Reciprocal Modulation of Tumour and Immune Cell Motility: Uncovering Dynamic Interplays and Therapeutic Approaches
Simple Summary
Abstract
1. Unravelling the Mechanisms Regulating Tumour Cell Motility
1.1. An Overview
1.2. The Role of EMT Transcription Factors in Tumour Cell Motility
1.3. The Role of Hypoxia
1.4. The Role of CAFs
1.5. The Role of Exosomes
1.6. Mesenchymal Stromal Cells (MSMCs)
1.7. The Role of ECM
1.8. TAM, Tumour-Associated Macrophages
1.9. Metabolism and Tumour Migration
2. Regulation of Intra-Tumour T-Cell Motility: Dynamics, Mechanisms, and Implications
2.1. Groundwork of Intra-Tumour T-Cell Motility: Concepts and Challenges
2.2. Chemokines and Their Role in the Regulation of Intra-Tumour T-Cell Migration
2.3. Modulation of Adhesion Processes Excludes Anti-Tumour T-Cells: LFA-1 as a Critical Mediator
2.4. Role of CD28 and CTLA-4: Key Regulators of T-Cell Activation and Migration
2.5. The Role of Stromal and ECM Components in Intra-Tumour T-Cell Motility
2.6. Tumour Vasculature Can Limit CD8+ T-Cell Infiltration into Tumours
2.7. Metabolic Control of CD8+ T-Cell Motility and Implications for Immunotherapy
2.8. Direct Impact of Tumour Features on T-Cell Intra-Tumour Infiltration
3. Therapeutic Strategies
3.1. Therapeutic Strategies to Contrast Tumour Cell Motility
3.2. Potential Approaches to Increase Intra-Tumour T-Cell Motility
4. Unveiling the Dynamic Interplay of Tumour and T-Cell Motility in the TME
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De la Fuente, I.M.; López, J.I. Cell Motility and Cancer. Cancers 2020, 12, 2177. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K. Uncoupling Metastasis from Tumourigenesis. N. Engl. J. Med. 2023, 388, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, X.; Huang, X.; Zhang, D.; Chen, Z.; Zhang, J.; Bai, R.; Zhang, S.; Zhao, H.; Xu, Z.; et al. Single cell transcriptomic analysis deciphers heterogenous cancer stem like cells in colorectal cancer and their organ specific metastasis. Gut 2024, 73, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Pietilä, M.; Ivaska, J.; Mani, S.A. Whom to blame for metastasis, the epithelial–mesenchymal transition or the tumour microenvironment? Cancer Lett. 2016, 380, 359–368. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, H.; Gao, Y.; Liang, Y.; Du, Y.; Hao, S.; Ni, T. The tumour microenvironment: Signal transduction. Biomolecules 2024, 14, 438. [Google Scholar]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumour progressionand metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Hu, A.; Sun, L.; Lin, H.; Liao, Y.; Yang, H.; Mao, Y. Harnessing innate immunepathways for therapeutic advancement in cancer. Signal Transduct. Target Ther. 2024, 9, 68. [Google Scholar] [CrossRef]
- Heerboth, S.; Housman, G.; Leary, M.; Longacre, M.; Byler, S.; Lapinska, K.; Willbanks, A.; Sarkar, S. EMT and tumour metastasis. Clin. Transl. Med. 2015, 4, 6. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Mladinich, M.; Ruan, D.; Chan, C.H. Tackling cancer stem cells via inhibition of EMT transcription factors. Stem Cells Int. 2016, 2016, 5285892. [Google Scholar] [CrossRef]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging ax-is of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef]
- Khan, A.Q.; Hasan, A.; Mir, S.S.; Rashid, K.; Uddin, S.; Steinhoff, M. Exploiting transcription factors to target EMT and cancer stem cells for tumour modulation and therapy. Semin. Cancer Biol. 2024, 100, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Peyre, L.; Meyer, M.; Hofman, P.; Roux, J. TRAIL receptor-induced features of epithelial-to-mesenchymal transition increase tumour phenotypic heterogeneity: Potential cell survival mechanisms. Br. J. Cancer 2021, 124, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.M.; Seligson, N.D.; Ahmad, S.M.; Rasool, R.U.; Gandhi, S.G.; Bhagat, M.; Goswami, A. Convergence of therapy-induced senescence (TIS) and EMT in multistep carcinogenesis: Current opinions and emerging perspectives. Cell Death Discov. 2020, 6, 51. [Google Scholar] [CrossRef]
- Pece, S.; Tosoni, D.; Confalonieri, S.; Mazzarol, G.; Vecchi, M.; Ronzoni, S.; Bernard, L.; Viale, G.; Pelicci, P.G.; Di Fiore, P.P. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 2010, 140, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Hirano, K.; Kanemori, M.; Sun, L.T.; Tada, T. Self-renewal and pluripotency acquired through somatic reprogramming to human cancer stem cells. PLoS ONE 2012, 7, e48699. [Google Scholar] [CrossRef]
- Shibue, T.R.; Weinberg, A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Cabarcas, S.M.; Mathews, L.A.; Farrar, W.L. The cancer stem cell niche—There goes the neighborhood? Int. J. Cancer 2011, 129, 2315–2327. [Google Scholar] [CrossRef]
- Tsai, J.H.; Donaher, J.L.; Murphy, D.A.; Chau, S.; Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012, 22, 725–736. [Google Scholar] [CrossRef]
- Ocana, O.H.; Córcoles, R.; Fabra, A.; Moreno-Bueno, G.; Acloque, H.; Vega, S.; Barrallo-Gimeno, A.; Cano, A.; Nieto, M.A. Metastatic colonization requires the repression of the epithelial mesenchymal transition inducer Prrx1. Cancer Cell 2012, 22, 709–724. [Google Scholar] [CrossRef]
- Terragno, M.; Vetrova, A.; Semenov, O.; Sayan, A.E.; Kriajevska, M.; Tulchinsky, E. Mesenchymal-epithelial transition and AXL inhibitor TP-0903 sensitise triple-negative breast cancer cells to the antimalarial compound, artesunate. Sci. Rep. 2024, 14, 425. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Eissner, G. Mesenchymal stromal cells “think” globally, but act locally. Cell 2023, 12, 509. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, J.; Li, L.; Liao, S.; He, J.; Zhou, S.; Zhou, Y. Pericytes in the tumour micro-environment. Cancer Lett. 2023, 556, 216074. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J.W. A timeline of tumour-associated macrophage biology. Nat. Rev. Cancer 2023, 23, 238–257. [Google Scholar] [CrossRef]
- Dikiy, S.; Rudensky, A.Y. Principles of regulatory T cell function. Immunity 2023, 56, 240–255. [Google Scholar] [CrossRef]
- Laumont, C.M.; Banville, A.C.; Gilardi, M.; Hollern, D.P.; Nelson, B.H. Tumour-infiltrating B cells: Immunological mechanisms, clinical impact and therapeutic opportunities. Nat. Rev. Cancer 2022, 22, 414–430. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, L.; Liu, L.; Li, X. NK cells are never alone: Crosstalk and communication in tumour microenvironments. Mol. Cancer 2023, 22, 34. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, M.; Niu, M.; Mei, Q.; Wu, K. Myeloid-derived suppressor cells: An emerging target for anticancer immunotherapy. Mol. Cancer 2022, 21, 184. [Google Scholar] [CrossRef]
- Yan, M.; Zheng, M.; Niu, R.; Yang, X.; Tian, S.; Fan, L.; Li, Y.; Zhang, S. Roles of tumour associated neutrophils in tumour metastasis and its clinical applications. Front. Cell Dev. Biol. 2022, 10, 938289. [Google Scholar] [CrossRef]
- Lei, X.; Khatri, I.; de Wit, T.; de Rink, I.; Nieuwland, M.; Kerkhoven, R.; van Eenennaam, H.; Sun, C.; Garg, A.D.; Borst, J.; et al. CD4+ helper T cells endow cDC1 with cancer-impeding functions in the human tumour microenvironment. Nat. Commun. 2023, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target Ther. 2021, 6, 153. [Google Scholar] [CrossRef]
- Lappano, R.; Todd, L.A.; Stanic, M.; Cai, Q.; Maggiolini, M.; Marincola, F.; Pietrobon, V. Multifaceted interplay between hormones, growth factors and hypoxia in the tumour microenvironment. Cancers 2022, 14, 539. [Google Scholar] [CrossRef]
- Martins-Lima, C.; Chianese, U.; Benedetti, R.; Altucci, L.; Jerónimo, C.; Correia, M.P. Tumour microenvironment and epithelial-mesenchymal transition in bladder cancer: Cytokines in the game? Front. Mol. Biosci. 2022, 9, 1070383. [Google Scholar]
- Bose, S.; Saha, P.; Chatterjee, B.; Srivastava, A.K. Chemokines driven ovarian cancer progression, metastasis and chemoresistance: Potential pharmacological targets for cancer therapy. Semin. Cancer Biol. 2022, 86, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Koran, S.; AlOmair, L. Insights into the role of matrix metalloproteinases in cancer and its various therapeutic aspects: A review. Front. Mol. Biosci. 2022, 9, 896099. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Gao, B.; Tong, D.; Huang, C. Crosstalk between extracellular vesicles and tumour-associated macrophage in the tumour microenvironment. Cancer Lett. 2023, 552, 215979. [Google Scholar] [CrossRef] [PubMed]
- Doodmani, S.M.; Safari, M.H.; Akbari, M.; Farahani, N.; Alimohammadi, M.; Aref, A.R.; Tajik, F.; Maghsoodlou, A.; Daneshi, S.; Tabari, T.; et al. Metastasis and chemoresistance in breast cancer: Crucial function of ZEB1/2 proteins. Pathol. Res. Pract. 2025, 267, 155838. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Garg, M. Epithelial-mesenchymal transition—Activating transcription factors—Multifunctional regulators in cancer. World J. Stem Cells. 2013, 26, 188–195. [Google Scholar] [CrossRef]
- Peinado, H.; Quintanilla, M.; Cano, A. Transforming growth factor β-1 induces snail transcription factor in epithelial cell lines mechanisms for epithelial mesenchymal transitions. J. Biol. Chem. 2003, 278, 21113–21123. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Nishida-Fukuda, H.; Nanba, D.; Nakashiro, K.-I.; Nakayama, H.; Kubota, H.; Higashiyama, S. Reversible interconversion and maintenance of mammary epithelial cell characteristics by the ligand-regulated EGFR system. Sci. Rep. 2016, 6, 20209. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.R.; Zhau, H.E.; Odero-Marah, V.A.; Osunkoya, A.O.; Kimbro, K.S.; Tighiouart, M.; Liu, T.; Simons, J.W.; O’Regan, R.M. Insulin-like growth factor-I–dependent up-regulation of ZEB1 drives epitheli-al-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008, 68, 2479–2488. [Google Scholar] [CrossRef]
- Jiao, D.; Wang, J.; Lu, W.; Tang, X.; Chen, J.; Mou, H.; Chen, Q.Y. Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signalling pathways in lung cancer. Mol. Ther.-Oncolytics 2016, 3, 16018. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Yang, L.; Chen, C.; Ashrafizadeh, M.; Tian, Y.; Sun, R. Wnt/β-catenindriven EMT regulation in human cancers. Cell Mol. Life Sci. 2024, 81, 79. [Google Scholar] [CrossRef]
- Fukusumi, T.; Guo, T.W.; Sakai, A.; Ando, M.; Ren, S.; Haft, S.; Liu, C.; Amornphimoltham, P.; Gutkind, J.S.; Califano, J.A.; et al. The NOTCH4–HEY1 pathway induces epithelial–mesenchymal transition in head and neck squamous cell carcinoma. Clin. Cancer Res. 2018, 24, 619–633. [Google Scholar] [CrossRef]
- Kaufhold, S.; Bonavida, B. Central role of Snail1 in the regulation of EMT and resistance in cancer: A target for therapeutic intervention. J. Exp. Clin. Cancer Res. 2014, 33, 62. [Google Scholar] [CrossRef]
- Comijn, J.; Berx, G.; Vermassen, P.; Verschueren, K.; van Grunsven, L.; Bruyneel, E.; Mareel, M.; Huylebroeck, D.; van Roy, F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell 2001, 7, 1267–1278. [Google Scholar] [CrossRef]
- Batlle, E.; Sancho, E.; Francí, C.; Domínguez, D.; Monfar, M.; Baulida, J.; García De Herreros, A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000, 2, 84–89. [Google Scholar] [CrossRef]
- Vandewalle, C.; Comijn, J.; De Craene, B.; Vermassen, P.; Bruyneel, E.; Andersen, H.; Tulchinsky, E.; Van Roy, F.; Berx, G. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res. 2005, 33, 6566–6578. [Google Scholar] [CrossRef]
- Martínez-Estrada, O.M.; Cullerés, A.; Soriano, F.X.; Peinado, H.; Bolós, V.; Martínez, F.O.; Reina, M.; Cano, A.; Fabre, M.; Vilaró, S. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem. J. 2006, 394, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, J.; Matsuda, M.; Furuse, M.; Tsukita, S. Regulation of tight junctions during the epithelium-mesenchyme transition: Direct repression of the gene expression of claudins/occludin by Snail. J. Cell Sci. 2003, 116, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, E.L.; Fan, S.; Harder, J.L.; Walton, K.D.; Liu, C.J.; Soofi, A.; Fogg, V.C.; Hershenson, M.B.; Dressler, G.; Deutsch, G.H.; et al. Crumbs3 is essential for proper epithelial development and viability. Mol. Cell Biol. 2014, 34, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Yook, J.I.; Li, X.Y.; Ota, I.; Hu, C.; Kim, H.; Kim, N.H.; Cha, S.Y.; Ryu, J.K.; Choi, Y.J.; Kim, J.; et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat. Cell Biol. 2006, 8, 1398–1406. [Google Scholar] [CrossRef]
- Min, A.L.; Choi, J.Y.; Woo, H.Y.; Kim, J.D.; Kwon, J.H.; Bae, S.H.; Yoon, S.K.; Shin, S.H.; Chung, Y.J.; Jung, C.K. High expression of Snail mRNA in blood from hepatocellular carcinoma patients with extra-hepatic metastasis. Clin. Exp. Metastasis 2009, 26, 759–767. [Google Scholar] [CrossRef]
- Guo, W.; Keckesova, Z.; Donaher, J.L.; Shibue, T.; Tischler, V.; Reinhardt, F.; Itzkovitz, S.; Noske, A.; Zürrer-Härdi, U.; Bell, G.; et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 2012, 148, 1015–1028. [Google Scholar] [CrossRef]
- Margetts, P.J.; Bonniaud, P.; Liu, L.; Hoff, C.M.; Holmes, C.J.; West-Mays, J.A.; Kelly, M.M. Transient overexpression of TGF-{beta}1 induces epithelial mesenchymal transition in the rodent peritoneum. J. Am. Soc. Nephrol. 2005, 16, 425–436. [Google Scholar] [CrossRef]
- Martínez-Alvarez, C.; Blanco, M.J.; Pérez, R.; Rabadán, M.A.; Aparicio, M.; Resel, E.; Martínez, T.; Nieto, M.A. Snail family members and cell survival in physiological and pathological cleft palates. Dev. Biol. 2004, 265, 207–218. [Google Scholar] [CrossRef]
- Blechschmidt, K.; Sassen, S.; Schmalfeldt, B.; Schuster, T.; Höfler, H.; Becker, K.F. The E-cadherin repressor Snail is associated with lower overall survival of ovarian cancer patients. Br. J. Cancer 2008, 98, 489–495. [Google Scholar] [CrossRef]
- Fujita, N.; Jaye, D.L.; Kajita, M.; Geigerman, C.; Moreno, C.S.; Wade, P.A. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell 2003, 113, 207–219. [Google Scholar] [CrossRef]
- Shioiri, M.; Shida, T.; Koda, K.; Oda, K.; Seike, K.; Nishimura, M.; Takano, S.; Miyazaki, M. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br. J. Cancer 2006, 94, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.G.; Vicente-Dueñas, C.; González-Herrero, I.; Anderson, C.; Flores, T.; Hughes, S.; Tselepis, C.; Ross, J.A.; Sánchez-García, I. Snail family transcription factors are implicated in thyroid carcinogenesis. Am. J. Pathol. 2007, 171, 1037–1046. [Google Scholar] [CrossRef]
- Hotz, B.; Arndt, M.; Dullat, S.; Bhargava, S.; Buhr, H.J.; Hotz, H.G. Epithelial to mesenchymal transition: Expression of the regulators snail, slug, and twist in pancreatic cancer. Clin. Cancer Res. 2007, 13, 4769–4776. [Google Scholar] [CrossRef] [PubMed]
- Kurrey, N.K.; Jalgaonkar, S.P.; Joglekar, A.V.; Ghanate, A.D.; Chaskar, P.D.; Doiphode, R.; Bapat, S.A. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 2009, 27, 2059–2068. [Google Scholar] [CrossRef]
- Bruyere, F.; Namdarian, B.; Corcoran, N.M.; Pedersen, J.; Ockrim, J.; Voelzke, B.B.; Mete, U.; Costello, A.J.; Hovens, C.M. Snail expression is an independent predictor of tumour recurrence in superficial bladder cancers. Urol. Oncol. 2010, 28, 591–596. [Google Scholar] [CrossRef]
- Kim, N.O.; Cha, Y.H.; Lee, J.; Lee, S.H.; Yang, J.; Yun, J.; Cho, E.S.; Zhang, X.; Nam, M.; Kim, N.; et al. Snail reprograms glucose metabolism by repressing phosphofructokinase PFKP allowing cancer cell survival under metabolic stress. Nat. Commun. 2017, 8, 14374. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, D.; Montserrat-Sentís, B.; Virgós-Soler, A.; Guaita, S.; Grueso, J.; Porta, M.; Puig, I.; Baulida, J.; Francí, C.; García de Herreros, A.; et al. Phosphorylation regulates the subcellular location and activity of the snail transcriptional repressor. Mol. Cell Biol. 2003, 23, 5078–5089. [Google Scholar] [CrossRef]
- Bachelder, R.E.; Yoon, S.O.; Franci, C.; de Herreros, A.; Mercurio, A.M. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: Implications for the epithelial-mesenchymal transition. J. Cell Biol. 2005, 168, 29–33. [Google Scholar] [CrossRef]
- Barrallo-Gimeno, A.; Nieto, M.A. The Snail genes as inducers of cell movement and survival: Implications in development and cancer. Development 2005, 132, 3151–3161. [Google Scholar] [CrossRef]
- Wu, Y.; Evers, B.M.; Zhou, B.P. Small C-terminal domain phosphatase enhances snail activity through dephosphorylation. J. Biol. Chem. 2009, 284, 640–648. [Google Scholar] [CrossRef]
- Franco, H.L.; Casasnovas, J.; Rodríguez-Medina, J.R.; Cadilla, C.L. Redundant or separate entities? Roles of Twist1 and Twist2 as molecular switches during gene transcription. Nucleic Acids Res. 2011, 39, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Chen, S.; Han, J.X.; Qian, B.; Wang, X.R.; Zhong, W.L.; Qin, Y.; Zhang, H.; Gao, W.F.; Lei, Y.Y.; et al. Twist1 Regulates Vimentin through Cul2 Circular RNA to Promote EMT in Hepatocellular Carcinoma. Cancer Res. 2018, 78, 4150–4162. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Chen, H.C.; Zhang, D.; Fu, J. Twist: A molecular target in cancer therapeutics. Tumour Biol. 2013, 34, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.J.; Zhang, W.; Xu, X.M.; Zhang, F.; Tao, W.P.; Ye, J.J.; Ge, W. Twist mediates an aggressive phenotype in human colorectal cancer cells. Int. J. Oncol. 2016, 48, 1117–1124. [Google Scholar] [CrossRef]
- Hong, J.; Zhou, J.; Fu, J.; He, T.; Qin, J.; Wang, L.; Liao, L.; Xu, J. Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness. Cancer Res. 2011, 71, 3980–3990. [Google Scholar] [CrossRef]
- Tang, H.; Massi, D.; Hemmings, B.A.; Mandalà, M.; Hu, Z.; Wicki, A.; Xue, G. AKT-ions with a TWIST between EMT and MET. Oncotarget 2016, 7, 62767–62777. [Google Scholar] [CrossRef]
- Eger, A.; Aigner, K.; Sonderegger, S.; Dampier, B.; Oehler, S.; Schreiber, M.; Berx, G.; Cano, A.; Beug, H.; Foisner, R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 2005, 24, 2375–2385. [Google Scholar] [CrossRef]
- Wang, L.; Ni, X.; Covington, K.R.; Yang, B.Y.; Shiu, J.; Zhang, X.; Xi, L.; Meng, Q.; Langridge, T.; Drummond, J.; et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signalling and differentiation genes. Nat. Genet. 2015, 47, 1426–1434. [Google Scholar] [CrossRef]
- Dillner, N.B.; Sanders, M.M. The zinc finger/homeodomain protein deltaEF1 mediates estrogen-specific induction of the ovalbumin gene. Mol. Cell Endocrinol. 2002, 192, 85–91. [Google Scholar] [CrossRef]
- Heldin, C.H.; Vanlandewijck, M.; Moustakas, A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012, 586, 1959–1970. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015, 14, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Dave, N.; Guaita-Esteruelas, S.; Gutarra, S.; Frias, À.; Beltran, M.; Peiró, S.; de Herreros, A.G. Functional cooperation between Snail1 and twist in the regulation of ZEB1 ex-pression during epithelial to mesenchymal transition. J. Biol. Chem. 2011, 286, 12024–12032. [Google Scholar] [CrossRef] [PubMed]
- Spaderna, S.; Schmalhofer, O.; Wahlbuhl, M.; Dimmler, A.; Bauer, K.; Sultan, A.; Hlubek, F.; Jung, A.; Strand, D.; Eger, A.; et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008, 68, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Marjanovic, N.D.; Lee, T.; Bell, G.; Kleer, C.G.; Reinhardt, F.; D’Alessio, A.C.; Young, R.A.; Weinberg, R.A. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumourigenicity. Cell 2013, 154, 61–74. [Google Scholar] [CrossRef]
- Zhang, G.J.; Zhou, T.; Tian, H.P.; Liu, Z.L.; Xia, S.S. High expression of ZEB1 correlates with liver metastasis and poor prognosis in colorectal cancer. Oncol Lett. 2013, 5, 564–568. [Google Scholar] [CrossRef]
- Jang, M.H.; Kim, H.J.; Kim, E.J.; Chung, Y.R.; Park, S.Y. Expression of epithelial-mesenchymal transition-related markers in triple-negative breast cancer: ZEB1 as a potential biomarker for poor clinical outcome. Hum. Pathol. 2015, 46, 1267–1274. [Google Scholar] [CrossRef]
- Pouyafar, A.; Heydarabad, M.Z.; Abdolalizadeh, J.; Zade, J.A.; Rahbarghazi, R.; Talebi, M. Modulation of lipolysis and glycolysis pathways in cancer stem cells changed multipotentiality and differentiation capacity toward endothelial lineage. Cell Biosci. 2019, 9, 30. [Google Scholar]
- Kurahara, H.; Takao, S.; Maemura, K.; Mataki, Y.; Kuwahata, T.; Maeda, K.; Ding, Q.; Sakoda, M.; Iino, S.; Ishigami, S.; et al. Epithelial-mesenchymal transition and mesenchymal-epithelial transition via regulation of ZEB-1 and ZEB-2 expression in pancreatic cancer. J. Surg. Oncol. 2012, 105, 655–661. [Google Scholar] [CrossRef]
- Chen, B.; Chen, B.; Zhu, Z.; Ye, W.; Zeng, J.; Liu, G.; Wang, S.; Gao, J.; Xu, G.; Huang, Z. Prognostic value of ZEB-1 in solid tumours: A meta-analysis. BMC Cancer 2019, 9, 635. [Google Scholar]
- Gheldof, A.; Hulpiau, P.; van Roy, F.; De Craene, B.; Berx, G. Evolutionary functional analysis and molecular regulation of the ZEB transcription factors. Cell Mol. Life Sci. 2012, 69, 2527–2541. [Google Scholar] [CrossRef]
- Chen, H.; Lu, W.; Huang, C.; Ding, K.; Xia, D.; Wu, Y.; Cai, M. Prognostic significance of ZEB1 and ZEB2 in digestive cancers: A cohort-based analysis and secondary analysis. Oncotarget 2017, 8, 31435–31448. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. The hypoxic tumour microenvironment: A driving force for breast cancer progression. Biochim. Biophys. Acta 2016, 183, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Z.; Tsai, Y.P.; Yang, M.H.; Huang, C.H.; Chang, S.Y.; Chang, C.C.; Teng, S.C.; Wu, K.J. Interplay between HDAC3 and WDR5 is essential for hypoxia-induced epithelial-mesenchymal transition. Mol. Cell 2011, 43, 811–822. [Google Scholar] [CrossRef]
- Xu, M.; Pang, Q.; Xu, S.; Ye, C.; Lei, R.; Shen, Y.; Xu, J. Hypoxia-inducible factor-1α activates transforming growth factor-β1/ Smad signalling and in-creases collagen deposition in dermal fibroblasts. Oncotarget 2018, 9, 3188. [Google Scholar]
- Lester, R.D.; Jo, M.; Montel, V.; Takimoto, S.; Gonias, S.L. uPAR induces epithelial- mesenchymal transition in hypoxic breast cancer cells. J. Cell Biol. 2007, 178, 425–436. [Google Scholar] [CrossRef]
- Hapke, R.Y.; Haake, S.M. Hypoxia-induced epithelial to mesenchymal transition in cancer. Cancer Lett. 2020, 487, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Wu, M.-Z.; Chiou, S.-H.; Chen, P.-M.; Chang, S.-Y.; Liu, C.J.; Teng, S.C.; Wu, K.J. Direct regulation of TWIST by HIF-1α promotes metastasis. Nat. Cell Biol. 2008, 10, 295–305. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, X.; Peng, Y.; Wu, M.; Zhang, P.; Xie, R.; Wu, Y.; Yan, Q.; Liu, S.; Wang, J.; et al. HIF-1α promotes epithelial-mesenchymal transition and metastasis through direct regulation of ZEB1 in colorectal cancer. PLoS ONE. 2015, 10, e0129603. [Google Scholar] [CrossRef]
- Bogdanova, D.; Kolosova, E.D.; Pukhalskaia, T.V.; Levchuk, K.A.; Demidov, O.N.; Belotserkovskaya, E.V. The differential effect of senolytics on sasp cytokine secretion regulation of EMT by CAFs. Int. J. Mol. Sci. 2024, 25, 4031. [Google Scholar] [CrossRef]
- Xiang, H.; Ramil, C.P.; Hai, J.; Zhang, C.; Wang, H.; Watkins, A.A.; Afshar, R.; Georgiev, P.; Sze, M.A.; Song, X.S.; et al. Cancer associated fibroblasts promote immunosuppression by inducing ROS-generating monocytic MDSCs in lung squamous cell carcinoma. Cancer Immunol. Res. 2020, 8, 436–450. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Zhang, Y.; Pradhan, R.N.; Ganguly, D.; Chandra, R.; Murimwa, G.; Wright, S.; Gu, X.; Maddipati, R.; et al. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of reg-ulatory T cells in pancreatic cancer. Cancer Cell 2022, 40, 656–673.e7. [Google Scholar] [CrossRef] [PubMed]
- Kerdidani, D.; Aerakis, E.; Verrou, K.M.; Angelidis, I.; Douka, K.; Maniou, M.A.; Stamoulis, P.; Goudevenou, K.; Prados, A.; Tzaferis, C.; et al. Lung tumour MHCII immunity depends on in situ antigen presentation by fibroblasts. J. Exp. Med. 2022, 219, e20210815. [Google Scholar] [CrossRef]
- Wen, S.; Hou, Y.; Fu, L.; Xi, L.; Yang, D.; Zhao, M.; Qin, Y.; Sun, K.; Teng, Y.; Liu, M. Cancer-associated fibroblast (CAF)-derived IL32 promotes breast cancer cell invasion and metastasis via integrin beta3-p38 MAPK signalling. Cancer Lett. 2019, 442, 320–332. [Google Scholar] [CrossRef]
- Liao, H.; Li, H.; Dong, J.; Song, J.; Chen, H.; Si, H.; Wang, J.; Bai, X. Melatonin blunts the tumour-promoting effect of cancer-associated fibroblasts by reducing IL-8 expression and reversing epithelial-mesenchymal transition. Int. Immunopharmacol. 2023, 119, 110194. [Google Scholar] [CrossRef] [PubMed]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumour growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, C.; Tan, L.; Wang, Q.; Li, X.; Feng, Y. Cancer-associated fibroblasts induce epithelial–mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer 2014, 110, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, C. Difference of TGF-β/Smads signalling pathway in epithelial-mesenchymal transition of normal colonic epithelial cells induced by tumour-associated fibroblasts and colon cancer cells. Mol. Biol. Rep. 2019, 46, 2749–2759. [Google Scholar] [CrossRef]
- Wang, L.; Cao, L.; Wang, H.; Liu, B.; Zhang, Q.; Meng, Z.; Wu, X.; Zhou, Q.; Xu, K. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signalling pathway. Oncotarget 2017, 8, 76116. [Google Scholar] [CrossRef]
- Qu, Z.; Wu, J.; Ji, A.; Qiang, G.; Jiang, Y.; Jiang, C.; Ding, Y. Exosomal miR-665 as a novel minimally invasive biomarker for hepatocellular carcinoma diagnosis and prognosis. Oncotarget 2017, 8, 80666–80678. [Google Scholar] [CrossRef]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef]
- Abulaiti, A.; Shintani, Y.; Funaki, S.; Nakagiri, T.; Inoue, M.; Sawabata, N.; Minami, M.; Okumura, M. Interaction between non-small-cell lung cancer cells and fibroblastsvia enhancement of TGF-β signalling by IL-6. Lung Cancer 2013, 82, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, W.; Sun, X.; Lin, Y.; Chen, W. Cancer-associated fibroblasts induce epithelial-mesenchymal transition through secreted cytokines in endometrial cancer cells. Oncol. Lett. 2018, 15, 5694–5702. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, X.; Zhang, Q.; Shi, X.; Xu, G.; Zou, C. Cancer-associated fibroblasts secrete FGF-1 to promote ovarian proliferation, migration, and invasion through the activation of FGF-1/FGFR4 signalling. Tumour Biol. 2017, 39, 1010428317712592. [Google Scholar] [CrossRef]
- Takatsu, F.; Suzawa, K.; Tomida, S.; Thu, Y.M.; Sakaguchi, M.; Toji, T.; Ohki, M.; Tsudaka, S.; Date, K.; Matsuda, N.; et al. Periostin secreted by cancer-associated fibroblasts promotes cancer progression and drug resistance in non-small cell lung cancer. J. Mol. Med. 2023, 101, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Chen, S.; Chen, X.; Lin, W.R.; Li, W.; Ma, J.; Wu, T.; Ji, H.; Li, Y.; Cui, X.; et al. Prometastatic mechanisms of CAF-mediated EMT regulation in pancreatic cancer cells. Int. J. Oncol. 2017, 50, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, M.; Zhuravleva, E.; Satriano, L.; Martinez, M.B.; Bhatt, D.K.; Oliveira, D.V.N.P.; Antoku, Y.; Keggenhoff, F.L.; Castven, D.; Marquardt, J.U.; et al. Fibroblast-derived lysyl oxidase increases oxidative phosphorylation and stemness in cholangiocarcinoma. Gastroenterology 2024, 166, 886–901.e7. [Google Scholar] [CrossRef]
- Makutani, Y.; Kawakami, H.; Tsujikawa, T.; Yoshimura, K.; Chiba, Y.; Ito, A.; Kawamura, J.; Haratani, K.; Nakagawa, K. Contribution of MMP14-expressing cancer-associated fibroblasts in the tumour immune microenvironment to progression of colorectal cancer. Front. Oncol. 2022, 12, 956270. [Google Scholar] [CrossRef]
- Wright, K.; Ly, T.; Kriet, M.; Czirok, A.; Thomas, S.M. Cancer-associated fibroblasts: Master tumour microenvironment modifiers. Cancers 2023, 15, 1899. [Google Scholar] [CrossRef]
- Fiori, M.E.; Di Franco, S.; Villanova, L.; Bianca, P.; Stassi, G.; De Maria, R. Cancer-associated fibroblasts as abettors of tumour progression at the crossroads of EMT and therapy resistance. Mol. Cancer 2019, 18, 70. [Google Scholar] [CrossRef]
- Jo, H.; Shim, K.; Jeoung, D. Exosomes: Diagnostic and therapeutic implications in cancer. Pharmaceutics 2023, 15, 1465. [Google Scholar] [CrossRef]
- Fang, X.; Lan, H.; Jin, K.; Qian, J. Pancreatic cancer and exosomes: Role in progression, diagnosis, monitoring, and treatment. Front. Oncol. 2023, 13, 1149551. [Google Scholar] [CrossRef]
- Rizwan, M.N.; Ma, Y.; Nenkov, M.; Jin, L.; Schröder, D.C.; Westermann, M.; Gabler, N.; Chen, Y. Tumour-derived exosomes: Key players in non-small cell lung cancer metastasis and their implication for targeted therapy. Mol. Carcinog. 2022, 61, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, Y.; Yan, M.; Wang, J.; Wang, M.; Xuan, Y.; Cheng, H.; Ma, J.; Chai, C.; Li, M.; et al. Exosomes derived from cancer-associated fibroblasts promote tumourigenesis, metastasis and chemoresistance of colorectal cancer by upregulating circ_0067557 to target Lin28. BMC Cancer 2024, 24, 64. [Google Scholar]
- Li, Y.-Y.; Tao, Y.-W.; Gao, S.; Li, P.; Zheng, J.-M.; Zhang, S.-E.; Liang, J.; Zhang, Y. Cancer associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 2018, 36, 209–220. [Google Scholar] [CrossRef]
- You, J.; Li, M.; Cao, L.; Gu, Q.; Deng, P.; Tan, Y.; Hu, C.P. Snail1-dependent cancer-associated fibroblasts induce epithelial-mesenchymal transition in lung cancer cells via exosomes. QJM Int. J. Med. 2019, 112, 581–590. [Google Scholar] [CrossRef]
- Ariston Gabriel, A.N.; Wang, F.; Jiao, Q.; Yvette, U.; Yang, X.; Al-Ameri, S.A.; Du, L.; Wang, Y.S.; Wang, C. The involvement of exosomes in the diagnosis and treatment of pancreatic cancer. Mol. Cancer 2020, 19, 132. [Google Scholar] [CrossRef]
- Mastronikolis, N.S.; Kyrodimos, E.; Spyropoulou, D.; Delides, A.; Giotakis, E.; Piperigkou, Z.; Karamanos, N.K. The role of exosomes in epithelial-to-mesenchymal transition and cell functional properties in head and neck cancer. Cancers 2023, 15, 2156. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, E.; Fiore, D.; Nappa, M.; Roscigno, G.; Adamo, A.; Iaboni, M.; Russo, V.; Affinito, A.; Puoti, I.; Quintavalle, C.; et al. Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget 2017, 8, 19592. [Google Scholar] [CrossRef]
- Hu, J.; Wang, W.; Lan, X.; Zeng, Z.; Liang, Y.; Yan, Y.R.; Song, F.Y.; Wang, F.F.; Zhu, X.H.; Liao, W.J.; et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol. Cancer 2019, 18, 91. [Google Scholar] [CrossRef]
- Zhou, L.; Li, J.; Tang, Y.; Yang, M. Exosomal LncRNA LINC00659 transferred from cancer-associated fibroblasts promotes colorectal cancer cell progression via miR-342-3p/ANXA2 axis. J. Transl. Med. 2021, 19, 8. [Google Scholar] [CrossRef]
- Paunescu, V.; Bojin, F.M.; Tatu, C.A.; Gavriliuc, O.I.; Rosca, A.; Gruia, A.T.; Tanasie, G.; Bunu, C.; Crisnic, D.; Gherghiceanu, M.; et al. Tumour-associated fibroblasts and mesenchymal stem cells: More similarities than differ-ences. J. Cell Mol. Med. 2011, 15, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Kabashima-Niibe, A.; Higuchi, H.; Takaishi, H.; Masugi, Y.; Matsuzaki, Y.; Mabuchi, Y.; Funakoshi, S.; Adachi, M.; Hamamoto, Y.; Kawachi, S.; et al. Mesenchymal stem cells regulate epithelial-mesenchymal transition and tumour progression of pancreatic cancer cells. Cancer Sci. 2013, 104, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.T.; Dwyer, R.M.; Kelly, J.; Khan, S.; Murphy, J.M.; Curran, C.; Miller, N.; Hennessy, E.; Dockery, P.; Barry, F.P.; et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: Stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res. Treat. 2010, 124, 317–326. [Google Scholar] [CrossRef]
- Schinköthe, T.; Bloch, W.; Schmidt, A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev. 2008, 17, 199–205. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, K.S.; Bae, S.; Son, H.K.; Myung, P.K.; Hong, H.J.; Kim, H. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int. J. Stem Cells 2009, 2, 59–68. [Google Scholar] [CrossRef]
- Ries, C.; Egea, V.; Karow, M.; Kolb, H.; Jochum, M.; Neth, P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: Differential regulation by inflammatory cytokines. Blood 2007, 109, 4055–4063. [Google Scholar] [CrossRef] [PubMed]
- Lozito, T.P.; Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Human mesenchymal stem cells generate a distinct pericellular zone of MMP activities via binding of MMPs and secretion of high levels of TIMPs. Matrix Biol. 2014, 34, 132–143. [Google Scholar] [CrossRef]
- Dittmer, A.; Hohlfeld, K.; Lützkendorf, J.; Müller, L.P.; Dittmer, J. Human mesenchymal stem cells induce E-cadherin degradation in breast carcinoma spheroids by activating AD-AM10. Cell Mol. Life Sci. 2009, 66, 3053–3065. [Google Scholar] [CrossRef]
- Makinoshima, H.; Dezawa, M. Pancreatic cancer cells activate CCL5 expression in mesenchymal stromal cells through the insulin-like growth factor-I pathway. FEBS Lett. 2009, 583, 3697–3703. [Google Scholar] [CrossRef]
- Fregni, G.; Quinodoz, M.; Möller, E.; Vuille, J.; Galland, S.; Fusco, C.; Martin, P.; Letovanec, I.; Provero, P.; Rivolta, C.; et al. Reciprocal modulation of mesenchymal stem cells and tumour cells promotes lung cancer metastasis. EBioMedicine 2018, 29, 128–145. [Google Scholar] [CrossRef]
- Isert, L.; Mehta, A.; Loiudice, G.; Oliva, A.; Roidl, A.; Merkel, O.M. An In Vitro Approach to Model EMT in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 7757. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.V.; Jücker, M. The functional role of extracellular matrix proteins in cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Shintani, Y.; Maeda, M.; Chaika, N.; Johnson, K.R.; Wheelock, M.J. Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signalling. Am. J. Respi. Cel. Mol. Biol. 2008, 38, 95–104. [Google Scholar] [CrossRef]
- Koenig, A.; Mueller, C.; Hasel, C.; Adler, G.; Menke, A. Collagen type I induces disruption of E-cadherin-mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 2006, 66, 4662–4671. [Google Scholar] [CrossRef]
- Menke, A.; Philippi, C.; Vogelmann, R.; Seidel, B.; Lutz, M.P.; Adler, G.; Wedlich, D. Down-regulation of E-cadherin gene expression by collagen type I and type III in pancreatic cancer cell lines. Cancer Res. 2001, 61, 3508–3517. [Google Scholar]
- Zhou, F.; Sun, J.; Ye, L.; Jiang, T.; Li, W.; Su, C.; Ren, S.; Wu, F.; Zhou, C.; Gao, G. Fibronectin promotes tumour angiogenesis and progression of non-small-cell lung cancer by elevating WISP3 expression via FAK/MAPK/ HIF-1α axis and activating wnt signalling pathway. Exp. Hema-tol. Oncol. 2023, 12, 61. [Google Scholar] [CrossRef]
- Li, C.L.; Yang, D.; Cao, X.; Wang, F.; Hong, D.Y.; Wang, J.; Shen, X.C.; Chen, Y. Fibronectin induces epithelial-mesenchymal transition in human breast cancer MCF-7 cells via activation of calpain. Oncol. Lett. 2017, 13, 3889–3895. [Google Scholar] [CrossRef]
- Nguyen, N.; Kumar, A.; Chacko, S.; Ouellette, R.J.; Ghosh, A. Human hyaluronic acid synthase-1 promotes malignant transformation via epithelial-to-mesenchymal transition, micronucleation and centrosome abnormalities. Cell Commun. Signal. 2017, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Chow, G.; Tauler, J.; Mulshine, J.L. Cytokines and growth factors stimulate hyaluronan production: Role of hyaluronan in epithelial to mesenchymal-like transition in non-small cell lung cancer. J. Biomed. Biotechnol. 2010, 1, 485468. [Google Scholar] [CrossRef]
- El-Haibi, C.P.; Bell, G.W.; Zhang, J.; Collmann, A.Y.; Wood, D.; Scherber, C.M.; Csizmadia, E.; Mariani, O.; Zhu, C.; Campagne, A.; et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc. Natl. Acad. Sci. USA 2012, 109, 17460–17465. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Herndon, J.M.; Sojka, D.K.; Kim, K.-W.; Knolhoff, B.L.; Zuo, C.; Cullinan, D.R.; Luo, J.; Bearden, A.R.; Lavine, K.J.; et al. Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumour progression. Immunity 2017, 47, 597. [Google Scholar] [CrossRef]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Walens, A.; DiMarco, A.V.; Lupo, R.; Kroger, B.R.; Damrauer, J.S.; Alvarez, J.V. CCL5 pro-motes breast cancer recurrence through macrophage recruitment in residual tumours. eLife 2019, 8, e43653. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Deng, Y.R.; Wang, Z.C.; Wei, W.F.; Zhou, C.F.; Zhang, Y.M.; Yan, R.M.; Liang, L.J.; Zhong, M.; Liang, L.; et al. Hypoxia-induced ZEB1 promotes cervical cancer progression via CCL8-dependent tumour-associated macrophage recruitment. Cell Death Dis. 2019, 10, 508. [Google Scholar] [CrossRef]
- Friedman-DeLuca, M.; Karagiannis, G.S.; Condeelis, J.S.; Oktay, M.H.; Entenberg, D. Macrophages in tumour cell migration and metastasis. Front. Immunol. 2024, 15, 1494462. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-b-mediated epithelial-mesenchymal transition and cancer metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Ling, Z.; Yang, X.; Chen, X.; Xia, J.; Cheng, B.; Tao, X. CCL2 promotes cell migration by inducing epithelial-mesenchymal transition in oral squamous cell carcinoma. J. Oral Pathol. Med. 2019, 48, 477–482. [Google Scholar] [CrossRef]
- Rokavec, M.; Öner, M.G.; Li, H.; Jackstadt, R.; Jiang, L.; Lodygin, D.; Kaller, M.; Horst, D.; Ziegler, P.K.; Schwitalla, S.; et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Investig. 2014, 124, 1853–1867. [Google Scholar] [CrossRef]
- Weng, Y.-S.; Tseng, H.-Y.; Chen, Y.-A.; Shen, P.-C.; Al Haq, A.T.; Chen, L.-M.; Tung, Y.C.; Hsu, H.L. MCT- 1/miR-34a/IL-6/IL-6R signalling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol. Cancer 2019, 18, 42. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, H.S.; Li, X.-Y.; Lee, I.; Choi, H.-S.; Kang, S.E.; Cha, S.Y.; Ryu, J.K.; Yoon, D.; Fearon, E.; et al. A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J. Cell Biol. 2011, 195, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Liu, Q.; Dou, R.; Xiong, B. Crosstalk between cancer cells and tumour associated macrophages is required for mesenchymal circulating tumour cell-mediated colorectal cancer metastasis. Mol. Cancer 2019, 18, 64. [Google Scholar] [CrossRef]
- Fu, X.-L.; Duan, W.; Su, C.-Y.; Mao, F.-Y.; Lv, Y.-P.; Teng, Y.-S.; Yu, P.W.; Zhuang, Y.; Zhao, Y.L. Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol. Immunother. 2017, 66, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Liu, Q.; Chen, J.; Chen, J.; Chen, F.; He, C.; Huang, D.; Wu, W.; Lin, L.; Huang, W.; et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 2014, 25, 605–620. [Google Scholar] [CrossRef]
- Huang, B.; Lang, X.; Li, X. The role of IL-6/JAK2/STAT3 signalling pathway in cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef]
- Yang, C.; Dou, R.; Wei, C.; Liu, K.; Shi, D.; Zhang, C.; Liu, Q.; Wang, S.; Xiong, B. Tumour-derived exosomal microRNA-106b-5p activates EMT-cancer cell and M2-subtype TAM interaction to facilitate CRC metastasis. Mol. Ther. 2021, 29, 2088–2107. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Tai, S.-K.; Yang, M.-H. Snail-overexpressing cancer cells promote M2-like polarization of tumour-associated macrophages by delivering miR-21-abundant exosomes. Neoplasia 2018, 20, 775–788. [Google Scholar] [CrossRef]
- Pires, B.R.; Mencalha, A.L.; Ferreira, G.M.; de Souza, W.F.; Morgado-Díaz, J.A.; Maia, A.M.; Corrêa, S.; Abdelhay, E.S. NF-kappaB Is Involved in the Regulation of EMT Genes in Breast Cancer Cells. PLoS ONE 2017, 12, e0169622. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, W.; Zhang, H.; Wu, W.; Peng, Y.; Zeng, Y.; Wan, Y.; Wang, J.; Ouyang, N. CCL18/PITPNM3 enhances migration, invasion, and EMT through the NF-kB signalling pathway in hepatocellular carcinoma. Tumour Biol. 2016, 37, 3461–3468. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, B.; Sun, X.; Zhang, X.; Lv, S.; Li, H.; Wang, X.; Zhao, C.; Zhang, H.; Xie, X.; et al. CC chemokine ligand 18(CCL18) promotes migration and invasion of lung cancer cells by binding to Nir1 through Nir1-ELMO1/DOC180 signalling pathway. Mol. Carcinog. 2016, 55, 2051–2062. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Wu, F.; Zhou, Y.; Bao, Z.; Li, H.; Zheng, P.; Zhao, S. Gastric cancer-derived mesen-chymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis. 2019, 10, 918. [Google Scholar] [CrossRef] [PubMed]
- Che, D.; Zhang, S.; Jing, Z.; Shang, L.; Jin, S.; Liu, F.; Shen, J.; Li, Y.; Hu, J.; Meng, Q.; et al. Macrophages induce EMT to promote invasion of lung cancer cells through the IL-6-mediated COX-2/PGE. Mol. Immunol. 2017, 90, 197–210. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, W.; Zhong, W.Q.; Liu, Z.J.; Li, H.M.; Yu, Z.L.; Li, H.M.; Yu, Z.L.; Zhao, Y.F. Tumour associated macrophages induce epithelial to mesenchymal transition via the EGFR/ERK1/2 pathway in head and neck squamous cell carcinoma. Oncol. Rep. 2018, 40, 2558–2572. [Google Scholar]
- Kuziel, G.; Moore, B.N.; Arendt, L.M. Obesity and fibrosis: Setting the stage for breast cancer. Cancers 2023, 15, 2929. [Google Scholar] [CrossRef] [PubMed]
- LaRue, M.M.; Parker, S.; Puccini, J.; Cammer, M.; Kimmelman, A.C.; Bar-Sagi, D. Metabolic reprogramming of tumour-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2119168119. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.-H.; Jia, Y.; Guo, F.-J.; Chen, J.; Zhang, X.-W.; Cui, M.-H. Phosphorylation of STAT3 at Tyr705 regulates MMP-9 production in epithelial ovarian cancer. PLoS ONE. 2017, 12, e0183622. [Google Scholar] [CrossRef]
- Liu, X.; Lv, Z.; Zou, J.; Liu, X.; Ma, J.; Sun, C.; Sa, N.; Xu, W. Elevated AEG-1 expression in macro-phages promotes hypopharyngeal cancer invasion through the STAT3-MMP-9 signalling pathway. Oncotarget 2016, 7, 77244–77256. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Fan, Y.; Xu, Q.; Ji, W.; Tian, R.; Niu, R. Elevated STAT3 signalling-mediated upregulation of MMP-2/9 confers enhanced invasion ability in multidrug-resistant breast cancer cells. Int. J. Mol. Sci. 2015, 16, 24772–24790. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, R.; Lan, T.; Ding, D.; Huang, S.; Shao, J.; Zheng, Z.; Chen, T.; Huang, Y.; Liu, J.; et al. Tumour-associated macro-phage-specific CD155 contributes to M2-phenotype transition, immunosuppression, and tumour progression in colorectal cancer. J. Immunother Cancer 2022, 10, e004219. [Google Scholar] [CrossRef]
- Yamanaka, N.; Morisaki, T.; Nakashima, H.; Tasaki, A.; Kubo, M.; Kuga, H.; Nakahara, C.; Nakamura, K.; Noshiro, H.; Yao, T.; et al. Interleukin 1beta enhances invasive ability of gastric carcinoma through nuclear factor kappa B activation. Clin. Cancer Res. 2004, 10, 1853–1859. [Google Scholar] [CrossRef]
- Kang, S.U.; Cho, S.Y.; Jeong, H.; Han, J.; Chae, H.Y.; Yang, H.; Sung, C.O.; Choi, Y.L.; Shin, Y.K.; Kwon, M.J. Matrix metalloproteinase 11 (MMP11) in macrophages promotes the migration of HER2- positive breast cancer cells and monocyte recruitment through CCL2-CCR2 signalling. Lab. Invest. 2022, 102, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Du, G.; Wang, X.; Wu, X.; Li, L.; Liu, W.; Wu, R. MMP-9 secreted by M2-type macrophages promotes Wilms’ tumour metastasis through the PI3K/AKT pathway. Mol. Biol. Rep. 2022, 49, 3469–3480. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ye, Y.; Zhu, X. MMP-9 secreted by tumour associated macrophages promoted gastric cancer metastasis through a PI3K/AKT/Snail pathway. BioMed. Pharmacother. 2019, 117, 109096. [Google Scholar] [CrossRef] [PubMed]
- Arwert, E.N.; Harney, A.S.; Entenberg, D.; Wang, Y.; Sahai, E.; Pollard, J.W.; Condeelis, J.S. A unidirectional transition from migratory to perivascular macrophage is required for tumour cell intravasation. Cell Rep. 2018, 23, 1239–1248. [Google Scholar] [CrossRef]
- Harney, A.S.; Arwert, E.N.; Entenberg, D.; Wang, Y.; Guo, P.; Qian, B.-Z.; Oktay, M.H.; Pollard, J.W.; Jones, J.G.; Condeelis, J.S. Realtime imaging reveals local, transient vascular permeability, and tumour cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov. 2015, 5, 932–943. [Google Scholar] [CrossRef]
- Roh-Johnson, M.; Bravo-Cordero, J.J.; Patsialou, A.; Sharma, V.P.; Guo, P.; Liu, H.; Hodgson, L.; Condeelis, J. Macrophage contact induces RhoA GTPase signalling to trigger tumour cell intravasation. Oncogene 2014, 33, 4203–4212. [Google Scholar] [CrossRef]
- Pignatelli, J.; Bravo-Cordero, J.J.; Roh-Johnson, M.; Gandhi, S.J.; Wang, Y.; Chen, X.; Eddy, R.J.; Xue, A.; Singer, R.H.; Hodgson, L.; et al. Macrophage-dependent tumour cell transendothelial migration is mediated by Notch1/Mena(INV)-initiated invadopodium formation. Sci. Rep. 2016, 6, 37874. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Lorenz, M.; Kempiak, S.; Sarmiento, C.; Coniglio, S.; Symons, M.; Segall, J.; Eddy, R.; Miki, H.; Takenawa, T.; et al. Molecular mechanisms of invadopodium formation: The role of the N-WASP-Arp2/3 com-plex pathway and cofilin. J. Cell Biol. 2005, 168, 441–452. [Google Scholar] [CrossRef]
- Jeannot, P.; Besson, A. Cortactin function in invadopodia. Small GTPases 2020, 11, 256–270. [Google Scholar] [CrossRef]
- Weidmann, M.D.; Surve, C.; Eddy, R.; Chen, X.; Gertler, F.; Sharma, V.; Condeelis, J.S. Mena (INV) dysregulates cortactin phosphorylation to promote invadopodium maturation. Sci. Rep. 2016, 6, 36142. [Google Scholar] [CrossRef]
- Goswami, S.; Sahai, E.; Wyckoff, J.B.; Cammer, M.; Cox, D.; Pixley, F.J.; Stanley, E.R.; Segall, J.E.; Condeelis, J.S. Macrophages promote the invasion of breast carcinoma cells via a colony stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005, 65, 5278–5283. [Google Scholar] [CrossRef] [PubMed]
- Roussos, E.T.; Balsamo, M.; Alford, S.K.; Wyckoff, J.B.; Gligorijevic, B.; Wang, Y.; Pozzuto, M.; Stobezki, R.; Goswami, S.; Segall, J.E.; et al. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J. Cell Sci. 2011, 124, 2120–2131. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, Q.; Xu, L.; Zhang, W.; Zhu, Y.; Liu, H.; Xu, J.; Gu, J. Increased expression of colony stimulating factor-1 is a predictor of poor prognosis in patients with clear-cell renal cell carcinoma. BMC Cancer 2015, 15, 67. [Google Scholar] [CrossRef]
- Mroczko, B.; Groblewska, M.; Wereszczyńska-Siemiatkowska, U.; Okulczyk, B.; Kedra, B.; Łaszewicz, W.; Dabrowski, A.; Szmitkowski, M. Serum macrophage-colony stimulating factor levels incolorectal cancer patients correlate with lymph node metastasis and poor prognosis. Clin. Chim. Acta. 2007, 380, 208–212. [Google Scholar] [CrossRef]
- Lo, H.-W.; Xia, W.; Wei, Y.; Ali-Seyed, M.; Huang, S.-F.; Hung, M.-C. Novel prognostic value of nuclear epidermal growth factor receptor in breast cancer. Cancer Res. 2005, 65, 338–348. [Google Scholar] [CrossRef]
- Leung, E.; Xue, A.; Wang, Y.; Rougerie, P.; Sharma, V.P.; Eddy, R.; Cox, D.; Condeelis, J. Blood vessel endothelium-directed tumour cell streaming in breast tumours requires the HGF/C-Met signalling pathway. Oncogene 2017, 36, 2680–2692. [Google Scholar] [CrossRef]
- Katopodi, T.; Petanidis, S.; Anestakis, D.; Charalampidis, C.; Chatziprodromidou, I.; Floros, G.; Eskitzis, P.; Zarogoulidis, P.; Koulouris, C.; Sevva, C.; et al. Tumour cell metabolic reprogramming and hypoxic immunosuppression: Driving carcinogenesis to metastatic colonization. Front. Immunol. 2023, 14, 1325360. [Google Scholar]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Baiey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumour microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Xiang, L.; Mou, J.; Shao, B.; Wei, Y.; Liang, H.; Takano, N.; Semenza, G.L.; Xie, G. Glutaminase 1 expression in colorectal cancer cells is induced by hypoxia and required for tumour growth, invasion, and metastatic colonization. Cell Death Dis. 2019, 10, 40. [Google Scholar] [CrossRef]
- Du, F.; Chen, J.; Liu, H.; Cai, Y.; Cao, T.; Han, W.; Qian, M.; Tian, D.; Nie, Y.; Wu, K.; et al. SOX12 promotes colorectal cancer cell proliferation and metastasis by regulating asparagine synthesis. Cell Death Dis. 2019, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pu, X.; Wang, X.; Xu, M. Reprogramming of lipid metabolism in the tumour microenvironment: A strategy for tumour immunotherapy. Lipids Health Dis. 2024, 23, 35. [Google Scholar] [CrossRef]
- Jin, H.-R.; Wang, J.; Wang, Z.-J.; Xi, M.-J.; Xia, B.-H.; Deng, K.; Yang, J.-L. Lipid metabolic reprogramming in tumour microenvironment: From mechanisms to therapeutics. J. Hematol. Oncol. 2023, 16, 103. [Google Scholar] [CrossRef]
- Torino, F.; Fuggetta, M.P.; Aquino, A.; Roselli, M.; Bonmassar, E.; Giuliani, A.; D’Atri, S. Tumour Immunotherapy: Drug-Induced Neoantigens (Xenogenization) and Immune Checkpoint Inhibitors. Oncotarget 2017, 8, 41641–41669. [Google Scholar]
- Geraud, A.; Gougis, P.; Vozy, A.; Anquetil, C.; Allenbach, Y.; Romano, E.; Funck-Brentano, E.; Moslehi, J.J.; Johnson, D.B.; Salem, J.E. Clinical Pharmacology and Interplay of Immune Checkpoint Agents: A Yin-Yang Balance. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Franzese, O. Tumour Microenvironment Drives the Cross-Talk Between Co-Stimulatory and Inhibitory Molecules in Tumour-Infiltrating Lymphocytes: Implications for Optimizing Immunotherapy Outcomes. Int. J. Mol. Sci. 2024, 25, 12848. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Q.; Shan, Q.; Liang, T.; Forde, P.; Zheng, L. Clinical Development of Immuno-Oncology Therapeutics. Cancer Lett. 2025, 617, 217616. [Google Scholar] [CrossRef]
- Khosravi, G.R.; Mostafavi, S.; Bastan, S.; Ebrahimi, N.; Gharibvand, R.S.; Eskandari, N. Immunologic Tumour Microenvironment Modulators for Turning Cold Tumours Hot. Cancer Commun. 2024, 44, 521–553. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, W.; Shen, L.; Yao, Y.; Xia, W.; Ni, C. Tumour Battlefield within In-flamed, Excluded or Desert Immune Phenotypes: The Mechanisms and Strategies. Exp. Hematol. Oncol. 2024, 13, 80. [Google Scholar] [CrossRef]
- Yang, W.; Liu, S.; Mao, M.; Gong, Y.; Li, X.; Lei, T.; Liu, C.; Wu, S.; Hu, Q. T-Cell Infiltration and Its Regulatory Mechanisms in Cancers: Insights at Single-Cell Resolution. J. Exp. Clin. Cancer Res. 2024, 43, 38. [Google Scholar] [CrossRef]
- Ouyang, P.; Wang, L.; Wu, J.; Tian, Y.; Chen, C.; Li, D.; Yao, Z.; Chen, R.; Xiang, G.; Gong, J.; et al. Overcoming Cold Tumours: A Combination Strategy of Immune Checkpoint Inhibitors. Front. Immunol. 2024, 15, 1344272. [Google Scholar] [CrossRef]
- Ma, K.; Wang, L.; Li, W.; Tang, T.; Ma, B.; Zhang, L.; Zhang, L. Turning Cold into Hot: Emerging Strategies to Fire Up the Tumour Microenvironment. Trends Cancer 2025, 11, 117–134. [Google Scholar] [CrossRef]
- Melssen, M.M.; Sheybani, N.D.; Leick, K.M.; Slingluff, C.L., Jr. Barriers to Immune Cell Infiltration in Tumours. J. Immunother. Cancer 2023, 11, e006401. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Coupland, S.E.; Aittokallio, T.; Figueiredo, C.R. Resistance to Immune Checkpoint Therapies by Tumour-Induced T-Cell Desertification and Exclusion: Key Mechanisms, Prognostication and New Therapeutic Opportunities. Br. J. Cancer 2023, 129, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Kohli, K.; Pillarisetty, V.G.; Kim, T.S. Key Chemokines Direct Migration of Immune Cells in Solid Tumours. Cancer Gene Ther. 2022, 29, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.J.; Pesenacker, A.M.; Wang, A.Y.; Gillies, J.; Mojibian, M.; Morishita, K.; Tan, R.; Kieffer, T.J.; Verchere, C.B.; Panagiotopoulos, C.; et al. T Regulatory Cell Chemokine Production Mediates Pathogenic T Cell Attraction and Suppression. J. Clin. Investig. 2016, 126, 1039–1051. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Cavallo, F.; Defilippi, P.; Conti, L. The Crosstalk Between Tumour Cells and the Immune Microenvironment in Breast Cancer: Implications for Immunotherapy. Front. Oncol. 2021, 11, 610303. [Google Scholar] [CrossRef]
- Fejza, A.; Carobolante, G.; Poletto, E.; Camicia, L.; Schinello, G.; Di Siena, E.; Ricci, G.; Mongiat, M.; Andreuzzi, E. The Entanglement of Extracellular Matrix Molecules and Immune Checkpoint Inhibitors in Cancer: A Systematic Review of the Literature. Front. Immunol. 2023, 14, 1270981. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Eroglu, Z.; Zaretsky, J.M.; Hu-Lieskovan, S.; Kim, D.W.; Algazi, A.; Johnson, D.B.; Liniker, E.; Kong, B.; Munhoz, R.; Rapisuwon, S.; et al. High Response Rate to PD-1 Blockade in Desmoplastic Melanomas. Nature 2018, 553, 347–350. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A Guide to Chemokines and Their Receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Reynders, N.; Abboud, D.; Baragli, A.; Noman, M.Z.; Rogister, B.; Niclou, S.P.; Heveker, N.; Janji, B.; Hanson, J.; Szpakowska, M.; et al. The Distinct Roles of CXCR3 Variants and Their Ligands in the Tumour Microenvironment. Cells 2019, 8, 613. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Wang, S.; Agarwal, V.; Marcisak, E.F.; Zuo, A.; Jablonski, S.A.; Loth, M.; Fertig, E.J.; MacDougall, J.; Zhukovsky, E.; et al. DPP Inhibition Alters the CXCR3 Axis and Enhances NK and CD8+ T Cell Infiltration to Improve Anti-PD1 Efficacy in Murine Models of Pancreatic Ductal Adenocarcinoma. J. Immunother. Cancer 2021, 9, e002837. [Google Scholar] [CrossRef]
- Stoll, G.; Pol, J.; Soumelis, V.; Zitvogel, L.; Kroemer, G. Impact of Chemotactic Factors and Receptors on the Cancer Immune Infiltrate: A Bioinformatics Study Revealing Homogeneity and Heterogeneity Among Patient Cohorts. Oncoimmunology 2018, 7, e1484980. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Wang, S.; Ni, H.; Zhao, P.; Chen, G.; Xu, B.; Yuan, L. The Role of CXCR3 and Its Ligands in Cancer. Front. Oncol. 2022, 12, 1022688. [Google Scholar] [CrossRef] [PubMed]
- Groom, J.R.; Luster, A.D. CXCR3 ligands: Redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 2011, 89, 207–215. [Google Scholar] [CrossRef]
- Bikfalvi, A.; Billottet, C. The CC and CXC chemokines: Major regulators of tumour progression and the tumour microenvironment. American journal of physiology. Cell physiol. 2020, 318, C542–C554. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.T.; Ozga, A.J.; Servis, R.L.; Frederick, D.T.; Lo, J.A.; Fisher, D.E.; Freeman, G.J.; Boland, G.M.; Luster, A.D. Intratumoural Activity of the CXCR3 Chemokine System Is Re-quired for the Efficacy of Anti-PD-1 Therapy. Immunity 2019, 50, 1498–1512.e5. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the Tumour Microenvironment on NK Cell Function in Solid Tumours. Front. Immunol. 2020, 10, 3038. [Google Scholar] [CrossRef]
- Bonanni, V.; Antonangeli, F.; Santoni, A.; Bernardini, G. Targeting of CXCR3 Improves Anti-Myeloma Efficacy of Adoptively Transferred Activated Natural Killer Cells. J. Immunother. Cancer 2019, 7, 290. [Google Scholar] [CrossRef]
- Sektioglu, I.M.; Carretero, R.; Bulbuc, N.; Bald, T.; Tüting, T.; Rudensky, A.Y.; Hämmerling, G.J. Basophils Promote Tumour Rejection via Chemotaxis and Infiltration of CD8+ T Cells. Cancer Res. 2017, 77, 291–302. [Google Scholar] [CrossRef]
- Reschke, R.; Enk, A.H.; Hassel, J.C. Chemokines and Cytokines in Immunotherapy of Melanoma and Other Tumours: From Biomarkers to Therapeutic Targets. Int. J. Mol. Sci. 2024, 25, 6532. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Yu, X.; Xue, L.; Ge, X.; Zhao, W.; Peng, W. Intrinsic β-Catenin Signalling Suppresses CD8+ T-Cell Infiltration in Colorectal Cancer. Biomed. Pharmacother. 2019, 115, 108921. [Google Scholar] [CrossRef]
- Huang, B.; Han, W.; Sheng, Z.F.; Shen, G.L. Identification of Immune-Related Biomarkers Associated with Tumourigenesis and Prognosis in Cutaneous Melanoma Patients. Cancer Cell Int. 2020, 20, 195. [Google Scholar] [CrossRef] [PubMed]
- Fairfax, B.P.; Taylor, C.A.; Watson, R.A.; Nassiri, I.; Danielli, S.; Fang, H.; Mahé, E.A.; Cooper, R.; Woodcock, V.; Traill, Z.; et al. Peripheral CD8+ T Cell Characteristics Associated with Durable Responses to Immune Checkpoint Blockade in Patients with Metastatic Melanoma. Nat. Med. 2020, 26, 193–199. [Google Scholar] [CrossRef]
- Chen, R.; Ma, L.; Jiang, C.; Zhang, S. Expression and Potential Role of CCL4 in CD8+ T Cells in NSCLC. Clin. Transl. Oncol. 2022, 24, 2420–2431. [Google Scholar] [CrossRef]
- Williford, J.M.; Ishihara, J.; Ishihara, A.; Mansurov, A.; Hosseinchi, P.; Marchell, T.M.; Potin, L.; Swartz, M.A.; Hubbell, J.A. Recruitment of CD103+ Dendritic Cells via Tumour-Targeted Chemokine Delivery Enhances Efficacy of Checkpoint Inhibitor Immunotherapy. Sci. Adv. 2019, 5, eaay1357. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, X.L.; Wang, Y.J.; Fang, Y.; Li, M.L.; Liu, X.Y.; Luo, H.Y.; Tian, Y. The Regulatory Network of the Chemokine CCL5 in Colorectal Cancer. Ann. Med. 2023, 55, 2205168. [Google Scholar] [CrossRef]
- Halama, N.; Zoernig, I.; Berthel, A.; Kahlert, C.; Klupp, F.; Suarez-Carmona, M.; Suetter-lin, T.; Brand, K.; Krauss, J.; Lasitschka, F.; et al. Tumoural Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Ef-fectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell 2016, 29, 587–601. [Google Scholar] [CrossRef]
- Aldinucci, D.; Borghese, C.; Casagrande, N. The CCL5/CCR5 Axis in Cancer Progression. Cancers 2020, 12, 1765. [Google Scholar] [CrossRef]
- Harlin, H.; Meng, Y.; Peterson, A.C.; Zha, Y.; Tretiakova, M.; Slingluff, C.; McKee, M.; Gajewski, T.F. Chemokine Expression in Melanoma Metastases Associated with CD8+ T-Cell Recruitment. Cancer Res. 2009, 69, 3077–3085. [Google Scholar] [CrossRef]
- Shan, J.; Xu, Y.; Lun, Y. Comprehensive Analysis of the Potential Biological Significance of CCL5 in Pan-Cancer Prognosis and Immunotherapy. Sci. Rep. 2024, 14, 22138. [Google Scholar] [CrossRef] [PubMed]
- Dangaj, D.; Bruand, M.; Grimm, A.J.; Ronet, C.; Barras, D.; Duttagupta, P.A.; Lanitis, E.; Duraiswamy, J.; Tanyi, J.L.; Benencia, F.; et al. Cooperation between Constitutive and Inducible Chemokines Enables T Cell Engraftment and Immune Attack in Solid Tumours. Cancer Cell 2019, 35, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ-Related mRNA Profile Predicts Clinical Response to PD-1 Blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Jiao, X.; Nawab, O.; Patel, T.; Kossenkov, A.V.; Halama, N.; Jaeger, D.; Pestell, R.G. Recent Advances Targeting CCR5 for Cancer and Its Role in Immuno-Oncology. Cancer Res. 2019, 79, 4801–4807. [Google Scholar] [CrossRef] [PubMed]
- Röhrle, N.; Knott, M.M.L.; Anz, D. CCL22 Signalling in the Tumour Environment. Adv. Exp. Med. Biol. 2020, 1231, 79–96. [Google Scholar]
- Ozga, A.J.; Chow, M.T.; Luster, A.D. Chemokines and the immune response to cancer. Immunity 2021, 54, 859–874. [Google Scholar] [CrossRef]
- Klarquist, J.; Tobin, K.; Farhangi Oskuei, P.; Henning, S.W.; Fernandez, M.F.; Dellacecca, E.R.; Navarro, F.C.; Eby, J.M.; Chatterjee, S.; Mehrotra, S.; et al. CCL22 Di-verts T Regulatory Cells and Controls the Growth of Melanoma. Cancer Res. 2016, 76, 6230–6240. [Google Scholar] [CrossRef]
- Mizukami, Y.; Kono, K.; Kawaguchi, Y.; Akaike, H.; Kamimura, K.; Sugai, H.; Fujii, H. CCL17 and CCL22 chemokines within tumour microenvironment are related to accumula-tion of Foxp3+ regulatory T cells in gastric cancer. Int. J. Cancer 2008, 122, 2286–2293. [Google Scholar] [CrossRef]
- Wiedemann, G.M.; Knott, M.M.; Vetter, V.K.; Rapp, M.; Haubner, S.; Fesseler, J.; Kühnemuth, B.; Layritz, P.; Thaler, R.; Kruger, S.; et al. Cancer Cell-Derived IL-1α Induces CCL22 and the Recruitment of Regulatory T Cells. Cancer Res. 2023, 83, 123–134. [Google Scholar] [CrossRef]
- Adler, E.P.; Lemken, C.A.; Katchen, N.S.; Kurt, R.A. A dual role for tumour-derived chemokine RANTES (CCL5). Immunol. Lett. 2003, 90, 187–194. [Google Scholar] [CrossRef]
- Paolini, L.; Saldmann, A.; Tartour, E. CD8+ T Cell in Cancer Immunotherapy: Role and Value of Its Therapeutic Targeting. Bull. Acad. Natl. Med. 2021, 205, 354–363. [Google Scholar]
- Mami-Chouaib, F.; Blanc, C.; Corgnac, S.; Hans, S.; Malenica, I.; Granier, C.; Tihy, I.; Tartour, E. Resident Memory T Cells, Critical Components in Tumour Immunology. J. Immunother. Cancer 2018, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, N.; Tran, T.; Sam, I.; Pourmir, I.; Gruel, N.; Granier, C.; Pineau, J.; Gey, A.; Kob-old, S.; Fabre, E.; et al. CXCR6 Expressing T Cells: Functions and Role in the Control of Tumours. Front. Immunol. 2022, 13, 1022136. [Google Scholar] [CrossRef]
- Di Pilato, M.; Kfuri-Rubens, R.; Pruessmann, J.N.; Ozga, A.J.; Messemaker, M.; Cadilha, B.L.; Sivakumar, R.; Cianciaruso, C.; Warner, R.D.; Marangoni, F.; et al. CXCR6 Positions Cytotoxic T Cells to Receive Critical Survival Signals in the Tumour Microenvironment. Cell 2021, 184, 4512–4530. [Google Scholar] [CrossRef]
- Cullen, R.; Germanov, E.; Shimaoka, T.; Johnston, B. Enhanced Tumour Metastasis in Response to Blockade of the Chemokine Receptor CXCR6 Is Overcome by NKT Cell Activation. J. Immunol. 2009, 183, 5807–5815. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.S.; Wang, L.; Lim, B.; Deng, D.; Wu, H.; Wang, X.F.; Li, Q.J. Resident Memory T Cells in Tumour-Distant Tissues Fortify against Metastasis Formation. Cell Rep. 2021, 35, 109118. [Google Scholar] [CrossRef]
- Christo, S.N.; Park, S.L.; Mueller, S.N.; Mackay, L.K. The Multifaceted Role of Tissue-Resident Memory T Cells. Annu. Rev. Immunol. 2024, 42, 317–345. [Google Scholar] [CrossRef]
- Takamura, S.; Kato, S.; Motozono, C.; Shimaoka, T.; Ueha, S.; Matsuo, K.; Miyauchi, K.; Masumoto, T.; Katsushima, A.; Nakayama, T.; et al. Interstitial-Resident Memory CD8+ T Cells Sustain Frontline Epithelial Memory in the Lung. J. Exp. Med. 2019, 216, 2736–2747. [Google Scholar] [CrossRef]
- Djenidi, F.; Adam, J.; Gour, A.; Durgeau, A.; Meurice, G.; de Montpréville, V.; Validire, P.; Besse, B.; Mami-Chouaib, F. CD8+CD103+ Tumour-Infiltrating Lymphocytes Are Tumour-Specific Tissue-Resident Memory T Cells and a Prognostic Factor for Survival in Lung Cancer Patients. J. Immunol. 2015, 194, 3475–3486. [Google Scholar] [CrossRef] [PubMed]
- Ariotti, S.; Hogenbirk, M.A.; Dijkgraaf, F.E.; Visser, L.L.; Hoekstra, M.E.; Song, J.Y.; Jacobs, H.; Haanen, J.B.; Schumacher, T.N. Skin-Resident Memory CD8+ T Cells Trigger a State of Tissue-Wide Pathogen Alert. Science 2014, 346, 101–105. [Google Scholar] [CrossRef]
- Hartana, C.A.; Ahlén Bergman, E.; Broomé, A.; Berglund, S.; Johansson, M.; Alamdari, F.; Jakubczyk, T.; Huge, Y.; Aljabery, F.; Palmqvist, K.; et al. Tissue-Resident Memory T Cells Are Epigenetically Cytotoxic with Signs of Exhaus-tion in Human Urinary Bladder Cancer. Clin. Exp. Immunol. 2018, 194, 39–53. [Google Scholar] [CrossRef]
- Edwards, J.; Wilmott, J.S.; Madore, J.; Gide, T.N.; Quek, C.; Tasker, A.; Ferguson, A.; Chen, J.; Hewavisenti, R.; Hersey, P.; et al. CD103+ Tumour-Resident CD8+ T Cells Are Associated with Improved Survival in Immunotherapy-Naïve Melanoma Patients and Expand Significantly during Anti-PD-1 Treatment. Clin. Cancer Res. 2018, 24, 3036–3045. [Google Scholar] [CrossRef] [PubMed]
- Kurd, N.S.; He, Z.; Louis, T.L.; Milner, J.J.; Omilusik, K.D.; Jin, W.; Tsai, M.S.; Widjaja, C.E.; Kanbar, J.N.; Olvera, J.G.; et al. Early Precursors and Molecular Determinants of Tissue-Resident Memory CD8+ T Lymphocytes Revealed by Single-Cell RNA Sequencing. Sci. Immunol. 2020, 5, eaaz6894. [Google Scholar] [CrossRef] [PubMed]
- Palermo, B.; Franzese, O.; Frisullo, G.; D’Ambrosio, L.; Panetta, M.; Campo, G.; D’Andrea, D.; Sperduti, I.; De Nicola, F.; Goeman, F.; et al. CD28/PD1 Co-Expression: Dual Impact on CD8+ T Cells in Peripheral Blood and Tumour Tissue, and its Significance in NSCLC Patients’ Survival and ICB Response. J. Exp. Clin. Cancer Res. 2023, 42, 287. [Google Scholar] [CrossRef]
- Marceaux, C.; Weeden, C.E.; Gordon, C.L.; Asselin-Labat, M.L. Holding Our Breath: The Promise of Tissue-Resident Memory T Cells in Lung Cancer. Transl. Lung Cancer Res. 2021, 10, 2819–2829. [Google Scholar] [CrossRef]
- Pettersen, J.S.; Fuentes-Duculan, J.; Suárez-Fariñas, M.; Pierson, K.C.; Pitts-Kiefer, A.; Fan, L.; Belkin, D.A.; Wang, C.Q.; Bhuvanendran, S.; Johnson-Huang, L.M.; et al. Tumour-Associated Macrophages in the Cutaneous SCC Microenvironment Are Heterogeneously Activated. J. Investig. Dermatol. 2011, 131, 1322–1330. [Google Scholar] [CrossRef]
- Gabrusiewicz, K.; Rodriguez, B.; Wei, J.; Hashimoto, Y.; Healy, L.M.; Maiti, S.N.; Thom-as, G.; Zhou, S.; Wang, Q.; Elakkad, A.; et al. Glioblastoma-Infiltrated Innate Immune Cells Resemble M0 Macrophage Phenotype. JCI Insight 2016, 1, e85841. [Google Scholar] [CrossRef]
- Yang, C.M.; Ji, S.; Li, Y.; Fu, L.Y.; Jiang, T.; Meng, F.D. β-Catenin Promotes Cell Proliferation, Migration, and Invasion but Induces Apoptosis in Renal Cell Carcinoma. OncoTargets Ther. 2017, 10, 711–724. [Google Scholar] [CrossRef]
- Hong, R.; Shen, M.H.; Xie, X.H.; Ruan, S.M. Inhibition of Breast Cancer Metastasis via PITPNM3 by Pachymic Acid. Asian Pac. J. Cancer Prev. 2012, 13, 1877–1880. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Huang, Z.; Sun, X.; Zheng, X.; Liu, J.; Shen, J.; Jia, B.; Luo, H.; Mai, Z.; Chen, G.; et al. CCL18-NIR1 Promotes Oral Cancer Cell Growth and Metastasis by Activating the JAK2/STAT3 Signalling Pathway. BMC Cancer 2020, 20, 632. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Olbromski, M.; Dzięgiel, P. CCL18 in the Progression of Cancer. Int. J. Mol. Sci. 2020, 21, 7955. [Google Scholar] [CrossRef] [PubMed]
- Duluc, D.; Corvaisier, M.; Blanchard, S.; Catala, L.; Descamps, P.; Gamelin, E.; Ponsoda, S.; Delneste, Y.; Hebbar, M.; Jeannin, P. Interferon-Gamma Reverses the Immunosuppressive and Protumoural Properties and Prevents the Generation of Human Tumour-Associated Macrophages. Int. J. Cancer 2009, 125, 367–373. [Google Scholar] [CrossRef]
- Su, S.; Liao, J.; Liu, J.; Huang, D.; He, C.; Chen, F.; Yang, L.; Wu, W.; Chen, J.; Lin, L.; et al. Blocking the Recruitment of Naive CD4+ T Cells Reverses Immunosuppression in Breast Cancer. Cell Res. 2017, 27, 461–482. [Google Scholar] [CrossRef]
- Tiemessen, M.M.; Jagger, A.L.; Evans, H.G.; van Herwijnen, M.J.; John, S.; Taams, L.S. CD4+CD25+Foxp3+ Regulatory T Cells Induce Alternative Activation of Human Monocytes/Macrophages. Proc. Natl. Acad. Sci. USA 2007, 104, 19446–19451. [Google Scholar] [CrossRef]
- Hoves, S.; Krause, S.W.; Schütz, C.; Halbritter, D.; Schölmerich, J.; Herfarth, H.; Fleck, M. Monocyte-Derived Human Macrophages Mediate Anergy in Allogeneic T Cells and Induce Regulatory T Cells. J. Immunol. 2006, 177, 2691–2698. [Google Scholar] [CrossRef]
- Vulcano, M.; Struyf, S.; Scapini, P.; Cassatella, M.; Bernasconi, S.; Bonecchi, R.; Calleri, A.; Penna, G.; Adorini, L.; Luini, W.; et al. Unique Regulation of CCL18 Production by Maturing Dendritic Cells. J. Immunol. 2003, 170, 3843–3849. [Google Scholar] [CrossRef]
- Yu, G.; Fang, M.; Gong, M.; Liu, L.; Zhong, J.; Feng, W.; Xiong, P.; Wang, C.Y.; Gong, F. Steady-State Dendritic Cells with Forced IDO Expression Induce Skin Allograft Tolerance by Upregulation of Regulatory T Cells. Transpl. Immunol. 2008, 18, 208–219. [Google Scholar] [CrossRef]
- Meng, W.; Xue, S.; Chen, Y. The role of CXCL12 in tumor microenvironment. Gene 2018, 641, 105–110. [Google Scholar] [CrossRef]
- Mezzapelle, R.; Leo, M.; Caprioglio, F.; Colley, L.S.; Lamarca, A.; Sabatino, L.; Colantuoni, V.; Crippa, M.P.; Bianchi, M.E. CXCR4/CXCL12 Activities in the Tumour Microenvironment and Implications for Tumour Immunotherapy. Cancers 2022, 14, 2314. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the Immune System in Cancer: From Tumour Initiation to Metastatic Progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, G.; Cecchinato, V.; Uguccioni, M. Chemokine Heterocomplexes and Cancer: A Novel Chapter to Be Written in Tumour Immunity. Front. Immunol. 2018, 9, 2185. [Google Scholar] [CrossRef] [PubMed]
- Levoye, A.; Balabanian, K.; Baleux, F.; Bachelerie, F.; Lagane, B. CXCR7 Heterodimerizes with CXCR4 and Regulates CXCL12-Mediated G Protein Signalling. Blood 2009, 113, 6085–6093. [Google Scholar] [CrossRef]
- Gao, S.H.; Liu, S.Z.; Wang, G.Z.; Zhou, G.B. CXCL13 in Cancer and Other Diseases: Biological Functions, Clinical Significance, and Therapeutic Opportunities. Life 2021, 11, 1282. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.A.; Martinez, B.R.; Koppensteiner, L.; Mathieson, L.; Akram, A.R. Cancer-Associated Fibroblasts Drive CXCL13 Production in Activated T Cells via TGF-Beta. Front. Immunol. 2023, 14, 1221532. [Google Scholar] [CrossRef]
- Groeneveld, C.S.; Fontugne, J.; Cabel, L.; Bernard-Pierrot, I.; Radvanyi, F.; Allory, Y.; de Reyniès, A. Tertiary Lymphoid Structures Marker CXCL13 is Associated with Better Survival for Patients with Advanced-Stage Bladder Cancer Treated with Immunotherapy. Eur. J. Cancer 2021, 148, 181–189. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B Cells and Tertiary Lymphoid Structures Promote Immunotherapy Response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Yang, M.; Lu, J.; Zhang, G.; Wang, Y.; He, M.; Xu, Q.; Xu, C.; Liu, H. CXCL13 shapes im-munoactive tumour microenvironment and enhances the efficacy of PD-1 checkpoint blockade in high-grade serous ovarian cancer. J. Immunother. Cancer 2021, 9, e001136. [Google Scholar] [CrossRef]
- Vahidian, F.; Lamaze, F.C.; Bouffard, C.; Coulombe, F.; Gagné, A.; Blais, F.; Tonneau, M.; Orain, M.; Routy, B.; Manem, V.S.K.; et al. CXCL13 Positive Cells Localization Predict Response to Anti-PD-1/PD-L1 in Pulmonary Non-Small Cell Carcinoma. Cancers 2024, 16, 708. [Google Scholar] [CrossRef] [PubMed]
- Dustin, M.L. T-Cell Activation through Immunological Synapses and Kinapses. Immunol. Rev. 2008, 221, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Shakhar, G.; Lindquist, R.L.; Skokos, D.; Dudziak, D.; Huang, J.H.; Nussenzweig, M.C.; Dustin, M.L. Stable T Cell-Dendritic Cell Interactions Precede the Development of Both Tol-erance and Immunity in Vivo. Nat. Immunol. 2005, 6, 707–714. [Google Scholar] [CrossRef]
- Nourshargh, S.; Alon, R. Leukocyte migration into inflamed tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Comrie, W.A.; Babich, A.; Burkhardt, J.K. F-Actin Flow Drives Affinity Maturation and Spatial Organization of LFA-1 at the Immunological Synapse. J. Cell Biol. 2015, 208, 475–491. [Google Scholar] [CrossRef]
- Shi, H.; Shao, B. LFA-1 Activation in T-Cell Migration and Immunological Synapse Formation. Cells 2023, 12, 1136. [Google Scholar] [CrossRef]
- Shulman, Z.; Shinder, V.; Klein, E.; Grabovsky, V.; Yeger, O.; Geron, E.; Montresor, A.; Bolomini-Vittori, M.; Feigelson, S.W.; Kirchhausen, T.; et al. Lymphocyte Crawling and Transendothelial Migration Require Chemokine Triggering of High-Affinity LFA-1 Integrin. Immunity 2009, 30, 384–396. [Google Scholar] [CrossRef]
- Franzese, O.; Ancona, P.; Bianchi, N.; Aguiari, G. Apoptosis, a Metabolic “Head-to-Head” between Tumour and T Cells: Implications for Immunotherapy. Cells 2024, 13, 1430. [Google Scholar] [CrossRef]
- Beckermann, K.E.; Hongo, R.; Ye, X.; Young, K.; Carbonell, K.; Healey, D.C.C.; Siska, P.J.; Barone, S.; Roe, C.E.; Smith, C.C.; et al. CD28 Costimulation Drives Tumour-Infiltrating T Cell Glycolysis to Promote Inflammation. J. Clin. Investig. 2020, 5, e138729. [Google Scholar]
- Kong, K.F.; Yokosuka, T.; Canonigo-Balancio, A.J.; Isakov, N.; Saito, T.; Altman, A. A Motif in the V3 Domain of the Kinase PKC-θ Determines Its Localization in the Immuno-logical Synapse and Functions in T Cells via Association with CD28. Nat. Immunol. 2011, 12, 1105–1112. [Google Scholar] [CrossRef]
- Aquino, A.; Bianchi, N.; Terrazzan, A.; Franzese, O. Protein Kinase C at the Crossroad of Mutations, Cancer, Targeted Therapy, and Immune Response. Biology 2023, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Esensten, J.H.; Helou, Y.A.; Chopra, G.; Weiss, A.; Bluestone, J.A. CD28 Costimulation: From Mechanism to Therapy. Immunity 2016, 44, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Mirenda, V.; Jarmin, S.J.; David, R.; Dyson, J.; Scott, D.; Gu, Y.; Lechler, R.I.; Okkenhaug, K.; Marelli-Berg, F.M. Physiologic and Aberrant Regulation of Memory T-Cell Trafficking by the Costimulatory Molecule CD28. Blood 2007, 109, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Krummel, M.F.; Bartumeus, F.; Gérard, A. T Cell Migration, Search Strategies, and Mechanisms. Nat. Rev. Immunol. 2016, 16, 193–201. [Google Scholar] [CrossRef]
- Masteller, E.L.; Chuang, E.; Mullen, A.C.; Reiner, S.L.; Thompson, C.B. Structural Analysis of CTLA-4 Function in Vivo. J. Immunol. 2000, 164, 5319–5327. [Google Scholar] [CrossRef]
- Jain, N.; Nguyen, H.; Chambers, C.; Kang, J. Dual Function of CTLA-4 in Regulatory T Cells and Conventional T Cells to Prevent Multiorgan Autoimmunity. Proc. Natl. Acad. Sci. USA 2010, 107, 1524–1528. [Google Scholar] [CrossRef]
- Schneider, H.; Valk, E.; Leung, R.; Rudd, C.E. CTLA-4 Activation of Phosphatidylinositol 3-Kinase (PI 3-K) and Protein Kinase B (PKB/AKT) Sustains T-Cell Anergy without Cell Death. PLoS ONE 2008, 3, e3842. [Google Scholar] [CrossRef]
- Schneider, H.; Valk, E.; da Rocha Dias, S.; Wei, B.; Rudd, C.E. CTLA-4 Up-Regulation of Lymphocyte Function-Associated Antigen 1 Adhesion and Clustering as an Alternate Basis for Coreceptor Function. Proc. Natl. Acad. Sci. USA 2005, 102, 12861–12866. [Google Scholar] [CrossRef]
- Brunner-Weinzierl, M.C.; Rudd, C.E. CTLA-4 and PD-1 Control of T-Cell Motility and Migration: Implications for Tumour Immunotherapy. Front. Immunol. 2018, 9, 2737. [Google Scholar] [CrossRef]
- Pentcheva-Hoang, T.; Simpson, T.R.; Montalvo-Ortiz, W.; Allison, J.P. Cytotoxic T Lymphocyte Antigen-4 Blockade Enhances Antitumour Immunity by Stimulating Melanoma-Specific T-Cell Motility. Cancer Immunol. Res. 2014, 2, 970–980. [Google Scholar] [CrossRef]
- Lu, Y.; Schneider, H.; Rudd, C.E. Murine Regulatory T Cells Differ from Conventional T Cells in Resisting the CTLA-4 Reversal of TCR Stop-Signal. Blood 2012, 120, 4560–4570. [Google Scholar] [CrossRef] [PubMed]
- Fife, B.T.; Pauken, K.E.; Eagar, T.N.; Obu, T.; Wu, J.; Tang, Q.; Azuma, M.; Krummel, M.F.; Bluestone, J.A. Interactions between PD-1 and PD-L1 Promote Tolerance by Blocking the TCR-Induced Stop Signal. Nat. Immunol. 2009, 10, 1185–1192. [Google Scholar] [CrossRef]
- Gimsa, U.; ØRen, A.; Pandiyan, P.; Teichmann, D.; Bechmann, I.; Nitsch, R.; Brunner-Weinzierl, M.C. Astrocytes Protect the CNS: Antigen-Specific T Helper Cell Responses Are Inhibited by Astrocyte-Induced Upregulation of CTLA-4 (CD152). J. Mol. Med. 2004, 82, 364–372. [Google Scholar] [CrossRef]
- Gimsa, U.; Mitchison, N.A.; Brunner-Weinzierl, M.C. Immune Privilege as an Intrinsic CNS Property: Astrocytes Protect the CNS against T-Cell-Mediated Neuroinflammation. Mediators Inflamm. 2013, 2013, 320519. [Google Scholar] [CrossRef]
- Ruocco, M.G.; Pilones, K.A.; Kawashima, N.; Cammer, M.; Huang, J.; Babb, J.S.; Liu, M.; Formenti, S.C.; Dustin, M.L.; Demaria, S. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumour effects. J. Clin. Investig. 2012, 122, 3718–3730. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Greenshields-Watson, A.; Jones, E.; Smart, K.; Lauder, S.N.; Somerville, M.; Mi-lutinovic, S.; Kendrick, H.; Hindley, J.P.; French, R.; et al. Immune remodeling of the extracellular matrix drives loss of cancer stem cells and tumour rejection. Cancer Immunol. Res. 2020, 8, 1520–1531. [Google Scholar] [CrossRef]
- Lv, D.; Fei, Y.; Chen, H.; Wang, J.; Han, W.; Cui, B.; Feng, Y.; Zhang, P.; Chen, J. Crosstalk between T lymphocytes and extracellular matrix in the tumour microenvironment. Front. Immunol. 2024, 15, 1340702. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.A.; Chariou, P.L.; Gameiro, S.R.; Qin, H.; Iida, M.; Fousek, K.; Meyer, T.J.; Cam, M.; Flies, D.; Langermann, S.; et al. Remodeling the tumour microenvironment via blockade of LAIR-1 and TGF-β signalling enables PD-L1-mediated tumour eradication. J. Clin. Investig. 2022, 132, e155148. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of extracellular matrix in develop-ment and cancer progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Ali, A.J.; Abuelela, A.F.; Merzaban, J.S. An analysis of trafficking receptors shows that CD44 and P-selectin glycoprotein ligand-1 collectively control the migration of activated human T-cells. Front. Immunol. 2017, 8, 492. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Zhao, Y. Targeting Collagen in “Armored & Cold” Tumours: Overcoming Barriers to Cancer Therapy. Cancer Pathog. Ther. 2024, in press. [Google Scholar]
- Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010, 10, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Carrasco, G.; Le Saout, C.; Fontanaud, P.; Michau, A.; Mollard, P.; Hernandez, J.; Schaeffer, M. Integrin β1 optimizes diabetogenic T cell migration and function in the pancreas. Front. Immunol. 2018, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix in tumour progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumour microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Huang, Y.; Yuan, J.; Righi, E.; Kamoun, W.S.; Ancukiewicz, M.; Nezivar, J.; Santosuosso, M.; Martin, J.D.; Martin, M.R.; Vianello, F.; et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumour microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. USA 2012, 109, 17561–17566. [Google Scholar] [CrossRef]
- Khan, K.A.; Kerbel, R.S. Improving Immunotherapy Outcomes with Anti-Angiogenic Treatments and Vice Versa. Nat. Rev. Clin. Oncol. 2018, 15, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Wachholz, G.E.; Akbari, P.; Huijbers, E.J.M.; Jalan, P.; van Beijnum, J.R.; Griffioen, A.W. Targeting Endothelial Cell Anergy to Improve CAR T Cell Therapy for Solid Tumours. Biochim. Biophys. Acta 2024, 1879, 189155. [Google Scholar]
- Peske, J.D.; Woods, A.B.; Engelhard, V.H. Control of CD8 T-cell infiltration into tumours by vasculature and microenvironment. Adv. Cancer Res. 2015, 128, 263–307. [Google Scholar]
- Sui, X.; Ma, J.; Han, W.; Wang, X.; Fang, Y.; Li, D.; Pan, H.; Zhang, L. The anticancer immune response of anti-PD-1/PD-L1 and the genetic determinants of response to anti-PD-1/PD-L1 antibodies in cancer patients. Oncotarget 2015, 6, 19393–19404. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Brinkman, C.C.; Peske, J.D.; Engelhard, V.H. Peripheral tissue homing receptor control of naïve, effector, and memory CD8 T cell localization in lymphoid and non-lymphoid tissues. Front. Immunol. 2013, 4, 241. [Google Scholar] [CrossRef]
- Woods, A.N.; Wilson, A.L.; Srivinisan, N.; Zeng, J.; Dutta, A.B.; Peske, J.D.; Tewalt, E.F.; Gregg, R.K.; Ferguson, A.R.; Engelhard, V.H. Differential expression of homing receptor ligands on tumour-associated vasculature that control CD8 effector T-cell entry. Cancer Immunol. Res. 2017, 5, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer. 2019, 19, 307–325. [Google Scholar] [CrossRef] [PubMed]
- van de Walle, T.; Vaccaro, A.; Ramachandran, M.; Pietilä, I.; Essand, M.; Dimberg, A. Tertiary lymphoid structures in the central nervous system: Implications for glioblastoma. Front. Immunol. 2021, 12, 724739. [Google Scholar] [CrossRef]
- Georganaki, M.; van Hooren, L.; Dimberg, A. Vascular targeting to increase the efficiency of immune checkpoint blockade in cancer. Front. Immunol. 2018, 9, 3081. [Google Scholar] [CrossRef] [PubMed]
- Magar, A.G.; Morya, V.K.; Kwak, M.K.; Oh, J.U.; Noh, K.C. A Molecular Perspective on HIF-1α and Angiogenic Stimulator Networks and Their Role in Solid Tumours: An Update. Int. J. Mol. Sci. 2024, 25, 3313. [Google Scholar] [CrossRef]
- Huang, H.; Langenkamp, E.; Georganaki, M.; Loskog, A.; Fuchs, P.F.; Dieterich, L.C.; Kreuger, J.; Dimberg, A. VEGF suppresses T-lymphocyte infiltration in the tumour microenvironment through inhibition of NF-κB-induced endothelial activation. FASEB J. 2015, 29, 227–238. [Google Scholar] [CrossRef]
- Yu, J.S.; Lee, P.K.; Ehtesham, M.; Samoto, K.; Black, K.L.; Wheeler, C.J. Intratumoural T cell subset ratios and Fas ligand expression on brain tumour endothelium. J. Neuro-Oncol. 2003, 64, 55–61. [Google Scholar] [CrossRef]
- Motz, G.T.; Santoro, S.P.; Wang, L.P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumour endothelium FasL establishes a selec-tive immune barrier promoting tolerance in tumours. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef]
- Simula, L.; Fumagalli, M.; Vimeux, L.; Rajnpreht, I.; Icard, P.; Birsen, G.; An, D.; Pendino, F.; Rouault, A.; Bercovici, N.; et al. Mitochondrial Metabolism Sustains CD8+ T Cell Migration for an Efficient Infiltration into Solid Tumours. Nat. Commun. 2024, 15, 2203. [Google Scholar] [CrossRef]
- Vuononvirta, J.; Marelli-Berg, F.M.; Poobalasingam, T. Metabolic Regulation of T Lymphocyte Motility and Migration. Mol. Asp. Med. 2021, 77, 100888. [Google Scholar] [CrossRef]
- Cham, C.M.; Driessens, G.; O’Keefe, J.P.; Gajewski, T.F. Glucose Deprivation Inhibits Multiple Key Gene Expression Events and Effector Functions in CD8+ T Cells. Eur. J. Immunol. 2008, 38, 2438–2450. [Google Scholar] [CrossRef]
- Marelli-Berg, F.M.; Jangani, M. Metabolic Regulation of Leukocyte Motility and Migration. J. Leukoc. Biol. 2018, 104, 285–293. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Haas, R.; Smith, J.; Rocher-Ros, V.; Nadkarni, S.; Montero-Melendez, T.; D’Acquisto, F.; Bland, E.J.; Bombardieri, M.; Pitzalis, C.; Perretti, M.; et al. Lactate Regulates Metabolic and Pro-Inflammatory Circuits in Control of T Cell Migration and Effector Functions. PLoS Biol. 2015, 13, e1002202. [Google Scholar] [CrossRef]
- Kishore, M.; Cheung, K.C.P.; Fu, H.; Bonacina, F.; Wang, G.; Coe, D.; Ward, E.J.; Colamatteo, A.; Jangani, M.; Baragetti, A.; et al. Regulatory T Cell Migration Is Dependent on Glucokinase-Mediated Glycolysis. Immunity 2017, 47, 875–889. [Google Scholar] [CrossRef]
- Norris, V.; Amar, P.; Legent, G.; Ripoll, C.; Thellier, M.; Ovádi, J. Sensor Potency of the Moonlighting Enzyme-Decorated Cytoskeleton: The Cytoskeleton as a Metabolic Sensor. BMC Biochem. 2013, 14, 3. [Google Scholar] [CrossRef]
- Real-Hohn, A.; Zancan, P.; Da Silva, D.; Martins, E.R.; Salgado, L.T.; Mermelstein, C.S.; Gomes, A.M.; Sola-Penna, M. Filamentous Actin and Its Associated Binding Proteins Are the Stimulatory Site for 6-Phosphofructo-1-Kinase Association within the Membrane of Human Erythrocytes. Biochimie 2010, 92, 538–544. [Google Scholar] [CrossRef]
- Barbieri, L.; Veliça, P.; Gameiro, P.A.; Cunha, P.P.; Foskolou, I.P.; Rullman, E.; Bargiela, D.; Johnson, R.S.; Rundqvist, H. Lactate Exposure Shapes the Metabolic and Tran-scriptomic Profile of CD8+ T Cells. Front. Immunol. 2023, 14, 1101433. [Google Scholar] [CrossRef]
- Watson, M.J.; Vignali, P.D.A.; Mullett, S.J.; Overacre-Delgoffe, A.E.; Peralta, R.M.; Grebinoski, S.; Menk, A.V.; Rittenhouse, N.L.; DePeaux, K.; Whetstone, R.D.; et al. Metabolic Support of Tumour-Infiltrating Regulatory T Cells by Lactic Acid. Nature 2021, 591, 645–651. [Google Scholar] [CrossRef]
- Piconese, S.; Campello, S.; Natalini, A. Recirculation and Residency of T Cells and Tregs: Lessons Learnt in Anacapri. Front. Immunol. 2020, 11, 682. [Google Scholar] [CrossRef]
- Cipolat, S.; Martins de Brito, O.; Dal Zilio, B.; Scorrano, L. OPA1 Requires Mitofusin 1 to Promote Mitochondrial Fusion. Proc. Natl. Acad. Sci. USA 2004, 101, 15927–15932. [Google Scholar] [CrossRef]
- Eura, Y.; Ishihara, N.; Yokota, S.; Mihara, K. Two Mitofusin Proteins, Mammalian Homo-logues of FZO, with Distinct Functions Are Both Required for Mitochondrial Fusion. J. Biochem. 2003, 134, 333–344. [Google Scholar] [CrossRef]
- Kingnate, C.; Charoenkwan, K.; Kumfu, S.; Chattipakorn, N.; Chattipakorn, S.C. Possible Roles of Mitochondrial Dynamics and the Effects of Pharmacological Interventions in Chemoresistant Ovarian Cancer. EBioMedicine 2018, 34, 256–266. [Google Scholar] [CrossRef]
- Wang, R.; Dillon, C.P.; Shi, L.Z.; Milasta, S.; Carter, R.; Finkelstein, D.; McCormick, L.L.; Fitzgerald, P.; Chi, H.; Munger, J.; et al. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity 2011, 35, 871–882. [Google Scholar] [CrossRef]
- Campello, S.; Lacalle, R.A.; Bettella, M.; Mañes, S.; Scorrano, L.; Viola, A. Orchestration of Lymphocyte Chemotaxis by Mitochondrial Dynamics. J. Exp. Med. 2006, 203, 2879–2886. [Google Scholar] [CrossRef]
- Feng, Z.; Yin, Y.; Liu, B.; Zheng, Y.; Shi, D.; Zhang, H.; Qin, J. Prognostic and Immunological Role of FAT Family Genes in Non-Small Cell Lung Cancer. Cancer Control. 2022, 29, 10732748221076682. [Google Scholar] [CrossRef]
- Chanoch-Myers, R.; Wider, A.; Suva, M.L.; Tirosh, I. Elucidating the Diversity of Malignant Mesenchymal States in Glioblastoma by Integrative Analysis. Genome Med. 2022, 14, 106. [Google Scholar] [CrossRef]
- Huang, X.; Chen, C.; Xu, Y.; Shen, L.; Chen, Y.; Su, H. Infiltrating T-cell abundance combined with EMT-related gene expression as a prognostic factor of colon cancer. Bioengineered 2021, 12, 2688–2701. [Google Scholar] [CrossRef]
- Chae, Y.K.; Chang, S.; Ko, T.; Anker, J.; Agte, S.; Iams, W.; Choi, W.M.; Lee, K.; Cruz, M. Epithelial-mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci. Rep. 2018, 8, 2918. [Google Scholar] [CrossRef]
- Jerby-Arnon, L.; Shah, P.; Cuoco, M.S.; Rodman, C.; Su, M.J.; Melms, J.C.; Leeson, R.; Kanodia, A.; Mei, S.; Lin, J.R.; et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 2018, 175, 984–997.e24. [Google Scholar] [CrossRef]
- Gunderson, A.J.; Yamazaki, T.; McCarty, K.; Fox, N.; Phillips, M.; Alice, A.; Blair, T.; White-ford, M.; O’Brien, D.; Ahmad, R.; et al. TGFβ Suppresses CD8+ T Cell Expression of CXCR3 and Tumour Trafficking. Nat. Commun. 2020, 11, 1749. [Google Scholar] [CrossRef]
- Wang, C.; Tan, J.Y.M.; Chitkara, N.; Bhatt, S. TP53 Mutation-Mediated Immune Evasion in Cancer: Mechanisms and Therapeutic Implications. Cancers 2024, 16, 3069. [Google Scholar] [CrossRef]
- Krenz, B.; Lee, J.; Kannan, T.; Eilers, M. Immune Evasion: An Imperative and Consequence of MYC Deregulation. Mol. Oncol. 2024, 18, 2338–2355. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, M.; Chung, Y.R.; Woo, J.W.; Choi, H.Y.; Park, S.Y. Expression of HLA Class I Is Associated with Immune Cell Infiltration and Patient Outcome in Breast Cancer. Sci. Rep. 2022, 12, 20367. [Google Scholar] [CrossRef]
- Riesenberg, S.; Groetchen, A.; Siddaway, R.; Bald, T.; Reinhardt, J.; Smorra, D.; Kohlmeyer, J.; Renn, M.; Phung, B.; Aymans, P.; et al. MITF and c-Jun Antagonism Interconnects Melanoma Dedifferentiation with Pro-Inflammatory Cytokine Responsiveness and Myeloid Cell Recruitment. Nat. Commun. 2015, 6, 8755. [Google Scholar] [CrossRef]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef]
- Yang, D.; Liu, J.; Qian, H.; Zhuang, Q. Cancer-associated fibroblasts: From basic science to anticancer therapy. Exp. Mol. Med. 2023, 55, 1322–1332. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, T.; Xia, R.; Wei, Y.; Wei, X. Targeting the tumour stroma for cancer therapy. Mol. Cancer 2022, 21, 208. [Google Scholar] [CrossRef]
- Wu, H.; Xiang, Z.; Huang, G.; He, Q.; Song, J.; Dou, R.; Yang, C.; Wang, S.; Xiong, B. BGN/FAP/STAT3 positive feedback loop mediated mutual interaction between tumour cells and mesothelial cells contributes to peritoneal metastasis of gastric cancer. Int. J. Biol Sci. 2023, 19, 465–483. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic targeting of the tumour microenvi-ronment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef]
- Scott, A.M.; Wiseman, G.; Welt, S.; Adjei, A.; Lee, F.-T.; Hopkins, W.; Divgi, C.R.; Hanson, L.H.; Mitchell, P.; Gansen, D.N.; et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res. 2003, 9, 1639–1647. [Google Scholar]
- Hofheinz, R.D.; al-Batran, S.E.; Hartmann, F.; Hartung, G.; Jäger, D.; Renner, C.; Tanswell, P.; Kunz, U.; Amelsberg, A.; Kuthan, H.; et al. Stromal antigen targeting by a humanised monoclonal antibody: An early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie 2003, 26, 44–48. [Google Scholar] [CrossRef]
- Loeffler, M.; Krüger, J.A.; Niethammer, A.G.; Reisfeld, R.A. Targeting tumourassociated fibroblasts improves cancer chemotherapy by increasing intratumoural drug uptake. J. Clin. Investig. 2006, 116, 1955–1962. [Google Scholar] [CrossRef]
- Shintani, Y.; Kimura, T.; Funaki, S.; Ose, N.; Kanou, T.; Fukui, E. Therapeutic targeting of cancer-associated fibroblasts in the non-small cell lung cancer tumour microenvironment. Cancers 2023, 15, 335. [Google Scholar] [CrossRef]
- Mori, Y.; Okimoto, Y.; Sakai, H.; Kanda, Y.; Ohata, H.; Shiokawa, D.; Suzuki, M.; Yoshida, H.; Ueda, H.; Sekizuka, T.; et al. Targeting PDGF signalling of cancer-associated fibroblasts blocks feedback activation of HIF-1α and tumour progression of clear cell ovarian cancer. Cell Rep. Med. 2024, 5, 101532. [Google Scholar] [CrossRef]
- Su, C.; Mo, J.; Dong, S.; Liao, Z.; Zhang, B.; Zhu, P. Integrinβ-1 in disorders and cancers: Molecular mechanisms and therapeutic targets. Cell Commun. Signal. 2024, 22, 71. [Google Scholar] [CrossRef]
- Sayegh, N.; Tripathi, N.; Agarwal, N.; Swami, U. Clinical evidence and selecting patients for treatment with erdafitinib in advanced urothelial carcinoma. Oncol. Targets Ther. 2022, 15, 1047–1055. [Google Scholar] [CrossRef]
- Frampton, J.E.; Basset-Séguin, N. Vismodegib: A review in advanced basal cell carci-noma. Drugs 2018, 78, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Rixe, O.; Bukowski, R.M.; Michaelson, M.D.; Wilding, G.; Hudes, G.R.; Bolte, O.; Motzer, R.J.; Bycott, P.; Liau, K.F.; Freddo, J.; et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: A phase II study. Lancet Oncol. 2007, 8, 975–984. [Google Scholar] [CrossRef]
- Gorchs, L.; Ahmed, S.; Mayer, C.; Knauf, A.; Fernández Moro, C.; Svensson, M.; Heuchel, R.; Rangelova, E.; Bergman, P.; Kaipe, H.; et al. The vitamin D analogue calcipotriol promotes an anti-tumourigenic phenotype of human pan-creatic CAFs but reduces T cell mediated immunity. Sci. Rep. 2020, 10, 1744. [Google Scholar] [CrossRef]
- Kelley, R.; Gane, E.; Assenat, E.; Siebler, J.; Galle, P.R.; Merle, P.; Hourmand, I.O.; Cleverly, A.; Zhao, Y.; Gueorguieva, I.; et al. A phase 2 study of galunisertib (TGF-β1 receptor type I inhibitor) and sorafenib in patients with advanced hepatocellular carcinoma. Clin. Transl. Gastroenterol. 2019, 10, e00056. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Gunderson, A.J.; Gilchrist, M.; Whiteford, M.; Kiely, M.X.; Hayman, A.; O’Brien, D.; Ahmad, R.; Manchio, J.V.; Fox, N.; et al. Galunisertib plus neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: A single-arm, phase 2 trial. Lancet Oncol. 2022, 23, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- DiPersio, J.; Stadtmauer, E.A.; Nademanee, A.P.; Stiff, P.; Micallef, I.; Angell, J.; Bridger, G.; Calandra, G. A phase III multicenter randomized, double-blind placebo controlled comparative trial of AMD3100 (Plerixafor)+G-CSF vs G-CSF+placebo for mobilization in multiple myeloma (MM) patients for autologous hematopoietic stem cell (aHSC) transplantation (Abstract). Blood 2007, 110, 445. [Google Scholar] [CrossRef]
- DiPersio, J.F.; Micallef, I.N.; Stiff, P.J.; Bolwell, B.J.; Maziarz, R.T.; Jacobsen, E.; Nademanee, A.; McCarty, J.; Bridger, G.; Calandra, G. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granu-locyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J. Clin. Oncol. 2009, 27, 4767–4773. [Google Scholar]
- Kumar, R.; Mateo, J.; Smith, A.D.; Khan, K.H.; Ruddle, R.; Swales, K.E.; Decordova, S.; Backholer, Z.; Jones, P.; Tran, C.; et al. First-in-human, first-in-class phase 1 study of a novel oral multi-AGC kinase inhibitor AT13148 in patients (pts) with advanced solid tumours (Abstract). J. Clin. Oncol. 2014, 32, 15. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, L.; Li, R.; Zhu, G. Preliminary results of the efficacy and safety of all-trans retinoic acid combined with low-dose apatinib in the treatment of patients with recurrent/metastatic adenoid cystic carcinoma of the head and neck (Abstract). J. Clin. Oncol. 2021, 39, 6026. [Google Scholar] [CrossRef]
- Platzbecker, U.; Avvisati, G.; Cicconi, L.; Thiede, C.; Paoloni, F.; Vignetti, M.; Ferrara, F.; Divona, M.; Albano, F.; Efficace, F.; et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: Final results of the random-ized Italian-German APL0406 trial. J. Clin. Oncol. 2017, 35, 605–612. [Google Scholar] [CrossRef]
- Cheng, L.; Hill, A.F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cao, Y.; Chen, L.; Lai, X.; Zhang, S.; Wang, S. Role of exosomes in cancer and aptamer-modified exosomes as a promising platform for cancer targeted therapy. Biol. Proced. Online 2024, 26, 15. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, S.; Najafi, H.; Afzali, B.; Doroudian, M. Extracellular vesicles and exosomes: Novel insights and perspectives on lung cancer from early detection to targeted treatment. Biomedicines 2024, 12, 123. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvi-ronmental pH is a key factor for exosome traffic in tumour cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef]
- Bobrie, A.; Krumeich, S.; Reyal, F.; Recchi, C.; Moita, L.F.; Seabra, M.C.; Ostrowski, M.; Théry, C. Rab27a supports exosome-dependent and-independent mechanisms that modify the tumour mi-croenvironment and can promote tumour progression. Cancer Res. 2012, 72, 4920–4930. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Sento, S.; Sasabe, E.; Yamamoto, T. Application of a persistent heparin treatment in-hibits the malignant potential of oral squamous carcinoma cells induced by tumour cell-derived exosomes. PLoS ONE. 2016, 11, e0148454. [Google Scholar] [CrossRef]
- Xu, S.; Wang, C.; Yang, L.; Wu, J.; Li, M.; Xiao, P.; Xu, Z.; Xu, Y.; Wang, K. Targeting immune checkpoints on tumour-associated macrophages in tumour immunotherapy. Front. Immunol. 2023, 14, 1199631. [Google Scholar]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef]
- Truxova, I.; Cibula, D.; Spisek, R.; Fucikova, J. Targeting tumour-associated macro-phages for successful immunotherapy of ovarian carcinoma. J. Immunother Cancer 2023, 11, e005968. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.Y.; Zhang, H.; Li, X.F.; Zhang, C.B.; Selli, C.; Tagliavini, G.; Lam, A.D.; Prost, S.; Sims, A.H.; Hu, H.Y.; et al. Monocyte derived macrophages promote breast cancer bone metastasis outgrowth. J. Exp. Med. 2020, 217, e20191820. [Google Scholar] [CrossRef] [PubMed]
- Nywening, T.M.; Wang-Gillam, A.; Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Cusworth, B.M.; Toriola, A.T.; Nieman, R.K.; Worley, L.A.; Yano, M.; et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: A single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016, 17, 651–662. [Google Scholar] [CrossRef]
- Brana, I.; Calles, A.; LoRusso, P.M.; Yee, L.K.; Puchalski, T.A.; Seetharam, S.; Zhong, B.; de Boer, C.J.; Tabernero, J.; Calvo, E.; et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, incombination with four chemotherapy regimens for the treatment of patients with solid tumours: An open-label, multicenter phase 1b study. Target Oncol. 2015, 10, 111–123. [Google Scholar] [CrossRef]
- Zhao, L.; Lim, S.Y.; Gordon-Weeks, A.N.; Tapmeier, T.T.; Im, J.H.; Cao, Y.; Beech, J.; Allen, D.; Smart, S.; Muschel, R.J. Recruit-ment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the develop-ment ofcolorectal cancer liver metastasis. Hepatology 2013, 57, 829–839. [Google Scholar] [CrossRef]
- Bonapace, L.; Coissieux, M.M.; Wyckoff, J.; Mertz, K.D.; Varga, Z.; Junt, T.; Bentires-Alj, M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 2014, 515, 130–133. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and tar-gets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Wen, J.; Wang, S.; Guo, R.; Liu, D. CSF1R inhibitors are emerging immunotherapeutic drugs for cancer treatment. Eur. J. Med. Chem. 2023, 245, 114884. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perdigon, M.; Haeni, L.; Rothen-Rutishauser, B.; Rüegg, C. Dual CSF1R inhi-bition and CD40 activation demonstrates anti-tumour activity in a 3D macrophage- HER2. Front. Bioeng. Biotechnol. 2023, 11, 1159819. [Google Scholar] [CrossRef]
- Moeller, A.; Kurzrock, R.; Botta, G.P.; Adashek, J.J.; Patel, H.; Lee, S.; Pabla, S.; Nesline, M.K.; Conroy, J.; Sicklick, J.K.; et al. Challenges and prospects of CSF1R targeting for advanced malignancies. Am. J. Cancer Res. 2023, 13, 3257–3265. [Google Scholar]
- Zhou, B.; Yang, Y.; Kang, Y.; Hou, J. Targeting the macrophage immunocheckpoint: A novel insight into solid tumour immunotherapy. Cell Commun. Signal. 2024, 22, 66. [Google Scholar] [CrossRef]
- Cersosimo, F.; Lonardi, S.; Ulivieri, C.; Martini, P.; Morrione, A.; Vermi, W.; Giordano, A.; Giurisato, E. CSF-1R in cancer: More than a myeloid cell receptor. Cancers 2024, 16, 282. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Kornete, M.; Joyce, J.A. Re-education of macrophages as a therapeutic strat-egy in cancer. Immunotherapy. 2019, 11, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef]
- Xiang, X.; Wang, J.; Lu, D.; Xu, X. Targeting tumour-associated macrophages to syner-gize tumour immunotherapy. Signal Transduct. Target Ther. 2021, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Perdiguero, E.G.; Geissmann, F. The development and maintenance of resident macro-phages. Nat. Immunol. 2016, 17, 2–8. [Google Scholar] [CrossRef]
- Yu, M.; Wu, Y.; Li, Q.; Hong, W.; Yang, Y.; Hu, X.; Yang, Y.; Lu, T.; Zhao, X.; Wei, X. Colony-stimulating factor-1 re-ceptor inhibition combined with paclitaxel exerts effective antitumour effects in the treat-ment of ovarian cancer. Genes Dis. 2024, 11, 100989. [Google Scholar] [CrossRef]
- Yang, T.; Ke, H.; Liu, J.; An, X.; Xue, J.; Ning, J.; Hao, F.; Xiong, L.; Chen, C.; Wang, Y.; et al. Narazaciclib, a novel multikinase inhibitor with potent activity against CSF1R, FLT3 and CDK6, shows strong anti-AML activ-ity in defined preclinical models. Sci. Rep. 2024, 14, 9032. [Google Scholar]
- Akkari, L.; Bowman, R.L.; Tessier, J.; Klemm, F.; Handgraaf, S.M.; de Groot, M.; Quail, D.F.; Tillard, L.; Gadiot, J.; Huse, J.T.; et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci. Transl. Med. 2020, 12, eaaw7843. [Google Scholar] [CrossRef]
- Yan, D.; Kowal, J.; Akkari, L.; Schuhmacher, A.J.; Huse, J.T.; West, B.L.; Joyce, J.A. Inhibition of colony stimulating factor-1 receptor abrogates microenvironment- mediated therapeutic resistance in gliomas. Oncogene 2017, 36, 6049–6058. [Google Scholar] [CrossRef]
- Olson, O.C.; Kim, H.; Quail, D.F.; Foley, E.A.; Joyce, J.A. Tumour-associated macrophages suppress the cytotoxic activity of antimitotic agents. Cell Rep. 2017, 19, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Tap, W.D.; Gelderblom, H.; Palmerini, E.; Desai, J.; Bauer, S.; Blay, J.Y.; Alcindor, T.; Ganjoo, K.; Martín-Broto, J.; Ryan, C.W.; et al. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): A randomised phase 3 trial. Lancet 2019, 394, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Pexidartinib: First approval. Drugs 2019, 79, 1805–1812. [Google Scholar] [CrossRef]
- Butowski, N.; Colman, H.; De Groot, J.F.; Omuro, A.M.; Nayak, L.; Wen, P.Y.; Cloughesy, T.F.; Marimuthu, A.; Haidar, S.; Perry, A.; et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblas-toma: An Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro-Oncology 2016, 18, 557–564. [Google Scholar] [CrossRef]
- Beatty, G.L.; Chiorean, E.G.; Fishman, M.P.; Saboury, B.; Teitelbaum, U.R.; Sun, W.; Huhn, R.D.; Song, W.; Li, D.; Sharp, L.L.; et al. CD40 agonists alter tumour stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011, 331, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Hoves, S.; Ooi, C.H.; Wolter, C.; Sade, H.; Bissinger, S.; Schmittnaegel, M.; Ast, O.; Giusti, A.M.; Wartha, K.; Runza, V.; et al. Rapid activation of tumour-associated macrophages boosts preexisting tumour immunity. J. Exp. Med. 2018, 215, 859–876. [Google Scholar] [CrossRef]
- Vonderheide, R.H.; Burg, J.M.; Mick, R.; Trosko, J.A.; Li, D.; Shaik, M.N.; Tolcher, A.W.; Hamid, O. Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumours. Oncoimmunology 2013, 2, e23033. [Google Scholar] [CrossRef]
- O’Hara, M.H.; O’Reilly, E.M.; Varadhachary, G.; Wolff, R.A.; Wainberg, Z.A.; Ko, A.H.; Fisher, G.; Rahma, O.; Lyman, J.P.; Cabanski, C.R.; et al. CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: An open-label, multicentre, phase 1b study. Lancet Oncol. 2021, 22, 118–131. [Google Scholar] [CrossRef]
- Vonderheide, R.H.; Dutcher, J.P.; Anderson, J.E.; Eckhardt, S.G.; Stephans, K.F.; Razvillas, B.; Garl, S.; Butine, M.D.; Perry, V.P.; Armitage, R.J.; et al. Phase I study of recombinant human CD40 ligand in cancer patients. J. Clin. Oncol. 2001, 19, 3280–3287. [Google Scholar] [CrossRef]
- Kotowicz, K.; Dixon, G.L.; Klein, N.J.; Peters, M.J.; Callard, R.E. Biological Function of CD40 on Human Endothelial Cells: Costimulation with CD40 Ligand and Interleukin-4 Selectively Induces Expression of Vascular Cell Adhesion Molecule-1 and P-Selectin Resulting in Preferential Adhesion of Lymphocytes. Immunology 2000, 100, 441–448. [Google Scholar] [CrossRef]
- Eriksson, E.; Moreno, R.; Milenova, I.; Liljenfeldt, L.; Dieterich, L.C.; Christiansson, L.; Karlsson, H.; Ullenhag, G.; Mangsbo, S.M.; Dimberg, A.; et al. Activation of Myeloid and Endothelial Cells by CD40L Gene Therapy Supports T-Cell Expansion and Migra-tion into the Tumour Microenvironment. Gene Ther. 2017, 24, 92–103. [Google Scholar] [CrossRef] [PubMed]
- van Hooren, L.; Georganaki, M.; Huang, H.; Mangsbo, S.M.; Dimberg, A. Sunitinib Enhances the Antitumour Responses of Agonistic CD40-Antibody by Reducing MDSCs and Synergistically Improving Endothelial Activation and T-Cell Recruitment. Oncotarget 2016, 7, 50277–50289. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; Hamilton, E.P.; Winer, I.S.; Tan, W.; Hubbard, J.M.; Schenk, E.L.; Sonbol, M.B.; Jahchan, N.; Pierce, K.; Li, Y.; et al. A phase 1a dose-escalation study of PY314, a TREM2 (triggering receptor expressed on macrophages 2) targeting monoclonal antibody. J. Clin. Oncol. 2022, 40, 2648. [Google Scholar] [CrossRef]
- Lanahan, S.M.; Wymann, M.P.; Lucas, C.L. The role of PI3Kγ in the immune system: New insights and translational implications. Nat. Rev. Immunol. 2022, 22, 687–700. [Google Scholar] [CrossRef]
- Hong, D.S.; Postow, M.; Chmielowski, B.; Sullivan, R.; Patnaik, A.; Cohen, E.E.W.; Shapiro, G.; Steuer, C.; Gutierrez, M.; Yeckes-Rodin, H.; et al. Eganelisib, a First-in-Class PI3Kγ Inhibitor, in Patients with Advanced Solid Tumours: Re-sults of the Phase 1/1b MARIO-1 Trial. Clin. Cancer Res. 2023, 29, 2210–2219. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Hingorani, S.R. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br. J. Cancer 2013, 108, 1–8. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Zheng, L.; Bullock, A.J.; Seery, T.E.; Harris, W.P.; Sigal, D.S.; Braiteh, F.; Ritch, P.S.; Zalupski, M.M.; Bahary, N.; et al. HALO202: Randomized phase IIstudy of PEGPH20 plus nab-paclitaxel/ gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated metastatic pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2018, 36, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; Tempero, M.; Corrie, P.G. HALO-109-301: A Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future Oncol. 2018, 14, 13–22. [Google Scholar] [CrossRef]
- Benson, A.B.; Wainberg, Z.A.; Hecht, J.R.; Vyushkov, D.; Dong, H.; Bendell, J.; Kudrik, F. A phase II randomized, double-blind, placebo-controlled study of simtuzumab or placebo in combination with gemcitabine for the first-line treatment of pancreatic adenocarcinoma. Oncologist 2017, 22, 241-e15. [Google Scholar] [CrossRef]
- Hecht, J.R.; Benson, A.B.; Vyushkov, D.; Yang, Y.; Bendell, J.; Verma, U. A phase II, ran-domized, double-blind, placebo-controlled study of simtuzumab in combination with FOLFIRI for the second-line treatment of metastatic KRAS mutant colorectal adenocarcinoma. Oncologist 2017, 22, 243-e23. [Google Scholar] [CrossRef]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Clark, J.W.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Ly, L.; Baglini, C.V.; Blaszkowsky, L.S.; et al. Total neoad ju-vant therapy with FOLFIRINOX in combination with losartan followed by chemoradiother-apy for locally advanced pancreatic cancer: A phase 2 clinical trial. JAMA Oncol. 2019, 5, 1020–1027. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Chen, B.E.; Gelmon, K.A.; Whelan, T.J.; Ennis, M.; Lemieux, J.; Ligibel, J.A.; Hershman, D.L.; Mayer, I.A.; Hobday, T.J.; et al. Effect of t metformin vs placebo on invasive disease free survival in patients with breast cancer: The MA32 randomized clinical trial. JAMA 2022, 327, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Wang, S.Q.; Shang, H.L.; Lv, H.F.; Chen, B.B.; Gao, S.G.; Chen, X.B. Roles and inhibi-tors of FAK in cancer: Current advances and future directions. Front. Pharmacol. 2024, 15, 1274209. [Google Scholar]
- Lampi, M.C.; Reinhart-King, C.A. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci. Transl. Med. 2018, 10, eaao0475. [Google Scholar] [CrossRef] [PubMed]
- Bergonzini, C.; Kroese, K.; Zweemer, A.J.M.; Danen, E.H.J. Targeting integrins for cancer therapy—disappointments and opportunities. Front. Cell Dev. Biol. 2022, 10, 863850. [Google Scholar] [CrossRef]
- Patnaik, A.; Calvo, E.; Piha-Paul, S.A.A.; Hollebecque, A.; Galvao, V.; Lopez, J.S. A phase 1 study of SGN-B6A, an antibody-drug conjugate targeting integrin beta-6, in patients with advanced solid tumours (SGNB6A-001, Trial in Progress). J. Clin. Oncol. 2021, 39, TPS3144. [Google Scholar] [CrossRef]
- Hosen, N.; Matsunaga, Y.; Hasegawa, K.; Matsuno, H.; Nakamura, Y.; Makita, M.; Watanabe, K.; Yoshida, M.; Satoh, K.; Morimoto, S.; et al. The activated conformation of integrin β7 is a novel multiple myeloma–specific target for CAR T cell therapy. Nat. Med. 2017, 23, 1436–1443. [Google Scholar] [CrossRef]
- Chuang, H.H.; Zhen, Y.Y.; Tsai, Y.C.; Chuang, C.H.; Hsiao, M.; Huang, M.S.; Yang, C.J. FAK in cancer: From mechanisms to therapeutic strategies. Int. J. Mol. Sci. 2022, 23, 1726. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Lim, K.H.; McWilliams, R.; Suresh, R.; Lockhart, A.C.; Brown, A.; Breden, M.; Belle, J.I.; Herndon, J.; Bogner, S.J.; et al. Defactinib, pembrolizumab, and gemcitabine in patients with advanced treatment refracto-ry pancreatic cancer: A phase i dose escalation and expansion study. Clin. Cancer Res. 2022, 28, 5254–5262. [Google Scholar] [CrossRef]
- Zhang, S.; Kohli, K.; Black, R.G.; Yao, L.; Spadinger, S.M.; He, Q.; Pillarisetty, V.G.; Cranmer, L.D.; Van Tine, B.A.; Yee, C.; et al. Systemic Interferon-γ Increases MHC Class I Expression and T-cell Infiltration in Cold Tumours: Results of a Phase 0 Clinical Trial. Cancer Immunol. Res. 2019, 7, 1237–1243. [Google Scholar] [CrossRef]
- Arenberg, D.A.; White, E.S.; Burdick, M.D.; Strom, S.R.; Strieter, R.M. Improved Survival in Tumour-Bearing SCID Mice Treated with Interferon-Gamma-Inducible Protein 10 (IP-10/CXCL10). Cancer Immunol. Immunother. 2001, 50, 533–538. [Google Scholar] [CrossRef]
- Peng, W.; Liu, C.; Xu, C.; Lou, Y.; Chen, J.; Yang, Y.; Yagita, H.; Overwijk, W.W.; Lizée, G.; Radvanyi, L.; et al. PD-1 Blockade Enhances T-cell Migration to Tumours by Elevating IFN-γ Inducible Chemokines. Cancer Res. 2012, 72, 5209–5218. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Chasalow, S.D.; Wang, L.; Hamid, O.; Schmidt, H.; Cogswell, J.; Alaparthy, S.; Berman, D.; Jure-Kunkel, M.; Siemers, N.O.; et al. An Immune-Active Tumour Microenvironment Favors Clinical Response to Ipilimumab. Cancer Immunol. Immunother. 2012, 61, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Srivastava, M.K.; Harris-White, M.; Huang, M.; Zhu, L.; Elashoff, D.; Stri-eter, R.M.; Dubinett, S.M.; Sharma, S. Role of CXCR3 Ligands in IL-7/IL-7Rα-Fc-Mediated Antitumour Activity in Lung Cancer. Clin. Cancer Res. 2011, 17, 3660–3672. [Google Scholar] [CrossRef] [PubMed]
- Parkes, E.E.; Walker, S.M.; Taggart, L.E.; McCabe, N.; Knight, L.A.; Wilkinson, R.; McCloskey, K.D.; Buckley, N.E.; Savage, K.I.; Salto-Tellez, M.; et al. Activation of STING-Dependent Innate Immune Signalling By S-Phase-Specific DNA Damage in Breast Cancer. J. Nat. Cancer Inst. 2016, 109, djw199. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.; Cong, Z.; Amoozgar, Z.; Kiner, E.; Xing, D.; Orsulic, S.; Matu-lonis, U.; Goldberg, M.S. The PARP1 inhibitor BMN 673 exhibits immunoregulatory ef-fects in a Brca1(-/-) murine model of ovarian cancer. Biochem. Biophys Res. Comm. 2015, 463, 551–556. [Google Scholar] [CrossRef]
- Franzese, O.; Graziani, G. Role of PARP Inhibitors in Cancer Immunotherapy: Potential Friends to Immune Activating Molecules and Foes to Immune Checkpoints. Cancers 2022, 14, 5633. [Google Scholar] [CrossRef]
- Strickland, K.C.; Howitt, B.E.; Shukla, S.A.; Rodig, S.; Ritterhouse, L.L.; Liu, J.F.; Garber, J.E.; Chowdhury, D.; Wu, C.J.; D’Andrea, A.D.; et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumour-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget 2016, 7, 13587–13598. [Google Scholar] [CrossRef]
- Pantelidou, C.; Sonzogni, O.; De Oliveria Taveira, M.; Mehta, A.K.; Kothari, A.; Wang, D.; Visal, T.; Li, M.K.; Pinto, J.; Castrillon, J.A.; et al. PARP Inhibitor Ef-ficacy Depends on CD8+ T-cell Recruitment via Intratumoural STING Pathway Activa-tion in BRCA-Deficient Models of Triple-Negative Breast Cancer. Cancer Discov. 2019, 9, 722–737. [Google Scholar] [CrossRef]
- Staniszewska, A.D.; Armenia, J.; King, M.; Michaloglou, C.; Reddy, A.; Singh, M.; San Martin, M.; Prickett, L.; Wilson, Z.; Proia, T.; et al. PARP inhibition is a modulator of anti-tumour immune response in BRCA-deficient tumours. Oncoimmunology 2022, 11, 2083755. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, W.; Ju, Z.; Wang, L.; Peng, Y.; Labrie, M.; Yap, T.A.; Mills, G.B.; Peng, G. PARPi Triggers the STING-Dependent Immune Response and Enhances the Therapeutic Ef-ficacy of Immune Checkpoint Blockade Independent of BRCAness. Cancer Res. 2019, 79, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.R.; Nelson, M.H.; Majchrzak, K.; Bowers, J.S.; Wyatt, M.M.; Smith, A.S.; Neal, L.R.; Shirai, K.; Carpenito, C.; June, C.H.; et al. Human CD26 high T Cells Elicit Tumour Im-munity against Multiple Malignancies via Enhanced Migration and Persistence. Nat. Commun. 2017, 8, 1961. [Google Scholar] [CrossRef]
- Galati, D.; Zanotta, S.; Capone, M.; Madonna, G.; Mallardo, D.; Romanelli, M.; Sime-one, E.; Festino, L.; Sparano, F.; Azzaro, R.; et al. A PoPotential clinical implicationf CD4+CD26high T cells for nivolumab treated melanoma patients. J. Transl Med. 2023, 21, 318. [Google Scholar] [CrossRef]
- Franzese, O.; Palermo, B.; Frisullo, G.; Panetta, M.; Campo, G.; D’Andrea, D.; Sperduti, I.; Taje, R.; Visca, P.; Nisticò, P. ADA/CD26 axis increases intra-tumour PD-1+CD28+CD8+ T-cell fitness and affects NSCLC prognosis and response to ICB. Oncoimmunology 2024, 13, 2371051. [Google Scholar] [CrossRef] [PubMed]
- Torphy, R.J.; Sun, Y.; Lin, R.; Caffrey-Carr, A.; Fujiwara, Y.; Ho, F.; Miller, E.N.; McCarter, M.D.; Lyons, T.R.; Schulick, R.D.; et al. GPR182 limits antitumour immunity via chemokine scavenging in mouse melanoma models. Nat. Commun. 2022, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strat-egies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Uslu, U.; June, C.H. Beyond the blood: Expanding CAR T cell therapy to solid tu-mours. Nat. Biotechnol. 2025, 43, 506–515. [Google Scholar] [CrossRef]
- Foeng, J.; Comerford, I.; McColl, S.R. Harnessing the chemokine system to home CAR-T cells into solid tumours. Cell Rep. Med. 2022, 3, 100543. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, L.; Ping, Y.; Zhang, Z.; Yao, C.; Shen, C.; Li, F.; Wen, C.; Zhang, Y. CCR5 and IL-12 co-expression in CAR T cells improves antitumour efficacy by reprogramming tumour microenvironment in solid tumours. Cancer Immunol. Immunother. 2025, 74, 55. [Google Scholar] [CrossRef]
- Michaelides, S.; Obeck, H.; Kechur, D.; Endres, S.; Kobold, S. Migratory Engineering of T Cells for Cancer Therapy. Vaccines 2022, 10, 1845. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, B.; Liang, Y.; Reeves, P.M.; Qu, X.; Ran, C.; Liu, Q.; Callahan, M.V.; Sluder, A.E.; Gelfand, J.A.; et al. Dual blockade of CXCL12-CXCR4 and PD-1-PD-L1 pathways prolongs survival of ovarian tumour-bearing mice by prevention of immunosuppression in the tumour microenvironment. FASEB J. 2019, 33, 6596–6608. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xu, R.; Chen, X.; Lu, Y.; Zheng, J.; Lin, Y.; Lin, P.; Zhao, X.; Cui, L. Metabolic gatekeepers: Harnessing tumour-derived metabolites to optimize T cell-based immunotherapy efficacy in the tumour microenvironment. Cell Death Dis. 2024, 15, 775. [Google Scholar] [CrossRef]
- Liu, H.; Pan, M.; Liu, M.; Zeng, L.; Li, Y.; Huang, Z.; Guo, C.; Wang, H. Lactate: A rising star in tumours and inflammation. Fron. Immunol. 2024, 15, 1496390. [Google Scholar] [CrossRef]
- Doherty, J.R.; Cleveland, J.L. Targeting Lactate Metabolism for Cancer Therapeutics. J. Clin. Investig. 2013, 123, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Y.; Chen, Z.N. Metabolic Interaction: Tumour-Derived Lactate Inhibiting CD8+ T Cell Cytotoxicity in a Novel Route. Signal Transduct. Target. Ther. 2023, 8, 52. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, D.; Harding, J.; Chen, W.; Liu, X.; Wang, Z.; Wang, L.; Qi, T.; Chen, S.; Guo, X.; et al. Lactate oxidase nanocapsules boost T cell immunity and efficacy of cancer immunotherapy. Sci. Trans. Med. 2023, 15, eadd2712. [Google Scholar] [CrossRef] [PubMed]
- Rostamian, H.; Khakpoor-Koosheh, M.; Jafarzadeh, L.; Masoumi, E.; Fallah-Mehrjardi, K.; Tavassolifar, M.J.; MPawelek, J.; Mirzaei, H.R.; Hadjati, J. Restricting tumour lactic acid metabolism using dichloroacetate improves T cell functions. BMC Cancer 2022, 22, 39. [Google Scholar] [CrossRef]
- Corbin, A.; Hughes, S.; Quareshy, M.; Bopp, T.; Cipriani, B.; Miller, D.; Naylor, A.; Milne, G.; Turner, D.; Young, B.; et al. 1324 Inhibition of acid sensing by GPR65 normalises gene expression in macrophages, increases immune cell infiltration in tumours, and restrains subcutaneous MC38 growth in mice. J. Immunother. Cancer 2022, 10, A1375. [Google Scholar]
- Hack, S.P.; Zhu, A.X.; Wang, Y. Augmenting Anticancer Immunity through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front. Immunol. 2020, 11, 2813. [Google Scholar] [CrossRef]
- Shrimali, R.K.; Yu, Z.; Theoret, M.R.; Chinnasamy, D.; Restifo, N.P.; Rosenberg, S.A. Anti-angiogenic agents can increase lymphocyte infiltration into tumour and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010, 70, 6171–6180. [Google Scholar] [CrossRef]
- Avram, G.; Sánchez-Sendra, B.; Martín, J.M.; Terrádez, L.; Ramos, D.; Monteagudo, C. The density and type of MECA-79-positive high endothelial venules correlate with lymphocytic infiltration and tumour regression in primary cutaneous melanoma. Histopathology 2013, 63, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Martinet, L.; Le Guellec, S.; Filleron, T.; Lamant, L.; Meyer, N.; Rochaix, P.; Garrido, I.; Girard, J.P. High Endothelial Venules (HEVs) in Human Melanoma Lesions: Major Gateways for Tumour-Infiltrating Lymphocytes. Oncoimmunology 2012, 1, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Johansson-Percival, A.; Ganss, R. Therapeutic induction of tertiary lymphoid structures in cancer through stromal remodeling. Front. Immunol. 2021, 12, 674375. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Lee, Y.; Liu, W.; Chin, R.K.; Wang, J.; Wang, Y.; Schietinger, A.; Philip, M.; Schreiber, H.; Fu, Y.X. Priming of Naive T Cells Inside Tumours Leads to Eradication of Established Tumours. Nat. Immunol. 2004, 5, 141–149. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, H.; Liu, X.; Wang, Z.; Zhang, Q.; Wei, N.; Guo, X. Vascular Normalization: A New Window Opened for Cancer Therapies. Front. Oncol. 2021, 11, 719836. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumour Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef]
- Reina, M.; Espel, E. Role of LFA-1 and ICAM-1 in Cancer. Cancers 2017, 9, 153. [Google Scholar] [CrossRef]
- Du, W.; Nair, P.; Johnston, A.; Wu, P.H.; Wirtz, D. Cell Trafficking at the Intersection of the Tumor-Immune Compartments. Ann. Rev. Biomed. Intersec. 2022, 24, 275–305. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Sign. Transd. Targ. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Zanotelli, M.R.; Zhang, J.; Ortiz, I.; Wang, W.; Chada, N.C.; Reinhart-King, C.A. Highly motile cells are metabolically responsive to collagen density. Proc. Natl. Acad. Sci. USA 2022, 119, e2114672119. [Google Scholar] [CrossRef]
- Kao, K.C.; Vilbois, S.; Tsai, C.H.; Ho, P.C. Metabolic communication in the tumour-immune microenvironment. Nat. Cell Biol. 2022, 24, 1574–1583. [Google Scholar] [CrossRef]
- Marzban, S.; Srivastava, S.; Kartika, S.; Bravo, R.; Safriel, R.; Zarski, A.; Anderson, A.R.A.; Chung, C.H.; Amelio, A.L.; West, J. Spatial interactions modulate tumor growth and immune infiltration. NPJ Syst. Biol. Appl. 2024, 10, 106. [Google Scholar] [CrossRef]
- Seager, R.J.; Hajal, C.; Spill, F.; Kamm, R.D.; Zaman, M.H. Dynamic interplay between tumour, stroma and immune system can drive or prevent tumour progression. Conv. Sci. Phys. Oncol. 2017, 3, 034002. [Google Scholar] [CrossRef] [PubMed]
- Frascoli, F.; Flood, E.; Kim, P.S. A model of the effects of cancer cell motility and cellular adhesion properties on tumour-immune dynamics. Math. Med. Biol. 2017, 34, 215–240. [Google Scholar] [CrossRef] [PubMed]
- Gorbachev, A.V.; Fairchild, R.L. Regulation of chemokine expression in the tumor microenvironment. Crit. Rev. Immunol. 2014, 34, 103–120. [Google Scholar] [CrossRef]
- Peng, D.; Kryczek, I.; Nagarsheth, N.; Zhao, L.; Wei, S.; Wang, W.; Sun, Y.; Zhao, E.; Vatan, L.; Szeliga, W.; et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015, 527, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Tanese, K.; Grimm, E.A.; Ekmekcioglu, S. The role of melanoma tumor-derived nitric oxide in the tumor inflammatory microenvironment: Its impact on the chemokine expression profile, including suppression of CXCL10. Int. J. Cancer 2012, 131, 891–901. [Google Scholar] [CrossRef]
- Tseng, P.C.; Chen, C.L.; Lee, K.Y.; Feng, P.H.; Wang, Y.C.; Satria, R.D.; Lin, C.F. Epithelial-to-mesenchymal transition hinders IFN-γ-dependent immunosurveillance in lung cancer cells. Cancer Lett. 2022, 539, 215712. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomarker Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Wurzer, H.; Hoffmann, C.; Al Absi, A.; Thomas, C. Actin Cytoskeleton Straddling the Immunological Synapse between Cytotoxic Lymphocytes and Cancer Cells. Cells 2019, 8, 463. [Google Scholar] [CrossRef]
- Liang, H.; Huang, J.; Li, H.; He, W.; Ao, X.; Xie, Z.; Chen, Y.; Lv, Z.; Zhang, L.; Zhong, Y.; et al. Spatial Proximity of CD8+ T Cells to Tumor Cells Predicts Neoadjuvant Therapy Efficacy in Breast Cancer. NPJ Breast Cancer 2025, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and Analysis of Cell-Cell Communication Using CellChat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef] [PubMed]

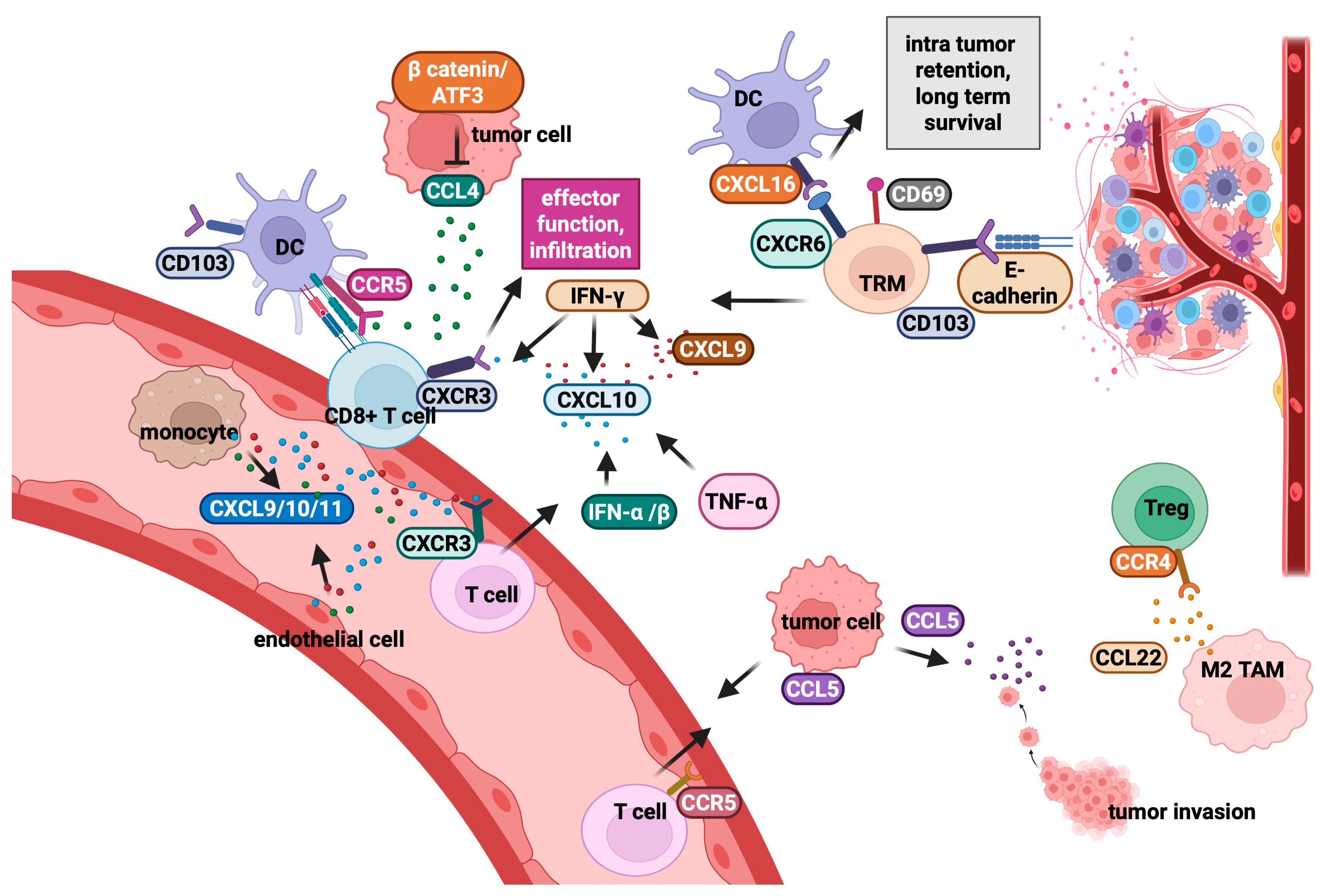
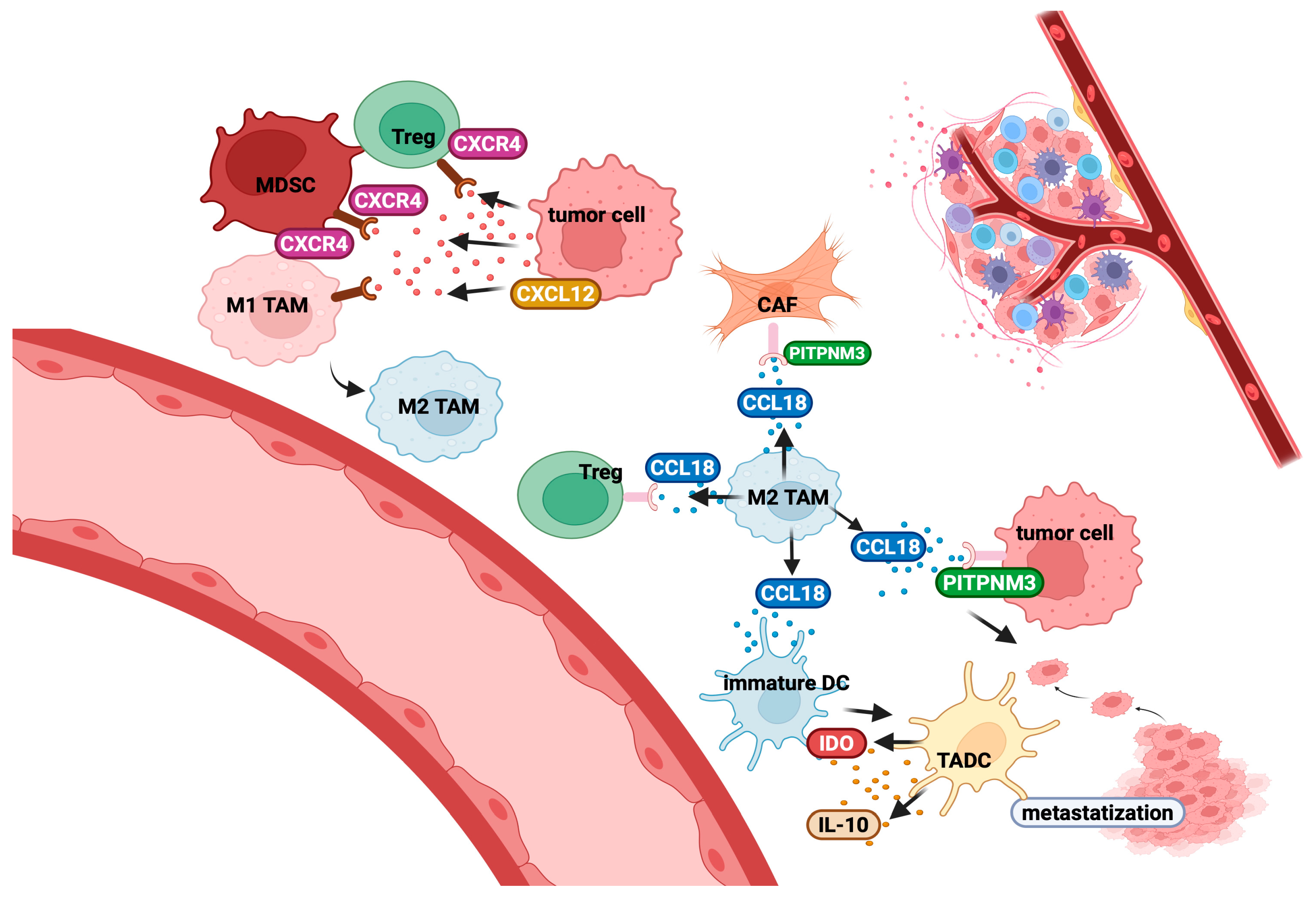
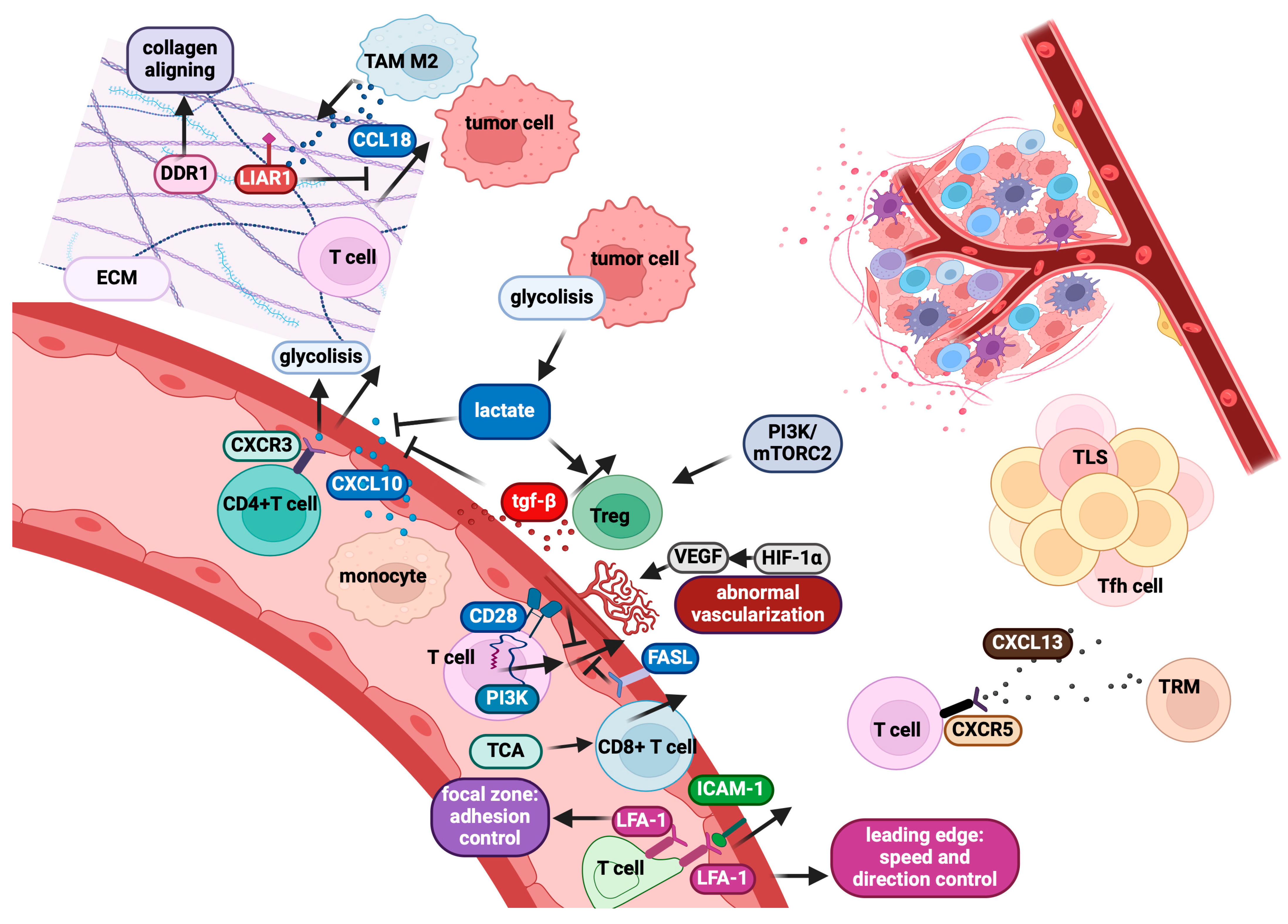
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino, A.; Franzese, O. Reciprocal Modulation of Tumour and Immune Cell Motility: Uncovering Dynamic Interplays and Therapeutic Approaches. Cancers 2025, 17, 1547. https://doi.org/10.3390/cancers17091547
Aquino A, Franzese O. Reciprocal Modulation of Tumour and Immune Cell Motility: Uncovering Dynamic Interplays and Therapeutic Approaches. Cancers. 2025; 17(9):1547. https://doi.org/10.3390/cancers17091547
Chicago/Turabian StyleAquino, Angelo, and Ornella Franzese. 2025. "Reciprocal Modulation of Tumour and Immune Cell Motility: Uncovering Dynamic Interplays and Therapeutic Approaches" Cancers 17, no. 9: 1547. https://doi.org/10.3390/cancers17091547
APA StyleAquino, A., & Franzese, O. (2025). Reciprocal Modulation of Tumour and Immune Cell Motility: Uncovering Dynamic Interplays and Therapeutic Approaches. Cancers, 17(9), 1547. https://doi.org/10.3390/cancers17091547



