Liquid Biopsy in Peritoneal Carcinomatosis from Colorectal Cancer: Current Evidence and Future Perspectives
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Circulating Tumor DNA: Definitions and Detection Assays
3.1. Pre-Analytical Issues
3.2. Analytical Methods
4. ctDNA in Early-Stage CRC: Detection of Minimal Residual Disease
5. ctDNA in Advanced Disease
5.1. Concordance Rates Between Tissue and Plasma Mutational Landscapes in PC-CRC
| Reference | No. Patients | No. PC-CRC | Study Design | Analytical Method | Samples Analyzed | Key Findings |
|---|---|---|---|---|---|---|
| Vidal Ann Oncol 2017 [87] | 115 | 27 | Retrospective/ Prospectice Observational | dPCR (OncoBEAM™) | Baseline Tissue and Plasma |
|
| Hofste Dis Colon Rectum 2023 [88] | 53 | 6 | Retrospective Observational | NGS (Illumina) | Pre- and post-op plasma |
|
| Baumgartner Ann Surg Oncol 2018 [89] | 80 | 11 | Prospective Observational | NGS (Guardant Health) | Pre-op Plasma |
|
| Kagawa Clin Cancer Res 2021 [90] | 221 | 25 | Retrospective Observational | dPCR (OncoBEAM™) | Baseline Tissue and Plasma |
|
| Sullivan Ann Surg Oncol 2023 [91] | 279 | 115 | Retrospective Observational | NGS (Guardant Health) | Pre-treatment plasma |
|
| Baumgartner Ann Surg Oncol 2020 [92] | 71 | 16 | Prospective Observational | NGS (Guardant Health) | Pre- and post-op plasma |
|
| Dhiman Ann Surg 2023 [93] | 33 | 13 | Retrospective Observational | NGS (Signatera) | Post-op plasma |
|
| Beagan J Clin Med 2020 [94] | 30 | 30 | Retrospective Observational | ddPCR (BioRad) | Surgical tissue; Pre- and post-op plasma |
|
| Loupakis JCO PO 2021 [95] | 112 | 16 | Retrospective Observational | NGS (Signatera) | Post-op plasma |
|
| Lopez-Rojo Ther Adv Med Oncol 2020 [96] | 26 | 8 | Prospective Observational | ddPCR (BioRad) |
Pre- and post-op plasma; Pre- and post-op peritoneal fluid |
|
| Van’t Eve J Pathol Clin Res 2021 [97] | 120 | 20 | Prospective Observational | ddPCR (BioRad) |
Pre-op plasma; pre-op peritoneal fluid |
|
5.2. The Prognostic Role of ctDNA in PC-CRC Patients
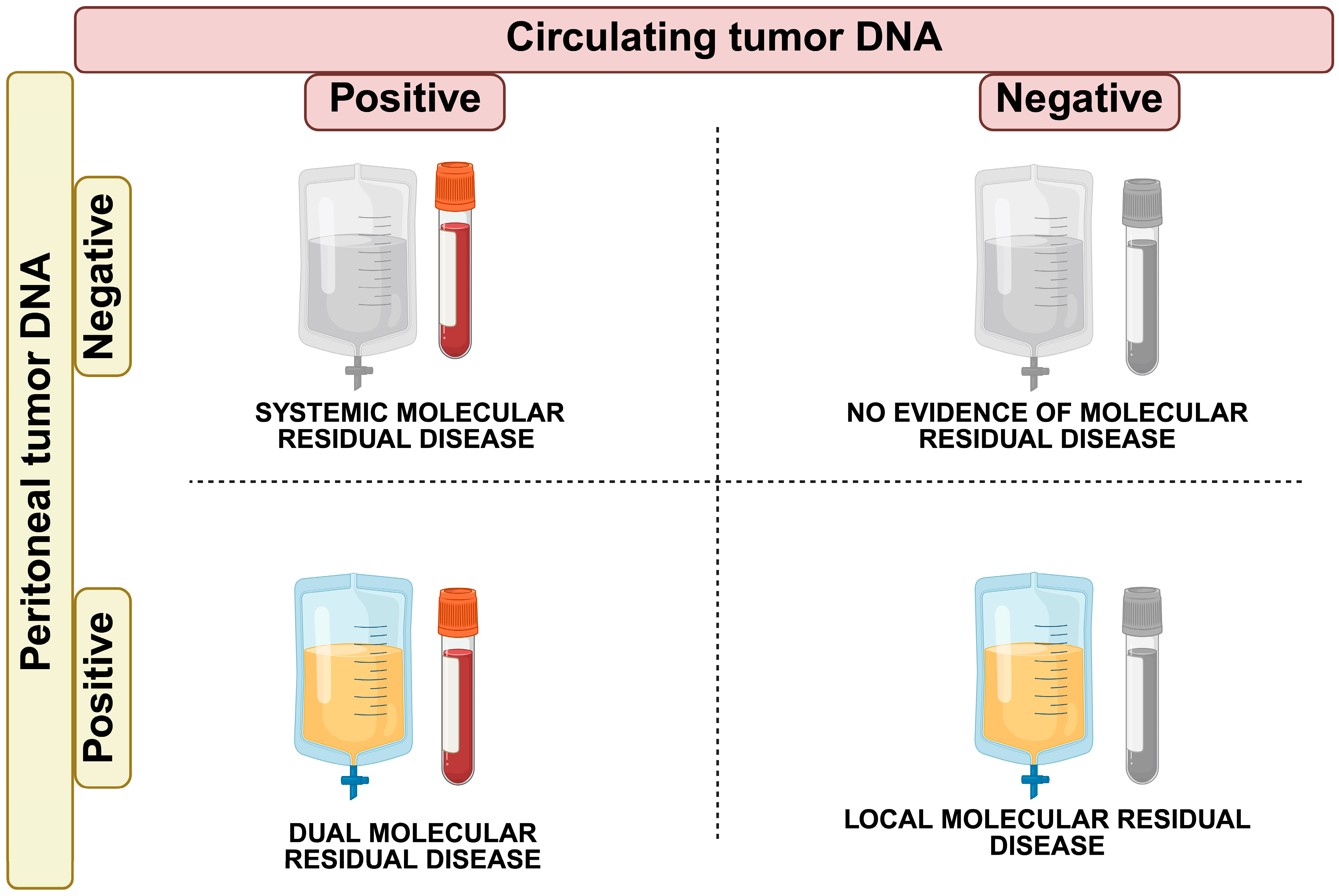
6. Peritoneal Tumor DNA: A Potential Biomarker in the Management of Peritoneal Carcinomatosis
- Treatment Response Monitoring: ptDNA can be used to monitor the efficacy of treatments, such as chemotherapy, targeted therapies, or immunotherapy. By providing real-time tracking of tumor molecular changes, ptDNA enables dynamic assessment of treatment response.
- Recurrence Prediction: ptDNA can be particularly useful for early detection of recurrence, even in patients who do not show clinical signs of progression. Since ptDNA directly reflects genetic alterations in the peritoneal microenvironment, it may be more sensitive than traditional imaging techniques (e.g., CT, MRI) in detecting residual disease. This sensitivity allows ptDNA to predict recurrences before they become clinically visible, enabling timely interventions.
- Personalized Treatment: another key advantage of ptDNA is its integration with ctDNA to provide a personalized approach to therapy. While ctDNA offers global insights into tumor dynamics, ptDNA provides more precise information on peritoneal disease, which is often the primary site of progression in PC-CRC. Combining these two biomarkers allows clinicians to better understand tumor burden and treatment response, leading to more informed therapeutic decisions, such as intensifying therapy for patients with developing recurrences or de-escalating treatment for those with a complete response.
7. Future Perspectives in the Management of Peritoneal Carcinomatosis from Colorectal Cancer
- Systemic Molecular Residual Disease (sMRD+; ctDNA positive, ptDNA negative): CRS + HIPEC successfully controlled peritoneal disease, but micrometastatic disease in the bloodstream may confer a risk of relapse in organs other than the peritoneum (i.e., liver metastases). Adjuvant systemic chemotherapy may be needed to eradicate micro-metastases and achieve complete disease control.
- Locoregional Molecular Residual Disease (lMRD+; ctDNA negative, ptDNA positive): CRS + HIPEC failed in controlling the peritoneal disease. This scenario may call for other localized interventions in the peritoneum, such as second-look surgery or alternative treatments like pressurized intraperitoneal aerosolized chemotherapy (PIPAC) [103].
- Dual Molecular Residual Disease (dMRD+; ctDNA positive, ptDNA positive): in this case, the presence of ctDNA and ptDNA indicates residual disease locally and micro-metastases systemically. A shift to systemic therapies could be necessary to address the minimal residual disease at both loco-regional and systemic levels.
- No Evidence of Molecular Residual Disease (MRD-; ctDNA negative, ptDNA negative): this is the ideal scenario where both ctDNA and ptDNA are undetectable, indicating complete disease clearance both locoregional and systemic. In this case, no further treatment may be needed, and the patient can be monitored through surveillance.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Rosello, S.; Arnold, D.; Normanno, N.; Taieb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Enblad, M.; Graf, W.; Birgisson, H. Risk factors for appendiceal and colorectal peritoneal metastases. Eur. J. Surg. Oncol. 2018, 44, 997–1005. [Google Scholar] [CrossRef]
- Segelman, J.; Granath, F.; Holm, T.; Machado, M.; Mahteme, H.; Martling, A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2012, 99, 699–705. [Google Scholar] [CrossRef]
- Lemmens, V.E.; Klaver, Y.L.; Verwaal, V.J.; Rutten, H.J.; Coebergh, J.W.; de Hingh, I.H. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: A population-based study. Int. J. Cancer 2011, 128, 2717–2725. [Google Scholar] [CrossRef]
- van Gestel, Y.R.; Thomassen, I.; Lemmens, V.E.; Pruijt, J.F.; van Herk-Sukel, M.P.; Rutten, H.J.; Creemers, G.J.; de Hingh, I.H. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur. J. Surg. Oncol. 2014, 40, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Kepenekian, V.; Bhatt, A.; Peron, J.; Alyami, M.; Benzerdjeb, N.; Bakrin, N.; Falandry, C.; Passot, G.; Rousset, P.; Glehen, O. Advances in the management of peritoneal malignancies. Nat. Rev. Clin. Oncol. 2022, 19, 698–718. [Google Scholar] [CrossRef]
- Kusamura, S.; Baratti, D.; Zaffaroni, N.; Villa, R.; Laterza, B.; Balestra, M.R.; Deraco, M. Pathophysiology and biology of peritoneal carcinomatosis. World J. Gastrointest. Oncol. 2010, 2, 12–18. [Google Scholar] [CrossRef]
- Coccolini, F.; Gheza, F.; Lotti, M.; Virzì, S.; Iusco, D.; Ghermandi, C.; Melotti, R.; Baiocchi, G.; Giulini, S.M.; Ansaloni, L.; et al. Peritoneal carcinomatosis. World J. Gastroenterol. 2013, 19, 6979–6994. [Google Scholar] [CrossRef]
- Koh, J.L.; Yan, T.D.; Glenn, D.; Morris, D.L. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann. Surg. Oncol. 2009, 16, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Ahmedzai, S.; Vora, V.; Hillam, S.; Paz, S. Supportive care for patients with gastrointestinal cancer. Cochrane Database Syst. Rev. 2004, 2004, Cd003445. [Google Scholar] [CrossRef]
- Brooks, G.A.; Abrams, T.A.; Meyerhardt, J.A.; Enzinger, P.C.; Sommer, K.; Dalby, C.K.; Uno, H.; Jacobson, J.O.; Fuchs, C.S.; Schrag, D. Identification of potentially avoidable hospitalizations in patients with GI cancer. J. Clin. Oncol. 2014, 32, 496–503. [Google Scholar] [CrossRef]
- Klaver, Y.L.; Lemmens, V.E.; Nienhuijs, S.W.; Luyer, M.D.; de Hingh, I.H. Peritoneal carcinomatosis of colorectal origin: Incidence, prognosis and treatment options. World J. Gastroenterol. 2012, 18, 5489–5494. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Rousset, P.; Benzerdjeb, N.; Kammar, P.; Mehta, S.; Parikh, L.; Goswami, G.; Shaikh, S.; Kepenekian, V.; Passot, G.; et al. Prospective correlation of the radiological, surgical and pathological findings in patients undergoing cytoreductive surgery for colorectal peritoneal metastases: Implications for the preoperative estimation of the peritoneal cancer index. Color. Dis. 2020, 22, 2123–2132. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [CrossRef] [PubMed]
- González-Moreno, S.; Kusamura, S.; Baratti, D.; Deraco, M. Postoperative residual disease evaluation in the locoregional treatment of peritoneal surface malignancy. J. Surg. Oncol. 2008, 98, 237–241. [Google Scholar] [CrossRef]
- Yurttas, C.; Hoffmann, G.; Tolios, A.; Haen, S.P.; Schwab, M.; Königsrainer, I.; Königsrainer, A.; Beckert, S.; Löffler, M.W. Systematic Review of Variations in Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Metastasis from Colorectal Cancer. J. Clin. Med. 2018, 7, 567. [Google Scholar] [CrossRef]
- González-Moreno, S.; González-Bayón, L.A.; Ortega-Pérez, G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J. Gastrointest. Oncol. 2010, 2, 68–75. [Google Scholar] [CrossRef]
- Glehen, O.; Cotte, E.; Kusamura, S.; Deraco, M.; Baratti, D.; Passot, G.; Beaujard, A.C.; Noel, G.F. Hyperthermic intraperitoneal chemotherapy: Nomenclature and modalities of perfusion. J. Surg. Oncol. 2008, 98, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; de Hingh, I.; Van Der Speeten, K.; Hubner, M.; Deraco, M.; Bakrin, N.; Villeneuve, L.; Kusamura, S.; Glehen, O. HIPEC Methodology and Regimens: The Need for an Expert Consensus. Ann. Surg. Oncol. 2021, 28, 9098–9113. [Google Scholar] [CrossRef] [PubMed]
- Piché, N.; Leblond, F.A.; Sidéris, L.; Pichette, V.; Drolet, P.; Fortier, L.P.; Mitchell, A.; Dubé, P. Rationale for heating oxaliplatin for the intraperitoneal treatment of peritoneal carcinomatosis: A study of the effect of heat on intraperitoneal oxaliplatin using a murine model. Ann. Surg. 2011, 254, 138–144. [Google Scholar] [CrossRef]
- Van der Speeten, K.; Lemoine, L.; Sugarbaker, P. Overview of the optimal perioperative intraperitoneal chemotherapy regimens used in current clinical practice. Pleura Peritoneum 2017, 2, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.H.C.; Shannon, N.B.; Chia, C.S.; Soo, K.C.; Teo, M.C.C. Platinum agents and mitomycin C-specific complications in cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Int. J. Hyperth. 2018, 34, 595–600. [Google Scholar] [CrossRef]
- Alyami, M.; Kim, B.J.; Villeneuve, L.; Vaudoyer, D.; Képénékian, V.; Bakrin, N.; Gilly, F.N.; Cotte, E.; Glehen, O.; Passot, G. Ninety-day post-operative morbidity and mortality using the National Cancer Institute’s common terminology criteria for adverse events better describe post-operative outcome after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int. J. Hyperth. 2018, 34, 532–537. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Cashin, P.; Sugarbaker, P.H. Hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal and appendiceal peritoneal metastases: Lessons learned from PRODIGE 7. J. Gastrointest. Oncol. 2021, 12, S120–S128. [Google Scholar] [CrossRef]
- Huang, C.Q.; Min, Y.; Wang, S.Y.; Yang, X.J.; Liu, Y.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: A systematic review and meta-analysis of current evidence. Oncotarget 2017, 8, 55657–55683. [Google Scholar] [CrossRef]
- Lenos, K.J.; Bach, S.; Ferreira Moreno, L.; Ten Hoorn, S.; Sluiter, N.R.; Bootsma, S.; Vieira Braga, F.A.; Nijman, L.E.; van den Bosch, T.; Miedema, D.M.; et al. Molecular characterization of colorectal cancer related peritoneal metastatic disease. Nat. Commun. 2022, 13, 4443. [Google Scholar] [CrossRef]
- Pascual, J.; Attard, G.; Bidard, F.C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef] [PubMed]
- Malla, M.; Loree, J.M.; Kasi, P.M.; Parikh, A.R. Using Circulating Tumor DNA in Colorectal Cancer: Current and Evolving Practices. J. Clin. Oncol. 2022, 40, 2846–2857. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.B.; Gale, R.P. Measurable residual disease in haematological and solid cancers. Leukemia 2024, 38, 1647–1648. [Google Scholar] [CrossRef]
- Mouliere, F.; El Messaoudi, S.; Pang, D.; Dritschilo, A.; Thierry, A.R. Multi-marker analysis of circulating cell-free DNA toward personalized medicine for colorectal cancer. Mol. Oncol. 2014, 8, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Mouliere, F.; Robert, B.; Arnau Peyrotte, E.; Del Rio, M.; Ychou, M.; Molina, F.; Gongora, C.; Thierry, A.R. High fragmentation characterizes tumour-derived circulating DNA. PLoS ONE 2011, 6, e23418. [Google Scholar] [CrossRef]
- Thierry, A.R.; Mouliere, F.; Gongora, C.; Ollier, J.; Robert, B.; Ychou, M.; Del Rio, M.; Molina, F. Origin and quantification of circulating DNA in mice with human colorectal cancer xenografts. Nucleic Acids Res. 2010, 38, 6159–6175. [Google Scholar] [CrossRef]
- El Messaoudi, S.; Mouliere, F.; Du Manoir, S.; Bascoul-Mollevi, C.; Gillet, B.; Nouaille, M.; Fiess, C.; Crapez, E.; Bibeau, F.; Theillet, C.; et al. Circulating DNA as a Strong Multimarker Prognostic Tool for Metastatic Colorectal Cancer Patient Management Care. Clin. Cancer Res. 2016, 22, 3067–3077. [Google Scholar] [CrossRef]
- Lo, Y.M.; Chan, K.C.; Sun, H.; Chen, E.Z.; Jiang, P.; Lun, F.M.; Zheng, Y.W.; Leung, T.Y.; Lau, T.K.; Cantor, C.R.; et al. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci. Transl. Med. 2010, 2, 61ra91. [Google Scholar] [CrossRef]
- Pittella-Silva, F.; Chin, Y.M.; Chan, H.T.; Nagayama, S.; Miyauchi, E.; Low, S.K.; Nakamura, Y. Plasma or Serum: Which Is Preferable for Mutation Detection in Liquid Biopsy? Clin. Chem. 2020, 66, 946–957. [Google Scholar] [CrossRef]
- Mattox, A.K.; Douville, C.; Wang, Y.; Popoli, M.; Ptak, J.; Silliman, N.; Dobbyn, L.; Schaefer, J.; Lu, S.; Pearlman, A.H.; et al. The Origin of Highly Elevated Cell-Free DNA in Healthy Individuals and Patients with Pancreatic, Colorectal, Lung, or Ovarian Cancer. Cancer Discov. 2023, 13, 2166–2179. [Google Scholar] [CrossRef]
- Underhill, H.R.; Kitzman, J.O.; Hellwig, S.; Welker, N.C.; Daza, R.; Baker, D.N.; Gligorich, K.M.; Rostomily, R.C.; Bronner, M.P.; Shendure, J. Fragment Length of Circulating Tumor DNA. PLoS Genet. 2016, 12, e1006162. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Mei, C.; Nan, X.; Hui, L. Evaluation and comparison of in vitro degradation kinetics of DNA in serum, urine and saliva: A qualitative study. Gene 2016, 590, 142–148. [Google Scholar] [CrossRef]
- To, E.W.; Chan, K.C.; Leung, S.F.; Chan, L.Y.; To, K.F.; Chan, A.T.; Johnson, P.J.; Lo, Y.M. Rapid clearance of plasma Epstein-Barr virus DNA after surgical treatment of nasopharyngeal carcinoma. Clin. Cancer Res. 2003, 9, 3254–3259. [Google Scholar] [PubMed]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Soueidy, C.; Zaanan, A.; Gelli, M.; Moati, E.; Gallois, C.; Taly, V.; Laurent-Puig, P.; Benhaim, L.; Taieb, J. Clinical impact of circulating tumor DNA to track minimal residual disease in colorectal cancer patients. Hopes and limitations. ESMO Gastrointest. Oncol. 2024, 4, 100068. [Google Scholar] [CrossRef]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Morris, V.K.; Allegra, C.J.; Atreya, C.; Benson, A.B., III; Boland, P.; Chung, K.; Copur, M.S.; Corcoran, R.B.; Deming, D.A.; et al. ctDNA applications and integration in colorectal cancer: An NCI Colon and Rectal-Anal Task Forces whitepaper. Nat. Rev. Clin. Oncol. 2020, 17, 757–770. [Google Scholar] [CrossRef]
- Meddeb, R.; Pisareva, E.; Thierry, A.R. Guidelines for the Preanalytical Conditions for Analyzing Circulating Cell-Free DNA. Clin. Chem. 2019, 65, 623–633. [Google Scholar] [CrossRef]
- Merker, J.D.; Oxnard, G.R.; Compton, C.; Diehn, M.; Hurley, P.; Lazar, A.J.; Lindeman, N.; Lockwood, C.M.; Rai, A.J.; Schilsky, R.L.; et al. Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J. Clin. Oncol. 2018, 36, 1631–1641. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Chen, P.; Li, S.; Luo, J.; Xia, H. Performance comparison of blood collection tubes as liquid biopsy storage system for minimizing cfDNA contamination from genomic DNA. J. Clin. Lab. Anal. 2019, 33, e22670. [Google Scholar] [CrossRef]
- Greytak, S.R.; Engel, K.B.; Parpart-Li, S.; Murtaza, M.; Bronkhorst, A.J.; Pertile, M.D.; Moore, H.M. Harmonizing Cell-Free DNA Collection and Processing Practices through Evidence-Based Guidance. Clin. Cancer Res. 2020, 26, 3104–3109. [Google Scholar] [CrossRef] [PubMed]
- Bellosillo, B.; Montagut, C. High-accuracy liquid biopsies. Nat. Med. 2019, 25, 1820–1821. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.T.; Nagayama, S.; Chin, Y.M.; Otaki, M.; Hayashi, R.; Kiyotani, K.; Fukunaga, Y.; Ueno, M.; Nakamura, Y.; Low, S.K. Clinical significance of clonal hematopoiesis in the interpretation of blood liquid biopsy. Mol. Oncol. 2020, 14, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Razavi, P.; Li, B.T.; Brown, D.N.; Jung, B.; Hubbell, E.; Shen, R.; Abida, W.; Juluru, K.; De Bruijn, I.; Hou, C.; et al. High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat. Med. 2019, 25, 1928–1937. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef]
- Postel, M.; Roosen, A.; Laurent-Puig, P.; Taly, V.; Wang-Renault, S.F. Droplet-based digital PCR and next generation sequencing for monitoring circulating tumor DNA: A cancer diagnostic perspective. Expert Rev. Mol. Diagn. 2018, 18, 7–17. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Bardelli, A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef]
- Cheng, D.T.; Mitchell, T.N.; Zehir, A.; Shah, R.H.; Benayed, R.; Syed, A.; Chandramohan, R.; Liu, Z.Y.; Won, H.H.; Scott, S.N.; et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J. Mol. Diagn. 2015, 17, 251–264. [Google Scholar] [CrossRef]
- Kinde, I.; Wu, J.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B. Detection and quantification of rare mutations with massively parallel sequencing. Proc. Natl. Acad. Sci. USA 2011, 108, 9530–9535. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabieres, C. Minimal residual disease as a target for liquid biopsy in patients with solid tumours. Nat. Rev. Clin. Oncol. 2025, 22, 65–77. [Google Scholar] [CrossRef]
- Wan, J.C.M.; Mughal, T.I.; Razavi, P.; Dawson, S.-J.; Moss, E.L.; Govindan, R.; Tan, I.B.; Yap, Y.-S.; Robinson, W.A.; Morris, C.D.; et al. Liquid biopsies for residual disease and recurrence. Med 2021, 2, 1292–1313. [Google Scholar] [CrossRef]
- Parikh, A.R.; Van Seventer, E.E.; Siravegna, G.; Hartwig, A.V.; Jaimovich, A.; He, Y.; Kanter, K.; Fish, M.G.; Fosbenner, K.D.; Miao, B.; et al. Minimal Residual Disease Detection using a Plasma-only Circulating Tumor DNA Assay in Patients with Colorectal Cancer. Clin. Cancer Res. 2021, 27, 5586–5594. [Google Scholar] [CrossRef]
- Cescon, D.W.; Bratman, S.V.; Chan, S.M.; Siu, L.L. Circulating tumor DNA and liquid biopsy in oncology. Nat. Cancer 2020, 1, 276–290. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Lo, S.N.; Wang, Y.; Li, L.; Christie, M.; Lee, M.; Wong, R.; Kosmider, S.; Skinner, I.; et al. Prognostic significance of postsurgery circulating tumor DNA in nonmetastatic colorectal cancer: Individual patient pooled analysis of three cohort studies. Int. J. Cancer 2021, 148, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, N.; Gimeno-Valiente, F.; Gambardella, V.; Zuñiga, S.; Rentero-Garrido, P.; Huerta, M.; Roselló, S.; Martinez-Ciarpaglini, C.; Carbonell-Asins, J.A.; Carrasco, F.; et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann. Oncol. 2019, 30, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Wang, Y.; Tomasetti, C.; Li, L.; Springer, S.; Kinde, I.; Silliman, N.; Tacey, M.; Wong, H.L.; Christie, M.; et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016, 8, 346ra392. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Cohen, J.D.; Wang, Y.; Christie, M.; Simons, K.; Lee, M.; Wong, R.; Kosmider, S.; Ananda, S.; McKendrick, J.; et al. Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer. JAMA Oncol. 2019, 5, 1710–1717. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Lo, S.N.; Lahouel, K.; Cohen, J.D.; Wong, R.; Shapiro, J.D.; Harris, S.J.; Khattak, A.; Burge, M.E.; et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer: Overall survival and updated 5-year results from the randomized DYNAMIC trial. J. Clin. Oncol. 2024, 42, 108. [Google Scholar] [CrossRef]
- Montagut, C.; Vidal, J. Liquid Biopsy for Precision Adjuvant Chemotherapy in Colon Cancer. N. Engl. J. Med. 2022, 386, 2330–2331. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.; Lahouel, K.; Lo, S.N.; Wang, Y.; Wong, R.; Shapiro, J.; Harris, S.; Khattak, A.; Burge, M.; et al. 318MO Circulating tumour DNA (ctDNA) dynamics, CEA and sites of recurrence for the randomised DYNAMIC study: Adjuvant chemotherapy (ACT) guided by ctDNA analysis in stage II colon cancer (CC). Ann. Oncol. 2022, 33, S683. [Google Scholar] [CrossRef]
- Lonardi, S.; Pietrantonio, F.; Tarazona Llavero, N.; Montagut Viladot, C.; Sartore Bianchi, A.; Zampino, M.G.; Elez Fernandez, M.E.; Santos Vivas, C.; Mandalà, M.; Tamberi, S.; et al. LBA28 The PEGASUS trial: Post-surgical liquid biopsy-guided treatment of stage III and high-risk stage II colon cancer patients. Ann. Oncol. 2023, 34, S1268–S1269. [Google Scholar] [CrossRef]
- Kotani, D.; Oki, E.; Nakamura, Y.; Yukami, H.; Mishima, S.; Bando, H.; Shirasu, H.; Yamazaki, K.; Watanabe, J.; Kotaka, M.; et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat. Med. 2023, 29, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Watanabe, J.; Akazawa, N.; Hirata, K.; Kataoka, K.; Yokota, M.; Kato, K.; Kotaka, M.; Kagawa, Y.; Yeh, K.H.; et al. ctDNA-based molecular residual disease and survival in resectable colorectal cancer. Nat. Med. 2024, 30, 3272–3283. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, T.V.; Demuth, C.; Frydendahl, A.; Nors, J.; Nesic, M.; Rasmussen, M.H.; Reinert, T.; Larsen, O.H.; Jaensch, C.; Love, U.S.; et al. Unraveling the potential clinical utility of circulating tumor DNA detection in colorectal cancer-evaluation in a nationwide Danish cohort. Ann. Oncol. 2024, 35, 229–239. [Google Scholar] [CrossRef]
- Kasi, P.M.; Aushev, V.N.; Ensor, J.; Langer, N.; Wang, C.G.; Cannon, T.L.; Berim, L.D.; Feinstein, T.; Grothey, A.; McCollom, J.W.; et al. Circulating tumor DNA (ctDNA) for informing adjuvant chemotherapy (ACT) in stage II/III colorectal cancer (CRC): Interim analysis of BESPOKE CRC study. J. Clin. Oncol. 2024, 42, 9. [Google Scholar] [CrossRef]
- Shah, P.K.; Aushev, V.N.; Ensor, J.; Sanchez, S.A.; Wang, C.G.; Cannon, T.L.; Berim, L.D.; Feinstein, T.; Grothey, A.; McCollom, J.W.; et al. Circulating tumor DNA for detection of molecular residual disease (MRD) in patients (pts) with stage II/III colorectal cancer (CRC): Final analysis of the BESPOKE CRC sub-cohort. J. Clin. Oncol. 2025, 43, 15. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Peritoneal-plasma barrier. Cancer Treat. Res. 1996, 82, 53–63. [Google Scholar] [CrossRef]
- Alese, O.B.; Cook, N.; Ortega-Franco, A.; Ulanja, M.B.; Tan, L.; Tie, J. Circulating Tumor DNA: An Emerging Tool in Gastrointestinal Cancers. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 1–20. [Google Scholar] [CrossRef]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B.; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 678–700. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Pietrantonio, F.; Lonardi, S.; Mussolin, B.; Rua, F.; Crisafulli, G.; Bartolini, A.; Fenocchio, E.; Amatu, A.; Manca, P.; et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: The phase 2 CHRONOS trial. Nat. Med. 2022, 28, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.A.; Huang, H.J.; Piha-Paul, S.A.; Call, S.G.; Karp, D.D.; Fu, S.; Naing, A.; Subbiah, V.; Pant, S.; Dustin, D.J.; et al. Longitudinal Monitoring of Circulating Tumor DNA to Predict Treatment Outcomes in Advanced Cancers. JCO Precis. Oncol. 2022, 6, e2100512. [Google Scholar] [CrossRef]
- Cortes-Guiral, D.; Hubner, M.; Alyami, M.; Bhatt, A.; Ceelen, W.; Glehen, O.; Lordick, F.; Ramsay, R.; Sgarbura, O.; Van Der Speeten, K.; et al. Primary and metastatic peritoneal surface malignancies. Nat. Rev. Dis. Primers 2021, 7, 91. [Google Scholar] [CrossRef]
- Tivey, A.; Church, M.; Rothwell, D.; Dive, C.; Cook, N. Circulating tumour DNA—Looking beyond the blood. Nat. Rev. Clin. Oncol. 2022, 19, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef]
- McMullen, J.R.W.; Selleck, M.; Wall, N.R.; Senthil, M. Peritoneal carcinomatosis: Limits of diagnosis and the case for liquid biopsy. Oncotarget 2017, 8, 43481–43490. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.; Muinelo, L.; Dalmases, A.; Jones, F.; Edelstein, D.; Iglesias, M.; Orrillo, M.; Abalo, A.; Rodriguez, C.; Brozos, E.; et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann. Oncol. 2017, 28, 1325–1332. [Google Scholar] [CrossRef]
- Hofste, L.S.M.; Geerlings, M.J.; Kamping, E.J.; Kouwenhoven, N.D.H.; von Rhein, D.; Jansen, E.A.M.; Garms, L.M.; Nagtegaal, I.D.; van der Post, R.S.; de Wilt, J.H.W.; et al. Clinical Validity of Tumor-Informed Circulating Tumor DNA Analysis in Patients Undergoing Surgery of Colorectal Metastases. Dis. Colon Rectum 2023, 66, 796–804. [Google Scholar] [CrossRef]
- Baumgartner, J.M.; Raymond, V.M.; Lanman, R.B.; Tran, L.; Kelly, K.J.; Lowy, A.M.; Kurzrock, R. Preoperative Circulating Tumor DNA in Patients with Peritoneal Carcinomatosis is an Independent Predictor of Progression-Free Survival. Ann. Surg. Oncol. 2018, 25, 2400–2408. [Google Scholar] [CrossRef]
- Kagawa, Y.; Elez, E.; Garcia-Foncillas, J.; Bando, H.; Taniguchi, H.; Vivancos, A.; Akagi, K.; Garcia, A.; Denda, T.; Ros, J.; et al. Combined Analysis of Concordance between Liquid and Tumor Tissue Biopsies for RAS Mutations in Colorectal Cancer with a Single Metastasis Site: The METABEAM Study. Clin. Cancer Res. 2021, 27, 2515–2522. [Google Scholar] [CrossRef]
- Sullivan, B.G.; Lo, A.; Yu, J.; Gonda, A.; Dehkordi-Vakil, F.; Dayyani, F.; Senthil, M. Circulating Tumor DNA is Unreliable to Detect Somatic Gene Alterations in Gastrointestinal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2023, 30, 278–284. [Google Scholar] [CrossRef]
- Baumgartner, J.M.; Riviere, P.; Lanman, R.B.; Kelly, K.J.; Veerapong, J.; Lowy, A.M.; Kurzrock, R. Prognostic Utility of Pre- and Postoperative Circulating Tumor DNA Liquid Biopsies in Patients with Peritoneal Metastases. Ann. Surg. Oncol. 2020, 27, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, A.; Kothary, V.; Witmer, H.D.D.; Bregio, C.; Sood, D.; Ong, C.T.; Polite, B.; Eng, O.S.; Shergill, A.; Turaga, K.K. Role of Tumor-informed Personalized Circulating Tumor DNA Assay in Informing Recurrence in Patients With Peritoneal Metastases From Colorectal and High-grade Appendix Cancer Undergoing Curative-intent Surgery. Ann. Surg. 2023, 278, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Beagan, J.J.; Sluiter, N.R.; Bach, S.; Eijk, P.P.; Vlek, S.L.; Heideman, D.A.M.; Kusters, M.; Pegtel, D.M.; Kazemier, G.; van Grieken, N.C.T.; et al. Circulating Tumor DNA as a Preoperative Marker of Recurrence in Patients with Peritoneal Metastases of Colorectal Cancer: A Clinical Feasibility Study. J. Clin. Med. 2020, 9, 1738. [Google Scholar] [CrossRef]
- Loupakis, F.; Sharma, S.; Derouazi, M.; Murgioni, S.; Biason, P.; Rizzato, M.D.; Rasola, C.; Renner, D.; Shchegrova, S.; Koyen Malashevich, A.; et al. Detection of Molecular Residual Disease Using Personalized Circulating Tumor DNA Assay in Patients with Colorectal Cancer Undergoing Resection of Metastases. JCO Precis. Oncol. 2021, 5, PO.21.00101. [Google Scholar] [CrossRef]
- Lopez-Rojo, I.; Olmedillas-Lopez, S.; Villarejo Campos, P.; Dominguez Prieto, V.; Barambio Buendia, J.; Cortes Guiral, D.; Garcia-Arranz, M.; Garcia-Olmo, D. Liquid biopsy in peritoneal fluid and plasma as a prognostic factor in advanced colorectal and appendiceal tumors after complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ther. Adv. Med. Oncol. 2020, 12, 1758835920981351. [Google Scholar] [CrossRef]
- Van’t Erve, I.; Rovers, K.P.; Constantinides, A.; Bolhuis, K.; Wassenaar, E.C.; Lurvink, R.J.; Huysentruyt, C.J.; Snaebjornsson, P.; Boerma, D.; van den Broek, D.; et al. Detection of tumor-derived cell-free DNA from colorectal cancer peritoneal metastases in plasma and peritoneal fluid. J. Pathol. Clin. Res. 2021, 7, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Husain, H.; Nykin, D.; Bui, N.; Quan, D.; Gomez, G.; Woodward, B.; Venkatapathy, S.; Duttagupta, R.; Fung, E.; Lippman, S.M.; et al. Cell-Free DNA from Ascites and Pleural Effusions: Molecular Insights into Genomic Aberrations and Disease Biology. Mol. Cancer Ther. 2017, 16, 948–955. [Google Scholar] [CrossRef]
- Hasovits, C.; Clarke, S. Pharmacokinetics and pharmacodynamics of intraperitoneal cancer chemotherapeutics. Clin. Pharmacokinet. 2012, 51, 203–224. [Google Scholar] [CrossRef]
- Cercek, A.; Cusack, J.C.; Ryan, D.P. Treatment of Peritoneal Carcinomatosis of Colorectal Origin; American Society of Clinical Oncology: Alexandria, VA, USA, 2015; pp. e208–e211. [Google Scholar] [CrossRef]
- Baraibar, I.; Ros, J.; Mulet, N.; Salva, F.; Argiles, G.; Martini, G.; Cuadra, J.L.; Sardo, E.; Ciardiello, D.; Tabernero, J.; et al. Incorporating traditional and emerging biomarkers in the clinical management of metastatic colorectal cancer: An update. Expert Rev. Mol. Diagn. 2020, 20, 653–664. [Google Scholar] [CrossRef]
- Baumgartner, J.M.; Botta, G.P. Role of Circulating Tumor DNA Among Patients with Colorectal Peritoneal Metastases. J. Gastrointest. Cancer 2024, 55, 41–46. [Google Scholar] [CrossRef]
- Kurtz, F.; Struller, F.; Horvath, P.; Solass, W.; Bösmüller, H.; Königsrainer, A.; Reymond, M.A. Feasibility, Safety, and Efficacy of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Peritoneal Metastasis: A Registry Study. Gastroenterol. Res. Pract. 2018, 2018, 2743985. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Kahle, M.; Vo, H.H.; Baysal, M.A.; Johnson, A.; Meric-Bernstam, F. Molecular tumour boards—Current and future considerations for precision oncology. Nat. Rev. Clin. Oncol. 2023, 20, 843–863. [Google Scholar] [CrossRef]
- Westphalen, C.B.; Boscolo Bielo, L.; Aftimos, P.; Beltran, H.; Benary, M.; Chakravarty, D.; Collienne, M.; Dienstmann, R.; El Helali, A.; Gainor, J.; et al. ESMO Precision Oncology Working Group recommendations on the structure and quality indicators for molecular tumour boards in clinical practice. Ann. Oncol. 2025. [Google Scholar] [CrossRef]
- Conca, V.; Ciraci, P.; Boccaccio, C.; Minelli, A.; Antoniotti, C.; Cremolini, C. Waiting for the “liquid revolution” in the adjuvant treatment of colon cancer patients: A review of ongoing trials. Cancer Treat. Rev. 2024, 126, 102735. [Google Scholar] [CrossRef]

| Reference | Stage | Median Follow-Up (Months) | Countries | Study Design | Analytical Method | No. Patients | No. Peritoneal Relapses | Key Findings on Peritoneal-Only Recurrence |
|---|---|---|---|---|---|---|---|---|
| Lonardi ESMO Congress 2023 [72] | II (high risk)–III | 21.7 | Spain, Italy | Non-randomized interventional | NGS (Guardant Health) | 135 | 2 |
|
| Nakamura Nat Med 2024 [74] | II–III–IV | 23 | Japan | Prospective observational | NGS (Guardant Health) | 2.240 | 115 |
|
| Henriksen Ann Oncol 2024 [75] | II–III | 26 | Denmark | Prospective observational | dPCR (BioRad) | 851 | 10 |
|
| Shah ASCO Gastrointestinal Cancers Symposium 2025 [77] | II–III | 23.2 | USA | Prospective observational | NGS (Signatera) | 1.166 | 20 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martelli, V.; Vidal, J.; Salvans, S.; Fernández, C.; Badia-Ramentol, J.; Linares, J.; Jiménez, M.; Sibilio, A.; Gibert, J.; Pérez, M.; et al. Liquid Biopsy in Peritoneal Carcinomatosis from Colorectal Cancer: Current Evidence and Future Perspectives. Cancers 2025, 17, 1461. https://doi.org/10.3390/cancers17091461
Martelli V, Vidal J, Salvans S, Fernández C, Badia-Ramentol J, Linares J, Jiménez M, Sibilio A, Gibert J, Pérez M, et al. Liquid Biopsy in Peritoneal Carcinomatosis from Colorectal Cancer: Current Evidence and Future Perspectives. Cancers. 2025; 17(9):1461. https://doi.org/10.3390/cancers17091461
Chicago/Turabian StyleMartelli, Valentino, Joana Vidal, Sílvia Salvans, Concepción Fernández, Jordi Badia-Ramentol, Jenniffer Linares, Marta Jiménez, Annarita Sibilio, Joan Gibert, Marina Pérez, and et al. 2025. "Liquid Biopsy in Peritoneal Carcinomatosis from Colorectal Cancer: Current Evidence and Future Perspectives" Cancers 17, no. 9: 1461. https://doi.org/10.3390/cancers17091461
APA StyleMartelli, V., Vidal, J., Salvans, S., Fernández, C., Badia-Ramentol, J., Linares, J., Jiménez, M., Sibilio, A., Gibert, J., Pérez, M., Bellosillo, B., Calon, A., Pietrantonio, F., Iglesias, M., Pascual, M., & Montagut, C. (2025). Liquid Biopsy in Peritoneal Carcinomatosis from Colorectal Cancer: Current Evidence and Future Perspectives. Cancers, 17(9), 1461. https://doi.org/10.3390/cancers17091461







