Identification of Adjustment Variables in Indirect Comparisons: A Rapid Review of CAR-T Therapies for Diffuse Large B-Cell Lymphoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
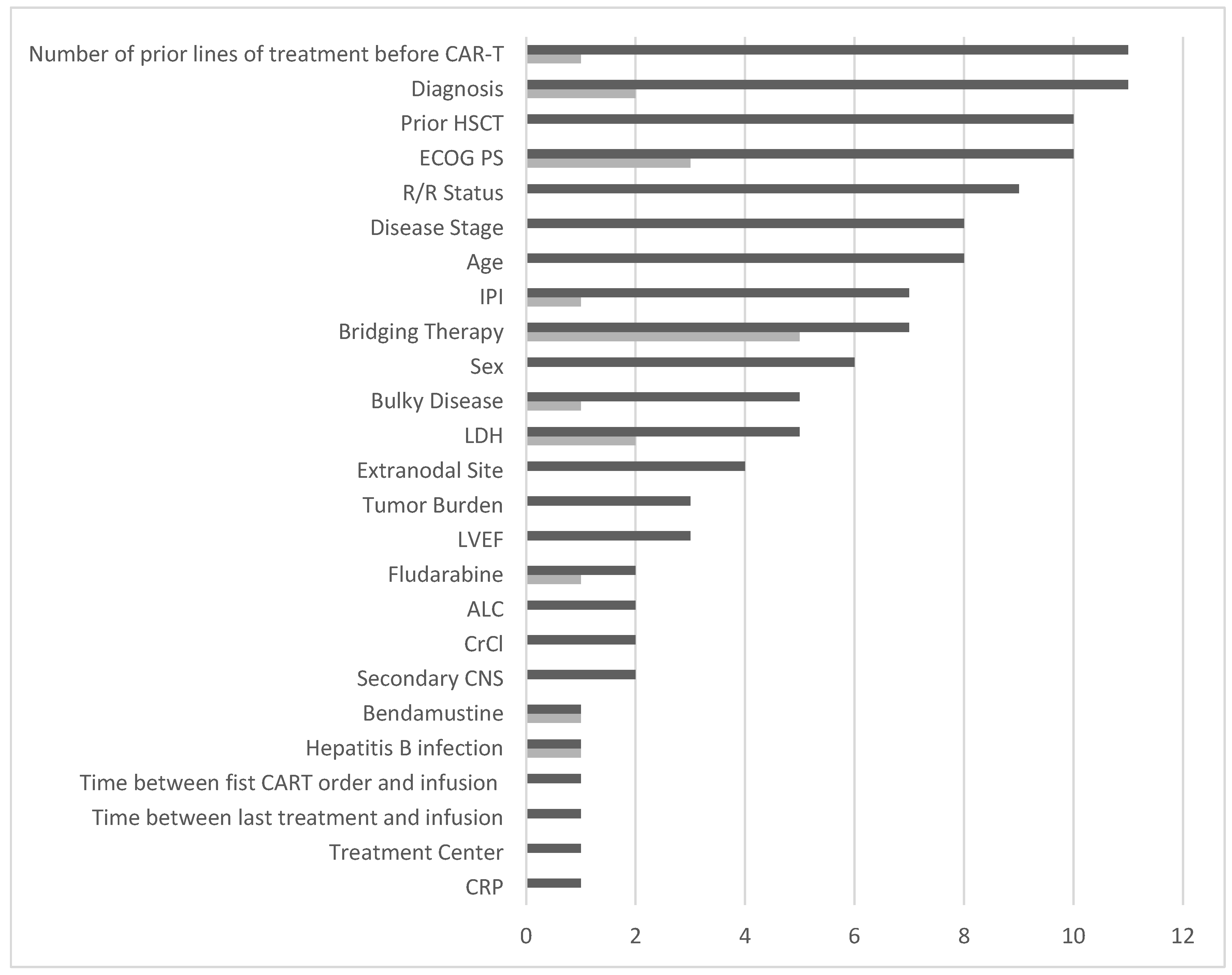
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gotti, M.; Defrancesco, I.; D’angelo, M.; Basso, S.; Crotto, L.; Marinelli, A.; Maccalli, C.; Iaconianni, V. Cancer Immunotherapy Using Chimeric Antigen Receptor Expressing T-Cells: Present and Future Needs of Clinical Cancer Centers. Front. Immunol. 2020, 11, 565236. [Google Scholar] [CrossRef] [PubMed]
- Boyiadzis, M.M.; Dhodapkar, M.V.; Brentjens, R.J.; Kochenderfer, J.N.; Neelapu, S.S.; Maus, M.V.; Porter, D.L.; Maloney, D.G.; Grupp, S.A.; Mackall, C.L.; et al. Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: Clinical perspective and significance. J. Immunother. Cancer. 2018, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Zelenetz, A.D.; Gordon, L.I.; Wierda, W.G.; Abramson, J.S.; Advani, R.H.; Andreadis, C.B.; Bartlett, N.; Byrd, J.C.; Fayad, L.E.; Fisher, R.I.; et al. Diffuse Large B-Cell Lymphoma Version 1.2016. J. Natl. Compr. Canc Netw. 2016, 14, 196–231. [Google Scholar] [CrossRef] [PubMed]
- Shargian, L.; Raanani, P.; Yeshurun, M.; Gafter-Gvili, A.; Gurion, R. Chimeric antigen receptor T-cell therapy is superior to standard of care as second-line therapy for large B-cell lymphoma: A systematic review and meta-analysis. Br. J. Haematol. 2022, 198, 838–846. [Google Scholar] [CrossRef]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef]
- Fiorenza, S.; Ritchie, D.S.; Ramsey, S.D.; Turtle, C.J.; Roth, J.A. Value and affordability of CAR T-cell therapy in the United States. Bone Marrow Transplant. 2020, 55, 1706–1715. [Google Scholar] [CrossRef]
- Jagannath, S.; Joseph, N.; Crivera, C.; Kharat, A.; Jackson, C.C.; Valluri, S.; Cost, P.; Phelps, H.; Slowik, R.; Klein, T.; et al. Component Costs of CAR-T Therapy in Addition to Treatment Acquisition Costs in Patients with Multiple Myeloma. Oncol. Ther. 2023, 11, 263–275. [Google Scholar] [CrossRef]
- Steinke, D. Essentials of Pharmacoepidemiology. In Clinical Pharmacy Education, Practice and Research; Dixon, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 203–214. [Google Scholar]
- Beneciuk, J.M.; George, S.Z.; Patterson, C.G.; Smith, C.N.; Brennan, G.P.; Wegener, S.T.; Roseen, E.J.; Saper, R.B.; Delitto, A. Treatment effect modifiers for individuals with acute low back pain: Secondary analysis of the TARGET trial. Pain 2023, 164, 171–179. [Google Scholar] [CrossRef]
- Phillippo, D.M.; Ades, A.E.; Dias, S.; Palmer, S.; Abrams, K.R.; Welton, N.J. Methods for Population-Adjusted Indirect Comparisons in Health Technology Appraisal. Med. Decis. Making. 2018, 38, 200–211. [Google Scholar] [CrossRef]
- Phillippo, D.M.; Dias, S.; Elsada, A.; Ades, A.E.; Welton, N.J. Population Adjustment Methods for Indirect Comparisons: A Review of National Institute for Health and Care Excellence Technology Appraisals. Int. J. Technol. Assess. Health Care 2019, 35, 221–228. [Google Scholar] [CrossRef]
- Pufulete, M.; Mahadevan, K.; Johnson, T.W.; Pithara, C.; Redwood, S.; Benedetto, U.; Higgins, J.P.; Reeves, B.C. Confounders and co-interventions identified in non-randomized studies of interventions. J. Clin. Epidemiol. 2022, 148, 115–123. [Google Scholar] [CrossRef] [PubMed]
- IQWIG General Methods—Version 7.0, Cologne. 2023. Available online: https://www.iqwig.de/methoden/general-methods_version-7-0.pdf (accessed on 2 December 2024).
- Vanier, A.; Fernandez, J.; Kelley, S.; Alter, L.; Semenzato, P.; Alberti, C.; Chevret, S.; Costagliola, D.; Cucherat, M.; Falissard, B.; et al. Rapid access to innovative medicinal products while ensuring relevant health technology assessment. Position of the French National Authority for Health. BMJ Evid. Based Med. 2024, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Borchmann, P.; Heger, J.-M.; Mahlich, J.; Papadimitrious, M.S.; Riou, S.; Werner, B. Healthcare Resource Utilization and Associated Costs of German Patients with Diffuse Large B-Cell Lymphoma: A Retrospective Health Claims Data Analysis. Oncol. Ther. 2023, 11, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Borchmann, P.; Heger, J.-M.; Mahlich, J.; Papadimitrious, M.S.; Riou, S.; Werner, B. Survival outcomes of patients newly diagnosed with diffuse large B-cell lymphoma: Real-world evidence from a German claims database. J. Cancer Res. Clin. Oncol. 2023, 149, 7091–7101. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wettersten, H.I.; Ganti, S.; Weiss, R.H. Metabolomic profiling of tumor-bearing mice. Methods Enzymol. 2014, 543, 275–296. [Google Scholar]
- Kent, P.; Cancelliere, C.; Boyle, E.; Cassidy, J.D.; Kongsted, A. A conceptual framework for prognostic research. BMC Med. Res. Methodol. 2020, 20, 172. [Google Scholar] [CrossRef]
- Bachy, E.; Le Gouill, S.; Di Blasi, R.; Sesques, P.; Manson, G.; Cartron, G.; Beauvais, D.; Roulin, L.; Gros, F.X.; Rubio, M.T.; et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat. Med. 2022, 28, 2145–2154. [Google Scholar] [CrossRef]
- Cartron, G.; Fox, C.P.; Liu, F.F.; Kostic, A.; Hasskarl, J.; Li, D.; Bonner, A.; Zhang, Y.; Maloney, D.G.; Kuruvilla, J. Matching-adjusted indirect treatment comparison of chimeric antigen receptor T-cell therapies for third-line or later treatment of relapsed or refractory large B-cell lymphoma: Lisocabtagene maraleucel versus tisagenlecleucel. Exp. Hematol. Oncol. 2022, 11, 17. [Google Scholar] [CrossRef]
- Maloney, D.G.; Kuruvilla, J.; Liu, F.F.; Kostic, A.; Kim, Y.; Bonner, A.; Zhang, Y.; Fox, C.P.; Cartron, G. Matching-adjusted indirect treatment comparison of liso-cel versus axi-cel in relapsed or refractory large B cell lymphoma. J. Hematol. Oncol. 2021, 14, 140. [Google Scholar] [CrossRef]
- Maziarz, R.T.; Zhang, J.; Yang, H.; Chai, X.; Yuan, C.; Schwarz, E.; Jakovac, M.; Martinez-Prieto, M.; Agarwal, A.; Degtyarev, E.; et al. Indirect comparison of tisagenlecleucel and historical treatments for relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2022, 6, 2536–2547. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, O.O.; Jansen, J.P.; Lin, V.W.; Chan, K.; Keeping, S.; Navale, L.; Locke, F.L. Comparing Efficacy, Safety, and Preinfusion Period of Axicabtagene Ciloleucel versus Tisagenlecleucel in Relapsed/Refractory Large B Cell Lymphoma. Biol. Blood Marrow Transplant. 2020, 26, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Spin, P.; Liu, F.F.; Garcia, J.; Kim, Y.; Hasskarl, J. Indirect Treatment Comparison of Liso-Cel vs. Salvage Chemotherapy in Diffuse Large B-Cell Lymphoma: TRANSCEND vs. SCHOLAR-1. Adv Ther. 2021, 38, 3266–3280. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.; Wang, M.L.; Arnason, J.E.; Purev, E.; Maloney, D.G.; Andreadis, C.B.; Sehgal, A.; et al. Two-year follow-up of lisocabtagene maraleucel in relapsed or refractory large B-cell lymphoma in TRANSCEND NHL 001. Blood 2024, 143, 404–416. [Google Scholar] [CrossRef]
- Schuster, S.J.; Zhang, J.; Yang, H.; Agarwal, A.; Tang, W.; Martinez-Prieto, M.; Bollu, V.; Kuzan, D.; Maziarz, R.T.; Kersten, M.J. Comparative efficacy of tisagenlecleucel and lisocabtagene maraleucel among adults with relapsed/refractory large B-cell lymphomas: An indirect treatment comparison. Leuk. Lymphoma 2022, 63, 845–854. [Google Scholar] [CrossRef]
- Weinstein, B.; Muresan, B.; Solano, S.; de Macedo, A.V.; Lee, Y.; Su, Y.-C.; Ahn, Y.; Henriquez, G.; Carmago, C.; Kim, G.-J.; et al. Efficacy and Safety of Innovative Experimental Chimeric Antigen Receptor (CAR) T-cells versus Axicabtagene ciloleucel (Yescarta) for the Treatment of Relapsed/Refractory Large B-Cell Lymphoma (LBCL): Matching Adjusted Indirect Comparisons (MAICs) and Systematic Review. Innov. Pharm. 2021, 12. [Google Scholar] [CrossRef]
- Oluwole, O.; Jansen, J.; Lin, V.; Chan, K.; Navale, L.; Kim, J.; Locke, F. PCN445 Indirect treatment comparison of axicabtagene ciloleucel (axi-cel) versus tisagen- lecleucel (tisa-cel) in relapsed/refractory large B cell lymphoma (RR-LBCL). Value Health 2019, 22 (Suppl. 3), S522. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Ma, Q.; Yang, H.; Signorovitch, J.; Wu, E. A Review of Two Regulatory Approved Anti-CD19 CAR T-Cell Therapies in Diffuse Large B-Cell Lymphoma: Why Are Indirect Treatment Comparisons Not Feasible? Adv. Ther. 2020, 37, 3040–3058. [Google Scholar] [CrossRef]
- Moradi-Lakeh, M.; Yaghoubi, M.; Seitz, P.; Javanbakht, M.; Brock, E. Cost-Effectiveness of Tisagenlecleucel in Paediatric Acute Lymphoblastic Leukaemia (pALL) and Adult Diffuse Large B-Cell Lymphoma (DLBCL) in Switzerland. Adv. Ther. 2021, 38, 3427–3443. [Google Scholar] [CrossRef]
- Oluwole, O.O.; Chen, J.M.; Chan, K.; Patel, A.R.; Jansen, J.P.; Keeping, S.; Zheng, Y.; Snider, J.T.; Locke, F.L. Matching-adjusted indirect comparison of axi-cel and liso-cel in relapsed or refractory large B-cell lymphoma. Leuk. Lymphoma 2022, 63, 3052–3062. [Google Scholar] [CrossRef]
- Shipp, M.A. International Non-Hodgkin’s Lymphoma Prognostic Factors Project Apredictive model for aggressive non-Hodgkin’s lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [Google Scholar]
- Ruppert, A.S.; Dixon, J.G.; Salles, G.A.; Wall, A.; Cunningham, D.; Poeschel, V.; Haioun, C.; Tilly, H.; Ghesquieres, H.; Ziepert, M.; et al. International prognostic indices in diffuse large B-cell lymphoma: A comparison of IPI, R-IPI, and NCCN-IPI. Blood 2020, 135, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Recio, M.; Wudhikarn, K.; Pennisi, M.; Alonso-Trillo, R.; Flynn, J.; Shouval, R.; Afuye, A.O.; Silverberg, M.L.; Batlevi, C.W.; Dahi, P.; et al. The International Prognostic Index Is Associated with Outcomes in Diffuse Large B Cell Lymphoma after Chimeric Antigen Receptor T Cell Therapy. Transplant. Cell Ther. 2021, 27, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, L.; Di Blasi, R.; Kanoun, S.; Tessoulin, B.; Rossi, C.; D’Aveni-Piney, M.; Obéric, L.; Bodet-Milin, C.; Bories, P.; Olivier, P.; et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020, 4, 5607–5615. [Google Scholar] [CrossRef]
- Di Blasi, R.; Le Gouill, S.; Bachy, E.; Cartron, G.; Beauvais, D.; Le Bras, F.; Gros, F.-X.; Choquet, S.; Bories, P.; Feugier, P.; et al. Outcomes of patients with aggressive B-cell lymphoma after failure of anti-CD19 CAR T-cell therapy: A DESCAR-T analysis. Blood 2022, 140, 2584–2593. [Google Scholar] [CrossRef]
- Duarte, C.; Kamdar, M. Management Considerations for Patients With Primary Refractory and Early Relapsed Diffuse Large B-Cell Lymphoma. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e390802. [Google Scholar] [CrossRef]
- Westin, J.; Sehn, L.H. CAR T cells as a second-line therapy for large B-cell lymphoma: A paradigm shift? Blood 2022, 139, 2737–2746. [Google Scholar] [CrossRef]
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef]
- Rosenwald, A.; Wright, G.; Chan, W.C.; Connors, J.M.; Campo, E.; Fisher, R.I.; Gascoyne, R.D.; Muller-Hermelink, H.K.; Smeland, E.B.; Giltnane, J.M.; et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 1937–1947. [Google Scholar] [CrossRef]
- Kuhnl, A.; Roddie, C.; Kirkwood, A.A.; Tholouli, E.; Menne, T.; Patel, A.; Besley, C.; Chaganti, S.; Sanderson, R.; O’Reilly, M.; et al. A national service for delivering CD19 CAR-Tin large B-cell lymphoma—The UK real-world experience. Br. J. Haematol. 2022, 198, 492–502. [Google Scholar] [CrossRef]
- Roddie, C.; Neill, L.; Osborne, W.; Iyengar, S.; Tholouli, E.; Irvine, D.; Chaganti, S.; Besley, C.; Bloor, A.; Jones, C.; et al. Effective bridging therapy can improve CD19 CAR-T outcomes while maintaining safety in patients with large B-cell lymphoma. Blood Adv. 2023, 7, 2872–2883. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Hüttmann, A.; Müller, S.P.; Hanoun, M.; Boellaard, R.; Brinkmann, M.; Jöckel, K.-H.; Dührsen, U.; Rekowski, J. Dynamic risk assessment based on positron emission tomography scanning in diffuse large B-cell lymphoma: Post-hoc analysis from the PETAL trial. Eur. J. Cancer. 2020, 124, 25–36. [Google Scholar] [CrossRef]
- Kostakoglu, L.; Mattiello, F.; Martelli, M.; Sehn, L.H.; Belada, D.; Ghiggi, C.; Chua, N.; González-Barca, E.; Hong, X.; Pinto, A.; et al. Total metabolic tumor volume as a survival predictor for patients with diffuse large B-cell lymphoma in the GOYA study. Haematologica 2022, 107, 1633–1642. [Google Scholar] [CrossRef]
- Kostakoglu, L.; Dalmasso, F.; Berchialla, P.; Pierce, L.A.; Vitolo, U.; Martelli, M.; Sehn, L.H.; Trněný, M.; Nielsen, T.G.; Bolen, C.R.; et al. A prognostic model integrating PET-derived metrics and image texture analyses with clinical risk factors from GOYA. EJHaem 2022, 3, 406–414. [Google Scholar] [CrossRef]
- Thieblemont, C.; Chartier, L.; Dührsen, U.; Vitolo, U.; Barrington, S.F.; Zaucha, J.M.; Vercellino, L.; Silva, M.G.; Patrocinio-Carvalho, I.; Decazes, P.; et al. A tumor volume and performance status model to predict outcome before treatment in diffuse large B-cell lymphoma. Blood Adv. 2022, 6, 5995–6004. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Ho, A.D.; Cavallin-Stahl, E.; Wolf, M.; Pettengell, R.; Vasova, I.; Belch, A.; Walewski, J.; Zinzani, P.-L.; Mingrone, W.; et al. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: An exploratory analysis of the MabThera International Trial Group (MInT) study. Lancet Oncol. 2008, 9, 435–444. [Google Scholar]
- Stella, F.; Pennisi, M.; Chiappella, A.; Casadei, B.; Bramanti, S.; Ljevar, S.; Chiusolo, P.; Di Rocco, A.; Tisi, M.C.; Angelillo, P.; et al. Prospective Validation of CAR-HEMATOTOX and a Simplified Version Predict Survival in Patients with Large B-Cell Lymphoma Treated with Anti-CD19 CAR T-Cells: Data from CART-SIE Study. Transplant. Cell Ther. 2025, 31, 240.e1–240.e9. [Google Scholar] [CrossRef]
- Aspioti, M.; Siciliano, P. CAR-T & Beyond: CGTs In Development in 2022, Cell & Gene. 8 September 2022. Available online: https://www.cellandgene.com/doc/car-t-beyond-cgts-in-development-in-0001 (accessed on 30 November 2024).

| Technique | Indirect comparison*, treatment comparison*, simulated treatment comparison*, STC, network meta analys*, NMA, MAIC, matching adjusted indirect comparison*, adjusted comparison*, comparing efficacy, real world comparison*, comparative efficacy |
| Drug | chimeric antigen receptor T-cell therap*, CAR T*, tisagenlecleucel, tisa-cel, Kymriah, axicabtagene ciloleucel, axi-cel, Yescarta, lisocabtagene maraleucel, liso-cel, Breyanzi |
| Indication | diffuse large B cell lymphoma, large B cell lymphoma, DLBCL, LBCL |
| Comparison | Confounders | Endpoints | Method | Publication |
|---|---|---|---|---|
| Axi-cel vs. tisa-cel (third line) |
| OS and PFS for all confounders | Univariate prognostic analyses Type of ITC: Propensity score matching (PSM) Inverse probability of treatment weighting was used to support the findings of PSM analysis and to allow for proper comparison between the two populations | Bachy, et al. [20] |
| Liso-cel vs. tisa-cel (third line) | Clinical factors included in the primary and sensitivity analyses:
| CR, ORR, OS, and PFS | Literature search reviewed by a panel of external clinical experts (ranked list using classification or survival-based random forest models) Type of ITC: unanchored MAIC + propensity score matching | Cartron, et al. [21] |
| Liso-cel vs. axi-cel (third line) | Clinical factors included in the primary and sensitivity analyses:
| Efficacy: ORR, CRR, PFS, and OSSafety: all grade and grade ≥ 3 CRS, neurological events, grade ≥ 3 infections, hypogammaglobulinemia, grade ≥ 3 prolonged cytopenia | Literature search and input from a panel of five external clinical experts (Canada, France, Germany, UK, US) (ranked list using statistical random forest models) Type of ITC: unanchored MAIC + Propensity score weighting | Maloney, et al. [22] |
| Tisa-cel vs. SOC (salvage therapy, CORAL cohort) (third line) | Confounders identified as very important and included in the matching analysis:
| OS and ORR for all confounders | Systemic literature search and clinical experts’ inputs (ranking with “not important”, “less important”, and “very important”) Type of ITC: Propensity score weighting based on both standardized mortality ratio weight (SMRW) and fine stratification weight (FSW) | Maziarz, et al. [23] |
| Axi-cel vs. tisa-cel (third line) |
| ORR, CR, and OS for all covariates | Inputs from clinical experts (identification and ranking) Type of ITC: unanchored MAIC + propensity score weighting | Oluwole, et al. [24] |
| Liso-cel vs. SOC (salvage therapy) (third line) |
| OS, CRR, and ORR for all covariates | Baseline characteristics reported in TRANSCEND and SCHOLAR-1 Type of ITC: unanchored MAIC + propensity score weighting | Salles, et al. [25] |
| Tisa-cel vs. liso-cel(3rd line) |
| ORR, CRR, OS, and PFS for all confounders | Input from clinical experts (identification and validation) and factors considered important by Abramson, et al. [26] Type of ITC: MAIC + propensity score weighting | Schuster, et al. [27] |
| Axi-cel vs. pooled experimental CAR-Ts (third line) |
| Efficacy: PFS for all covariates Safety: grade ≥3 CRS and neurotoxicity for all covariates | Selection of covariates following the NICE Guidelines [10] and confirmed by clinicians and experts Type of ITC:unanchored MAIC | Weinstein, et al. [28] |
| Tisa-cel vs. axi-cel (third line) |
| OS for all endpoints | Literature research (Oluwole, et al. [29]) and patient-level data from JULIET trial Type of ITC: MAIC + propensity score matching, and simulated treatment comparison (STC) | Zhang, et al. [30] |
| Tisa-cel vs. salvage chemo (third line) | Key prognostic factors include:
| OS | No methodology disclosed Type of ITC: MAIC | Moradi-Lakeh, et al. [31] |
| Axi-cel vs. Liso-cel (third line) | Effect modifiers included in the MAIC:
Other variables not included in the primary MAIC but included in the post hoc sensitivity analysis:
| Efficacy: DoR, PFS, and OS for all effect modifiers Safety: CRS, neurological events for all effect modifiers | Effect modifiers were ranked according to clinical relevance based on recommendations from clinical experts and data availability Type of ITC: MAIC + propensity score weighting | Oluwole, et al. [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riou, S.; Rungaldier, S.; Mahlich, J. Identification of Adjustment Variables in Indirect Comparisons: A Rapid Review of CAR-T Therapies for Diffuse Large B-Cell Lymphoma. Cancers 2025, 17, 1335. https://doi.org/10.3390/cancers17081335
Riou S, Rungaldier S, Mahlich J. Identification of Adjustment Variables in Indirect Comparisons: A Rapid Review of CAR-T Therapies for Diffuse Large B-Cell Lymphoma. Cancers. 2025; 17(8):1335. https://doi.org/10.3390/cancers17081335
Chicago/Turabian StyleRiou, Sybille, Stefanie Rungaldier, and Jörg Mahlich. 2025. "Identification of Adjustment Variables in Indirect Comparisons: A Rapid Review of CAR-T Therapies for Diffuse Large B-Cell Lymphoma" Cancers 17, no. 8: 1335. https://doi.org/10.3390/cancers17081335
APA StyleRiou, S., Rungaldier, S., & Mahlich, J. (2025). Identification of Adjustment Variables in Indirect Comparisons: A Rapid Review of CAR-T Therapies for Diffuse Large B-Cell Lymphoma. Cancers, 17(8), 1335. https://doi.org/10.3390/cancers17081335







