Arterial Resections in Pancreatic Cancer—An Updated Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Assessment
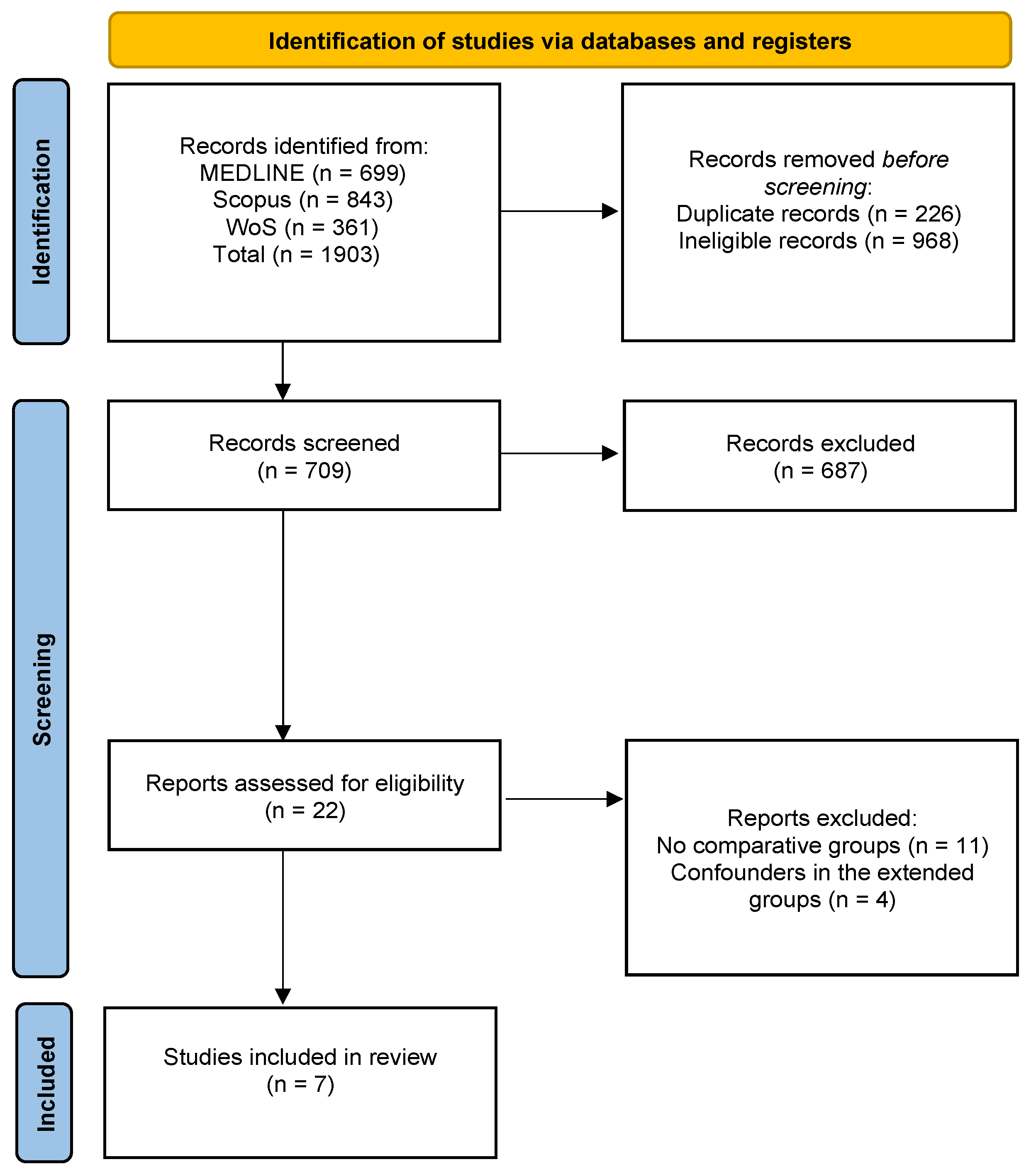
2.3. Study Selection
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
| First Author | Year | Study Type | Total | Country | Sex: M/F | Mean Age | Types of Operation (Surgery) | Type of Surgical Approach | Complications | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Loos [16] | 2024 | R | 2135 | Germany | 659/793 | 61.5/62.3 | DP (2135, 100%) | Open/LAP/ROB | POPF/DGE/NAT/PPH/CDC/LOS | 7 |
| Asano [17] | 2018 | R | 51 | Japan | 27/24 | 73/73 | PD (51, 100%) | NR | BL/CDC/LOS | 4 |
| Yang [11] | 2019 | R | 56 | China | 32/24 | 63.5/63.8 | PD (44, 78.5%), TP (12, 22.2%) | Open/LAP/ROB | POPF/DGE/BL/NAT/PPH/CDC/LOS/R0 | 8 |
| Li [15] | 2021 | R | 92 | China | 54/38 | 63.1/61.5 | DP (92, 100%) | NR | POPF/DGE/BL/NAT/PPH/CDC/LOS | 9 |
| Malinka [13] | 2020 | R | 40 | Germany | 22/18 | 65.05/60.75 | DP (40, 100%) | Open/LAP/ROB | POPF/DGE/NAT/PPH/CDC/LOS | 8 |
| Storkholm [14] | 2020 | P | 89 | Denmark | 50/39 | 62.3/58.3 | DP (89, 100%) | Open/LAP/ROB | POPF/DGE/BL/NAT/PPH/CDC/LOS/R0 | 6 |
| Zettervall [12] | 2019 | R | 3002 | USA | 1420/1198 | 64/63 | PD (3002, 100%) | Open/LAP/ROB | POPF/DGE | 6 |
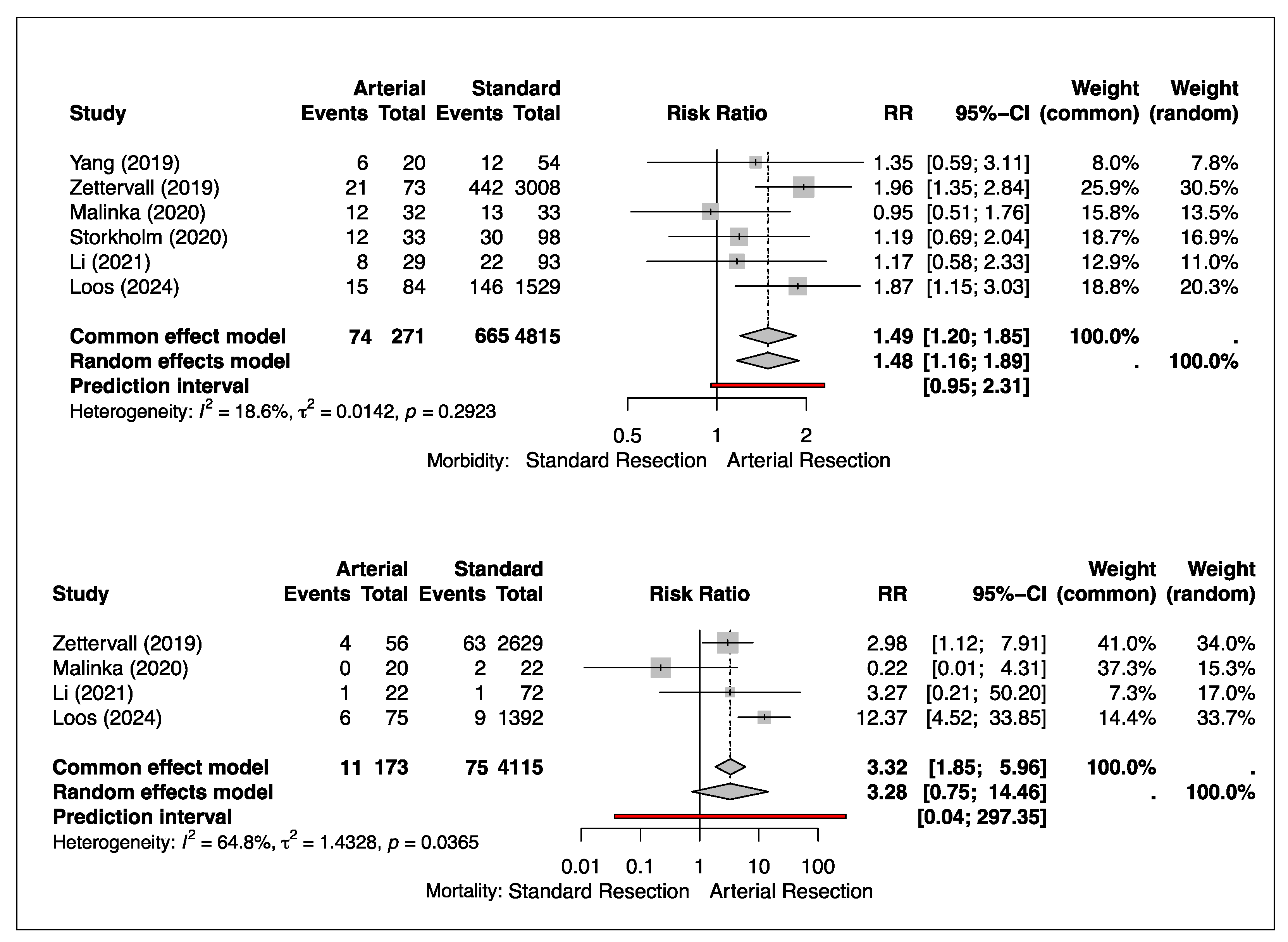
3.1. Morbidity
3.2. Mortality
3.3. Survival
3.4. Blood Loss
3.5. Clavien–Dindo Classification (CDC) ≥ 3
3.6. Hospital Length of Stay (LOS)
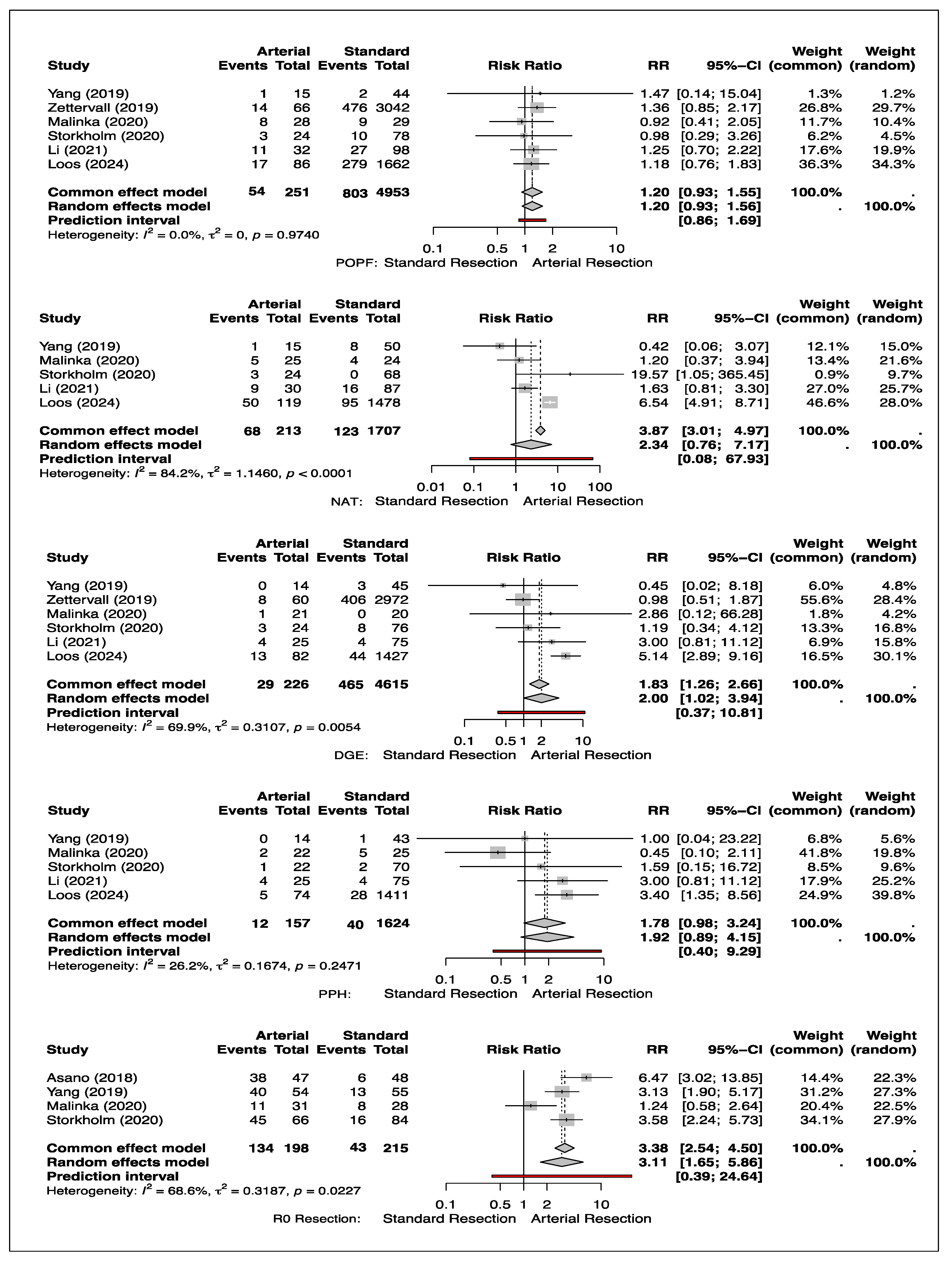
3.7. Neoadjuvant Chemotherapy Treatment (NAT)
3.8. Delayed Emptying (DGE)
3.9. Post-Pancreatectomy Hemorrhage (PPH)
3.10. Post-Operative Pancreatic Fistula (POPF)
3.11. R0 Resection
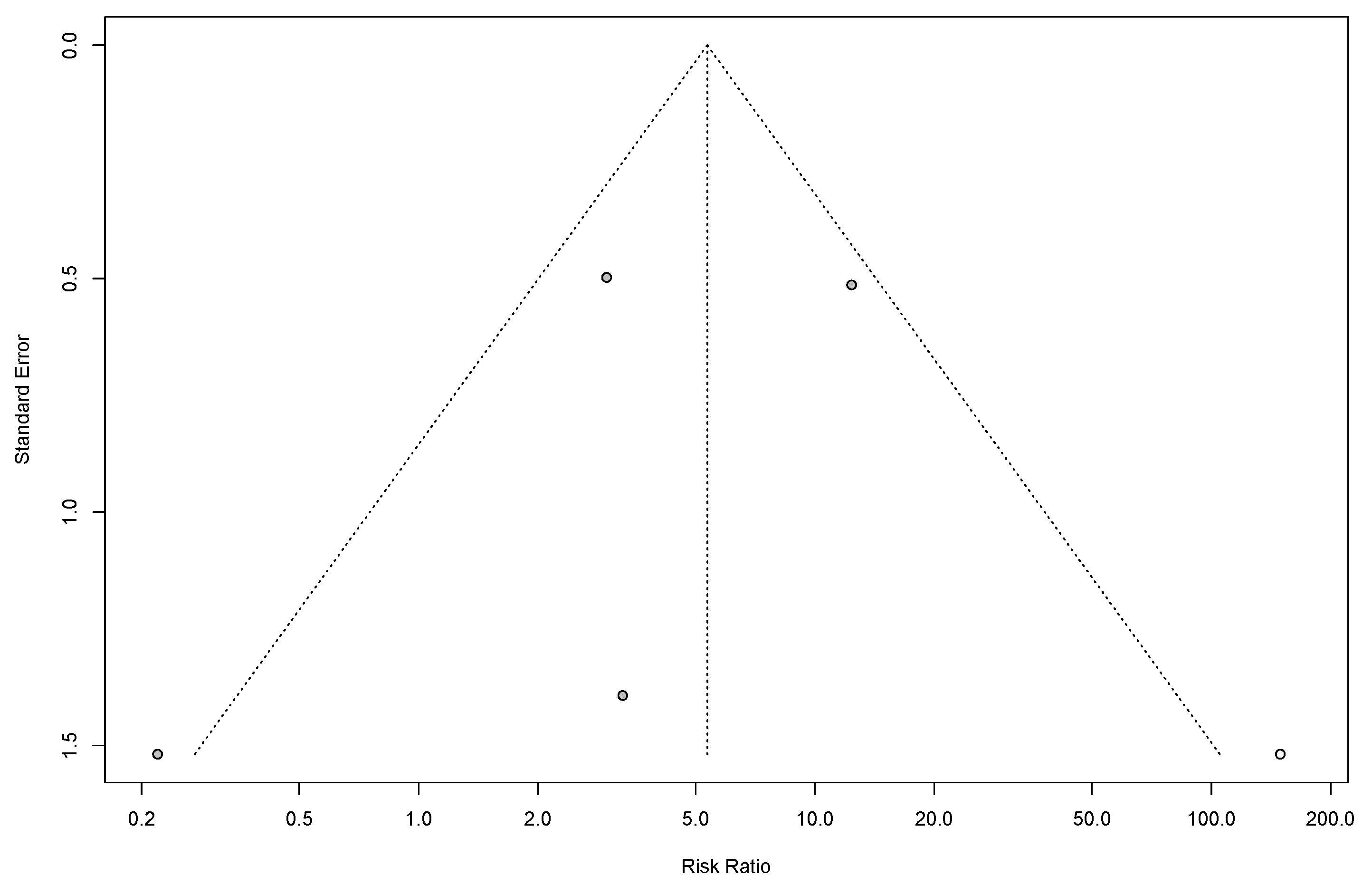
3.12. Heterogeneity and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Fortner, J.G. Regional resection of cancer of the pancreas: A new surgical approach. Surgery 1973, 73, 307–320. [Google Scholar]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. Available online: https://jnccn.org/view/journals/jnccn/19/4/article-p439.xml (accessed on 21 March 2025). [CrossRef]
- Papakonstantinou, M.; Fiflis, S.; Giakoustidis, A.; Christodoulidis, G.; Myriskou, A.; Louri, E.; Papalavrentios, L.; Papadopoulos, V.N.; Giakoustidis, D. Survival after vascular resections in patients with borderline resectable or locally advanced pancreatic head cancer: A systematic review. Ann. Hepato-Biliary-Pancreat. Surg. 2024, 28, 423–432. Available online: http://www.ahbps.org/journal/view.html?doi=10.14701/ahbps.24-118 (accessed on 18 February 2025). [CrossRef]
- Zwart, E.S.; Yilmaz, B.S.; Halimi, A.; Ahola, R.; Kurlinkus, B.; Laukkarinen, J.; Ceyhan, G.O. Venous resection for pancreatic cancer, a safe and feasible option? A systematic review and meta-analysis. Pancreatology 2022, 22, 803–809. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1424390322001685 (accessed on 27 February 2025). [CrossRef]
- Małczak, P.; Sierżęga, M.; Stefura, T.; Kacprzyk, A.; Droś, J.; Skomarovska, O.; Krzysztofik, M.; Major, P.; Pędziwiatr, M. Arterial resections in pancreatic cancer—Systematic review and meta-analysis. HPB 2020, 22, 961–968. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1365182X20301271 (accessed on 27 February 2025). [CrossRef]
- Salameh, J.-P.; Bossuyt, P.M.; McGrath, T.A.; Thombs, B.D.; Hyde, C.J.; Macaskill, P.; Deeks, J.J.; Leeflang, M.; Korevaar, D.A.; Whiting, P.; et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. BMJ 2020, 370, m2632. Available online: https://www.bmj.com/lookup/doi/10.1136/bmj.m2632 (accessed on 30 January 2025). [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. Available online: http://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-016-0384-4 (accessed on 30 January 2025). [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2021. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 23 January 2025).
- Lin, L. Comparison of four heterogeneity measures for meta-analysis. J. Eval. Clin. Pract. 2020, 26, 376–384. Available online: https://onlinelibrary.wiley.com/doi/10.1111/jep.13159 (accessed on 30 January 2025). [CrossRef]
- Yang, F.; Wang, X.; Jin, C.; He, H.; Fu, D. Pancreatectomy with Hepatic Artery Resection for Pancreatic Head Cancer. World J. Surg. 2019, 43, 2909–2919. Available online: https://onlinelibrary.wiley.com/doi/10.1007/s00268-019-05106-8 (accessed on 30 January 2025). [CrossRef]
- Zettervall, S.L.; Ju, T.; Holzmacher, J.L.; Huysman, B.; Werba, G.; Sidawy, A.; Lin, P.; Vaziri, K. Arterial, but Not Venous, Reconstruction Increases 30-Day Morbidity and Mortality in Pancreaticoduodenectomy. J. Gastrointest. Surg. 2020, 24, 578–584. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1091255X2301404X (accessed on 30 January 2025). [CrossRef] [PubMed]
- Malinka, T.; Timmermann, L.; Klein, F.; Geisel, D.; Pratschke, J.; Bahra, M. Is there a Role for the Appleby Procedure in 2020? Results from a Matched-Pair-Analysis. Anticancer Res. 2020, 40, 387–392. Available online: http://ar.iiarjournals.org/lookup/doi/10.21873/anticanres.13964 (accessed on 30 January 2025). [CrossRef] [PubMed]
- Storkholm, J.; Burgdorf, S.; Hansen, C. Distal pancreas–coeliac axis resection with preoperative selective embolization of the coeliac axis: A single high-volume centre experience. Langenbecks Arch. Surg. 2020, 405, 635–645. Available online: https://link.springer.com/10.1007/s00423-020-01919-7 (accessed on 30 January 2025). [CrossRef] [PubMed]
- Li, M.; Shen, R.; Wang, S.; Zhu, D.; Wang, X. Distal pancreatectomy with celiac artery resection acquires satisfactory survival for locally advanced pancreatic neck/body cancer. Asian J. Surg. 2022, 45, 137–142. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1015958421002050 (accessed on 30 January 2025). [CrossRef]
- Loos, M.; Mack, C.E.; Xu, A.T.L.; Hassenpflug, M.; Hinz, U.; Mehrabi, A.; Berchtold, C.; Schneider, M.; Al-Saeedi, M.; Roth, S.; et al. Distal Pancreatectomy: Extent of Resection Determines Surgical Risk Categories. Ann. Surg. 2023, 279, 479–485. Available online: https://journals.lww.com/10.1097/SLA.0000000000005935 (accessed on 30 January 2025). [CrossRef]
- Asano, T.; Nakamura, T.; Noji, T.; Okamura, K.; Tsuchikawa, T.; Nakanishi, Y.; Tanaka, K.; Murakami, S.; Ebihara, Y.; Kurashima, Y.; et al. Outcome of concomitant resection of the replaced right hepatic artery in pancreaticoduodenectomy without reconstruction. Langenbecks Arch. Surg. 2018, 403, 195–202. Available online: http://link.springer.com/10.1007/s00423-018-1650-9 (accessed on 22 January 2025). [CrossRef]
- Kwon, J.; Shin, S.H.; Yoo, D.; Hong, S.; Lee, J.W.; Youn, W.Y.; Hwang, K.; Lee, S.J.; Park, G.; Park, Y.; et al. Arterial resection during pancreatectomy for pancreatic ductal adenocarcinoma with arterial invasion: A single-center experience with 109 patients. Medicine 2020, 99, e22115. Available online: https://journals.lww.com/10.1097/MD.0000000000022115 (accessed on 21 March 2025). [CrossRef]
- Mollberg, N.; Rahbari, N.N.; Koch, M.; Hartwig, W.; Hoeger, Y.; Büchler, M.W.; Weitz, J. Arterial Resection During Pancreatectomy for Pancreatic Cancer: A Systematic Review and Meta-Analysis. Ann. Surg. 2011, 254, 882–893. Available online: https://journals.lww.com/00000658-201112000-00009 (accessed on 17 March 2025). [CrossRef]
- Rompen, I.F.; Marchetti, A.; Levine, J.; Swett, B.; Galimberti, V.; Han, J.; Riachi, M.E.; Habib, J.R.; Imam, R.; Kaplan, B.; et al. Impact of resection margin status on recurrence and survival in patients with resectable, borderline resectable, and locally advanced pancreatic cancer. Surgery 2025, 180, 109114. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0039606024011012 (accessed on 17 February 2025). [CrossRef]
- Hwang, J.W.; Kim, S.C.; Song, K.B.; Yoon, J.H.; Nam, J.S.; Lee, J.H.; Park, K.-M.; Lee, Y.-J. Significance of Radiologic Location and Extent of Portal Venous Involvement on Prognosis After Resection for Pancreatic Adenocarcinoma. Pancreas 2015, 44, 665–671. Available online: https://journals.lww.com/00006676-201505000-00024 (accessed on 17 March 2025). [CrossRef]
- Bachellier, P.; Rosso, E.; Fuchshuber, P.; Addeo, P.; David, P.; Oussoultzoglou, E.; Lucescu, I. Use of a temporary intraoperative mesentericoportal shunt for pancreatic resection for locally advanced pancreatic cancer with portal vein occlusion and portal hypertension. Surgery 2014, 155, 449–456. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0039606013004984 (accessed on 17 March 2025). [CrossRef] [PubMed]
- Bachellier, P.; Addeo, P.; Faitot, F.; Nappo, G.; Dufour, P. Pancreatectomy With Arterial Resection for Pancreatic Adenocarcinoma: How Can It Be Done Safely and With Which Outcomes?: A Single Institution’s Experience with 118 Patients. Ann. Surg. 2020, 271, 932–940. Available online: https://journals.lww.com/10.1097/SLA.0000000000003010 (accessed on 17 March 2025). [CrossRef] [PubMed]
- Del Chiaro, M.; Rangelova, E.; Halimi, A.; Ateeb, Z.; Scandavini, C.; Valente, R.; Segersvärd, R.; Arnelo, U.; Verbeke, C.S. Pancreatectomy with arterial resection is superior to palliation in patients with borderline resectable or locally advanced pancreatic cancer. HPB 2019, 21, 219–225. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1365182X18327047 (accessed on 21 March 2025). [CrossRef] [PubMed]
- Gurusamy, K.S.; Kumar, S.; Davidson, B.R.; Fusai, G. Resection versus other treatments for locally advanced pancreatic cancer. Cochrane Database Syst. Rev. 2014, 2014, CD010244. Available online: http://doi.wiley.com/10.1002/14651858.CD010244.pub2 (accessed on 21 March 2025). [CrossRef]
- Marchetti, A.; Corvino, G.; Perri, G.; Marchegiani, G.; De Luca, R. Reoperation for pancreatic fistula: A systematic review of completion pancreatectomy vs. pancreas-preserving-procedures and outcomes. HPB 2025, 27, 240–249. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1365182X24024201 (accessed on 17 March 2025). [CrossRef]
- Fancellu, A.; Petrucciani, N.; Porcu, A.; Deiana, G.; Sanna, V.; Ninniri, C.; Perra, T.; Celoria, V.; Nigri, G. The Impact on Survival and Morbidity of Portal–Mesenteric Resection During Pancreaticoduodenectomy for Pancreatic Head Adenocarcinoma: A Systematic Review and Meta-Analysis of Comparative Studies. Cancers 2020, 12, 1976. Available online: https://www.mdpi.com/2072-6694/12/7/1976 (accessed on 17 March 2025). [CrossRef]
- Tamagawa, H.; Aoyama, T.; Yamamoto, N.; Kamiya, M.; Murakawa, M.; Atsumi, Y.; Numata, M.; Kazama, K.; Hara, K.; Yukawa, N.; et al. The Impact of Intraoperative Blood Loss on the Survival of Patients With Stage II/III Pancreatic Cancer. Vivo 2020, 34, 1469–1474. Available online: http://iv.iiarjournals.org/lookup/doi/10.21873/invivo.11931 (accessed on 21 March 2025). [CrossRef]
- Preston, W.A.; Collins, M.L.; Gönen, M.; Murtha, T.; Rivera, V.; Lamm, R.; Schafer, M.; Yarmohammadi, H.; Covey, A.; Brody, L.A.; et al. Hemorrhage Sites and Mitigation Strategies After Pancreaticoduodenectomy. JAMA Surg. 2024, 159, 891–899. Available online: https://jamanetwork.com/journals/jamasurgery/fullarticle/2819031 (accessed on 21 March 2025). [CrossRef]
| Author | Surgery Type | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Concomitant Arterial Resection | Non-Arterial Standard Resections | p-Value | |||||||
| Median OS (Months) | 1-Year OS (%) | 2-Year OS (%) | 3-Year OS (%) | Median OS (Months) | 1-Year OS (%) | 2-Year OS (%) | 3-Year OS (%) | ||
| Yang et al. | NS | 81.8 | 63.6 | 42.4 | NS | 74 | 40 | 38 | <0.001 |
| Li et al. | 27.4 | 85 | 45 | 30 | 32.6 | 94.3 | 67.1 | 51.4 | <0.001 |
| Malinka et al. | NS | 88 | 78 | 78 | NS | 90 | 60 | 60 | <0.001 |
| Mean (1-, 2-, 3-year OS) | 84.9 | 62.2 | 50.1 | 86.1 | 55.7 | 49.8 | |||
| Variables | p | Mean Difference | SE Difference | Lower | Upper |
|---|---|---|---|---|---|
| 1-year survival | 0.836 | 1.167 | 4.954 | −22.482 | 20.148 |
| 2-year survival | 0.696 | 6.500 | 14.391 | −55.420 | 68.420 |
| 3-year survival | 0.980 | 0.333 | 11.554 | −49.380 | 50.047 |
| LOS | 0.874 | 0.383 | 2.289 | −0.736 | 0.866 |
| Blood loss (mL) | 0.709 | 32.683 | 79.564 | −285.890 | 220.525 |
| CDC ≥ 3 | 0.738 | 2.600 | 7.360 | −21.520 | 16.320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noel, C.; Azeez, A.; Du Preez, A.; Noel, K. Arterial Resections in Pancreatic Cancer—An Updated Systematic Review and Meta-Analysis. Cancers 2025, 17, 1540. https://doi.org/10.3390/cancers17091540
Noel C, Azeez A, Du Preez A, Noel K. Arterial Resections in Pancreatic Cancer—An Updated Systematic Review and Meta-Analysis. Cancers. 2025; 17(9):1540. https://doi.org/10.3390/cancers17091540
Chicago/Turabian StyleNoel, Colin, Adeboye Azeez, Annamarie Du Preez, and Kiera Noel. 2025. "Arterial Resections in Pancreatic Cancer—An Updated Systematic Review and Meta-Analysis" Cancers 17, no. 9: 1540. https://doi.org/10.3390/cancers17091540
APA StyleNoel, C., Azeez, A., Du Preez, A., & Noel, K. (2025). Arterial Resections in Pancreatic Cancer—An Updated Systematic Review and Meta-Analysis. Cancers, 17(9), 1540. https://doi.org/10.3390/cancers17091540







