Development and Validation of a Pre-Transplant Risk Score (LT-MVI Score) to Predict Microvascular Invasion in Hepatocellular Carcinoma Candidates for Liver Transplantation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Population
2.2. Outcomes, Data Collection, and Definitions
2.3. Statistical Analysis
3. Results
3.1. LT-MVI Score Creation
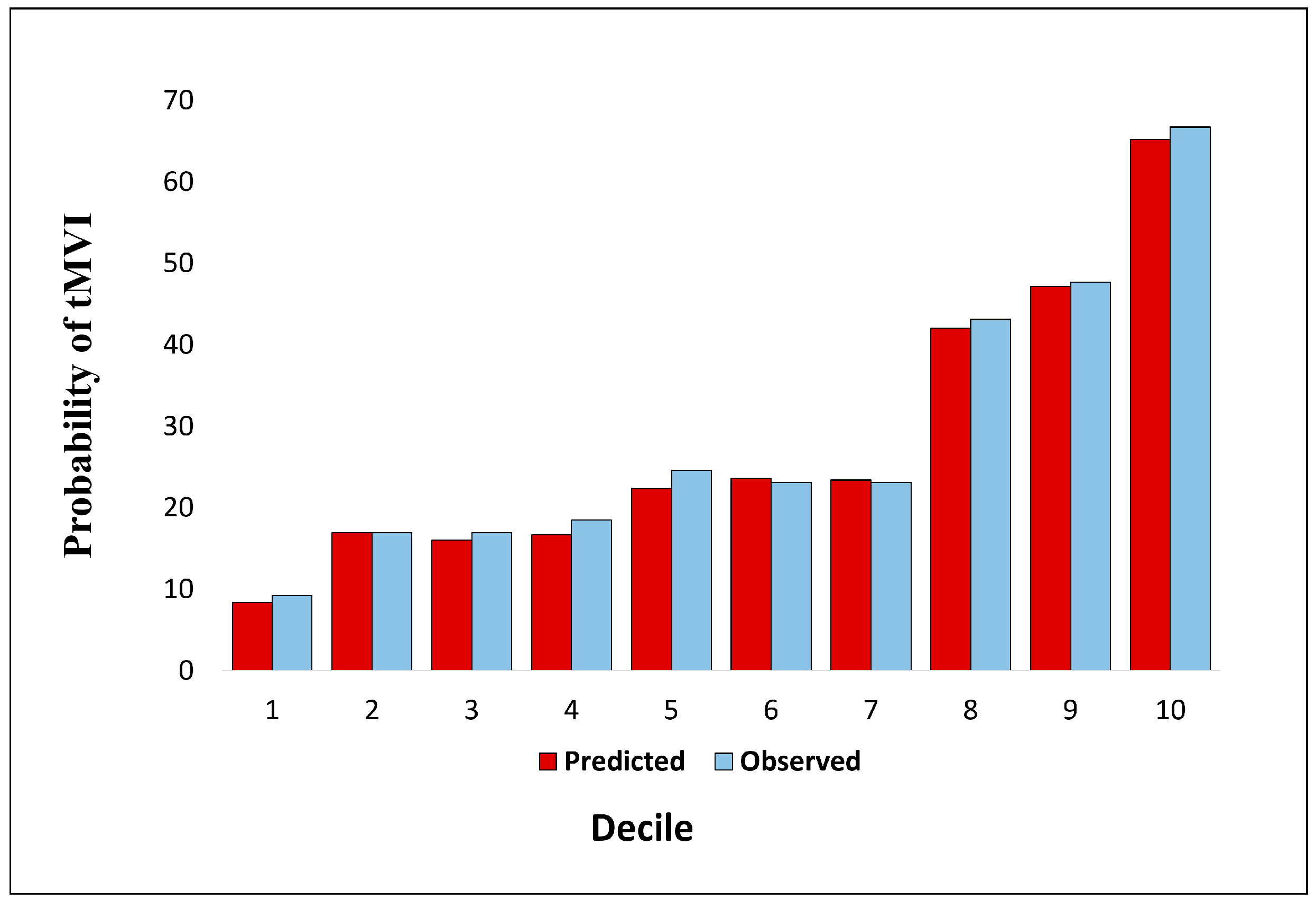
3.2. Calibration of the LT-MVI Score
3.3. Net Reclassification Index and Net Benefit of the LT-MVI Score
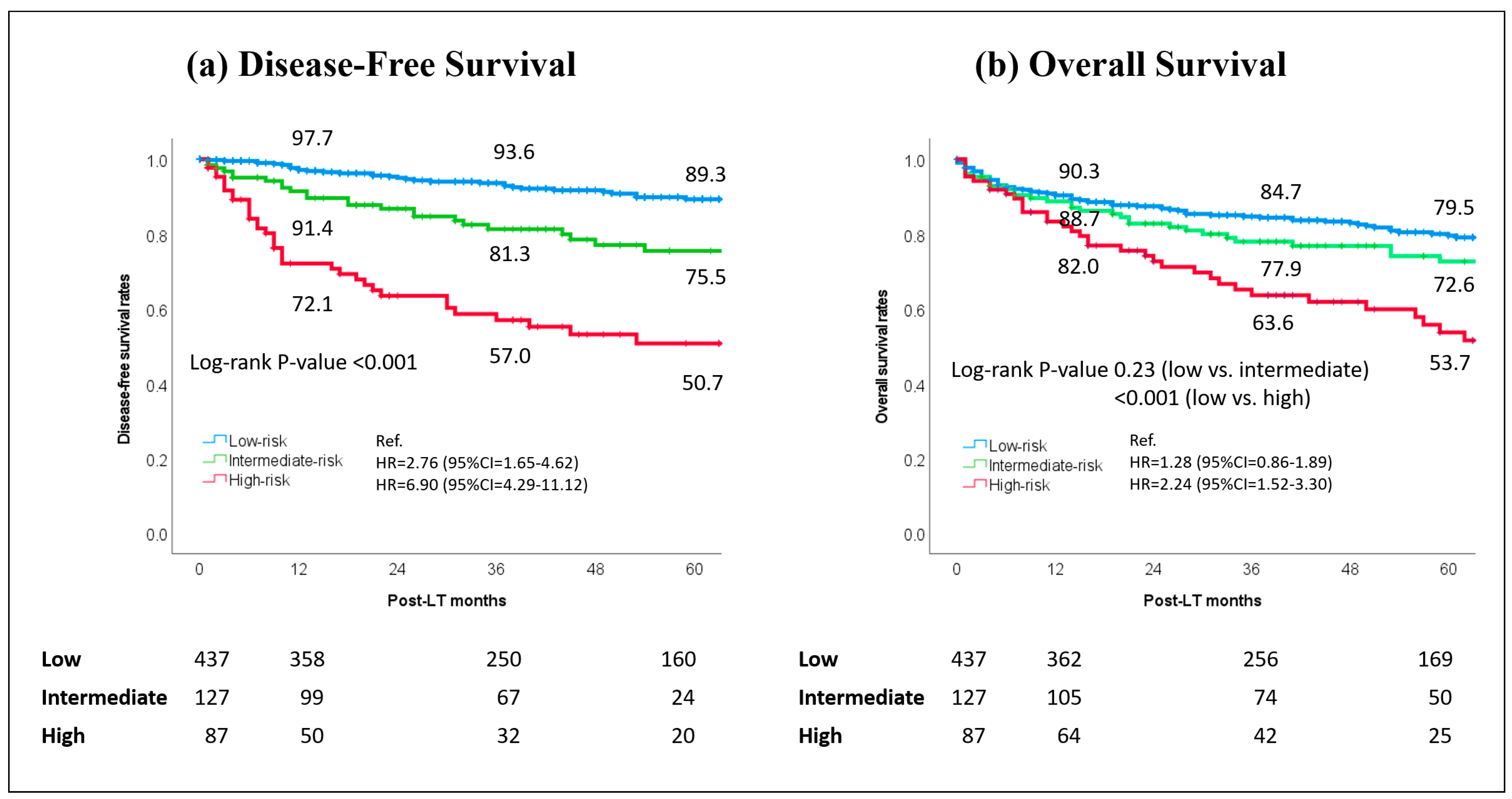
3.4. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 95%CI | 95% Confidence interval |
| AFP | Alpha-fetoprotein |
| AUC | Area under the curve |
| DOR | Diagnostic odds ratio |
| HCC | Hepatocellular carcinoma |
| LDLT | Living-donor liver transplantation |
| EHBH | Eastern Hepatobiliary Surgery Hospital |
| HALTHCC | Hazard Associated with Liver Transplantation for Hepatocellular Carcinoma |
| LT | Liver transplantation |
| MELD | Model for end-stage liver disease |
| mRECIST | Modified Response Evaluation Criteria in Solid Tumors |
| MVI | Microvascular invasion |
| NLR | Neutrophil-to-lymphocyte ratio |
| NRI | Net reclassification |
| OR | Odds ratio |
| Q1–Q3 | First–third quartiles |
| ROC | Receiver operating characteristic |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| TBS | Tumor burden score |
References
- Vitale, A.; Cabibbo, G.; Iavarone, M.; Viganò, L.; Pinato, D.J.; Ponziani, F.R.; Lai, Q.; Casadei-Gardini, A.; Celsa, C.; Galati, G.; et al. Personalised management of patients with hepatocellular carcinoma: A multiparametric therapeutic hierarchy concept. Lancet Oncol. 2023, 24, e312–e322. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Dodge, J.L.; Roberts, J.P.; Yao, F.Y. Validation of the prognostic power of the RETREAT score for hepatocellular carcinoma recurrence using the UNOS database. Am. J. Transplant. 2018, 18, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Halazun, K.J.; Rosenblatt, R.E.; Mehta, N.; Lai, Q.; Hajifathalian, K.; Gorgen, A.; Brar, G.; Sasaki, K.; Doyle, M.B.M.; Tabrizian, P.; et al. Dynamic α-Fetoprotein Response and Outcomes After Liver Transplant for Hepatocellular Carcinoma. JAMA Surg. 2021, 156, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; De Stefano, C.; Emond, J.; Bhangui, P.; Ikegami, T.; Schaefer, B.; Hoppe-Lotichius, M.; Mrzljak, A.; Ito, T.; Vivarelli, M.; et al. Development and validation of an artificial intelligence model for predicting post-transplant hepatocellular cancer recurrence. Cancer Commun. 2023, 43, 1381–1385. [Google Scholar] [CrossRef]
- Piñero, F.; Costentin, C.; Degroote, H.; Notarpaolo, A.; Boin, I.F.; Boudjema, K.; Baccaro, C.; Chagas, A.; Bachellier, P.; Ettorre, G.M.; et al. AFP score and metroticket 2.0 perform similarly and could be used in a “within-ALL” clinical decision tool. JHEP Rep. 2022, 5, 100644. [Google Scholar] [CrossRef]
- Costentin, C.; Piñero, F.; Degroote, H.; Notarpaolo, A.; Boudjema, K.; Baccaro, C.; Podestá, L.G.; Bachellier, P.; Ettorre, G.M.; Poniachik, J.; et al. R3-AFP score is a new composite tool to refine prediction of hepatocellular carcinoma recurrence after liver transplantation. JHEP Rep. 2022, 4, 100445. [Google Scholar] [CrossRef]
- Tran, B.V.; Moris, D.; Markovic, D.; Zaribafzadeh, H.; Henao, R.; Lai, Q.; Florman, S.S.; Tabrizian, P.; Haydel, B.; Ruiz, R.M.; et al. Development and validation of a REcurrent Liver cAncer Prediction ScorE (RELAPSE) following liver transplantation in patients with hepatocellular carcinoma: Analysis of the US Multicenter HCC Transplant Consortium. Liver Transplant. 2023, 29, 683–697. [Google Scholar] [CrossRef]
- Mazzaferro, V.M.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Cheng, J.-M.; Chen, T.-W.; Zhang, X.-M.; Ou, J.; Cao, J.-M.; Li, H.-J. CT radiomics for prediction of microvascular invasion in hepatocellular carcinoma: A systematic review and meta-analysis. Clinics 2023, 78, 100264. [Google Scholar] [CrossRef]
- Famularo, S.; Penzo, C.; Maino, C.; Milana, F.; Oliva, R.; Marescaux, J.; Diana, M.; Romano, F.; Giuliante, F.; Ardito, F.; et al. Preoperative detection of hepatocellular carcinoma’s microvascular invasion on CT-scan by machine learning and radiomics: A preliminary analysis. Eur. J. Surg. Oncol. 2025, 51, 108274. [Google Scholar] [CrossRef]
- Cucchetti, A.; Piscaglia, F.; Grigioni, A.D.; Ravaioli, M.; Cescon, M.; Zanello, M.; Grazi, G.L.; Golfieri, R.; Grigioni, W.F.; Pinna, A.D. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: A pilot study. J. Hepatol. 2010, 52, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Heimbach, J.; Harnois, D.M.; Sapisochin, G.; Dodge, J.L.; Lee, D.; Burns, J.M.; Sanchez, W.; Greig, P.D.; Grant, D.R.; et al. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017, 3, 493–500. [Google Scholar] [CrossRef]
- Berenguer, M.; Burra, P.; Ghobrial, M.; Hibi, T.; Metselaar, H.; Sapisochin, G.M.; Bhoori, S.; Man, N.K.; Mas, V.; Ohira, M.; et al. Posttransplant Management of Recipients Undergoing Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-P.; Wang, K.; Wei, X.-B.; Li, L.-Q.; Sun, H.-C.; Wen, T.-F.; Chai, Z.-T.; Chen, Z.-H.; Shi, J.; Guo, W.-X.; et al. An Eastern Hepatobiliary Surgery Hospital Microvascular Invasion Scoring System in Predicting Prognosis of Patients with Hepatocellular Carcinoma and Microvascular Invasion After R0 Liver Resection: A Large-Scale, Multicenter Study. Oncologist 2019, 24, e1476–e1488. [Google Scholar] [CrossRef]
- Lei, Z.; Li, J.; Wu, D.; Xia, Y.; Wang, Q.; Si, A.; Wang, K.; Wan, X.; Lau, W.Y.; Wu, M.; et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg. 2016, 151, 356–363. [Google Scholar] [CrossRef]
- Endo, Y.; Alaimo, L.; Lima, H.A.; Moazzam, Z.; Ratti, F.; Marques, H.P.; Soubrane, O.; Lam, V.; Kitago, M.; Poultsides, G.A.; et al. A Novel Online Calculator to Predict Risk of Microvascular Invasion in the Preoperative Setting for Hepatocellular Carcinoma Patients Undergoing Curative-Intent Surgery. Ann. Surg. Oncol. 2023, 30, 725–733. [Google Scholar] [CrossRef]
- Merani, S.; Majno, P.; Kneteman, N.M.; Berney, T.; Morel, P.; Mentha, G.; Toso, C. The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma. J. Hepatol. 2011, 55, 814–819. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Avolio, A.W.; Graziadei, I.; Otto, G.; Rossi, M.; Tisone, G.; Goffette, P.; Vogel, W.; Pitton, M.B.; Lerut, J. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after loco-regional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013, 19, 1108–1118. [Google Scholar] [CrossRef]
- Sasaki, K.; Morioka, D.; Conci, S.; Margonis, G.A.; Sawada, Y.; Ruzzenente, A.; Kumamoto, T.; Iacono, C.; Andreatos, N.; Guglielmi, A.; et al. The Tumor Burden Score: A New “Metro-ticket” Prognostic Tool For Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann. Surg. 2018, 267, 132–141. [Google Scholar] [CrossRef]
- Vitale, A.; Lai, Q.; Farinati, F.; Bucci, L.; Giannini, E.G.; Napoli, L.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; et al. Utility of Tumor Burden Score to Stratify Prognosis of Patients with Hepatocellular Cancer: Results of 4759 Cases from ITA.LI.CA Study Group. J. Gastrointest. Surg. 2018, 22, 859–871. [Google Scholar] [CrossRef]
- Firl, D.J.; Sasaki, K.; Agopian, V.G.; Gorgen, A.; Kimura, S.; Dumronggittigule, W.; McVey, J.C.; Iesari, S.; Mennini, G.; Vitale, A.; et al. Charting the Path Forward for Risk Prediction in Liver Transplant for Hepatocellular Carcinoma: International Validation of HALTHCC Among 4089 Patients. Hepatology 2020, 71, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Sapisochin, G.; Gorgen, A.; Vitale, A.; Halazun, K.J.; Iesari, S.; Schaefer, B.; Bhangui, P.; Mennini, G.; Wong, T.C.; et al. Evaluation of the Intention-to-Treat Benefit of Living Donation in Patients with Hepatocellular Carcinoma Awaiting a Liver Transplant. JAMA Surg. 2021, 156, e213112. [Google Scholar] [CrossRef]
- Jackson, W.E.; Malamon, J.S.; Kaplan, B.; Saben, J.L.; Schold, J.D.; Pomposelli, J.J.; Pomfret, E.A. Survival Benefit of Living-Donor Liver Transplant. JAMA Surg. 2022, 157, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Akamatsu, N.; Fujiyoshi, M.; Kawaguchi, A.; Morita, S.; Kawasaki, S.; Uemoto, S.; Kokudo, N.; Hasegawa, K.; Ohdan, H.; et al. Expanded living-donor liver transplantation criteria for patients with hepatocellular carcinoma based on the Japanese nationwide survey: The 5-5-500 rule—A retrospective study. Transpl. Int. 2019, 32, 356–368. [Google Scholar] [CrossRef]
- Lai, Q.; Avolio, A.W.; Lerut, J.; Singh, G.; Chan, S.C.; Berloco, P.B.; Tisone, G.; Agnes, S.; Chok, K.S.; Sharr, W.; et al. Recurrence of hepatocellular cancer after liver transplantation: The role of primary resection and salvage transplantation in East and West. J. Hepatol. 2012, 57, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, S.; Mittler, J.; Theurer, J.; Ridder, D.A.; Marquardt, J.U.; Weinmann, A.; Scheuermann, U.; Otto, G.; Galle, P.R.; Straub, B.K.; et al. Microvascular invasion of hepatocellular carcinoma predicts microvascular invasion of its recurrence: Potential implications for salvage liver transplantation? Hepatobiliary Surg. Nutr. 2023, 12, 183–191. [Google Scholar] [CrossRef]
- Lin, C.-C.; Elsarawy, A.M.; Li, W.-F.; Lin, T.-L.; Yong, C.-C.; Wang, S.-H.; Wang, C.-C.; Kuo, F.-Y.; Cheng, Y.-F.; Chen, C.-L. Liver Transplantation for High Risk Hepatocellular Carcinoma After Liver Resection: A Sequential or Salvage Approach? Ann. Transplant. 2017, 22, 602–610. [Google Scholar] [CrossRef]
- Gondolesi, G.E.; Roayaie, S.; Muñoz, L.; Kim-Schluger, L.; Schiano, T.; Fishbein, T.M.; Emre, S.; Miller, C.M.; Schwartz, M.E. Adult living donor liver transplantation for patients with hepatocellular carcinoma: Extending UNOS priority criteria. Ann. Surg. 2004, 239, 142–149. [Google Scholar] [CrossRef]
- Fisher, R.A.; Kulik, L.M.; Freise, C.E.; Lok, A.S.F.; Shearon, T.H.; Brown, R.S., Jr.; Ghobrial, R.M.; Fair, J.H.; Olthoff, K.M.; Kam, I.; et al. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am. J. Transplant. 2007, 7, 1601–1608. [Google Scholar] [CrossRef]
- Lo, C.M.; Fan, S.T.; Liu, C.L.; Chan, S.C.; Ng, I.O.; Wong, J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br. J. Surg. 2007, 94, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Halazun, K.J.; Patzer, R.E.; Rana, A.A.; Verna, E.C.; Griesemer, A.D.; Parsons, R.F.; Samstein, B.; Guarrera, J.V.; Kato, T.; Brown, R.S.; et al. Standing the test of time: Outcomes of a decade of prioritizing patients with hepatocellular carcinoma, results of the UNOS natural geographic experiment. Hepatology 2014, 60, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Goldaracena, N.; Gorgen, A.; Doyle, A.; Hansen, B.E.; Tomiyama, K.; Zhang, W.; Ghanekar, A.; Lilly, L.; Cattral, M.; Galvin, Z.; et al. Live donor liver transplantation for patients with hepatocellular carcinoma offers increased survival vs. deceased donation. J. Hepatol. 2019, 70, 666–673. [Google Scholar] [CrossRef]
- Norman, J.S.; Li, P.J.; Kotwani, P.; Shui, A.M.; Yao, F.; Mehta, N. AFP-L3 and DCP strongly predict early hepatocellular carcinoma recurrence after liver transplantation. J. Hepatol. 2023, 79, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Vitale, A.; Iesari, S.; Finkenstedt, A.; Mennini, G.; Onali, S.; Hoppe-Lotichius, M.; Manzia, T.M.; Nicolini, D.; Avolio, A.W.; et al. The Intention-to-Treat Effect of Bridging Treatments in the Setting of Milan Criteria—In Patients Waiting for Liver Transplantation. Liver Transpl. 2019, 25, 1023–1033. [Google Scholar] [CrossRef]
- Kardashian, A.; Florman, S.S.; Haydel, B.; Ruiz, R.M.; Klintmalm, G.B.; Lee, D.D.; Taner, C.B.; Aucejo, F.; Tevar, A.D.; Humar, A.; et al. Liver Transplantation Outcomes in a U.S. Multicenter Cohort of 789 Patients with Hepatocellular Carcinoma Presenting Beyond Milan Criteria. Hepatology 2020, 72, 2014–2028. [Google Scholar] [CrossRef]
- Qin, S.; Chen, M.; O Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.C.; Zhou, J.; Wang, L.; Wen, X.; Heo, J.; et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1835–1847. [Google Scholar] [CrossRef]
- Tabrizian, P.; Florman, S.S.; Schwartz, M.E. PD-1 inhibitor as bridge therapy to liver transplantation? Am. J. Transplant. 2021, 21, 1979–1980. [Google Scholar] [CrossRef]
- Llovet, J.M.; Pinyol, R.; Yarchoan, M.; Singal, A.G.; Marron, T.U.; Schwartz, M.; Pikarsky, E.; Kudo, M.; Finn, R.S. Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2024, 21, 294–311. [Google Scholar] [CrossRef]
- Park, G.C.; Hwang, S.; You, Y.K.; Choi, Y.; Kim, J.M.; Joo, D.J.; Ryu, J.H.; Choi, D.; Kim, B.W.; Kim, D.S.; et al. Quantitative Prediction of Posttransplant Hepatocellular Carcinoma Prognosis Using ADV Score: Validation with Korea-Nationwide Transplantation Registry Database. J. Gastrointest. Surg. 2023, 27, 1353–1366. [Google Scholar] [CrossRef]
- Lai, Q.; Ito, T.; Iesari, S.; Ikegami, T.; Nicolini, D.; Laureiro, Z.L.; Rossi, M.; Vivarelli, M.; Yoshizumi, T.; Hatano, E.; et al. Role of protein induced by vitamin-K absence-II in transplanted patients with HCC not producing alpha-fetoprotein. Liver Transpl. 2024, 30, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Ghinolfi, D.; Lai, Q.; Dondossola, D.; De Carlis, R.; Zanierato, M.; Patrono, D.; Baroni, S.; Bassi, D.; Ferla, F.; Lauterio, A.; et al. Machine Perfusions in Liver Transplantation: The Evidence-Based Position Paper of the Italian Society of Organ and Tissue Transplantation. Liver Transpl. 2020, 26, 1298–1315. [Google Scholar] [CrossRef] [PubMed]
| Variables | Entire Population (N = 2170; 100.0%) | Training Set (n = 1519; 70.0%) | Validation Set (n = 651; 30.0%) | p-Value |
|---|---|---|---|---|
| Median (Q1–Q3) or n (%) | ||||
| Age, years | 59 (53–63) | 59 (53–64) | 58 (54–63) | 0.43 |
| Male sex | 1719 (79.2) | 1199 (78.9) | 520 (79.9) | 0.64 |
| Live donation | 967 (44.6) | 667 (43.9) | 300 (46.1) | 0.37 |
| Waiting time duration, months | 3 (1–7) | 3 (1–8) | 3 (1–7) | 0.01 |
| Underlying liver disease * | ||||
| HCV | 1015 (46.8) | 693 (45.6) | 322 (49.5) | 0.10 |
| HBV | 582 (26.8) | 418 (27.5) | 164 (25.2) | 0.27 |
| Alcohol | 126 (19.4) | 307 (20.2) | 126 (19.4) | 0.68 |
| NASH | 160 (7.4) | 117 (7.7) | 43 (6.6) | 0.42 |
| Other | 114 (5.3) | 80 (5.3) | 34 (5.2) | 1.00 |
| Lab-MELD | 12 (8–16) | 12 (8–16) | 12 (9–16) | 0.30 |
| Radiological features at entry | ||||
| Diameter of target lesion, cm | 2.5 (1.8–3.5) | 2.5 (1.8–3.8) | 2.5 (1.0–3.0) | 0.66 |
| Number of nodules | 1 (1–3) | 1 (1–3) | 1 (1–3) | 0.22 |
| Milan-OUT status | 679 (31.3) | 473 (31.1) | 206 (31.6) | 0.84 |
| Radiological features at LT | ||||
| Diameter of target lesion, cm | 2.2 (1.3–3.3) | 2.2 (1.3–3.3) | 2.1 (1.2–3.2) | 0.60 |
| Number of nodules | 1 (1–3) | 1 (1–3) | 1 (1–3) | 0.77 |
| Milan-OUT status | 608 (28.0) | 424 (27.9) | 184 (28.3) | 0.88 |
| TBS | 3.2 (2.0–4.6) | 3.2 (2.0–4.6) | 3.2 (2.0–4.6) | 0.99 |
| LRT number of treatments | 2 (1–3) | 2 (1–3) | 1 (0–3) | 0.68 |
| Radiological response mRECIST | ||||
| CR | 259 (11.9) | 185 (12.2) | 74 (11.4) | 0.61 |
| PR | 514 (23.7) | 357 (23.5) | 157 (24.1) | 0.78 |
| SD | 480 (22.1) | 321 (21.1) | 159 (24.4) | 0.09 |
| PD | 376 (17.3) | 279 (18.4) | 97 (14.9) | 0.06 |
| No LRT | 541 (24.9) | 377 (24.8) | 164 (25.2) | 0.87 |
| AFP, ng/mL | ||||
| At entry | 14 (5–55) | 14 (5–55) | 14 (6–56) | 0.93 |
| At LT | 10 (4–40) | 10 (4–38) | 4 (10–44) | 0.35 |
| NLR at LT | 2.8 (1.9–4.4) | 2.8 (1.9–4.3) | 2.9 (2.0–4.5) | 0.52 |
| PLR at LT | 68 (39–107) | 68 (38–108) | 68 (41–104) | 0.99 |
| Pathological features | ||||
| Diameter of target lesion, cm | 2.5 (1.5–3.5) | 2.5 (1.5–3.5) | 2.5 (1.5–3.5) | 0.62 |
| Number of nodules | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.38 |
| Milan-OUT status | 774 (35.7) | 533 (35.1) | 241 (37.0) | 0.41 |
| Poor grading | 263 (12.1) | 180 (11.8) | 83 (12.7) | 0.57 |
| MVI | 586 (27.0) | 397 (26.1) | 189 (29.0) | 0.17 |
| Variable | Univariable Analysis | Multivariable Analysis | ||||||||
| Beta | SE | OR | 95%CI | p-Value | Beta | SE | OR | 95%CI | p-Value | |
| Fast-track before LDLT | 0.90 | 0.12 | 2.45 | 1.94–3.10 | <0.001 | 0.69 | 0.12 | 1.99 | 1.56–2.53 | <0.001 |
| lnTBS at last imaging | 0.68 | 0.09 | 1.97 | 1.65–2.34 | <0.001 | 0.51 | 0.09 | 1.66 | 1.39–1.99 | <0.001 |
| lnAFP | 0.23 | 0.03 | 1.26 | 1.19–1.34 | <0.001 | 0.18 | 0.03 | 1.19 | 1.13–1.27 | <0.001 |
| NASH | 0.54 | 0.20 | 1.72 | 1.16–2.55 | 0.007 | - | - | - | - | - |
| Age, years | −0.02 | 0.007 | 0.98 | 0.97–1.00 | 0.007 | - | - | - | - | - |
| Waiting time, months | −0.01 | 0.007 | 0.99 | 0.97–1.00 | 0.03 | - | - | - | - | - |
| Lab-MELD | −0.02 | 0.01 | 0.98 | 0.96–1.00 | 0.06 | - | - | - | - | - |
| lnPLR | 0.11 | 0.06 | 1.11 | 0.99–1.25 | 0.07 | - | - | - | - | - |
| Male sex | 0.25 | 0.15 | 1.28 | 0.96–1.72 | 0.10 | - | - | - | - | - |
| HBV | 0.20 | 0.13 | 1.22 | 0.95–1.57 | 0.13 | - | - | - | - | - |
| HCV | −0.15 | 0.12 | 0.86 | 0.68–1.08 | 0.19 | - | - | - | - | - |
| Alcohol | −0.05 | 0.15 | 0.95 | 0.72–1.27 | 0.75 | - | - | - | - | - |
| lnNLR | 0.02 | 0.08 | 1.02 | 0.87–1.19 | 0.81 | - | - | - | - | - |
| Constant | - | - | - | - | - | −2.50 | 0.16 | 0.08 | - | <0.001 |
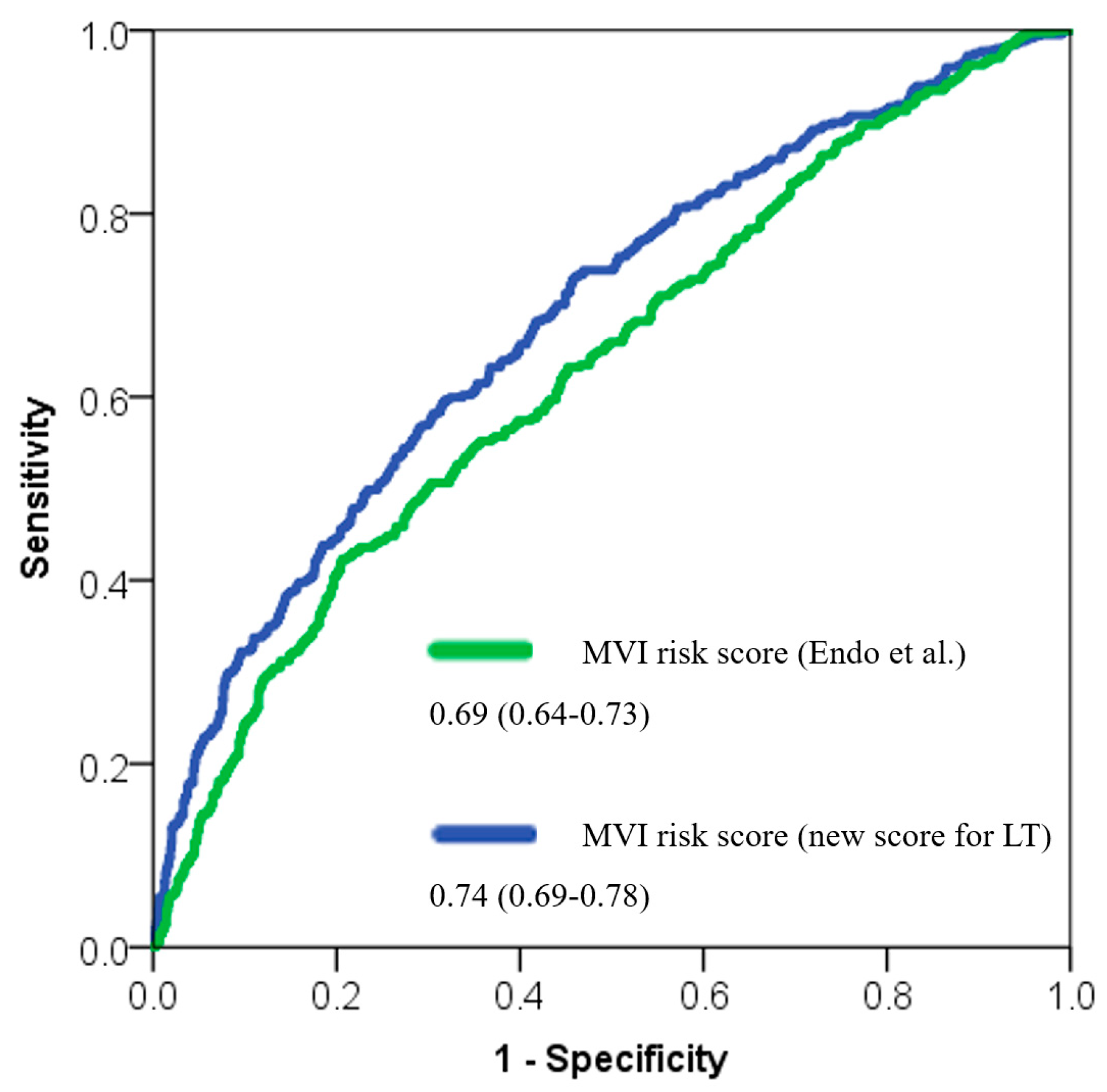
| Score | SE | AUC | 95%CI | p-Value | Brier Skill Score | Brier Skill Score (%) | |
|---|---|---|---|---|---|---|---|
| LT-MVI risk score | 0.02 | 0.74 | 0.69–0.78 | <0.001 | 0.2456 | Ref. | |
| MVI risk score by Endo et al. [16] | 0.02 | 0.69 | 0.64–0.73 | <0.001 | 0.4286 | −0.75 | |
| Nomogram by Lei et al. [15] | 0.02 | 0.69 | 0.65–0.73 | <0.001 | 0.2618 | −0.07 | |
| EHBH MVI score [14] | 0.02 | 0.68 | 0.64–0.73 | <0.001 | 0.2995 | −0.22 | |
| Different cut-offs of the LT-MVI score | |||||||
| Decile | % | Sens | Spec | DOR | |||
| 50 | 23.6 | 72.0 | 58.4 | 3.61 | |||
| 75 | 32.7 | 58.2 | 77.3 | 4.74 | |||
| 95 | 52.6 | 29.6 | 93.3 | 5.85 | |||
| NRI of the LT-MVI Score Versus the MVI Score Proposed by Endo et al. [16] |
|---|
| Events: (number of events with increased predicted risk − number of events with decreased predicted risk)/number of events 122-57/189 = 34.4% |
| Non-events: (number of non-events with decreased predicted risk − number of non-events with increased predicted risk)/number of non-events 284-161/462 = 26.6% |
| Overall NRI: event NRI + non-event NRI 0.344 + 0.266 = 0.610 |
| Net benefit |
| 50th percentile: (136–191)/651 × 0.236/(1 − 0.236) = −0.02610 (no net benefit) 75th percentile: (110–104)/651 × 0.327/(1 − 0.327) = 0.00448 (1 in 223) 95th percentile: (56–31)/651 × 0.526/(1 − 0.526) = 0.04262 (1 in 24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Q.; Pawlik, T.M.; Ajdini, S.; Emond, J.; Halazun, K.; Soin, A.S.; Bhangui, P.; Yoshizumi, T.; Toshima, T.; Panzer, M.; et al. Development and Validation of a Pre-Transplant Risk Score (LT-MVI Score) to Predict Microvascular Invasion in Hepatocellular Carcinoma Candidates for Liver Transplantation. Cancers 2025, 17, 1418. https://doi.org/10.3390/cancers17091418
Lai Q, Pawlik TM, Ajdini S, Emond J, Halazun K, Soin AS, Bhangui P, Yoshizumi T, Toshima T, Panzer M, et al. Development and Validation of a Pre-Transplant Risk Score (LT-MVI Score) to Predict Microvascular Invasion in Hepatocellular Carcinoma Candidates for Liver Transplantation. Cancers. 2025; 17(9):1418. https://doi.org/10.3390/cancers17091418
Chicago/Turabian StyleLai, Quirino, Timothy M. Pawlik, Suela Ajdini, Jean Emond, Karim Halazun, Arvinder S. Soin, Prashant Bhangui, Tomoharu Yoshizumi, Takeo Toshima, Marlene Panzer, and et al. 2025. "Development and Validation of a Pre-Transplant Risk Score (LT-MVI Score) to Predict Microvascular Invasion in Hepatocellular Carcinoma Candidates for Liver Transplantation" Cancers 17, no. 9: 1418. https://doi.org/10.3390/cancers17091418
APA StyleLai, Q., Pawlik, T. M., Ajdini, S., Emond, J., Halazun, K., Soin, A. S., Bhangui, P., Yoshizumi, T., Toshima, T., Panzer, M., Schaefer, B., Hoppe-Lotichius, M., Mittler, J., Ito, T., Hatano, E., Rossi, M., Chan, A. C. Y., Wong, T., Chen, C.-L., ... Lerut, J. P. (2025). Development and Validation of a Pre-Transplant Risk Score (LT-MVI Score) to Predict Microvascular Invasion in Hepatocellular Carcinoma Candidates for Liver Transplantation. Cancers, 17(9), 1418. https://doi.org/10.3390/cancers17091418










