Liquid Biopsy as a New Tool for Diagnosis and Monitoring in Renal Cell Carcinoma
Simple Summary
Abstract
1. Introduction
2. MicroRNA (miRNAs)
3. Extracellular Vesicles and Exosomes
4. Circulating Tumor Cells
5. Circulating Tumor DNA
6. Other Potential Protein, Metabolic, and Molecular Biomarkers for Diagnosis and Monitoring of Renal Cell Carcinoma
7. Microbiota in Renal Cell Carcinoma
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ccRCC | clear cell Renal Cell Carcinoma |
| cfDNA | circulating free DNA |
| CTCs | Circulating Tumor Cells |
| ctDNA | cell-free tumor DNA |
| EV | Extracellular Vesicles |
| FISH | Fluorescence In Situ Hybridization |
| miRNAs | microRNAs |
| RCC | Renal Cell Carcinoma |
| SRMs | Small Renal Masses |
| VHL | Von Hippel Lindau |
| WBC | White Blood Cells |
| WHO | World Health Organization |
References
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal Cell Carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Zieren, R.C.; Zondervan, P.J.; Pienta, K.J.; Bex, A.; de Reijke, T.M.; Bins, A.D. Diagnostic Liquid Biopsy Biomarkers in Renal Cell Cancer. Nat. Rev. Urol. 2023, 21, 133–157. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef]
- Ferro, M.; Crocetto, F.; Barone, B.; Del Giudice, F.; Maggi, M.; Lucarelli, G.; Busetto, G.M.; Autorino, R.; Marchioni, M.; Cantiello, F.; et al. Artificial Intelligence and Radiomics in Evaluation of Kidney Lesions: A Comprehensive Literature Review. Ther. Adv. Urol. 2023, 15, 17562872231164804. [Google Scholar] [CrossRef]
- Tataru, O.S.; Marchioni, M.; Crocetto, F.; Barone, B.; Lucarelli, G.; Del Giudice, F.; Busetto, G.M.; Veccia, A.; Lo Giudice, A.; Russo, G.I.; et al. Molecular Imaging Diagnosis of Renal Cancer Using (99m)Tc-Sestamibi SPECT/CT and Girentuximab PET-CT-Current Evidence and Future Development of Novel Techniques. Diagnostics 2023, 13, 593. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Zheng, J.; Li, Z.; Li, S.; Wang, K.; Chen, X. Liquid Biopsy at the Frontier in Renal Cell Carcinoma: Recent Analysis of Techniques and Clinical Application. Mol. Cancer 2023, 22, 37. [Google Scholar] [CrossRef]
- Spadaccino, F.; Netti, G.S.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Ranieri, E. Diagnostic and Prognostic Markers of Renal Cell Carcinoma. G. Ital. Nefrol. 2020, 37, 2020-vol2. [Google Scholar]
- Gigante, M.; Lucarelli, G.; Divella, C.; Netti, G.S.; Pontrelli, P.; Cafiero, C.; Grandaliano, G.; Castellano, G.; Rutigliano, M.; Stallone, G.; et al. Soluble Serum Aklotho Is a Potential Predictive Marker of Disease Progression in Clear Cell Renal Cell Carcinoma. Medicine 2015, 94, e1917. [Google Scholar] [CrossRef]
- Netti, G.S.G.S.; Lucarelli, G.; Spadaccino, F.; Castellano, G.; Gigante, M.; Divella, C.; Rocchetti, M.T.M.T.; Rascio, F.; Mancini, V.; Stallone, G.; et al. PTX3 Modulates the Immunoflogosis in Tumor Microenvironment and Is a Prognostic Factor for Patients with Clear Cell Renal Cell Carcinoma. Aging 2020, 12, 7585–7602. [Google Scholar] [CrossRef]
- Cimadamore, A.; Gasparrini, S.; Massari, F.; Santoni, M.; Cheng, L.; Lopez-Beltran, A.; Scarpelli, M.; Montironi, R. Emerging Molecular Technologies in Renal Cell Carcinoma: Liquid Biopsy. Cancers 2019, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Barone, B.; Ferro, M.; Busetto, G.M.; La Civita, E.; Buonerba, C.; Di Lorenzo, G.; Terracciano, D.; Schalken, J.A. Liquid Biopsy in Bladder Cancer: State of the Art and Future Perspectives. Crit. Rev. Oncol. Hematol. 2022, 170, 103577. [Google Scholar] [CrossRef] [PubMed]
- Muljo, S.A.; Kanellopoulou, C.; Aravind, L. MicroRNA Targeting in Mammalian Genomes: Genes and Mechanisms. WIREs Syst. Biol. Med. 2009, 2, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Stavast, C.; Erkeland, S. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells 2019, 8, 1465. [Google Scholar] [CrossRef]
- Spadaccino, F.; Gigante, M.; Netti, G.S.; Rocchetti, M.T.; Franzin, R.; Gesualdo, L.; Castellano, G.; Stallone, G.; Ranieri, E. The Ambivalent Role of MiRNAs in Carcinogenesis: Involvement in Renal Cell Carcinoma and Their Clinical Applications. Pharmaceuticals 2021, 14, 322. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of MRNA Translation and Stability by MicroRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Cardinali, B.; Tasso, R.; Piccioli, P.; Ciferri, M.C.; Quarto, R.; Del Mastro, L. Circulating MiRNAs in Breast Cancer Diagnosis and Prognosis. Cancers 2022, 14, 2317. [Google Scholar] [CrossRef]
- Wani, J.A.; Majid, S.; Imtiyaz, Z.; Rehman, M.U.; Alsaffar, R.M.; Shah, N.N.; Alshehri, S.; Ghoneim, M.M.; Imam, S.S. MiRNAs in Lung Cancer: Diagnostic, Prognostic, and Therapeutic Potential. Diagnostics 2022, 12, 1610. [Google Scholar] [CrossRef]
- Mehrgou, A.; Ebadollahi, S.; Seidi, K.; Ayoubi-Joshaghani, M.H.; Ahmadieh Yazdi, A.; Zare, P.; Jaymand, M.; Jahanban-Esfahlan, R. Roles of MiRNAs in Colorectal Cancer: Therapeutic Implications and Clinical Opportunities. Adv. Pharm. Bull. 2021, 11, 233–247. [Google Scholar] [CrossRef]
- Gujrati, H.; Ha, S.; Wang, B.-D. Deregulated MicroRNAs Involved in Prostate Cancer Aggressiveness and Treatment Resistance Mechanisms. Cancers 2023, 15, 3140. [Google Scholar] [CrossRef]
- Kartikasari, A.E.R.; Michel-Lara, P.; Exton, H.; Tekin-Sari, K.; Alnefai, E.M.M.; Mitchell, A.; Sanchez-Huertas, C.; Plebanski, M. Circulating MicroRNAs as Diagnostic Biomarkers to Detect Specific Stages of Ovarian Cancer: A Comprehensive Meta-Analysis. Cancers 2024, 16, 4190. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ha, S.E.; Yu, T.Y.; Ro, S. Dual Roles of MiR-10a-5p and MiR-10b-5p as Tumor Suppressors and Oncogenes in Diverse Cancers. Int. J. Mol. Sci. 2025, 26, 415. [Google Scholar] [CrossRef] [PubMed]
- Voropaeva, E.N.; Orlov, Y.L.; Loginova, A.B.; Seregina, O.B.; Maksimov, V.N.; Pospelova, T.I. Deregulation Mechanisms and Therapeutic Opportunities of P53-Responsive MicroRNAs in Diffuse Large B-Cell Lymphoma. PeerJ 2025, 13, e18661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, B.; Chen, J.; Li, T. Role of Exosomal MiRNAs and Macrophage Polarization in Gastric Cancer: A Novel Therapeutic Strategy. Eur. J. Pharmacol. 2025, 177268. [Google Scholar] [CrossRef]
- Dilsiz, N. Role of Exosomes and Exosomal MicroRNAs in Cancer. Future Sci. OA 2020, 6, FSO465. [Google Scholar] [CrossRef]
- Xiao, C.-T.; Lai, W.-J.; Zhu, W.-A.; Wang, H. MicroRNA Derived from Circulating Exosomes as Noninvasive Biomarkers for Diagnosing Renal Cell Carcinoma. Onco Targets Ther. 2020, 13, 10765–10774. [Google Scholar] [CrossRef]
- Langevin, S.M.; Kuhnell, D.; Orr-Asman, M.A.; Biesiada, J.; Zhang, X.; Medvedovic, M.; Thomas, H.E. Balancing Yield, Purity and Practicality: A Modified Differential Ultracentrifugation Protocol for Efficient Isolation of Small Extracellular Vesicles from Human Serum. RNA Biol. 2019, 16, 5–12. [Google Scholar] [CrossRef]
- Gowrishankar, B.; Ibragimova, I.; Zhou, Y.; Slifker, M.J.; Devarajan, K.; Al-Saleem, T.; Uzzo, R.G.; Cairns, P. MicroRNA Expression Signatures of Stage, Grade, and Progression in Clear Cell RCC. Cancer Biol. Ther. 2014, 15, 329–341. [Google Scholar] [CrossRef]
- Outeiro-Pinho, G.; Barros-Silva, D.; Aznar, E.; Sousa, A.-I.; Vieira-Coimbra, M.; Oliveira, J.; Gonçalves, C.S.; Costa, B.M.; Junker, K.; Henrique, R.; et al. MicroRNA-30a-5pme: A Novel Diagnostic and Prognostic Biomarker for Clear Cell Renal Cell Carcinoma in Tissue and Urine Samples. J. Exp. Clin. Cancer Res. 2020, 39, 98. [Google Scholar] [CrossRef]
- Cochetti, G.; Cari, L.; Nocentini, G.; Maulà, V.; Suvieri, C.; Cagnani, R.; Rossi De Vermandois, J.A.; Mearini, E. Detection of Urinary MiRNAs for Diagnosis of Clear Cell Renal Cell Carcinoma. Sci. Rep. 2020, 10, 21290. [Google Scholar] [CrossRef]
- Lu, L.; Li, Y.; Wen, H.; Feng, C. Overexpression of MiR-15b Promotes Resistance to Sunitinib in Renal Cell Carcinoma. J. Cancer 2019, 10, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, C.; Ellinger, J.; Kristiansen, G.; Hatzichristodoulou, G.; Kübler, H.; Kneitz, B.; Busch, J.; Fendler, A. Identification of MiR-21-5p and MiR-210-3p Serum Levels as Biomarkers for Patients with Papillary Renal Cell Carcinoma: A Multicenter Analysis. Transl. Androl. Urol. 2020, 9, 1314–1322. [Google Scholar] [CrossRef]
- Huang, G.; Li, X.; Chen, Z.; Wang, J.; Zhang, C.; Chen, X.; Peng, X.; Liu, K.; Zhao, L.; Lai, Y.; et al. A Three-MicroRNA Panel in Serum: Serving as a Potential Diagnostic Biomarker for Renal Cell Carcinoma. Pathol. Oncol. Res. 2020, 26, 2425–2434. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, L.; Wang, G.; Xiao, Y.; Ju, L.; Wang, X. Identification of a Three-miRNA Signature as a Novel Potential Prognostic Biomarker in Patients with Clear Cell Renal Cell Carcinoma. J. Cell Biochem. 2019, 120, 13751–13764. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and Secretion of Exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef]
- Junker, K.; Heinzelmann, J.; Beckham, C.; Ochiya, T.; Jenster, G. Extracellular Vesicles and Their Role in Urologic Malignancies. Eur. Urol. 2016, 70, 323–331. [Google Scholar] [CrossRef]
- Merchant, M.L.; Rood, I.M.; Deegens, J.K.J.; Klein, J.B. Isolation and Characterization of Urinary Extracellular Vesicles: Implications for Biomarker Discovery. Nat. Rev. Nephrol. 2017, 13, 731–749. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed. Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Chen, C.; Wu, Z.; Bai, P.; Li, S.; Chen, B.; Liu, R.; Zhang, K.; Li, W.; et al. Serum Exosomal MiR-210 as a Potential Biomarker for Clear Cell Renal Cell Carcinoma. J. Cell Biochem. 2018, 120, 1492–1502. [Google Scholar] [CrossRef]
- Crentsil, V.C.; Liu, H.; Sellitti, D.F. Comparison of Exosomal MicroRNAs Secreted by 786-O Clear Cell Renal Carcinoma Cells and HK-2 Proximal Tubule-Derived Cells in Culture Identifies MicroRNA-205 as a Potential Biomarker of Clear Cell Renal Carcinoma. Oncol. Lett. 2018, 16, 1285–1290. [Google Scholar] [CrossRef]
- Ivanova, E.; Asadullina, D.; Gilyazova, G.; Rakhimov, R.; Izmailov, A.; Pavlov, V.; Khusnutdinova, E.; Gilyazova, I. Exosomal MicroRNA Levels Associated with Immune Checkpoint Inhibitor Therapy in Clear Cell Renal Cell Carcinoma. Biomedicines 2023, 11, 801. [Google Scholar] [CrossRef]
- Li, Y.; Quan, J.; Chen, F.; Pan, X.; Zhuang, C.; Xiong, T.; Zhuang, C.; Li, J.; Huang, X.; Ye, J.; et al. MiR-31-5p Acts as a Tumor Suppressor in Renal Cell Carcinoma by Targeting Cyclin-Dependent Kinase 1 (CDK1). Biomed. Pharmacother. 2019, 111, 517–526. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles Released from Human Renal Cancer Stem Cells Stimulate Angiogenesis and Formation of Lung Premetastatic Niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef]
- Kulus, M.; Farzaneh, M.; Bryja, A.; Zehtabi, M.; Azizidoost, S.; Abouali Gale Dari, M.; Golcar-Narenji, A.; Ziemak, H.; Chwarzyński, M.; Piotrowska–Kempisty, H.; et al. Phenotypic Transitions the Processes Involved in Regulation of Growth and Proangiogenic Properties of Stem Cells, Cancer Stem Cells and Circulating Tumor Cells. Stem Cell Rev. Rep. 2024, 20, 967–979. [Google Scholar] [CrossRef]
- Kalli, M.; Stylianopoulos, T. Toward Innovative Approaches for Exploring the Mechanically Regulated Tumor-Immune Microenvironment. APL Bioeng. 2024, 8, 011501. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Shi, L.; Li, Z.; Liu, W.; Zhao, B.; Qiu, Y.; Zhao, Y.; Li, K.; Li, Y.; Zhu, Q. Circ_0039569 Promotes Renal Cell Carcinoma Growth and Metastasis by Regulating MiR-34a-5p/CCL22. Am. J. Transl. Res. 2019, 11, 4935. [Google Scholar] [PubMed]
- Massari, F.; Santoni, M.; Ciccarese, C.; Santini, D.; Alfieri, S.; Martignoni, G.; Brunelli, M.; Piva, F.; Berardi, R.; Montironi, R.; et al. PD-1 Blockade Therapy in Renal Cell Carcinoma: Current Studies and Future Promises. Cancer Treat. Rev. 2015, 41, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.I.; Dutcher, J.P.; Daniels, G.A.; Curti, B.D.; Patel, S.P.; Holtan, S.G.; Miletello, G.P.; Fishman, M.N.; Gonzalez, R.; Clark, J.I.; et al. Therapy with High-Dose Interleukin-2 (HD IL-2) in Metastatic Melanoma and Renal Cell Carcinoma Following PD1 or PDL1 Inhibition. J. Immunother. Cancer 2019, 7, 49. [Google Scholar] [CrossRef]
- Yin, J.; Yan, X.; Yao, X.; Zhang, Y.; Shan, Y.; Mao, N.; Yang, Y.; Pan, L. Secretion of Annexin A3 from Ovarian Cancer Cells and Its Association with Platinum Resistance in Ovarian Cancer Patients. J. Cell Mol. Med. 2012, 16, 337–348. [Google Scholar] [CrossRef]
- Shi, D.; Che, J.; Yan, Y.; Peng, B.; Yao, X.; Guo, C. Expression and Clinical Value of CD105 in Renal Cell Carcinoma Based on Data Mining in The Cancer Genome Atlas. Exp. Ther. Med. 2019, 17, 4499–4505. [Google Scholar] [CrossRef]
- Dias, F.; Teixeira, A.L.; Nogueira, I.; Morais, M.; Maia, J.; Bodo, C.; Ferreira, M.; Vieira, I.; Silva, J.; Lobo, J.; et al. Plasma Extracellular Vesicle-Derived TIMP-1 MRNA as a Prognostic Biomarker in Clear Cell Renal Cell Carcinoma: A Pilot Study. Int. J. Mol. Sci. 2020, 21, 4624. [Google Scholar] [CrossRef]
- Campbell, L.; Al-Jayyoussi, G.; Gutteridge, R.; Gumbleton, N.; Griffiths, R.; Gumbleton, S.; Smith, M.W.; Griffiths, D.F.; Gumbleton, M. Caveolin-1 in Renal Cell Carcinoma Promotes Tumour Cell Invasion, and in Co-Operation with PERK Predicts Metastases in Patients with Clinically Confined Disease. J. Transl. Med. 2013, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Veiga, T.; Schneegans, S.; Pantel, K.; Wikman, H. Circulating Tumor Cell-Blood Cell Crosstalk: Biology and Clinical Relevance. Cell Rep. 2022, 40, 111298. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, S.; Wang, Y.; Shi, D. Circulating Tumor Cell Isolation for Cancer Diagnosis and Prognosis. EBioMedicine 2022, 83, 104237. [Google Scholar] [CrossRef]
- Guan, Y.; Xu, F.; Tian, J.; Gao, K.; Wan, Z.; Wang, Y.; Gao, M.; Wang, Z.; Chong, T. The Prognostic Value of Circulating Tumour Cells (CTCs) and CTC White Blood Cell Clusters in Patients with Renal Cell Carcinoma. BMC Cancer 2021, 21, 826. [Google Scholar] [CrossRef] [PubMed]
- Vona, G.; Sabile, A.; Louha, M.; Sitruk, V.; Romana, S.; Schütze, K.; Capron, F.; Franco, D.; Pazzagli, M.; Vekemans, M.; et al. Isolation by Size of Epithelial Tumor Cells. Am. J. Pathol. 2000, 156, 57–63. [Google Scholar] [CrossRef] [PubMed]
- van der Toom, E.E.; Verdone, J.E.; Gorin, M.A.; Pienta, K.J. Technical Challenges in the Isolation and Analysis of Circulating Tumor Cells. Oncotarget 2016, 7, 62754–62766. [Google Scholar] [CrossRef]
- Chen, C.-F.; Chen, Y.-C.; Fu, Y.-S.; Tsai, S.-W.; Wu, P.-K.; Chen, C.-M.; Chen, W.-M.; Wu, H.-T.H.; Lee, C.-H.; Chang, C.-L.; et al. Safety and Tolerability of Intra-Articular Injection of Adipose-Derived Mesenchymal Stem Cells GXCPC1 in 11 Subjects With Knee Osteoarthritis: A Nonrandomized Pilot Study Without a Control Arm. Cell Transplant. 2024, 33. [Google Scholar] [CrossRef]
- Russo, G.; Musso, N.; Romano, A.; Caruso, G.; Petralia, S.; Lanzanò, L.; Broggi, G.; Camarda, M. The Role of Dielectrophoresis for Cancer Diagnosis and Prognosis. Cancers 2021, 14, 198. [Google Scholar] [CrossRef]
- Sperger, J.M.; Strotman, L.N.; Welsh, A.; Casavant, B.P.; Chalmers, Z.; Horn, S.; Heninger, E.; Thiede, S.M.; Tokar, J.; Gibbs, B.K.; et al. Integrated Analysis of Multiple Biomarkers from Circulating Tumor Cells Enabled by Exclusion-Based Analyte Isolation. Clin. Cancer Res. 2017, 23, 746–756. [Google Scholar] [CrossRef]
- Crocetto, F.; Cimmino, A.; Ferro, M.; Terracciano, D. Circulating Tumor Cells in Bladder Cancer: A New Horizon of Liquid Biopsy for Precision Medicine. J. Basic. Clin. Physiol. Pharmacol. 2021, 33, 525–527. [Google Scholar] [CrossRef]
- Hu, F.; Mao, X.; Zhang, Y.; Zheng, X.; Gu, P.; Wang, H.; Zhang, X. Reliability of Using Circulating Tumor Cells for Detecting Epidermal Growth Factor Receptor Mutation Status in Advanced Non-Small-Cell Lung Cancer Patients: A Meta-Analysis and Systematic Review. Onco Targets Ther. 2018, 11, 1373–1384. [Google Scholar] [CrossRef]
- Tan, Y.; Wu, H. The Significant Prognostic Value of Circulating Tumor Cells in Colorectal Cancer: A Systematic Review and Meta-Analysis. Curr. Probl. Cancer 2018, 42, 95–106. [Google Scholar] [CrossRef]
- Crocetto, F.; Russo, G.; Di Zazzo, E.; Pisapia, P.; Mirto, B.F.; Palmieri, A.; Pepe, F.; Bellevicine, C.; Russo, A.; La Civita, E.; et al. Liquid Biopsy in Prostate Cancer Management-Current Challenges and Future Perspectives. Cancers 2022, 14, 3272. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Lin, H.; Zhu, Y.; Huang, D.; Lai, M.; Xi, X.; Huang, J.; Zhang, W.; Zhong, T. Research Progress of CTC, CtDNA, and EVs in Cancer Liquid Biopsy. Front. Oncol. 2024, 14, 1303335. [Google Scholar] [CrossRef] [PubMed]
- Palmela Leitão, T.; Miranda, M.; Polido, J.; Morais, J.; Corredeira, P.; Alves, P.; Oliveira, T.; Pereira e Silva, R.; Fernandes, R.; Ferreira, J.; et al. Circulating Tumor Cell Detection Methods in Renal Cell Carcinoma: A Systematic Review. Crit. Rev. Oncol. Hematol. 2021, 161, 103331. [Google Scholar] [CrossRef]
- Ferro, M.; Chiujdea, S.; Vartolomei, M.D.; Bove, P.; Porreca, A.; Busetto, G.M.; del Giudice, F.; Antonelli, A.; Foschi, N.; Racioppi, M.; et al. Advanced Age Impacts Survival After Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma. Clin. Genitourin. Cancer 2024, 22, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Basso, U.; Facchinetti, A.; Rossi, E.; Maruzzo, M.; Conteduca, V.; Aieta, M.; Massari, F.; Fraccon, A.P.; Mucciarini, C.; Sava, T.; et al. Prognostic Role of Circulating Tumor Cells in Metastatic Renal Cell Carcinoma: A Large, Multicenter, Prospective Trial. Oncologist 2021, 26, 740–750. [Google Scholar] [CrossRef]
- Liu, S.; Tian, Z.; Zhang, L.; Hou, S.; Hu, S.; Wu, J.; Jing, Y.; Sun, H.; Yu, F.; Zhao, L.; et al. Combined Cell Surface Carbonic Anhydrase 9 and CD147 Antigens Enable High-Efficiency Capture of Circulating Tumor Cells in Clear Cell Renal Cell Carcinoma Patients. Oncotarget 2016, 7, 59877–59891. [Google Scholar] [CrossRef]
- Bootsma, M.; McKay, R.R.; Emamekhoo, H.; Bade, R.M.; Schehr, J.L.; Mannino, M.C.; Singh, A.; Wolfe, S.K.; Schultz, Z.D.; Sperger, J.; et al. Longitudinal Molecular Profiling of Circulating Tumor Cells in Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2022, 40, 3633–3641. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Zhang, P.; Li, H.-C.; Yang, X.-J.; Zhang, Y.-P.; Li, Z.-L.; Xue, L.; Xue, Y.-Q.; Li, H.-L.; Chen, Q.; et al. Dynamic Changes of Different Phenotypic and Genetic Circulating Tumor Cells as a Biomarker for Evaluating the Prognosis of RCC. Cancer Biol. Ther. 2019, 20, 505–512. [Google Scholar] [CrossRef]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid Biopsy: Monitoring Cancer-Genetics in the Blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Zou, B.; Wang, Z.; Li, P.; Wang, H.; Ou, Y.; Cui, K.; Bian, J.; Li, S.; Xu, X. Comparison of Two Detection Systems for Circulating Tumor Cells among Patients with Renal Cell Carcinoma. Int. Urol. Nephrol. 2018, 50, 1801–1809. [Google Scholar] [CrossRef]
- Bilkenroth, U.; Taubert, H.; Riemann, D.; Rebmann, U.; Heynemann, H.; Meye, A. Detection and Enrichment of Disseminated Renal Carcinoma Cells from Peripheral Blood by Immunomagnetic Cell Separation. Int. J. Cancer 2001, 92, 577–582. [Google Scholar] [CrossRef]
- Ashida, S.; Okuda, H.; Chikazawa, M.; Tanimura, M.; Sugita, O.; Yamamoto, Y.; Nakamura, S.; Moriyama, M.; Shuin, T. Detection of Circulating Cancer Cells with von Hippel-Lindau Gene Mutation in Peripheral Blood of Patients with Renal Cell Carcinoma. Clin. Cancer Res. 2000, 6, 3817–3822. [Google Scholar]
- Liu, M.; Li, R.; Tang, Y.; Chang, J.; Han, R.; Zhang, S.; Jiang, N.; Ma, F. New Applications of the Acridine Orange Fluorescence Staining Method: Screening for Circulating Tumor Cells. Oncol. Lett. 2017, 13, 2221–2229. [Google Scholar] [CrossRef]
- de Martino, M.; Klatte, T.; Haitel, A.; Marberger, M. Serum Cell-free DNA in Renal Cell Carcinoma. Cancer 2012, 118, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhu, L.; Jiang, Z.; Cheng, K. Monitoring of Plasma Cell-Free DNA in Predicting Postoperative Recurrence of Clear Cell Renal Cell Carcinoma. Urol. Int. 2013, 91, 273–278. [Google Scholar] [CrossRef]
- Sumiyoshi, T.; Yamasaki, T.; Takeda, M.; Mizuno, K.; Utsunomiya, N.; Sakamoto, H.; Nakamura, E.; Ogawa, O.; Akamatsu, S. Detection of von Hippel-Lindau Gene Mutation in Circulating Cell-Free DNA for Clear Cell Renal Cell Carcinoma. Cancer Sci. 2021, 112, 3363–3374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Xu, B.; Li, F.; Wang, Y.; Li, M.; Du, R.; Zhou, Y.; Salgia, M.; Yang, L.; et al. Circulating Tumor DNA Analysis of Metastatic Renal Cell Carcinoma. Mol. Clin. Oncol. 2021, 14, 16. [Google Scholar] [CrossRef]
- Hoque, M.O.; Begum, S.; Topaloglu, O.; Jeronimo, C.; Mambo, E.; Westra, W.H.; Califano, J.A.; Sidransky, D. Quantitative Detection of Promoter Hypermethylation of Multiple Genes in the Tumor, Urine, and Serum DNA of Patients with Renal Cancer. Cancer Res. 2004, 64, 5511–5517. [Google Scholar] [CrossRef]
- Basu, A.; Kollipara, R.; Sudhaman, S.; Mahmood, T.; Pajak, N.; Carson, C.; Dutta, P.; Calhoun, M.; ElNaggar, A.; Liu, M.C.; et al. Longitudinal Detection of Circulating Tumor DNA in Patients with Advanced Renal Cell Carcinoma. J. Clin. Oncol. 2023, 41, 715. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [PubMed]
- Corrò, C.; Hejhal, T.; Poyet, C.; Sulser, T.; Hermanns, T.; Winder, T.; Prager, G.; Wild, P.J.; Frew, I.; Moch, H.; et al. Detecting Circulating Tumor DNA in Renal Cancer: An Open Challenge. Exp. Mol. Pathol. 2017, 102, 255–261. [Google Scholar] [CrossRef]
- Smith, C.G.; Moser, T.; Mouliere, F.; Field-Rayner, J.; Eldridge, M.; Riediger, A.L.; Chandrananda, D.; Heider, K.; Wan, J.C.M.; Warren, A.Y.; et al. Comprehensive Characterization of Cell-Free Tumor DNA in Plasma and Urine of Patients with Renal Tumors. Genome Med. 2020, 12, 23. [Google Scholar] [CrossRef]
- Wan, J.C.M.; Heider, K.; Gale, D.; Murphy, S.; Fisher, E.; Mouliere, F.; Ruiz-Valdepenas, A.; Santonja, A.; Morris, J.; Chandrananda, D.; et al. CtDNA Monitoring Using Patient-Specific Sequencing and Integration of Variant Reads. Sci. Transl. Med. 2020, 12, eaaz8084. [Google Scholar] [CrossRef]
- Lasseter, K.; Nassar, A.H.; Hamieh, L.; Berchuck, J.E.; Nuzzo, P.V.; Korthauer, K.; Shinagare, A.B.; Ogorek, B.; McKay, R.; Thorner, A.R.; et al. Plasma Cell-Free DNA Variant Analysis Compared with Methylated DNA Analysis in Renal Cell Carcinoma. Genet. Med. 2020, 22, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.W.; Gill, D.M.; Maughan, B.; Agarwal, A.; Arjyal, L.; Gupta, S.; Streeter, J.; Bailey, E.; Pal, S.K.; Agarwal, N. Correlation of Genomic Alterations Assessed by Next-Generation Sequencing (NGS) of Tumor Tissue DNA and Circulating Tumor DNA (CtDNA) in Metastatic Renal Cell Carcinoma (MRCC): Potential Clinical Implications. Oncotarget 2017, 8, 33614–33620. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Sonpavde, G.; Agarwal, N.; Vogelzang, N.J.; Srinivas, S.; Haas, N.B.; Signoretti, S.; McGregor, B.A.; Jones, J.; Lanman, R.B.; et al. Evolution of Circulating Tumor DNA Profile from First-Line to Subsequent Therapy in Metastatic Renal Cell Carcinoma. Eur. Urol. 2017, 72, 557–564. [Google Scholar] [CrossRef]
- Maia, M.C.; Bergerot, P.G.; Dizman, N.; Hsu, J.; Jones, J.; Lanman, R.B.; Banks, K.C.; Pal, S.K. Association of Circulating Tumor DNA (CtDNA) Detection in Metastatic Renal Cell Carcinoma (MRCC) with Tumor Burden. Kidney Cancer 2017, 1, 65–70. [Google Scholar] [CrossRef]
- Bacon, J.V.W.; Annala, M.; Soleimani, M.; Lavoie, J.-M.; So, A.; Gleave, M.E.; Fazli, L.; Wang, G.; Chi, K.N.; Kollmannsberger, C.K.; et al. Plasma Circulating Tumor DNA and Clonal Hematopoiesis in Metastatic Renal Cell Carcinoma. Clin. Genitourin. Cancer 2020, 18, 322–331.e2. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Uemura, M.; Fujita, M.; Maejima, K.; Koh, Y.; Matsushita, M.; Nakano, K.; Hayashi, Y.; Wang, C.; Ishizuya, Y.; et al. Clinical Significance of the Mutational Landscape and Fragmentation of Circulating Tumor DNA in Renal Cell Carcinoma. Cancer Sci. 2019, 110, 617–628. [Google Scholar] [CrossRef]
- Jung, M.; Ellinger, J.; Gevensleben, H.; Syring, I.; Lüders, C.; de Vos, L.; Pützer, S.; Bootz, F.; Landsberg, J.; Kristiansen, G.; et al. Cell-Free SHOX2 DNA Methylation in Blood as a Molecular Staging Parameter for Risk Stratification in Renal Cell Carcinoma Patients: A Prospective Observational Cohort Study. Clin. Chem. 2019, 65, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-L.; Wang, Y.-P.; Li, H.-Z.; Zhang, X. Aberrant Promoter Methylation of PCDH17 (Protocadherin 17) in Serum and Its Clinical Significance in Renal Cell Carcinoma. Med. Sci. Monit. 2017, 23, 3318–3323. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kang, Y.; Kim, J.S.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Park, D.; et al. Potential of Circulating Tumor DNA as a Predictor of Therapeutic Responses to Immune Checkpoint Blockades in Metastatic Renal Cell Carcinoma. Sci. Rep. 2021, 11, 5600. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, H.; Jang, W.S.; Kim, J.; Ham, W.S.; Lee, S.-T. CtDNA Predicts Clinical T1a to Pathological T3a Upstaging after Partial Nephrectomy. Cancer Sci. 2024, 115, 1680–1687. [Google Scholar] [CrossRef]
- Iisager, L.; Ahrenfeldt, J.; Keller, A.K.; Nielsen, T.K.; Fristrup, N.; Lyskjær, I. KIDNEY-PAGER: Analysis of Circulating Tumor DNA as a Biomarker in Renal Cancer—An Observational Trial. Study Protocol. Acta Oncol. 2024, 63, 51–55. [Google Scholar] [CrossRef]
- Kapitsinou, P.P.; Haase, V.H. The VHL Tumor Suppressor and HIF: Insights from Genetic Studies in Mice. Cell Death Differ. 2008, 15, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yoshizato, T.; Shiraishi, Y.; Maekawa, S.; Okuno, Y.; Kamura, T.; Shimamura, T.; Sato-Otsubo, A.; Nagae, G.; Suzuki, H.; et al. Integrated Molecular Analysis of Clear-Cell Renal Cell Carcinoma. Nat. Genet. 2013, 45, 860–867. [Google Scholar] [CrossRef]
- Hakimi, A.A.; Chen, Y.-B.; Wren, J.; Gonen, M.; Abdel-Wahab, O.; Heguy, A.; Liu, H.; Takeda, S.; Tickoo, S.K.; Reuter, V.E.; et al. Clinical and Pathologic Impact of Select Chromatin-Modulating Tumor Suppressors in Clear Cell Renal Cell Carcinoma. Eur. Urol. 2013, 63, 848–854. [Google Scholar] [CrossRef]
- Peña-Llopis, S.; Vega-Rubín-de-Celis, S.; Liao, A.; Leng, N.; Pavía-Jiménez, A.; Wang, S.; Yamasaki, T.; Zhrebker, L.; Sivanand, S.; Spence, P.; et al. BAP1 Loss Defines a New Class of Renal Cell Carcinoma. Nat. Genet. 2012, 44, 751–759. [Google Scholar] [CrossRef]
- Varela, I.; Tarpey, P.; Raine, K.; Huang, D.; Ong, C.K.; Stephens, P.; Davies, H.; Jones, D.; Lin, M.-L.; Teague, J.; et al. Exome Sequencing Identifies Frequent Mutation of the SWI/SNF Complex Gene PBRM1 in Renal Carcinoma. Nature 2011, 469, 539–542. [Google Scholar] [CrossRef]
- Hakimi, A.A.; Ostrovnaya, I.; Reva, B.; Schultz, N.; Chen, Y.B.; Gonen, M.; Liu, H.; Takeda, S.; Voss, M.H.; Tickoo, S.K.; et al. Adverse Outcomes in Clear Cell Renal Cell Carcinoma with Mutations of 3p21 Epigenetic Regulators BAP1 and SETD2: A Report by MSKCC and the KIRC TCGA Research Network. Clin. Cancer Res. 2013, 19, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Kapur, P.; Peña-Llopis, S.; Christie, A.; Zhrebker, L.; Pavía-Jiménez, A.; Rathmell, W.K.; Xie, X.J.; Brugarolas, J. Effects on Survival of BAP1 and PBRM1 Mutations in Sporadic Clear-Cell Renal-Cell Carcinoma: A Retrospective Analysis with Independent Validation. Lancet Oncol. 2013, 14, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.I.; Grosser, M.; Herr, A.; Aust, D.E.; Meye, A.; Hoefling, C.; Fuessel, S.; Wuttig, D.; Wirth, M.P.; Baretton, G.B. Loss of Heterozygosity and Copy Number Abnormality in Clear Cell Renal Cell Carcinoma Discovered by High-Density Affymetrix 10K Single Nucleotide Polymorphism Mapping Array. Neoplasia 2008, 10, 634–642. [Google Scholar] [CrossRef] [PubMed]
- White, N.M.A.; Masui, O.; DeSouza, L.V.; Krakovska, O.; Metias, S.; Romaschin, A.D.; Honey, R.J.; Stewart, R.; Pace, K.; Lee, J.; et al. Quantitative Proteomic Analysis Reveals Potential Diagnostic Markers and Pathways Involved in Pathogenesis of Renal Cell Carcinoma. Oncotarget 2014, 5, 506–518. [Google Scholar] [CrossRef]
- Steffens, S.; Köhler, A.; Rudolph, R.; Eggers, H.; Seidel, C.; Janssen, M.; Wegener, G.; Schrader, M.; Kuczyk, M.A.; Schrader, A.J. Validation of CRP as Prognostic Marker for Renal Cell Carcinoma in a Large Series of Patients. BMC Cancer 2012, 12, 399. [Google Scholar] [CrossRef]
- de Martino, M.; Klatte, T.; Seemann, C.; Waldert, M.; Haitel, A.; Schatzl, G.; Remzi, M.; Weibl, P. Validation of Serum C-reactive Protein (CRP) as an Independent Prognostic Factor for Disease-free Survival in Patients with Localised Renal Cell Carcinoma (RCC). BJU Int. 2013, 111, E348–E353. [Google Scholar] [CrossRef]
- Ngo, T.C.; Wood, C.G.; Karam, J.A. Biomarkers of Renal Cell Carcinoma. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 243–251. [Google Scholar] [CrossRef]
- Toiyama, D.; Takaha, N.; Shinnoh, M.; Ueda, T.; Kimura, Y.; Nakamura, T.; Hongo, F.; Mikami, K.; Kamoi, K.; Kawauchi, A.; et al. Significance of Serum Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand as a Prognostic Biomarker for Renal Cell Carcinoma. Mol. Clin. Oncol. 2013, 1, 69–74. [Google Scholar] [CrossRef]
- Pastore, A.L.; Palleschi, G.; Silvestri, L.; Moschese, D.; Ricci, S.; Petrozza, V.; Carbone, A.; Di Carlo, A. Serum and Urine Biomarkers for Human Renal Cell Carcinoma. Dis. Markers 2015, 2015, 251403. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Gao, Y.; Zhao, L.; Liu, L.; Qin, Y.; Wang, X.; Song, T.; Huang, C. Identification of Potential Serum Proteomic Biomarkers for Clear Cell Renal Cell Carcinoma. PLoS ONE 2014, 9, e111364. [Google Scholar] [CrossRef]
- Nizioł, J.; Bonifay, V.; Ossoliński, K.; Ossoliński, T.; Ossolińska, A.; Sunner, J.; Beech, I.; Arendowski, A.; Ruman, T. Metabolomic Study of Human Tissue and Urine in Clear Cell Renal Carcinoma by LC-HRMS and PLS-DA. Anal. Bioanal. Chem. 2018, 410, 3859–3869. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.S.; Carvalho, M.; de Lourdes Bastos, M.; de Pinho, P.G. Biomarkers in Renal Cell Carcinoma: A Metabolomics Approach. Metabolomics 2014, 10, 1210–1222. [Google Scholar] [CrossRef]
- Arendowski, A.; Ossoliński, K.; Nizioł, J.; Ruman, T. Gold Nanostructures—Assisted Laser Desorption/Ionization Mass Spectrometry for Kidney Cancer Blood Serum Biomarker Screening. Int. J. Mass. Spectrom. 2020, 456, 116396. [Google Scholar] [CrossRef]
- Nizioł, J.; Copié, V.; Tripet, B.P.; Nogueira, L.B.; Nogueira, K.O.P.C.; Ossoliński, K.; Arendowski, A.; Ruman, T. Metabolomic and Elemental Profiling of Human Tissue in Kidney Cancer. Metabolomics 2021, 17, 30. [Google Scholar] [CrossRef]
- Zira, A.N.; Theocharis, S.E.; Mitropoulos, D.; Migdalis, V.; Mikros, E. (1)H NMR Metabonomic Analysis in Renal Cell Carcinoma: A Possible Diagnostic Tool. J. Proteome Res. 2010, 9, 4038–4044. [Google Scholar] [CrossRef]
- Yong, C.; Stewart, G.D.; Frezza, C. Oncometabolites in Renal Cancer. Nat. Rev. Nephrol. 2020, 16, 156–172. [Google Scholar] [CrossRef]
- Leonard, S.; Montanaro, F.; Leopold, Z.; Helstrom, E.; Abbosh, P.; Fulmes, A.; Correa, A.; Barata, P.; Nizam, A.; Weight, C.; et al. Microbiome in RCC—A Systematic Review of the Literature. Kidney Cancer 2024, 8, 158–170. [Google Scholar] [CrossRef]
- Wu, K.; Li, Y.; Ma, K.; Zhao, W.; Yao, Z.; Zheng, Z.; Sun, F.; Mu, X.; Liu, Z.; Zheng, J. The Microbiota and Renal Cell Carcinoma. Cell. Oncol. 2024, 47, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.B.; Young, V.B.; Skufca, J.; Ginty, F.; Testerman, T.; Pearson, A.T.; Macklin, P.; Mitchell, A.; Shmulevich, I.; Xie, L.; et al. The Cancer Microbiome: Distinguishing Direct and Indirect Effects Requires a Systemic View. Trends Cancer 2020, 6, 192–204. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Dong, Y.; Yang, Z.; Zhao, N.; Liu, Q.; Zhai, W.; Zheng, J. Characteristics of Gut Microbiota in Patients With Clear Cell Renal Cell Carcinoma. Front. Microbiol. 2022, 13, 913718. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Kawashima, A.; Jingushi, K.; Motooka, D.; Saito, T.; Nesrine, S.; Oka, T.; Okuda, Y.; Yamamoto, A.; Yamamichi, G.; et al. Bacteria-Derived DNA in Serum Extracellular Vesicles Are Biomarkers for Renal Cell Carcinoma. Heliyon 2023, 9, e19800. [Google Scholar] [CrossRef]
- Kovaleva, O.V.; Podlesnaya, P.; Sorokin, M.; Mochalnikova, V.; Kataev, V.; Khlopko, Y.A.; Plotnikov, A.O.; Stilidi, I.S.; Kushlinskii, N.E.; Gratchev, A. Macrophage Phenotype in Combination with Tumor Microbiome Composition Predicts RCC Patients’ Survival: A Pilot Study. Biomedicines 2022, 10, 1516. [Google Scholar] [CrossRef] [PubMed]
- Ninkov, M.; Schmerk, C.L.; Moradizadeh, M.; Parvathy, S.N.; Figueredo, R.; Burton, J.P.; Silverman, M.S.; Fernandes, R.; Maleki Vareki, S.; Haeryfar, S.M.M. Improved MAIT Cell Functions Following Fecal Microbiota Transplantation for Metastatic Renal Cell Carcinoma. Cancer Immunol. Immunother. 2023, 72, 1247–1260. [Google Scholar] [CrossRef]
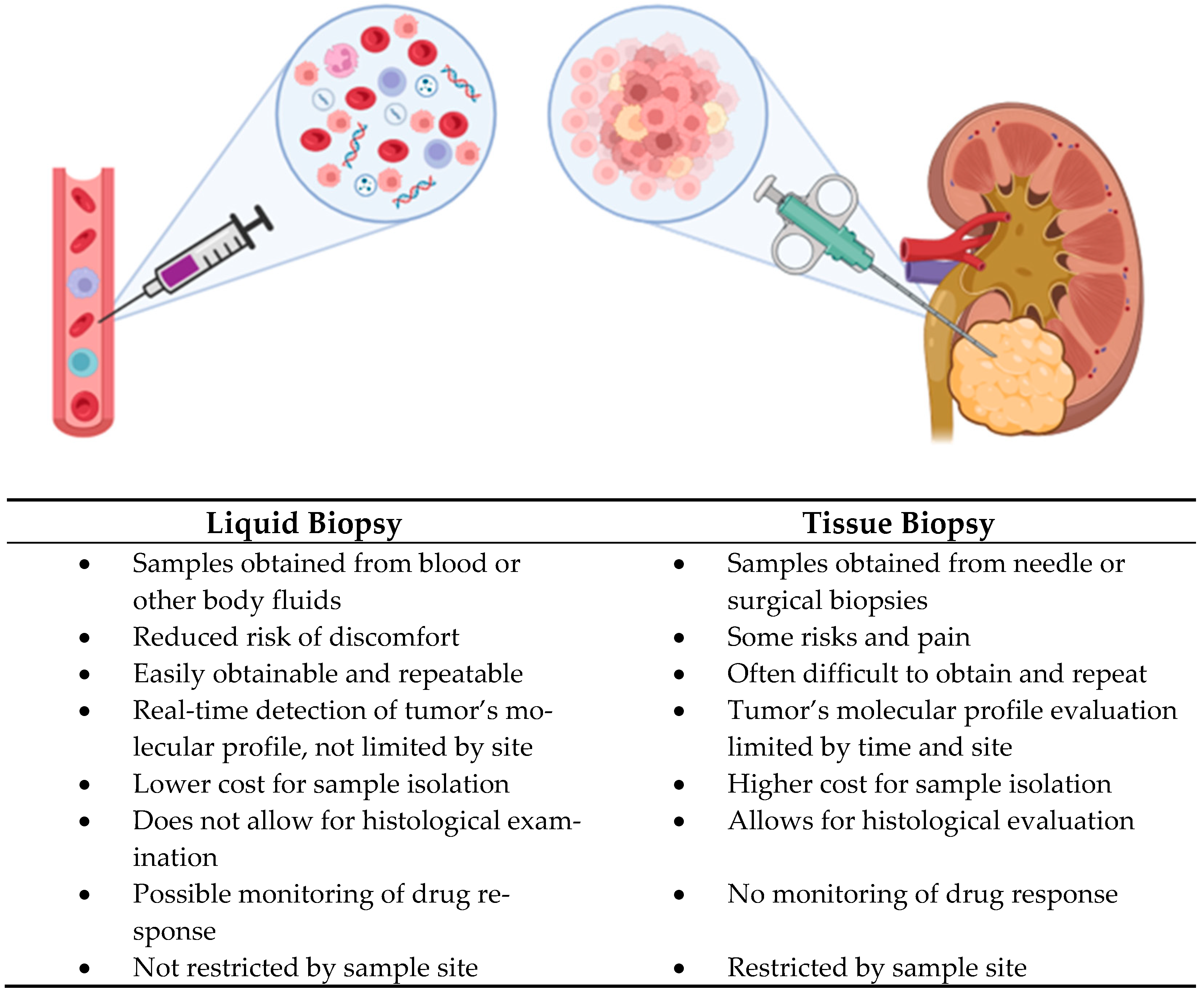
| Biomarker | Type | Sample | Clinical Relevance | Reference(s) |
|---|---|---|---|---|
| miR-122 | Molecular | Urine | Promotes malignant phenotypes | Cochetti, G. et al. [30] |
| miR-1271 | Molecular | Urine | Altered expression in RCC patients | Cochetti, G. et al. [30] |
| miR-15b | Molecular | Urine, Tissue | Anti cancer therapy resistance | Cochetti, G. et al. [30] Lu et al. [31] |
| miR-21-5p/miR-210-3p | Molecular | Serum | Potential biomarkers of pRCC | Kalogirou, C. et al. [32] |
| miR-224-5p/miR-34b-3p and miR-182-5p | Molecular | Serum | Potential biomarkers of pRCC | Huang, G. et al. [33] |
| miR-130b/miR-18a/miR-223 | Molecular | Serum | Prediction of the overall survival of patients with RCC | Luo, Y. et al. [34] |
| Exosomes | Molecular, protein | Serum, Tissue | Cancer detection, prognosis and response to therapy | Wang, X. et al. [39] Crentsil, V.C. et al. [40] Xiao, C.-T. et al. [26] Ivanova, E. et al. [41] |
| Circulating tumor cells | Whole tumor cells | Serum | Negative prognostic factor, diagnosis and monitoring | Guan, Y. [55] |
| Circulating tumor DNA | Tumor-derived DNA fragments | Plasma | Biomarker for both the diagnosis, monitoring and response to therapy | Hahn, A.W. et al. [88] Pal, S.K. [89] Maia, M.C. [90] Park, J.S. [96] |
| Hsp27 | Protein | Serum, Urine | Potential biomarkers of RCC | White, N.M.A. et al. [106] |
| SAA | Protein | Serum | Positive correlation with tumor stage | Steffens, S. et al. [107] de Martino, M. et al. [108] |
| PD-L1 | Immune checkpoint protein | Tumor tissue, Blood | Immune escape; target for immunotherapy | Ngo, T.C. et al. [109] |
| TRAIL (TNF-related apoptosis-inducing ligand) | Apoptotic mediator | Blood | Decreased in RCC; correlates with survival | Toiyama, D. et al. [110] |
| NGAL, KIM-1, MMPs, Aquaporins | Kidney injury and matrix markers | Urine | Altered levels in RCC | Pastore, A.L. et al. [111] |
| β-Tubulin peptides, Zinc Finger, RNA-binding proteins | Peptides Proteins | Serum | Upregulated in RCC; potential for diagnosis and monitoring | Yang, J. et al. [112] |
| Hydroxybutyrylcarnitine, Decanoylcarnitine, Carnitine, Dodecanoylcarnitine, Norepinephrine sulfate | Metabolites | Urine, Tissue, Blood | Elevated in RCC; identified via metabolomics | Nizioł, J. et al. [113] Monteiro, M.S. et al. [114] Arendowski, A. et al. [115] |
| Oncometabolites (L-2-hydroxyglutarate, Succinate, Fumarate) | Metabolites | Tumor tissue, Blood | Promote tumor progression; associated with aggressive subtypes | Yong, C. et al. [118] |
| NMR-based Metabolomic Profile | Metabolomic Signature | Tissue, Urine, Blood | Altered expression in RCC patients | Nizioł, J. et al. [116] Zira, A.N. et al. [117] |
| Microbial DNA | Microbial-derived DNA fragments | Plasma | RCC development and metastasis | Leonard, S. et al. [119] Wu, K. et al. [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netti, G.S.; De Luca, F.; Camporeale, V.; Khalid, J.; Leccese, G.; Troise, D.; Sanguedolce, F.; Stallone, G.; Ranieri, E. Liquid Biopsy as a New Tool for Diagnosis and Monitoring in Renal Cell Carcinoma. Cancers 2025, 17, 1442. https://doi.org/10.3390/cancers17091442
Netti GS, De Luca F, Camporeale V, Khalid J, Leccese G, Troise D, Sanguedolce F, Stallone G, Ranieri E. Liquid Biopsy as a New Tool for Diagnosis and Monitoring in Renal Cell Carcinoma. Cancers. 2025; 17(9):1442. https://doi.org/10.3390/cancers17091442
Chicago/Turabian StyleNetti, Giuseppe Stefano, Federica De Luca, Valentina Camporeale, Javeria Khalid, Giorgia Leccese, Dario Troise, Francesca Sanguedolce, Giovanni Stallone, and Elena Ranieri. 2025. "Liquid Biopsy as a New Tool for Diagnosis and Monitoring in Renal Cell Carcinoma" Cancers 17, no. 9: 1442. https://doi.org/10.3390/cancers17091442
APA StyleNetti, G. S., De Luca, F., Camporeale, V., Khalid, J., Leccese, G., Troise, D., Sanguedolce, F., Stallone, G., & Ranieri, E. (2025). Liquid Biopsy as a New Tool for Diagnosis and Monitoring in Renal Cell Carcinoma. Cancers, 17(9), 1442. https://doi.org/10.3390/cancers17091442







