Magrolimab Therapy in Conjunction with Conventional Chemotherapeutics Slows Disease Progression in Pediatric Acute Myeloid Leukemia Patient-Derived Xenograft Models
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
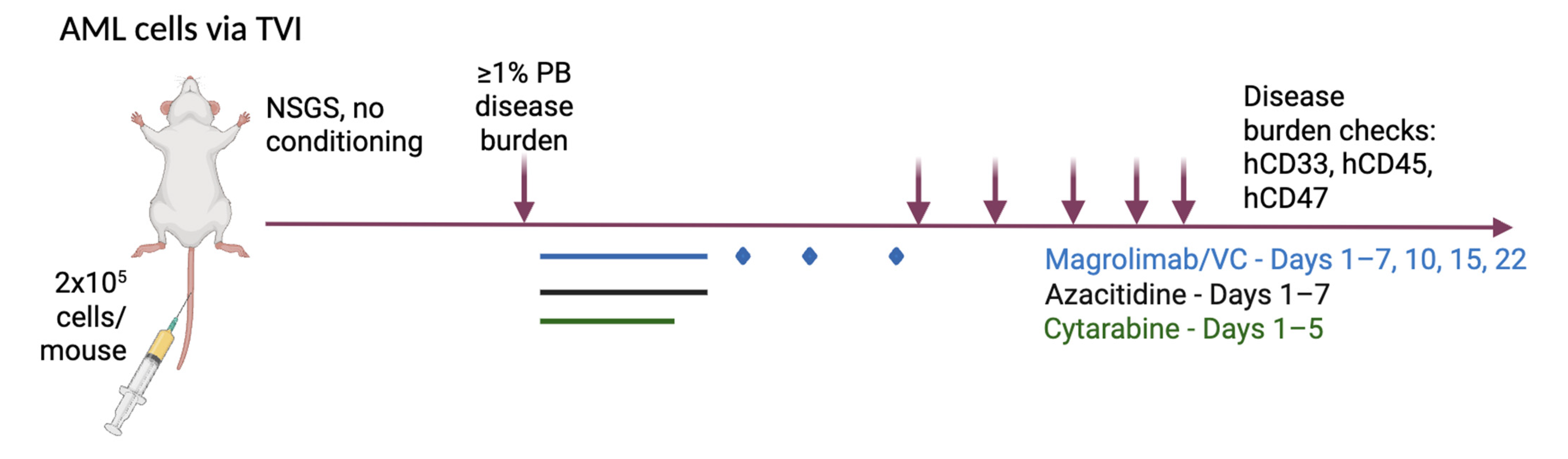
2.1. Patient-Derived Xenograft (PDX) Models
2.2. Magro and Chemotherapy Agents
2.3. Treatment
2.4. Disease and Clinical Monitoring
2.5. Humane Euthanasia and Tissue Collection
2.6. Data Analysis Methods
3. Results
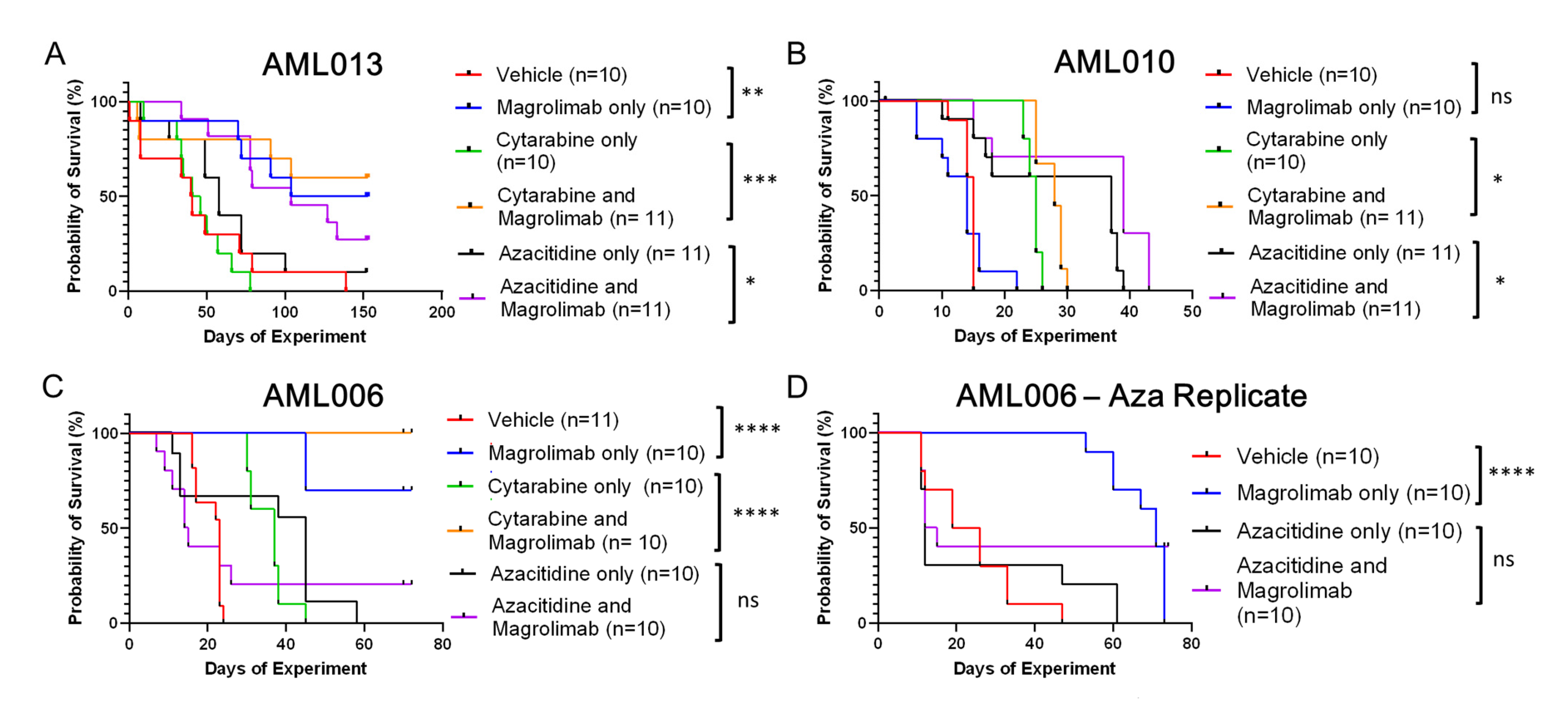
3.1. Tracking Survival Across Treatments and Models
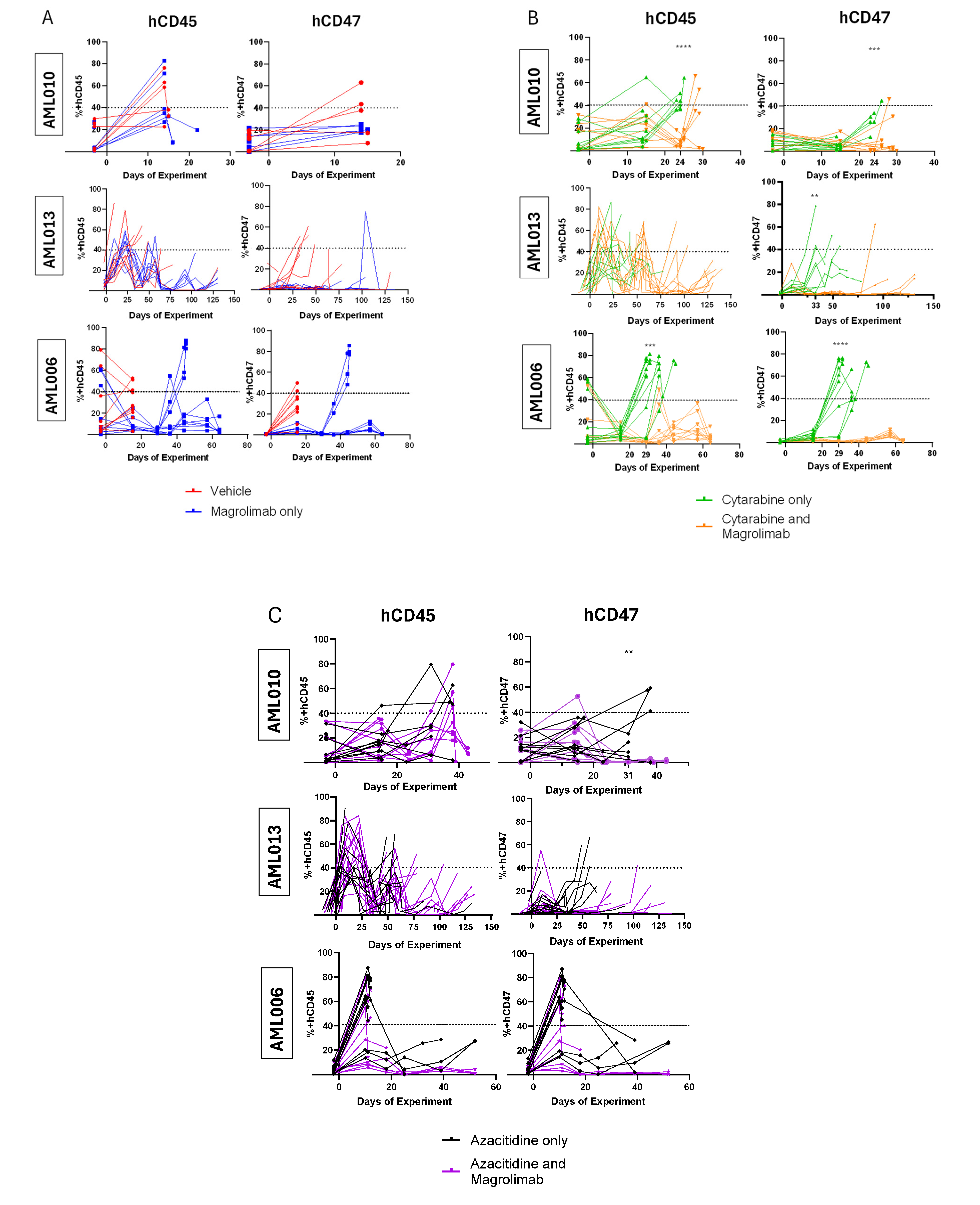
3.2. Tracking Disease Burden and CD47 Expression
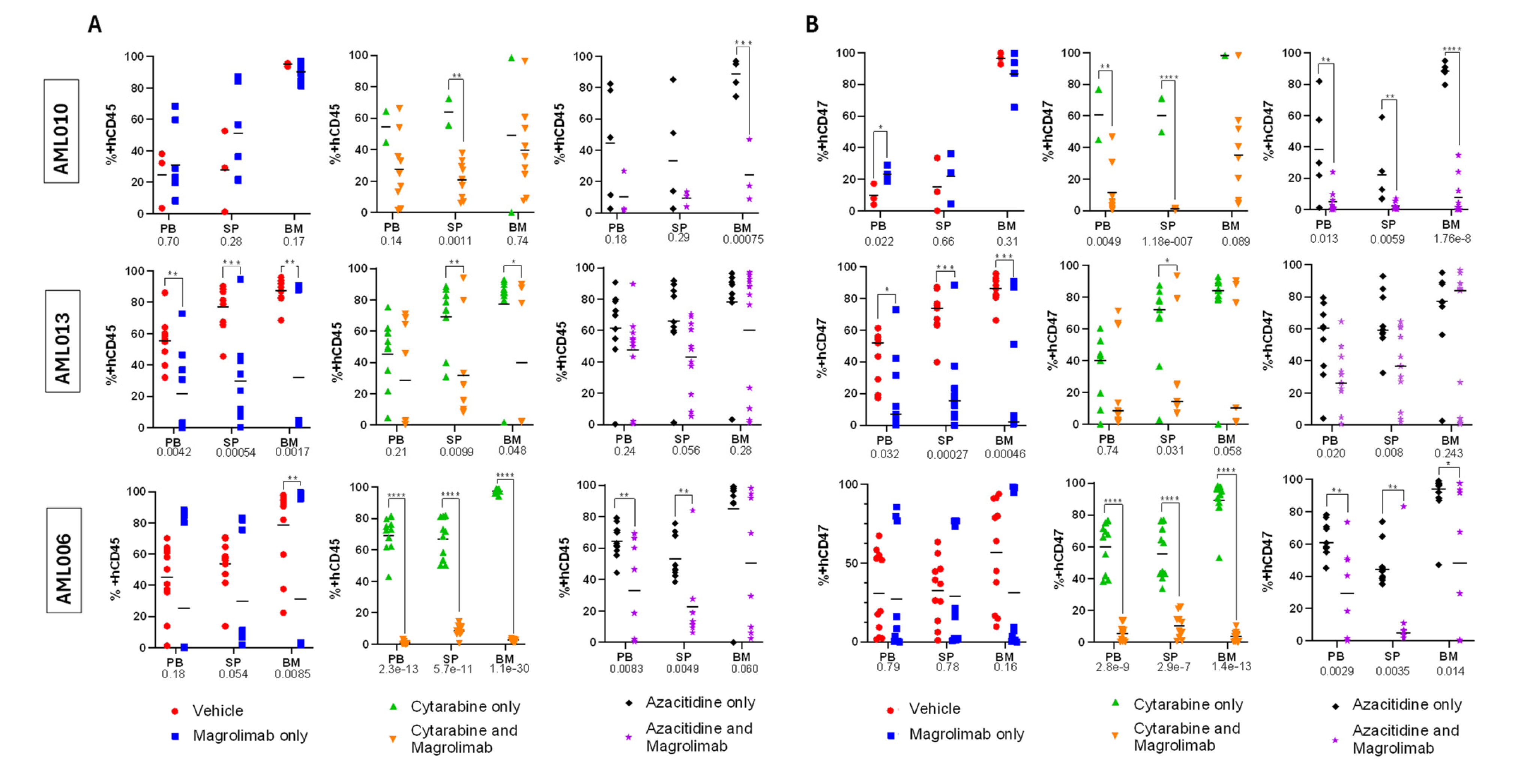
3.3. Disease Burden at Harvest
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| pAML | Pediatric acute myeloid leukemia |
| ALL | Acute lymphoblastic leukemia |
| OS | Overall survival |
| CD47 | Cluster of differentiation 47 |
| hCD47 | Human cluster of differentiation 47 |
| Magro | Magrolimab |
| DLBCL | Diffuse large B-cell lymphoma |
| CRR | Complete response rate |
| ORR | Objective response rate |
| LSCs | Leukemic stem cells |
| MF | Myelofibrosis |
| CD34 | Cluster of differentiation 34 |
| Aza | Azacitidine |
| MDS | Myelodysplastic syndromes |
| Ven | Venetoclax |
| Ara-C | Cytarabine |
| PDX | Patient-derived xenograft |
| VC | Vehicle control |
| PB | Peripheral blood |
| IP | Intraperitoneal |
| SP | Spleen |
| BM | Bone marrow |
| hCD45 | Human cluster of differentiation 45 |
Appendix A
Explanation for Repeating Azacitidine + Magro Arms for AML006
| Treatment Groups | AML010 | AML013 | AML006 | AML006 (Aza Replicate) |
| Date of experiment | NA | 152–153 | 70–72 | 73–75 |
| VC | 0 | 1 | 0 | 0 |
| Magrolimab | 0 | 5 | 6 | 6 |
| Ara-C + VC | 0 | 0 | 0 | NA |
| Ara-C + Magrolimab | 0 | 6 | 10 | NA |
| Aza + VC | 0 | 1 | 0 | 0 |
| Aza + Magrolimab | 0 | 3 | 2 | 4 |
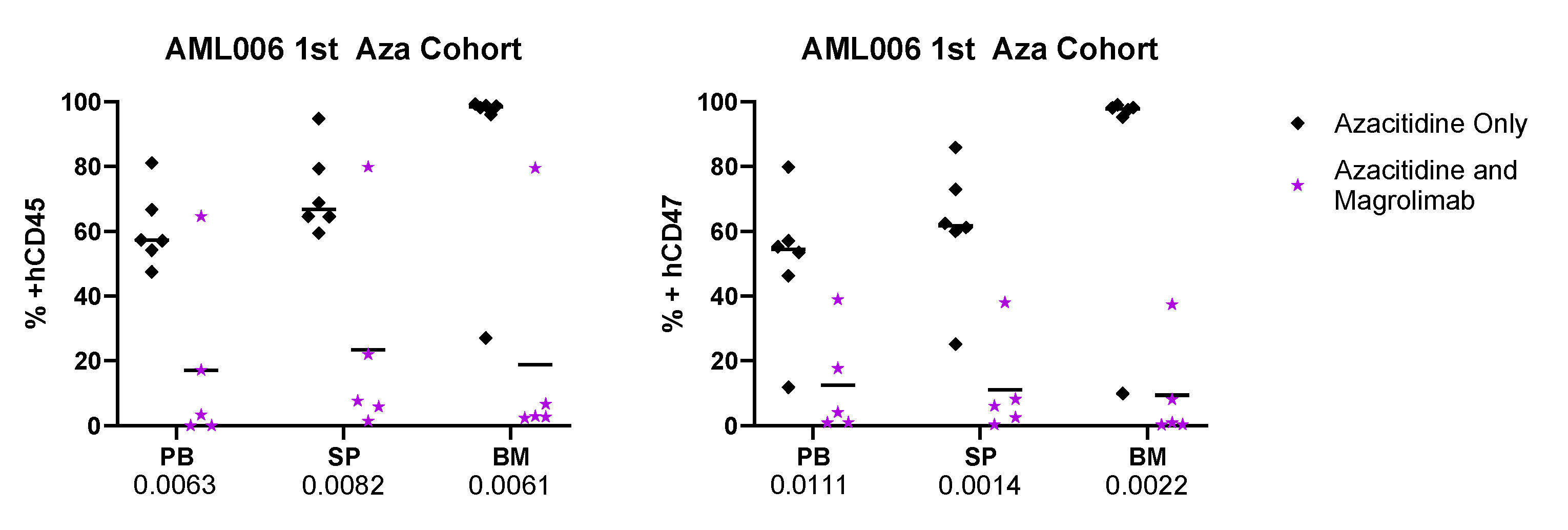
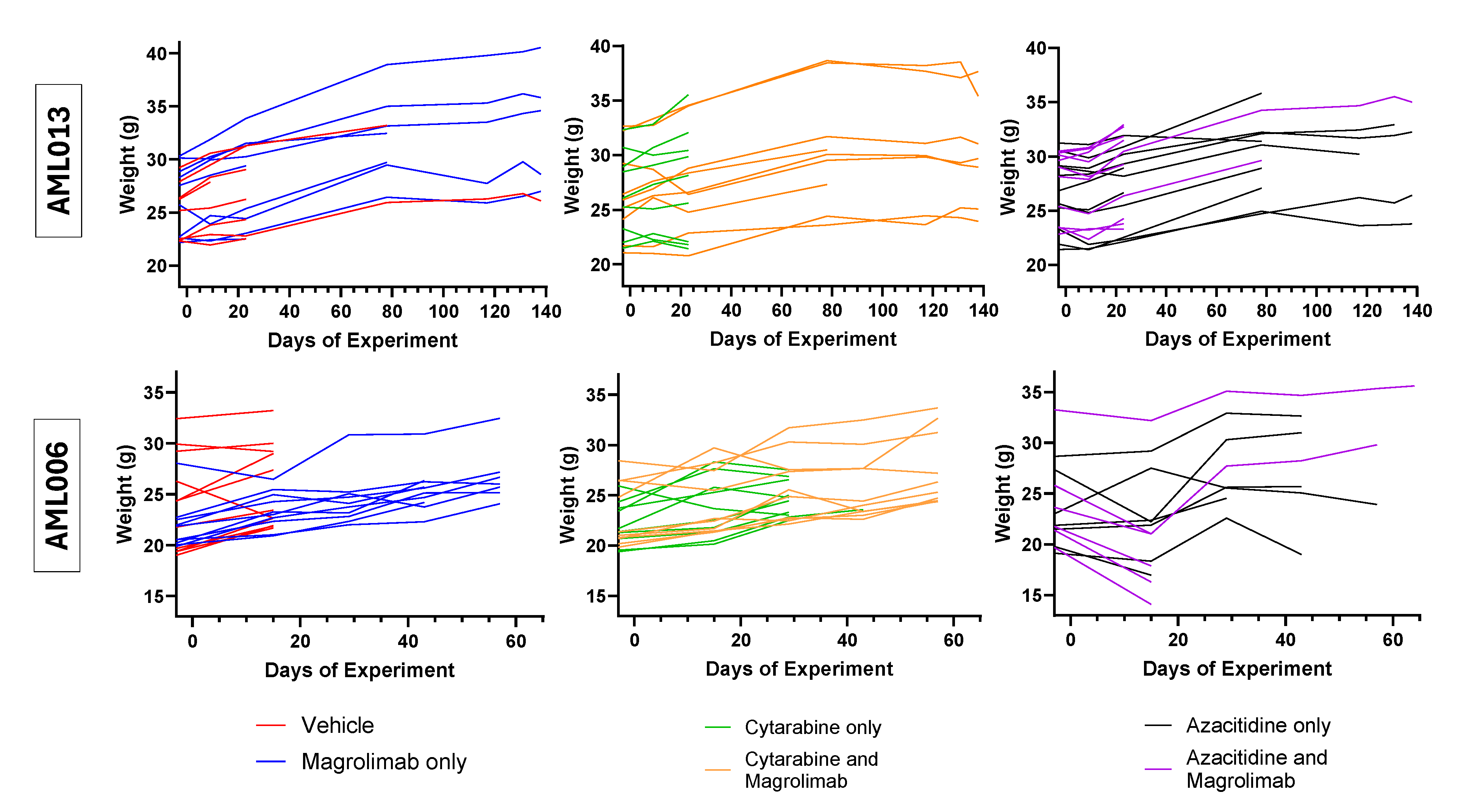
Appendix B
References
- Zwaan, C.M.; Kolb, E.A.; Reinhardt, D.; Abrahamsson, J.; Adachi, S.; Aplenc, R.; De Bont, E.S.; De Moerloose, B.; Dworzak, M.; Gibson, B.E.; et al. Collaborative Efforts Driving Progress in Pediatric Acute Myeloid Leukemia. J. Clin. Oncol. 2015, 33, 2949–2962. [Google Scholar] [CrossRef] [PubMed]
- Conneely, S.E.; Stevens, A.M. Acute Myeloid Leukemia in Children: Emerging Paradigms in Genetics and New Approaches to Therapy. Curr. Oncol. Rep. 2021, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.M.; Alonzo, T.A.; Tasian, S.K.; Kutny, M.A.; Hitzler, J.; Pollard, J.A.; Aplenc, R.; Meshinchi, S.; Kolb, E.A. Children’s Oncology Group’s 2023 blueprint for research: Myeloid neoplasms. Pediatr Blood Cancer 2023, 70, e30584. [Google Scholar] [CrossRef]
- Bolouri, H.; Farrar, J.E.; Triche, T., Jr.; Ries, R.E.; Lim, E.L.; Alonzo, T.A.; Ma, Y.; Moore, R.; Mungall, A.J.; Marra, M.A.; et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat. Med. 2018, 24, 103–112. [Google Scholar] [CrossRef]
- Ley, T.J.; Miller, C.; Ding, L.; Raphael, B.J.; Mungall, A.J.; Robertson, A.; Hoadley, K.; Triche, T.J., Jr.; Laird, P.W.; Baty, J.D.; et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Zhao, F.; Tseng, S.; Narayanan, C.; Shura, L.; Willingham, S.; Howard, M.; Prohaska, S.; Volkmer, J.; et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS ONE 2015, 10, e0137345. [Google Scholar] [CrossRef]
- Huang, J.; Liu, F.; Li, C.; Liang, X.; Li, C.; Liu, Y.; Yi, Z.; Zhang, L.; Fu, S.; Zeng, Y. Role of CD47 in tumor immunity: A potential target for combination therapy. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D., Jr.; van Rooijen, N.; Weissman, I.L. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef]
- Kiss, B.; Volkmer, A.K.; Feng, D.; McKenna, G.W.; Mihardja, S.; Chao, M.; Takimoto, C.H.; Liao, J.C.; Volkmer, J.-P.; Weissman, I.L. Magrolimab and gemcitabine-cisplatin combination enhance phagocytic elimination of bladder cancer. J. Clin. Oncol. 2020, 38, e17035. [Google Scholar] [CrossRef]
- Feng, D.; Gip, P.; McKenna, K.M.; Zhao, F.; Mata, O.; Choi, T.S.; Duan, J.; Sompalli, K.; Majeti, R.; Weissman, I.L.; et al. Combination Treatment with 5F9 and Azacitidine Enhances Phagocytic Elimination of Acute Myeloid Leukemia. Blood 2018, 132, 2729. [Google Scholar] [CrossRef]
- Rinaldi, C.R.; Boasman, K.; Simmonds, M. Effects of ruxolitinib and magrolimab on calreticulin in myelofibrosis CD34+ cells in vitro: Proof of concept for combination therapy. J. Clin. Oncol. 2023, 41. [Google Scholar] [CrossRef]
- A Sallman, D.; Asch, A.S.; Al Malki, M.M.; Lee, D.J.; Donnellan, W.B.; Marcucci, G.; Kambhampati, S.; Daver, N.G.; Garcia-Manero, G.; Komrokji, R.S.; et al. The First-in-Class Anti-CD47 Antibody Magrolimab (5F9) in Combination with Azacitidine Is Effective in MDS and AML Patients: Ongoing Phase 1b Results. Blood 2019, 134, 569. [Google Scholar] [CrossRef]
- Daver, N.; Senapati, J.; Maiti, A.; Loghavi, S.; Kadia, T.M.; DiNardo, C.D.; Pemmaraju, N.; Jabbour, E.; Montalban-Bravo, G.; Tang, G.; et al. Phase I/II Study of Azacitidine (AZA) with Venetoclax (VEN) and Magrolimab (Magro) in Patients (pts) with Newly Diagnosed (ND) Older/Unfit or High-Risk Acute Myeloid Leukemia (AML) and Relapsed/Refractory (R/R) AML. Blood 2022, 140, 141–144. [Google Scholar] [CrossRef]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef]
- Mehta, A.; Popplewell, L.; Collins, G.P.; Smith, S.M.; Flinn, I.W.; Bartlett, N.L.; Ghosh, N.; Hacohen-Kleiman, G.; Huo, Y.; Su-Feher, L.; et al. Magrolimab plus rituximab in relapsed/refractory indolent non-Hodgkin lymphoma: 3-year follow-up of a phase 1/2 trial. Blood Adv. 2024, 8, 5855–5863. [Google Scholar] [CrossRef]
- Maakaron, J.E.; Asch, A.; Popplewell, L.; Collins, G.P.; Flinn, I.W.; Ghosh, N.; Keane, C.; Ku, M.; Mehta, A.; Roschewski, M.; et al. Magrolimab plus rituximab with or without chemotherapy in patients with relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2024, 8, 5864–5874. [Google Scholar] [CrossRef]
- Paul, B.; Minarík, J.; Cottini, F.; Gasparetto, C.; Khouri, J.; Gandhi, M.; Hillengass, J.; Levy, M.; Liedtke, M.; Manda, S.; et al. Safety and Tolerability of Magrolimab Combinations in Patients with Relapsed/Refractory Multiple Myeloma (RRMM): Safety Run-in Results from a Phase 2 Study. Blood 2023, 142, 3383. [Google Scholar] [CrossRef]
- Colevas, A.; Kerrigan, K.; Chin, V.; Rainey, N.; Park, J.J.; Fang, B.; Costa, D.A.; Dinis, J.; Phan, M.; Lin, L.; et al. Safety and Tolerability of Magrolimab Combination Therapy in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (RM-HNSCC). Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, e85–e86. [Google Scholar] [CrossRef]
- Fisher, G.A.; Lakhani, N.J.; Eng, C.; Hecht, J.R.; Bendell, J.C.; Philip, P.A.; O’Dwyer, P.J.; Johnson, B.; Kardosh, A.; Ippolito, T.M.; et al. A phase Ib/II study of the anti-CD47 antibody magrolimab with cetuximab in solid tumor and colorectal cancer patients. J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- Juan-Videl, O.; Chiu, J.; Vaishampayan, U.N.; Joshi, R.; Furqan, M.; Rainey, N.; Fang, B.; Lawler, W.; Vicuna, B.; Xu, W.; et al. 698 Safety and tolerability of magrolimab in combination with taxanes in patients with solid tumors. J. Immuno Therapy Cancer 2023, 11, A792–A793. [Google Scholar] [CrossRef]
- Subbiah, V.; Vaishampayan, U.N.; Puri, S.; Lin, L.; Chao, M.; Ramsingh, G.; Kummar, S.; Strauss, J.F.; Patel, S.P. A phase 2, multiarm study of anti-CD47 antibody, magrolimab, in combination with docetaxel in patients with locally advanced or metastatic solid tumors. J. Clin. Oncol. 2022, 40. [Google Scholar] [CrossRef]
- Daver, N.; Vyas, P.; Chao, M.; Xing, G.; Renard, C.; Ramsingh, G.; Sallman, D.A.; Wei, A.H. A Phase 3, Randomized, Open-Label Study Evaluating the Safety and Efficacy of Magrolimab in Combination with Azacitidine in Previously Untreated Patients with TP53-Mutant Acute Myeloid Leukemia. Blood 2021, 138, 3426. [Google Scholar] [CrossRef]
- Daver, N.G.; Liu, K.; Kuwahara, S.B.; Caldwell, K.; Vyas, P. AML-577 A Phase III, Randomized Trial of Magrolimab in Combination with Venetoclax and Azacitidine in Previously Untreated Patients with Acute Myeloid Leukemia Who Are Ineligible for Intensive Chemotherapy (ENHANCE-3). Clin. Lymphoma Myeloma Leuk. 2023, 23, S313–S314. [Google Scholar] [CrossRef]
- Dolgin, E. Safety Concerns Prompt Pause of Magrolimab Trials. Cancer Discov. 2022, 12, 877–878. [Google Scholar] [CrossRef]
- Desai, P.N.; Wang, B.; Reville, P.K.; Jelloul, F.Z.; Borges, P.; Senapati, J.; Castillo, M.; Gunaratne, P.; Pemmaraju, N.; Kadia, T.M.; et al. Single-Cell Characterization of TP53-Mutated AML Patients Treated with Frontline Azacitidine, Venetoclax, and Magrolimab Reveals Mechanisms of Response and Resistance. Blood 2023, 142, 64. [Google Scholar] [CrossRef]
- Stevens, A.M.; Terrell, M.; Rashid, R.; Fisher, K.E.; Marcogliese, A.N.; Gaikwad, A.; Rao, P.; Vrana, C.; Krueger, M.; Loken, M.; et al. Addressing a Pre-Clinical Pipeline Gap: Development of the Pediatric Acute Myeloid Leukemia Patient-Derived Xenograft Program at Texas Children’s Hospital at Baylor College of Medicine. Biomedicines 2024, 12, 394. [Google Scholar] [CrossRef]
- Johnson, L.D.; Tang, S.; Zhang, Y.; Metzner, M.; Aviles, L.; Kavelaars, F.; Morrow, E.; Vitters, D.; Caldwell, K.; Valk, P.J.M.; et al. Mutational Landscape and Depth of Response in Patients with Acute Myeloid Leukemia (AML) Treated with Magrolimab in Combination with Venetoclax and Azacitidine Compared to Placebo. Blood 2024, 144, 2914. [Google Scholar] [CrossRef]
- Stelmach, P.; Trumpp, A. Leukemic stem cells and therapy resistance in acute myeloid leukemia. Haematologica 2023, 108, 353–366. [Google Scholar] [CrossRef]
- Jaiswal, S.; Jamieson, C.H.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef]
- Johnson, L.; Zhang, Y.; Su-Feher, L.; Lee, Y.; Sallman, D.A.; Asch, A.S.; Al Malki, M.M.; Kim, H.R. MDS-488 Magrolimab Alters the Tumor Microenvironment to Improve Bone Marrow Functions in Patients with Acute Myeloid Leukemia (AML) and Higher-Risk Myelodysplastic Syndromes (HR-MDS). Clin. Lymphoma Myeloma Leuk. 2023, 23, S368–S369. [Google Scholar] [CrossRef]
| Public Model Name | Age/ Sex | Race/ Ethnicity | Timepoint | Risk Group/ Clinical Outcome | Major Cytogenetic or Molecular Features | % CD47+ |
|---|---|---|---|---|---|---|
| AML006 | 9/M | B/NH | Relapse | High risk (fusion); DD | KMT2A::MLLT1, KRAS, SETD2, WT1 | 98.40% |
| AML010 | 2/M | W/NH | Diagnosis | Low risk (MRD negative, non-prognostic genetics); alive | +10, WT1 | 95.10% |
| AML013 | 13/M | B/NH | Diagnosis | High risk (fusion); DD | KMT2A::MLLT4, BRAF, BCOR | 92.80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.G.; Sandhu, S.K.; Dontula, R.V.; Cooper, J.J.; Sherman, J.; Rochette, M.; Siddiqui, R.; Kim, L.E.; Redell, M.S.; Stevens, A.M. Magrolimab Therapy in Conjunction with Conventional Chemotherapeutics Slows Disease Progression in Pediatric Acute Myeloid Leukemia Patient-Derived Xenograft Models. Cancers 2025, 17, 1509. https://doi.org/10.3390/cancers17091509
Kim JG, Sandhu SK, Dontula RV, Cooper JJ, Sherman J, Rochette M, Siddiqui R, Kim LE, Redell MS, Stevens AM. Magrolimab Therapy in Conjunction with Conventional Chemotherapeutics Slows Disease Progression in Pediatric Acute Myeloid Leukemia Patient-Derived Xenograft Models. Cancers. 2025; 17(9):1509. https://doi.org/10.3390/cancers17091509
Chicago/Turabian StyleKim, Julia G., Sohani K. Sandhu, Ritesh V. Dontula, Josh J. Cooper, Jaden Sherman, Max Rochette, Rehan Siddiqui, Lana E. Kim, Michelle S. Redell, and Alexandra M. Stevens. 2025. "Magrolimab Therapy in Conjunction with Conventional Chemotherapeutics Slows Disease Progression in Pediatric Acute Myeloid Leukemia Patient-Derived Xenograft Models" Cancers 17, no. 9: 1509. https://doi.org/10.3390/cancers17091509
APA StyleKim, J. G., Sandhu, S. K., Dontula, R. V., Cooper, J. J., Sherman, J., Rochette, M., Siddiqui, R., Kim, L. E., Redell, M. S., & Stevens, A. M. (2025). Magrolimab Therapy in Conjunction with Conventional Chemotherapeutics Slows Disease Progression in Pediatric Acute Myeloid Leukemia Patient-Derived Xenograft Models. Cancers, 17(9), 1509. https://doi.org/10.3390/cancers17091509





