal carcinomatosis in patients with brain metastases treated with stereotactic radiosurgery. J. Neuro-Oncol. 2018, 136, 395–401. [Google Scholar] [CrossRef]
- Chiang, C.L.; Yang, H.C.; Luo, Y.H.; Chen, C.J.; Wu, H.M.; Chen, Y.M.; Hu, Y.S.; Lin, C.J.; Chung, W.Y.; Shiau, C.Y.; et al. Leptomeningeal metastasis in patients with non-small cell lung cancer after stereotactic radiosurgery for brain metastasis. J. Neurosurg. 2022, 139, 385–392. [Google Scholar] [CrossRef]
- Asher, A.L.; Burri, S.H.; Wiggins, W.F.; Kelly, R.P.; Boltes, M.O.; Mehrlich, M.; Norton, H.J.; Fraser, R.W. A new treatment paradigm: Neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 899–906. [Google Scholar] [CrossRef]
- Prabhu, R.S.; Dhakal, R.; Vaslow, Z.K.; Dan, T.; Mishra, M.V.; Murphy, E.S.; Patel, T.R.; Asher, A.L.; Yang, K.; Manning, M.A.; et al. Preoperative Radiosurgery for Resected Brain Metastases: The PROPS-BM Multicenter Cohort Study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 764–772. [Google Scholar] [CrossRef]
- Prabhu, R.S.; Miller, K.R.; Asher, A.L.; Heinzerling, J.H.; Moeller, B.J.; Lankford, S.P.; McCammon, R.J.; Fasola, C.E.; Patel, K.R.; Press, R.H.; et al. Preoperative stereotactic radiosurgery before planned resection of brain metastases: Updated analysis of efficacy and toxicity of a novel treatment paradigm. J. Neurosurg. 2019, 131, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Burri, S.H.; Boselli, D.; Symanowski, J.T.; Asher, A.L.; Sumrall, A.; Fraser, R.W.; Press, R.H.; Zhong, J.; Cassidy, R.J.; et al. Comparing pre-operative stereotactic radiosurgery (SRS) to post-operative whole brain radiation therapy (WBRT) for resectable brain metastases: A multi-institutional analysis. J. Neuro-Oncol. 2017, 131, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Burri, S.H.; Asher, A.L.; Crocker, I.R.; Fraser, R.W.; Zhang, C.; Chen, Z.; Kandula, S.; Zhong, J.; Press, R.H.; et al. Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: A multi-institutional analysis. Neurosurgery 2016, 79, 279–285. [Google Scholar] [CrossRef]
- El Shafie, R.A.; Tonndorf-Martini, E.; Schmitt, D.; Weber, D.; Celik, A.; Dresel, T.; Bernhardt, D.; Lang, K.; Hoegen, P.; Adeberg, S.; et al. Pre-operative versus post-operative radiosurgery of brain metastases—Volumetric and dosimetric impact of treatment sequence and margin concept. Cancers 2019, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Palmisciano, P.; Ferini, G.; Khan, R.; Bin-Alamer, O.; Umana, G.E.; Yu, K.; Cohen-Gadol, A.A.; El Ahmadieh, T.Y.; Haider, A.S. Neoadjuvant Stereotactic Radiotherapy for Brain Metastases: Systematic Review and Meta-Analysis of the Literature and Ongoing Clinical Trials. Cancers 2022, 14, 4328. [Google Scholar] [CrossRef]
- Patel, A.R.; Nedzi, L.; Lau, S.; Barnett, S.L.; Mickey, B.E.; Moore, W.; Bindal, S.; Wardak, Z.; Dan, T.; Timmerman, R.; et al. Neoadjuvant Stereotactic Radiosurgery Before Surgical Resection of Cerebral Metastases. World Neurosurg. 2018, 120, e480–e487. [Google Scholar] [CrossRef]

| Characteristic | Overall (n = 147) |
|---|---|
| Sex | |
| Male | 55 (37%) |
| Female | 92 (63%) |
| Age at BM diagnosis | |
| Median (range) | 62 years (28–83) |
| Karnofsky Performance Status | |
| 100 | 11 (8%) |
| 90 | 59 (40%) |
| 80 | 35 (24%) |
| 70 | 33 (22%) |
| 60 | 5 (3%) |
| Unknown | 4 (3%) |
| Primary tumor histology | |
| Breast cancer | 24 (16%) |
| NSCLC (adenocarcinoma) | 59 (40%) |
| NSCLC (non-adenocarcinoma) | 16 (11%) |
| Colorectal cancer | 11 (8%) |
| Other | 37 (25%) |
| Number of lesions | |
| 1 | 92 (63%) |
| 2 | 32 (22%) |
| 3 or more | 23 (15%) |
| Max preoperative diameter of resected BM | |
| Median (range), all metastases | 38 mm (15–75) |
| Median (range), supratentorial metastases | 38 mm (15–75) |
| Median (range), infratentorial metastases | 36 mm (17–56) |
| Presence of extracranial metastases | |
| Yes | 67 (46%) |
| No | 80 (54%) |
| Location of resected BM | |
| Cerebral (supratentorial) | 107 (73%) |
| Cerebellar (infratentorial) | 40 (27%) |
| Proximity to CSF space | |
| Contact or involvement | 99 (67%) |
| Separated | 43 (29%) |
| Unknown | 5 (3%) |
| Resection method | |
| Piecemeal | 62 (42%) |
| En bloc | 63 (43%) |
| Unknown | 22 (15%) |
| Extent of resection | |
| Total | 101 (69%) |
| Subtotal | 45 (31%) |
| Unknown | 1 (1%) |
| Systemic therapy within 2 months of SRT | |
| Chemotherapy | 37 (25%) |
| Hormonal therapy | 7 (5%) |
| Targeted therapy | 18 (12%) |
| Immunotherapy | 15 (10%) |
| None | 79 (54%) |
| Unknown | 7 (5%) |
| Radiation fractionation and dose | |
| 1 × 15 Gy | 1 (1%) |
| 1 × 18 Gy | 9 (6%) |
| 1 × 21 Gy | 10 (7%) |
| 3 × 8 Gy | 59 (40%) |
| 3 × 8.5 Gy | 65 (44%) |
| 7 × 5 Gy | 1 (1%) |
| 13 × 3 Gy | 2 (1%) |
| Treatment Type | Number of Patients |
|---|---|
| Local recurrence (n = 20) | |
| Re-SRT | 11 (55%) |
| Re-resection | 6 (30%) |
| Whole-brain radiotherapy | 3 (15%) |
| Regional recurrence (n = 80) | |
| SRT | 43 (54%) |
| Resection | 6 (8%) |
| Whole-brain radiotherapy | 20 (25%) |
| Leptomeningeal disease (n = 21) | |
| Whole-brain radiotherapy | 9 (43%) |
| Systemic treatment | 8 (38%) |
| Characteristic | HR (95% CI, p) |
|---|---|
| Sex | |
| Male | 1.0 (reference) |
| Female | 0.66 (0.28–1.56, 0.34) |
| Age (years) | 1.00 (0.96–1.05, 0.87) |
| Primary tumor histology | |
| Breast cancer 1 | 1.0 (reference) |
| Lung cancer (adenocarcinoma) | 2.14 (0.47–9.77, 0.33) |
| Other | 2.25 (0.48–10.5, 0.30) |
| Number of lesions | |
| 1 | 1.0 (reference) |
| 2 | 1.30 (0.50–3.40, 0.59) |
| 3 or more | 0.27 (0.04–2.45, 0.27) |
| Max preoperative diameter of resected BM (mm) | 1.02 (0.98–1.06, 0.31) |
| Presence of extracranial metastases | |
| Yes | 1.0 (reference) |
| No | 0.81 (0.34–1.92, 0.63) |
| Location of resected BM | |
| Cerebral (supratentorial) | 1.0 (reference) |
| Cerebellar (infratentorial) | 2.54 (1.07–6.04, 0.034) |
| Tumor proximity to CSF space | |
| Separated | 1.0 (reference) |
| Contact or involvement | 2.16 (0.72–6.46, 0.17) |
| Resection method | |
| Piecemeal | 1.0 (reference) |
| En bloc | 0.74 (0.30–1.84, 0.51) |
| Extent of resection | |
| Total | 1.0 (reference) |
| Subtotal | 1.15 (0.46–2.81, 0.76) |
| Systemic therapy within 2 months of SRT | |
| Yes | 1.0 (reference) |
| No | 0.48 (0.20–1.16, 0.10) |
| Radiation fractionation | |
| 3× | 1.0 (reference) |
| 1× | 0.23 (0.03–1.72, 0.15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crouzen, J.A.; Petoukhova, A.L.; Hakstege, M.; van Schaik, E.E.M.W.; Nandoe Tewarie, R.D.S.; Nabuurs, R.J.A.; Vos, M.J.; Kerkhof, M.; van der Vaart, T.; Koekkoek, J.A.F.; et al. Patterns of Recurrence After Postoperative Stereotactic Radiotherapy for Brain Metastases. Cancers 2025, 17, 1557. https://doi.org/10.3390/cancers17091557
Crouzen JA, Petoukhova AL, Hakstege M, van Schaik EEMW, Nandoe Tewarie RDS, Nabuurs RJA, Vos MJ, Kerkhof M, van der Vaart T, Koekkoek JAF, et al. Patterns of Recurrence After Postoperative Stereotactic Radiotherapy for Brain Metastases. Cancers. 2025; 17(9):1557. https://doi.org/10.3390/cancers17091557
Chicago/Turabian StyleCrouzen, Jeroen A., Anna L. Petoukhova, Martijn Hakstege, Elise E. M. W. van Schaik, Rishi D. S. Nandoe Tewarie, Rob J. A. Nabuurs, Maaike J. Vos, Melissa Kerkhof, Thijs van der Vaart, Johan A. F. Koekkoek, and et al. 2025. "Patterns of Recurrence After Postoperative Stereotactic Radiotherapy for Brain Metastases" Cancers 17, no. 9: 1557. https://doi.org/10.3390/cancers17091557
APA StyleCrouzen, J. A., Petoukhova, A. L., Hakstege, M., van Schaik, E. E. M. W., Nandoe Tewarie, R. D. S., Nabuurs, R. J. A., Vos, M. J., Kerkhof, M., van der Vaart, T., Koekkoek, J. A. F., Hagenbeek, R. E., Yildirim, F. M., Wiltink, L. M., van der Voort van Zyp, N. C. M. G., Kiderlen, M., Broekman, M. L. D., Mast, M. E., & Zindler, J. D. (2025). Patterns of Recurrence After Postoperative Stereotactic Radiotherapy for Brain Metastases. Cancers, 17(9), 1557. https://doi.org/10.3390/cancers17091557






