Increased Risk of Early-Onset Endometrial Cancer in Women Aged 20–39 Years with Non-Alcoholic Fatty Liver Disease: A Nationwide Cohort Study
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Population
2.3. Anthropometrics and Laboratory Measurements
2.4. Definition of NAFLD
2.5. Study Outcome
2.6. Clinical Variables
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
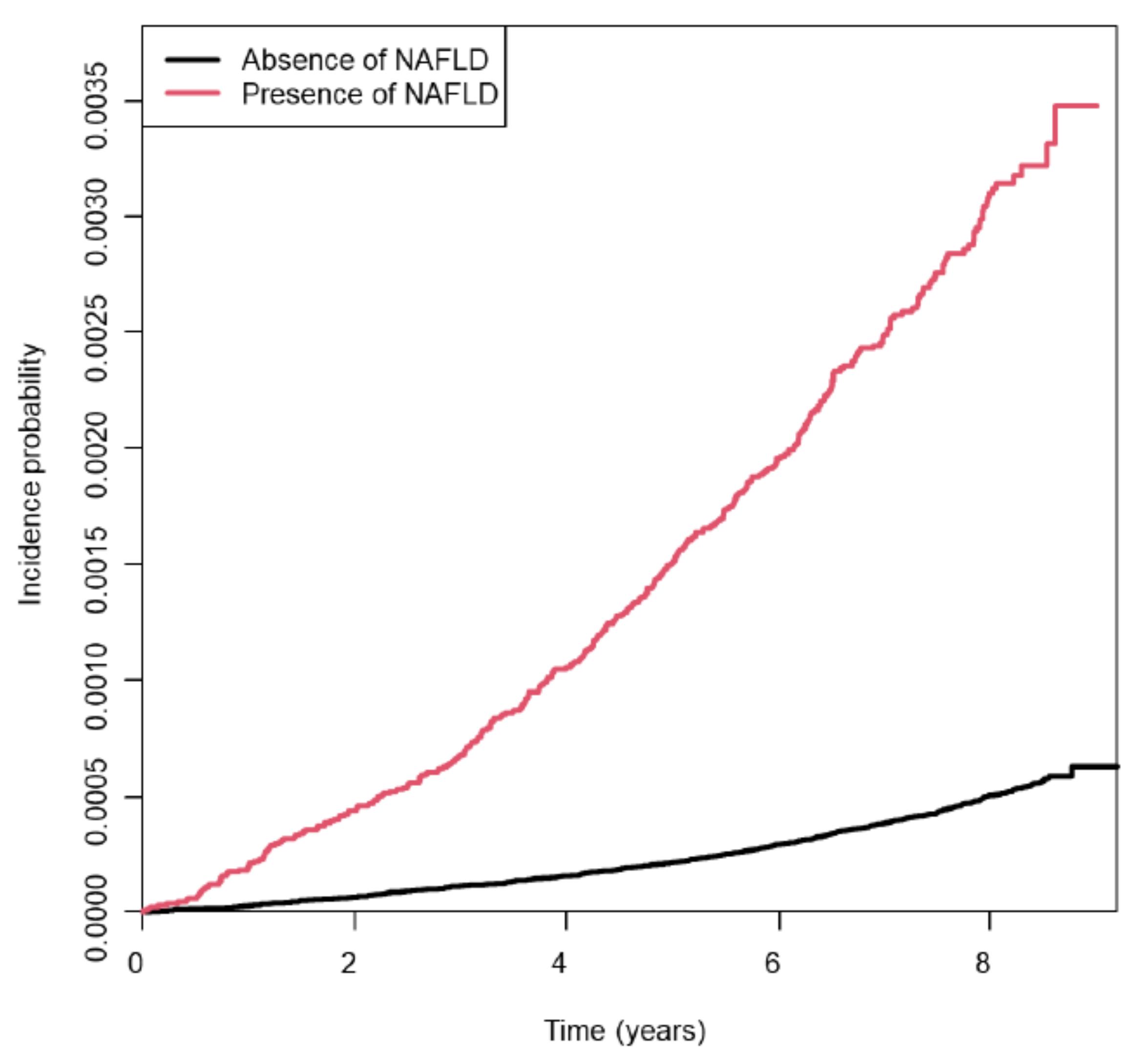
3.2. Risk of Young-Onset Endometrial Cancer According to NAFLD Status
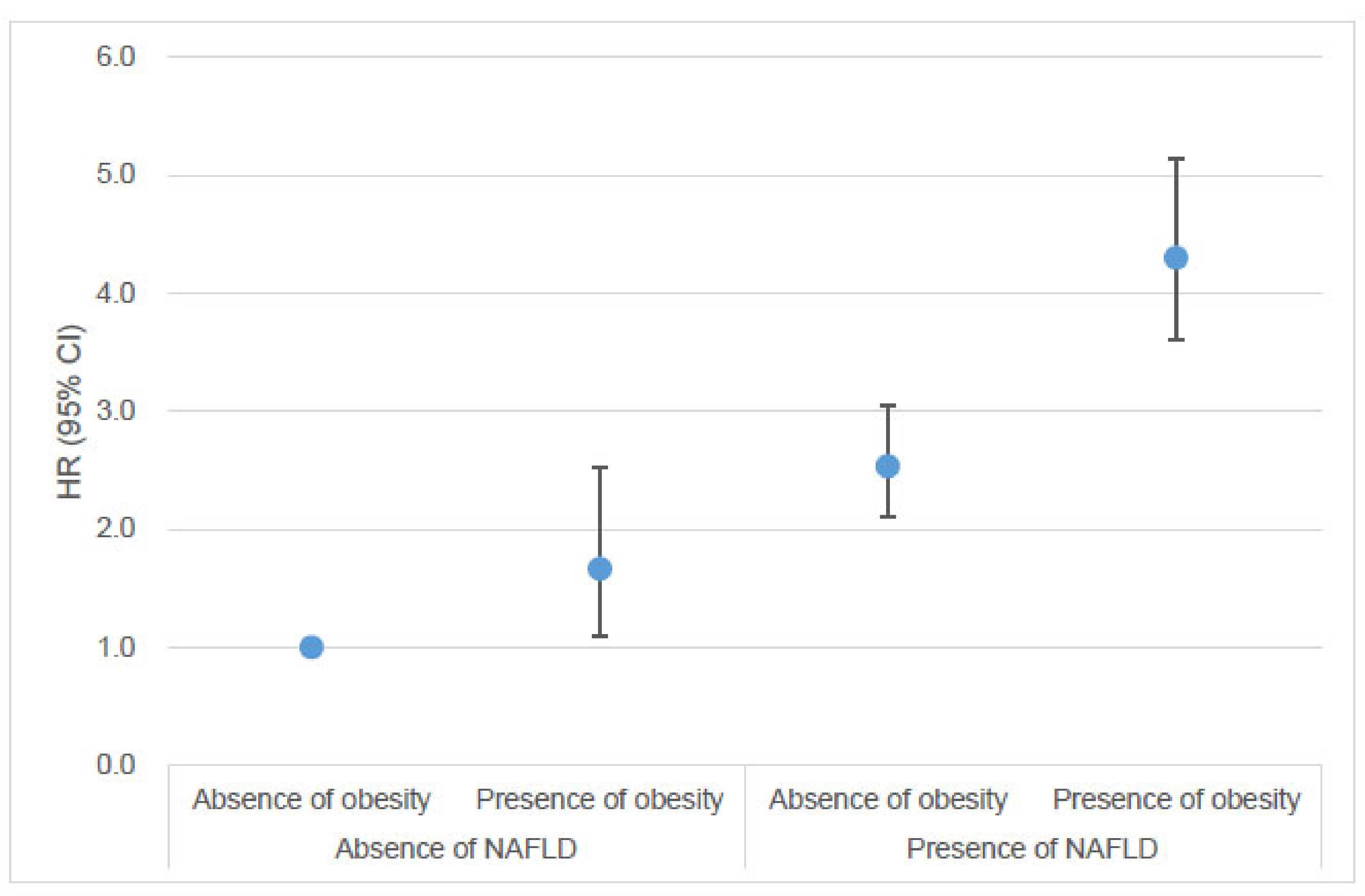
3.3. Risk of Young-Onset Endometrial Cancer According to the Combination of NAFLD and Obesity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated projection of US cancer incidence and death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Broaddus, R.R. Endometrial cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Rosenberg, P.S.; Jemal, A. Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry. Lancet Public Health 2019, 4, e137–e147. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef]
- Ugai, T.; Sasamoto, N.; Lee, H.Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E.; et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef]
- Bassette, E.; Ducie, J.A. Endometrial cancer in reproductive-aged females: Etiology and pathogenesis. Biomedicines 2024, 12, 886. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Soslow, R.A. Endometrial carcinoma in women aged 40 years and younger. Arch. Pathol. Lab. Med. 2014, 138, 335–342. [Google Scholar] [CrossRef]
- Son, J.; Carr, C.; Yao, M.; Radeva, M.; Priyadarshini, A.; Marquard, J.; Michener, C.M.; AlHilli, M. Endometrial cancer in young women: Prognostic factors and treatment outcomes in women aged ≤40 years. Int. J. Gynecol. Cancer 2020, 30, 631–639. [Google Scholar] [CrossRef]
- Matsuo, K.; Cripe, J.C.; Kurnit, K.C.; Kaneda, M.; Garneau, A.S.; Glaser, G.E.; Nizam, A.; Schillinger, R.M.; Kuznicki, M.L.; Yabuno, A.; et al. Recurrence, death, and secondary malignancy after ovarian conservation for young women with early-stage low-grade endometrial cancer. Gynecol. Oncol. 2019, 155, 39–50. [Google Scholar] [CrossRef]
- Dunberger, G.; Lind, H.; Steineck, G.; Waldenström, A.C.; Nyberg, T.; Al-Abany, M.; Nyberg, U.; Vall-Lundqvist, E. Self-reported symptoms of faecal incontinence among long-term gynaecological cancer survivors and population-based controls. Eur. J. Cancer 2010, 46, 606–615. [Google Scholar] [CrossRef]
- Lind, H.; Waldenström, A.C.; Dunberger, G.; al-Abany, M.; Alevronta, E.; Johansson, K.A.; Olsson, C.; Nyberg, T.; Wilderäng, U.; Steineck, G.; et al. Late symptoms in long-term gynaecological cancer survivors after radiation therapy: A population-based cohort study. Br. J. Cancer 2011, 105, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Sutradhar, R.; Kurdyak, P.; Aktar, S.; Pole, J.D.; Baxter, N.; Nathan, P.C.; Gupta, S. Incidence and predictors of mental health outcomes among survivors of adolescent and young adult cancer: A population-based study using the IMPACT cohort. J. Clin. Oncol. 2021, 39, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Smrz, S.A.; Calo, C.; Fisher, J.L.; Salani, R. An ecological evaluation of the increasing incidence of endometrial cancer and the obesity epidemic. Am. J. Obstet. Gynecol. 2021, 224, 506.e501–506.e508. [Google Scholar] [CrossRef]
- Dikaiou, P.; Edqvist, J.; Lagergren, J.; Adiels, M.; Björck, L.; Rosengren, A. Body mass index and risk of cancer in young women. Sci. Rep. 2024, 14, 6245. [Google Scholar] [CrossRef]
- Khalili, M. Endocrine manifestations of cirrhosis and liver disease. Int. J. Pediatr. 2014, 2, 16. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, J.Y.; Shen, J.J.; Han, K.; Park, J.O.; Park, Y.S.; Lim, H.Y. Increased risk of young-onset digestive tract cancers among young adults aged 20–39 years with nonalcoholic fatty liver disease: A nationwide cohort study. J. Clin. Oncol. 2023, 41, 3363–3373. [Google Scholar] [CrossRef]
- Liu, C.; Liu, T.; Zhang, Q.; Jia, P.; Song, M.; Zhang, Q.; Ruan, G.; Ge, Y.; Lin, S.; Wang, Z.; et al. New-onset age of nonalcoholic fatty liver disease and cancer risk. JAMA Netw. Open 2023, 6, e2335511. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Targher, G.; Tilg, H.; Byrne, C.D. Non-alcoholic fatty liver disease: A multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 2021, 6, 578–588. [Google Scholar] [CrossRef]
- Quiroz-Aldave, J.E.; Gamarra-Osorio, E.R.; Durand-Vásquez, M.D.C.; Rafael-Robles, L.D.P.; Gonzáles-Yovera, J.G.; Quispe-Flores, M.A.; Concepción-Urteaga, L.A.; Román-González, A.; Paz-Ibarra, J.; Concepción-Zavaleta, M.J. From liver to hormones: The endocrine consequences of cirrhosis. World J. Gastroenterol. 2024, 30, 1073–1095. [Google Scholar] [CrossRef]
- Allen, A.M.; Hicks, S.B.; Mara, K.C.; Larson, J.J.; Therneau, T.M. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity—A longitudinal cohort study. J. Hepatol. 2019, 71, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Björkström, K.; Widman, L.; Hagström, H. Risk of hepatic and extrahepatic cancer in NAFLD: A population-based cohort study. Liver Int. 2022, 42, 820–828. [Google Scholar] [CrossRef]
- Kim, G.A.; Lee, H.C.; Choe, J.; Kim, M.J.; Lee, M.J.; Chang, H.S.; Bae, I.Y.; Kim, H.K.; An, J.; Shim, J.H.; et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 2017, 68, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.T.; Mellemkjaer, L.; Jepsen, P.; Thulstrup, A.M.; Baron, J.; Olsen, J.H.; Vilstrup, H. Risk of cancer in patients hospitalized with fatty liver: A Danish cohort study. J. Clin. Gastroenterol. 2003, 36, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Roelstraete, B.; Sharma, R.; Khalili, H.; Hagström, H.; Ludvigsson, J.F. Cancer risk in patients with biopsy-confirmed nonalcoholic fatty liver disease: A population-based cohort study. Hepatology 2021, 74, 2410–2423. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ikeya, T.; Okuyama, S.; Fukuda, K.; Kobayashi, D. The association between non-alcoholic fatty liver disease (with or without metabolic syndrome) and extrahepatic cancer development. J. Gastroenterol. Hepatol. 2021, 36, 1971–1978. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Cho, E.J.; Jung, G.C.; Kwak, M.S.; Yang, J.I.; Yim, J.Y.; Yu, S.J.; Chung, G.E. Fatty liver index for predicting nonalcoholic fatty liver disease in an asymptomatic Korean population. Diagnostics 2021, 11, 2233. [Google Scholar] [CrossRef]
- Han, E.; Han, K.D.; Lee, Y.H.; Kim, K.S.; Hong, S.; Park, J.H.; Park, C.Y. Fatty liver and diabetes statistics in Korea: Nationwide data 2009 to 2017. Diabetes Metab. J. 2023, 47, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kwon, S.Y.; Lee, S.W.; Lee, C.H. Validation of fatty liver index and lipid accumulation product for predicting fatty liver in Korean population. Liver Int. 2011, 31, 1600–1601. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Hong, S.; Han, K.; Park, C.Y. Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: Nationwide population based study. BMJ 2024, 384, e076388. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, Y.-E.; Han, K.; Han, E.; Lee, B.W.; Kang, E.S.; Cha, B.-S.; Ko, S.-H.; Lee, Y.-H. Analysis of severe hypoglycemia among adults with type 2 diabetes and nonalcoholic fatty liver disease. JAMA Netw. Open 2022, 5, e220262. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Moon, S.M.; Choi, H.; Kim, S.H.; Kang, H.K.; Park, D.W.; Jung, J.H.; Han, K.; Shin, D.W.; Lee, H. Increased lung cancer risk and associated risk factors in tuberculosis survivors: A Korean population-based study. Clin. Infect. Dis. 2023, 77, 1329–1339. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, J.Y.; Han, K.; Park, Y.S.; Park, J.O. Light-to-moderate alcohol consumption increases the risk of biliary tract cancer in prediabetes and diabetes, but not in normoglycemic status: A nationwide cohort study. J. Clin. Oncol. 2022, 40, 3623–3632. [Google Scholar] [CrossRef]
- Seo, M.H.; Lee, W.Y.; Kim, S.S.; Kang, J.H.; Kang, J.H.; Kim, K.K.; Kim, B.Y.; Kim, Y.H.; Kim, W.J.; Kim, E.M.; et al. 2018 Korean Society for the Study of Obesity guideline for the management of obesity in Korea. J. Obes. Metab. Syndr. 2019, 28, 40–45. [Google Scholar] [CrossRef]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Knol, M.J.; VanderWeele, T.J.; Groenwold, R.H.; Klungel, O.H.; Rovers, M.M.; Grobbee, D.E. Estimating measures of interaction on an additive scale for preventive exposures. Eur. J. Epidemiol. 2011, 26, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: A meta-analysis of observational cohort studies. Gut 2022, 71, 778–788. [Google Scholar] [CrossRef]
- Kim, D.; Kim, W.R. Nonobese fatty liver disease. Clin. Gastroenterol. Hepatol. 2017, 15, 474–485. [Google Scholar] [CrossRef]
- Jin, Y.J.; Kim, K.M.; Hwang, S.; Lee, S.G.; Ha, T.Y.; Song, G.W.; Jung, D.H.; Kim, K.H.; Yu, E.; Shim, J.H.; et al. Exercise and diet modification in non-obese non-alcoholic fatty liver disease: Analysis of biopsies of living liver donors. J. Gastroenterol. Hepatol. 2012, 27, 1341–1347. [Google Scholar] [CrossRef]
- Kwak, M.S.; Kim, D.; Chung, G.E.; Kim, W.; Kim, J.S. The preventive effect of sustained physical activity on incident nonalcoholic fatty liver disease. Liver Int. 2017, 37, 919–926. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Nees, L.K.; Heublein, S.; Steinmacher, S.; Juhasz-Böss, I.; Brucker, S.; Tempfer, C.B.; Wallwiener, M. Endometrial hyperplasia as a risk factor of endometrial cancer. Arch. Gynecol. Obstet. 2022, 306, 407–421. [Google Scholar] [CrossRef]
- Hart-Unger, S.; Arao, Y.; Hamilton, K.J.; Lierz, S.L.; Malarkey, D.E.; Hewitt, S.C.; Freemark, M.; Korach, K.S. Hormone signaling and fatty liver in females: Analysis of estrogen receptor α mutant mice. Int. J. Obes. 2017, 41, 945–954. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, J.; Zhou, X.; Liu, J.; Wang, Q.; Zhang, B. Roles of estrogen receptor α and β in the regulation of proliferation in endometrial carcinoma. Pathol.-Res. Pract. 2020, 216, 153149. [Google Scholar] [CrossRef]
- Łaniewski, P.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. The microbiome and gynaecological cancer development, prevention and therapy. Nat. Rev. Urol. 2020, 17, 232–250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, G.; Gong, R.; Xi, Y. Gut microbiome dysbiosis in patients with endometrial cancer vs. healthy controls based on 16S rRNA gene sequencing. Curr. Microbiol. 2023, 80, 239. [Google Scholar] [CrossRef] [PubMed]
- Chase, D.; Goulder, A.; Zenhausern, F.; Monk, B.; Herbst-Kralovetz, M. The vaginal and gastrointestinal microbiomes in gynecologic cancers: A review of applications in etiology, symptoms and treatment. Gynecol. Oncol. 2015, 138, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Boutriq, S.; González-González, A.; Plaza-Andrades, I.; Laborda-Illanes, A.; Sánchez-Alcoholado, L.; Peralta-Linero, J.; Domínguez-Recio, M.E.; Bermejo-Pérez, M.J.; Lavado-Valenzuela, R.; Alba, E.; et al. Gut and endometrial microbiome dysbiosis: A new emergent risk factor for endometrial cancer. J. Pers. Med. 2021, 11, 659. [Google Scholar] [CrossRef]
- Yu, J.; Marsh, S.; Hu, J.; Feng, W.; Wu, C. The pathogenesis of nonalcoholic fatty liver disease: Interplay between diet, gut microbiota, and genetic background. Gastroenterol. Res. Pract. 2016, 2016, 2862173. [Google Scholar] [CrossRef]
- DiStefano, J.K.; Gerhard, G.S. NAFLD in normal weight individuals. Diabetol. Metab. Syndr. 2022, 14, 45. [Google Scholar] [CrossRef]
- D’Angelo, E.; Prat, J. Uterine sarcomas: A review. Gynecol. Oncol. 2010, 116, 131–139. [Google Scholar] [CrossRef]
| Early-Onset Endometrial Cancer | p Value | ||
|---|---|---|---|
| No | Yes | ||
| (n = 2,310,660) | (n = 1289) | ||
| Age (years), mean ± SD | 29.7 ± 5.2 | 32.3 ± 4.9 | <0.001 |
| Age groups (years), n (%) | <0.001 | ||
| <30 | 1,200,326 (52.0) | 408 (31.7) | |
| ≥30 | 1,110,334 (48.1) | 881 (68.4) | |
| BMI (kg/m2), mean ± SD | 21.3 ± 3.2 | 24.3 ± 5.6 | <0.001 |
| BMI category (kg/m2) | <0.001 | ||
| <18.5 | 343,329 (14.9) | 126 (9.8) | |
| 18.5–22.9 | 1,427,240 (61.8) | 524 (40.7) | |
| 23–24.9 | 268,752 (11.6) | 168 (13.0) | |
| 25–29.9 | 218,154 (9.4) | 269 (20.9) | |
| ≥30 | 53,185 (2.3) | 202 (15.7) | |
| Waist circumference (cm), mean ± SD | 70.6 ± 8.4 | 74.5 ± 10.7 | <0.001 |
| Systolic BP (mmHg), mean ± SD | 111.2 ± 11.5 | 115.6 ± 13.9 | <0.001 |
| Diastolic BP (mmHg), mean ± SD | 69.8 ± 8.5 | 72.8 ± 10.2 | <0.001 |
| Smoking status, n (%) | 0.93 | ||
| Never | 2,108,107 (91.2) | 1174 (91.1) | |
| Former | 80,346 (3.5) | 44 (3.4) | |
| Current | 122,207 (5.3) | 71 (5.5) | |
| Alcohol consumption a, n (%) | <0.001 | ||
| None | 1,282,782 (55.5) | 786 (61.0) | |
| Light to moderate | 1,027,878 (44.5) | 503 (39.0) | |
| Regular exercise, n (%) | 219,329 (9.5) | 140 (10.9) | 0.09 |
| Laboratory findings, mean ± SD | |||
| Fasting glucose (mg/dL) | 88.2 ± 13.1 | 92.4 ± 21.9 | <0.001 |
| HDL cholesterol (mg/dL) | 63.3 ± 27.4 | 59.9 ± 31.5 | <0.001 |
| LDL cholesterol (mg/dL) | 112.7 ± 306.8 | 118.9 ± 292.5 | 0.46 |
| Triglycerides b (mg/dL) | 71.6 (71.5–71.6) | 89.0 (86.4–91.8) | <0.001 |
| eGFR (mL/min/1.73 m2) | 98.2 ± 41.3 | 97.1 ± 50.2 | 0.34 |
| Low-income status, n (%) | 501,671 (21.7) | 284 (22.0) | 0.78 |
| Comorbidities, n (%) | |||
| Diabetes | 20,941 (0.9) | 49 (3.8) | <0.001 |
| Hypertension | 52,711 (2.3) | 99 (7.7) | <0.001 |
| Dyslipidemia | 85,084 (3.7) | 103 (8.0) | <0.001 |
| Chronic kidney disease | 63,638 (2.8) | 46 (3.6) | 0.07 |
| Obesity | 271,339 (11.7) | 471 (36.5) | <0.001 |
| NAFLD | n | Event, n | Person-Years a | IR b | HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| No | 2,179,497 | 941 | 15,910,429 | 0.59 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 132,452 | 348 | 952,646 | 3.65 | 6.24 (5.51–7.05) | 5.42 (4.79–6.14) | 3.19 (2.76–3.67) |
| Moderate | 92,236 | 155 | 666,101 | 2.33 | 3.96 (3.34–4.70) | 3.40 (2.86–4.03) | 2.38 (1.99–2.85) |
| Severe | 40,216 | 193 | 286,545 | 6.74 | 11.57 (9.91–13.50) | 10.32 (8.84–12.06) | 5.39 (4.44–6.53) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-H.; Hong, J.Y.; Han, K.; Kang, W.; Shen, J.J. Increased Risk of Early-Onset Endometrial Cancer in Women Aged 20–39 Years with Non-Alcoholic Fatty Liver Disease: A Nationwide Cohort Study. Cancers 2025, 17, 1322. https://doi.org/10.3390/cancers17081322
Park J-H, Hong JY, Han K, Kang W, Shen JJ. Increased Risk of Early-Onset Endometrial Cancer in Women Aged 20–39 Years with Non-Alcoholic Fatty Liver Disease: A Nationwide Cohort Study. Cancers. 2025; 17(8):1322. https://doi.org/10.3390/cancers17081322
Chicago/Turabian StylePark, Joo-Hyun, Jung Yong Hong, Kyungdo Han, Wonseok Kang, and Jay J. Shen. 2025. "Increased Risk of Early-Onset Endometrial Cancer in Women Aged 20–39 Years with Non-Alcoholic Fatty Liver Disease: A Nationwide Cohort Study" Cancers 17, no. 8: 1322. https://doi.org/10.3390/cancers17081322
APA StylePark, J.-H., Hong, J. Y., Han, K., Kang, W., & Shen, J. J. (2025). Increased Risk of Early-Onset Endometrial Cancer in Women Aged 20–39 Years with Non-Alcoholic Fatty Liver Disease: A Nationwide Cohort Study. Cancers, 17(8), 1322. https://doi.org/10.3390/cancers17081322






