[68Ga]-DOTATOC PET/CT Volumetric Parameters Reflect Metastatic Potential in Pancreatic Neuroendocrine Tumors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
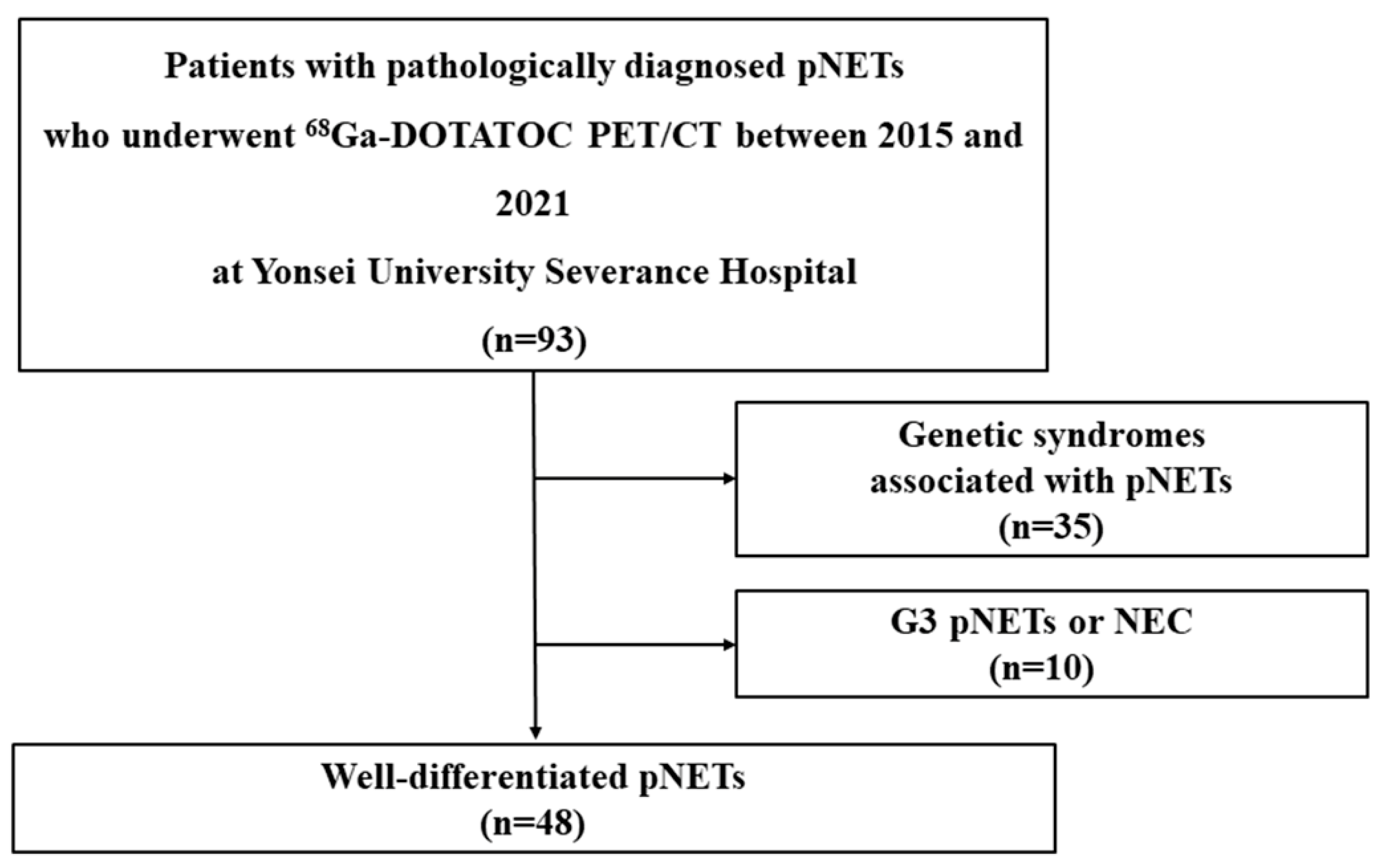
2.1. Patients and Study Design
2.2. Clinicopathologic Factors
2.3. PET/CT Imaging Protocol
2.4. [68Ga]-DOTATOC PET/CT Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Clinicopathologic Factors and [68Ga]-DOTATOC PET/CT Indices According to the Initial Metastasis of pNETs
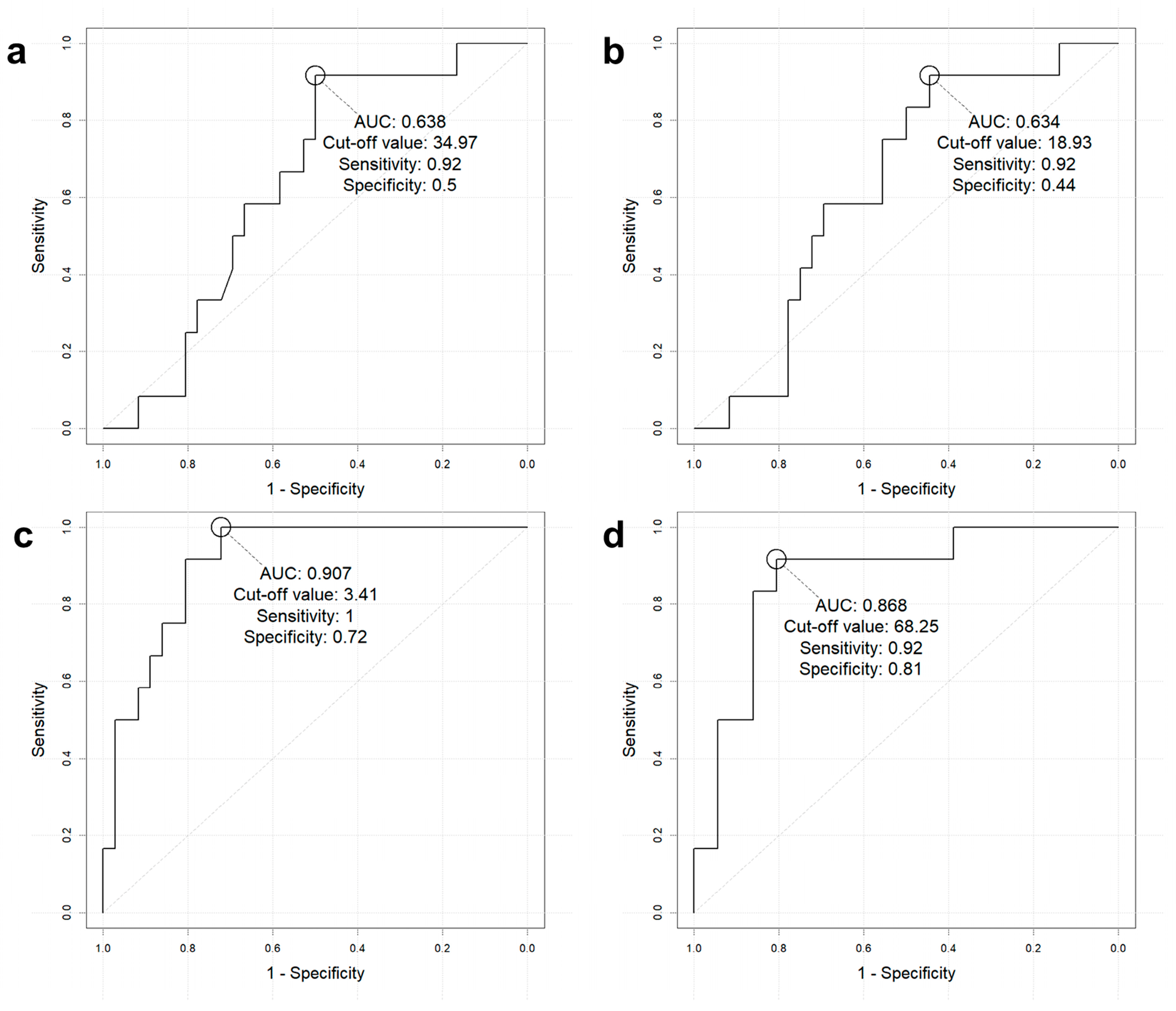
3.3. Diagnostic Performance of [68Ga]-DOTATOC PET/CT Indices to Predict Initial Metastasis
3.4. Subgroup Analysis of [68Ga]-DOTATOC PET/CT Indices to Predict Metastasis in pNETs Measuring > 20 mm
3.5. Clinicopathologic Factors and [68Ga]-DOTATOC Indices According to pNET WHO Grades
3.6. Relationship Between [68Ga]-DOTATOC PET/CT Indices, Proliferative Index, and Serum Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Oda, Y.; Tanaka, Y.; Naruse, T.; Sasanabe, R.; Tsubamoto, M.; Funahashi, H. Expression of somatostatin receptor and effects of somatostatin analog on pancreatic endocrine tumors. Surg. Today 2002, 32, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Papotti, M.; Bongiovanni, M.; Volante, M.; Allìa, E.; Landolfi, S.; Helboe, L.; Schindler, M.; Cole, S.L.; Bussolati, G. Expression of somatostatin receptor types 1–5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002, 440, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Zilli, A.; Fanetti, I.; Conte, D.; Massironi, S. A case of positive (68)Ga-DOTATOC-PET/CT pancreatic heterotopia mimicking an intestinal neuroendocrine tumor. Clin. Imaging 2018, 49, 156–158. [Google Scholar] [CrossRef]
- Reubi, J.C. Neuropeptide receptors in health and disease: The molecular basis for in vivo imaging. J. Nucl. Med. 1995, 36, 1825–1835. [Google Scholar] [PubMed]
- Reubi, J.C.; Schär, J.C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef]
- Miederer, M.; Seidl, S.; Buck, A.; Scheidhauer, K.; Wester, H.J.; Schwaiger, M.; Perren, A. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 48–52. [Google Scholar] [CrossRef]
- Tamm, E.P.; Bhosale, P.; Lee, J.H.; Rohren, E.M. State-of-the-art Imaging of Pancreatic Neuroendocrine Tumors. Surg. Oncol. Clin. N. Am. 2016, 25, 375–400. [Google Scholar] [CrossRef]
- Ambrosini, V.; Campana, D.; Polverari, G.; Peterle, C.; Diodato, S.; Ricci, C.; Allegri, V.; Casadei, R.; Tomassetti, P.; Fanti, S. Prognostic Value of 68Ga-DOTANOC PET/CT SUVmax in Patients with Neuroendocrine Tumors of the Pancreas. J. Nucl. Med. 2015, 56, 1843–1848. [Google Scholar] [CrossRef]
- Mapelli, P.; Partelli, S.; Salgarello, M.; Doraku, J.; Muffatti, F.; Schiavo Lena, M.; Pasetto, S.; Bezzi, C.; Bettinardi, V.; Andreasi, V.; et al. Dual Tracer 68Ga-DOTATOC and 18F-FDG PET Improve Preoperative Evaluation of Aggressiveness in Resectable Pancreatic Neuroendocrine Neoplasms. Diagnostics 2021, 11, 192. [Google Scholar] [CrossRef]
- Queiroz, M.M.; Lopes, C.D.H.; Salgues, A.C.R.; Barbosa, F.G.; Abe, E.S.; Silveira, T.P.; Machado, M.C.C.; Capareli, F.C. 18F-FDG PETCT and 68Ga-DOTA PETCT mismatch with in vivo histopathological characterization of low-grade neuroendocrine pancreatic tumor. Eur. J. Hybrid Imaging 2021, 5, 9. [Google Scholar] [CrossRef]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with (68)Ga-DOTA-conjugated somatostatin receptor targeting peptides and (18)F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Lau, W.F.; Hicks, R.J. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: Clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics 2015, 35, 500–516. [Google Scholar] [CrossRef]
- Kayani, I.; Bomanji, J.B.; Groves, A.; Conway, G.; Gacinovic, S.; Win, T.; Dickson, J.; Caplin, M.; Ell, P.J. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer 2008, 112, 2447–2455. [Google Scholar] [CrossRef]
- Campana, D.; Ambrosini, V.; Pezzilli, R.; Fanti, S.; Labate, A.M.; Santini, D.; Ceccarelli, C.; Nori, F.; Franchi, R.; Corinaldesi, R.; et al. Standardized uptake values of (68)Ga-DOTANOC PET: A promising prognostic tool in neuroendocrine tumors. J. Nucl. Med. 2010, 51, 353–359. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, D.; Saveanu, A.; Couvelard, A.; Gunz, G.; Enjalbert, A.; Jaquet, P.; Ruszniewski, P.; Barlier, A. The analysis of quantitative expression of somatostatin and dopamine receptors in gastro-entero-pancreatic tumours opens new therapeutic strategies. Eur. J. Endocrinol. 2006, 155, 849–857. [Google Scholar] [CrossRef]
- Yu, J.; Li, N.; Li, J.; Lu, M.; Leal, J.P.; Tan, H.; Su, H.; Fan, Y.; Zhang, Y.; Zhao, W.; et al. The Correlation Between [(68)Ga]DOTATATE PET/CT and Cell Proliferation in Patients With GEP-NENs. Mol. Imaging Biol. 2019, 21, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Paepegaey, A.C.; Gaujoux, S.; Meyer, C.; Rouquette, A.; Libé, R. Combined 68Ga-DOTATOC and 18F-FDG PET Predicts a Double Component With Different Grade of a Pancreatic Neuroendocrine Tumor in a Patient With Multiple Endocrine Neoplasia Type 1. Clin. Nucl. Med. 2020, 45, e281–e282. [Google Scholar] [CrossRef]
- Thuillier, P.; Liberini, V.; Grimaldi, S.; Rampado, O.; Gallio, E.; Santi, B.; Arvat, E.; Piovesan, A.; Filippi, R.; Abgral, R.; et al. Prognostic Value of Whole-Body PET Volumetric Parameters Extracted from (68)Ga-DOTATOC PET/CT in Well-Differentiated Neuroendocrine Tumors. J. Nucl. Med. 2022, 63, 1014–1020. [Google Scholar] [CrossRef]
- Toriihara, A.; Baratto, L.; Nobashi, T.; Park, S.; Hatami, N.; Davidzon, G.; Kunz, P.L.; Iagaru, A. Prognostic value of somatostatin receptor expressing tumor volume calculated from (68)Ga-DOTATATE PET/CT in patients with well-differentiated neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2244–2251. [Google Scholar] [CrossRef]
- Kaltsas, G.A.; Besser, G.M.; Grossman, A.B. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr. Rev. 2004, 25, 458–511. [Google Scholar] [CrossRef]
- Eriksson, B.; Oberg, K.; Stridsberg, M. Tumor markers in neuroendocrine tumors. Digestion 2000, 62 (Suppl. S1), 33–38. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.D.; Balci, S.; Saka, B.; Adsay, N.V. Neuroendocrine tumors of the pancreas: Current concepts and controversies. Endocr. Pathol. 2014, 25, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Tirosh, A.; Papadakis, G.Z.; Millo, C.; Hammoud, D.; Sadowski, S.M.; Herscovitch, P.; Pacak, K.; Marx, S.J.; Yang, L.; Nockel, P.; et al. Prognostic Utility of Total 68Ga-DOTATATE-Avid Tumor Volume in Patients With Neuroendocrine Tumors. Gastroenterology 2018, 154, 998–1008.e1. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Talamonti, M.S.; Tomlinson, J.S.; Stewart, A.K.; Winchester, D.P.; Ko, C.Y.; Bentrem, D.J. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: Analysis of 3851 patients. Ann. Surg. 2008, 247, 490–500. [Google Scholar] [CrossRef]
- Fischer, L.; Kleeff, J.; Esposito, I.; Hinz, U.; Zimmermann, A.; Friess, H.; Büchler, M.W. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br. J. Surg. 2008, 95, 627–635. [Google Scholar] [CrossRef]
- Jeune, F.; Taibi, A.; Gaujoux, S. Update on the Surgical Treatment of Pancreatic Neuroendocrine Tumors. Scand. J. Surg. 2020, 109, 42–52. [Google Scholar] [CrossRef]
- Tirosh, A.; Papadakis, G.Z.; Millo, C.; Sadowski, S.M.; Herscovitch, P.; Pacak, K.; Marx, S.J.; Yang, L.; Nockel, P.; Shell, J.; et al. Association between neuroendocrine tumors biomarkers and primary tumor site and disease type based on total (68)Ga-DOTATATE-Avid tumor volume measurements. Eur. J. Endocrinol. 2017, 176, 575–582. [Google Scholar] [CrossRef]

| Variable | Total (n = 48) | Non-Metastatic (n = 36) | Metastatic (n = 12) | p Value |
|---|---|---|---|---|
| Age ± SD, years | 54.8 ± 12.19 | 54.3 ± 12.63 | 56.4 ± 11.12 | 0.604 |
| Male sex, n (%) | 27 (56.3) | 21 (58.3) | 6 (50.0) | 0.614 |
| Histopathologic confirmation method, n (%) | <0.001 | |||
| Operation | 37 (77.1) | 34 (94.4) | 3 (25.0) | |
| Fine needle biopsy | 11 (22.9) | 2 (5.6) | 9 (75.0) | |
| Laboratory tests (serum) | ||||
| Chromogranin A, median (Q1, Q3), ng/mL | 82.75 (38.30, 125.30) | 69.70 (19.20, 93.20) | 104.15 (63.50, 160.80) | 0.079 * |
| CA 19-9, median (Q1, Q3), U/mL | 8.70 (3.70, 13.95) | 6.80 (3.70, 13.20) | 12.30 (7.90, 16.80) | 0.215 * |
| Characteristics of tumor | ||||
| Tumor size, median (Q1, Q3), mm | 18.00 (12.00, 27.00) | 14.50 (11.00, 20.00) | 34.50 (27.00, 40.00) | <0.001 * |
| Tumor size ≤20:>20, mm, n (%) | 30 (62.5):18 (37.5) | 29 (80.6):7 (19.4) | 1 (8.3):11 (91.7) | <0.001 |
| WHO G1:G2, n (%) | 30 (62.5):18 (37.5) | 27 (75.0):9 (25.0) | 3 (25.0):9 (75.0) | 0.002 |
| Mitotic count, median (Q1, Q3)/50 HPF | 1.00 (0.00, 1.00) | 0.00 (0.00, 1.00) | 1.00 (0.00, 2.00) | 0.604 * |
| Ki-67 index, median (Q1, Q3) | 1.98 (1.00, 4.69) | 1.49 (0.92, 2.43) | 4.90 (2.36, 12.00) | 0.002 * |
| Immunohistochemistry, n (%) † | ||||
| Chromogranin A | 32 (84.2) | 26 (89.7) | 6 (66.7) | 0.098 |
| CD 56 | 36 (85.7) | 29 (85.3) | 7 (87.5) | 0.873 |
| Synaptophysin | 44 (95.7) | 32 (94.1) | 12 (100.0) | 0.390 |
| [68Ga]-DOTATOC PET/CT indices in pancreatic tumor | ||||
| SUVmax, median (Q1, Q3) | 50.44 (23.37, 78.43) | 40.19 (17.96, 70.15) | 64.95 (42.72, 85.06) | 0.157 * |
| SUVmean, median (Q1, Q3) | 29.14 (13.35, 44.01) | 22.69 (9.58, 39.47) | 35.48 (24.70, 47.49) | 0.167 * |
| SRETV, median (Q1, Q3), mL | 3.25 (0.93, 7.22) | 1.50 (0.84, 3.78) | 11.80 (6.02, 16.01) | <0.001 * |
| TLSRE, median (Q1, Q3), g | 55.94 (25.82, 318.74) | 41.29 (16.94, 67.10) | 357.14 (290.16, 538.25) | <0.001 * |
| AUC [95% CI] | Group | Sensitivity | Specificity | Accuracy | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| SUVmax >34.97 | 0.638 (0.486–0.771) | All (n = 48) | 91.7% (11/12) | 50.0% (18/36) | 60.4% (29/48) | 37.9% (11/29) | 94.7% (18/19) |
| Size > 2 cm (n = 18) | 90.9% (10/11) | 42.9% (3/7) | 72.2% (13/18) | 71.4% (10/14) | 75% (3/4) | ||
| SUVmean >18.93 | 0.634 (0.483–0.768) | All (n = 48) | 91.7% (11/12) | 44.4% (16/36) | 56.3% (27/48) | 35.5% (11/31) | 94.1% (16/17) |
| Size > 2 cm (n = 18) | 90.9% (10/11) | 42.9% (3/7) | 72.2% (13/18) | 71.4% (10/14) | 75% (3/4) | ||
| SRETV >3.41 | 0.907 (0.788–0.972) | All (n = 48) | 100.0% (12/12) | 72.2% (26/36) | 79.2% (38/48) | 54.6% (12/22) | 100.0% (26/26) |
| Size > 2 cm (n = 18) | 100% (11/11) | 42.9% (3/7) | 77.8% (14/18) | 73.3% (11/15) | 100% (3/3) | ||
| TLSRE >68.25 | 0.868 (0.739–0.948) | All (n = 48) | 91.7% (11/12) | 80.6% (29/36) | 83.3% (40/48) | 61.1% (11/18) | 96.7% (29/30) |
| Size > 2 cm (n = 18) | 90.9% (10/11) | 42.9% (3/7) | 72.2% (13/18) | 71.4% (10/14) | 75% (3/4) |
| WHO Grade 1 (n = 30) | WHO Grade 2 (n = 18) | p Value | |
|---|---|---|---|
| Age ± SD, year | 55.50 ± 12.61 | 53.67 ± 11.73 | 0.619 |
| Male sex (%) | 21 (70.0) | 6 (33.3) | 0.013 |
| Histopathologic confirmation method, n (%) | 0.006 | ||
| Operation | 27 (90.0) | 10 (55.6) | |
| Fine needle biopsy | 3 (10.0) | 8 (44.4) | |
| Laboratory tests (serum) | |||
| Chromogranin A, median (Q1, Q3), ng/mL | 69.70 (35.90, 132.00) | 91.15 (42.60, 106.00) | 0.907 * |
| CA 19-9, median (Q1, Q3), U/mL | 8.70 (3.70, 14.50) | 8.75 (2.85, 13.40) | 0.820 * |
| Characteristics of tumor | |||
| Tumor size, median (Q1, Q3), mm | 13.00 (11.00, 20.00) | 23.50 (18.00, 35.00) | 0.002 * |
| Tumor size ≤20:>20, mm, n (%) | 23 (76.7):7 (23.3) | 7 (38.9):11 (61.1) | 0.009 |
| Metastasis, n (%) | 3 (10.0) | 9 (50.0) | 0.005 |
| Mitotic count, median (Q1, Q3)/50 HPF | 0.00 (0.00, 0.00) | 2.00 (1.00, 3.00) | <0.001 * |
| Ki-67 index, median (Q1, Q3) | 1.00 (0.79, 1.95) | 5.70 (4.00, 12.00) | <0.001 * |
| Immunohistochemistry, n (%) † | |||
| Chromogranin A | 23 (92.0) | 9 (69.2) | 0.154 ** |
| CD 56 | 24 (85.7) | 12 (85.7) | >0.999 |
| Synaptophysin | 29 (100.0) | 15 (88.2) | 0.131 ** |
| [68Ga]-DOTATOC PET/CT indices in pancreatic tumor | |||
| SUVmax, median (Q1, Q3) | 47.84 (22.76, 68.17) | 51.80 (31.72, 84.87) | 0.749 * |
| SUVmean, median (Q1, Q3) | 26.23 (12.79, 39.71) | 30.17 (17.08, 49.08) | 0.733 * |
| SRETV, median (Q1, Q3), mL | 1.83 (0.87, 5.81) | 5.31 (1.80, 9.20) | 0.092 * |
| TLSRE, median (Q1, Q3), g | 45.35 (24.84, 67.74) | 290.16 (35.72, 390.25) | 0.136 * |
| Mitotic Count * (n = 37) | Ki-67 (n = 48) | Chromogranin A * (n = 24) | CA 19-9 * (n = 36) | ||
|---|---|---|---|---|---|
| SUVmax | rho | 0.116 | −0.038 | −0.116 | 0.137 |
| p | 0.494 | 0.800 | 0.589 | 0.424 | |
| SUVmean | rho | 0.113 | −0.047 | −0.144 | 0.147 |
| p | 0.504 | 0.749 | 0.503 | 0.393 | |
| SRETV | rho | 0.155 | 0.263 | 0.133 | 0.126 |
| p | 0.361 | 0.071 | 0.537 | 0.463 | |
| TLSRE | rho | 0.166 | 0.199 | 0.094 | 0.217 |
| p | 0.327 | 0.175 | 0.662 | 0.205 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J.; Cha, J.; Lee, H.S.; Chung, M.J.; Park, J.Y.; Bang, S.; Park, S.W.; Song, S.Y.; Cho, A.; Jo, J.H. [68Ga]-DOTATOC PET/CT Volumetric Parameters Reflect Metastatic Potential in Pancreatic Neuroendocrine Tumors. Cancers 2025, 17, 1487. https://doi.org/10.3390/cancers17091487
Kim SJ, Cha J, Lee HS, Chung MJ, Park JY, Bang S, Park SW, Song SY, Cho A, Jo JH. [68Ga]-DOTATOC PET/CT Volumetric Parameters Reflect Metastatic Potential in Pancreatic Neuroendocrine Tumors. Cancers. 2025; 17(9):1487. https://doi.org/10.3390/cancers17091487
Chicago/Turabian StyleKim, So Jeong, Jongtae Cha, Hee Seung Lee, Moon Jae Chung, Jeong Youp Park, Seungmin Bang, Seung Woo Park, Si Young Song, Arthur Cho, and Jung Hyun Jo. 2025. "[68Ga]-DOTATOC PET/CT Volumetric Parameters Reflect Metastatic Potential in Pancreatic Neuroendocrine Tumors" Cancers 17, no. 9: 1487. https://doi.org/10.3390/cancers17091487
APA StyleKim, S. J., Cha, J., Lee, H. S., Chung, M. J., Park, J. Y., Bang, S., Park, S. W., Song, S. Y., Cho, A., & Jo, J. H. (2025). [68Ga]-DOTATOC PET/CT Volumetric Parameters Reflect Metastatic Potential in Pancreatic Neuroendocrine Tumors. Cancers, 17(9), 1487. https://doi.org/10.3390/cancers17091487





