Phyto-Sesquiterpene Lactones Prevent the Development of Multidrug Resistance in TNBC via ABC Transporters Inhibition and STAT3/MYC Signaling
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Cell-Cycle Analysis
2.5. Apoptosis Assay
2.6. Reactive Oxygen Species (ROS) Level Detection
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. siRNA Gene Silencing
2.9. Western Blot Assays
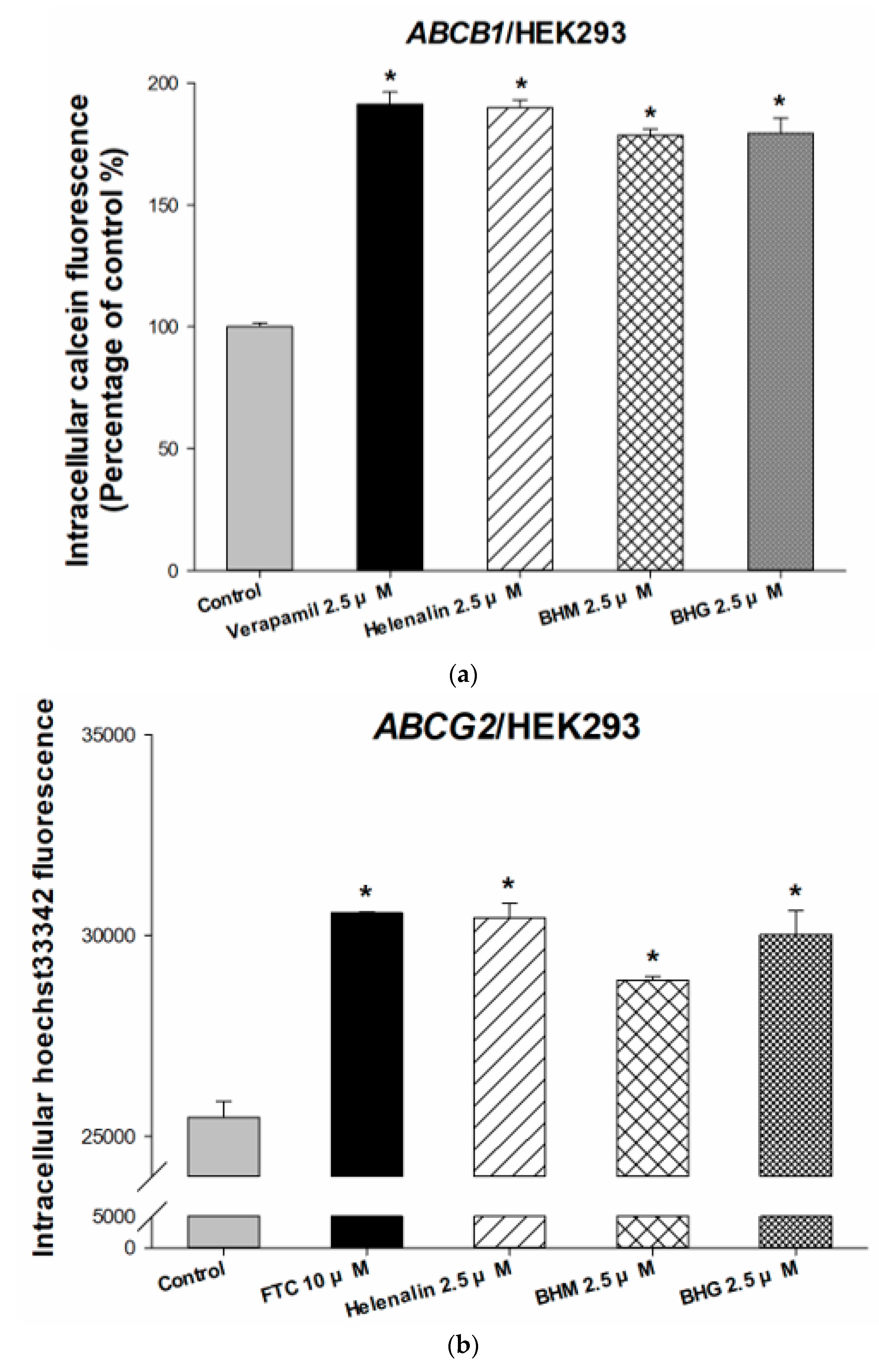
2.10. P-gp and BCRP Fuctional Assay
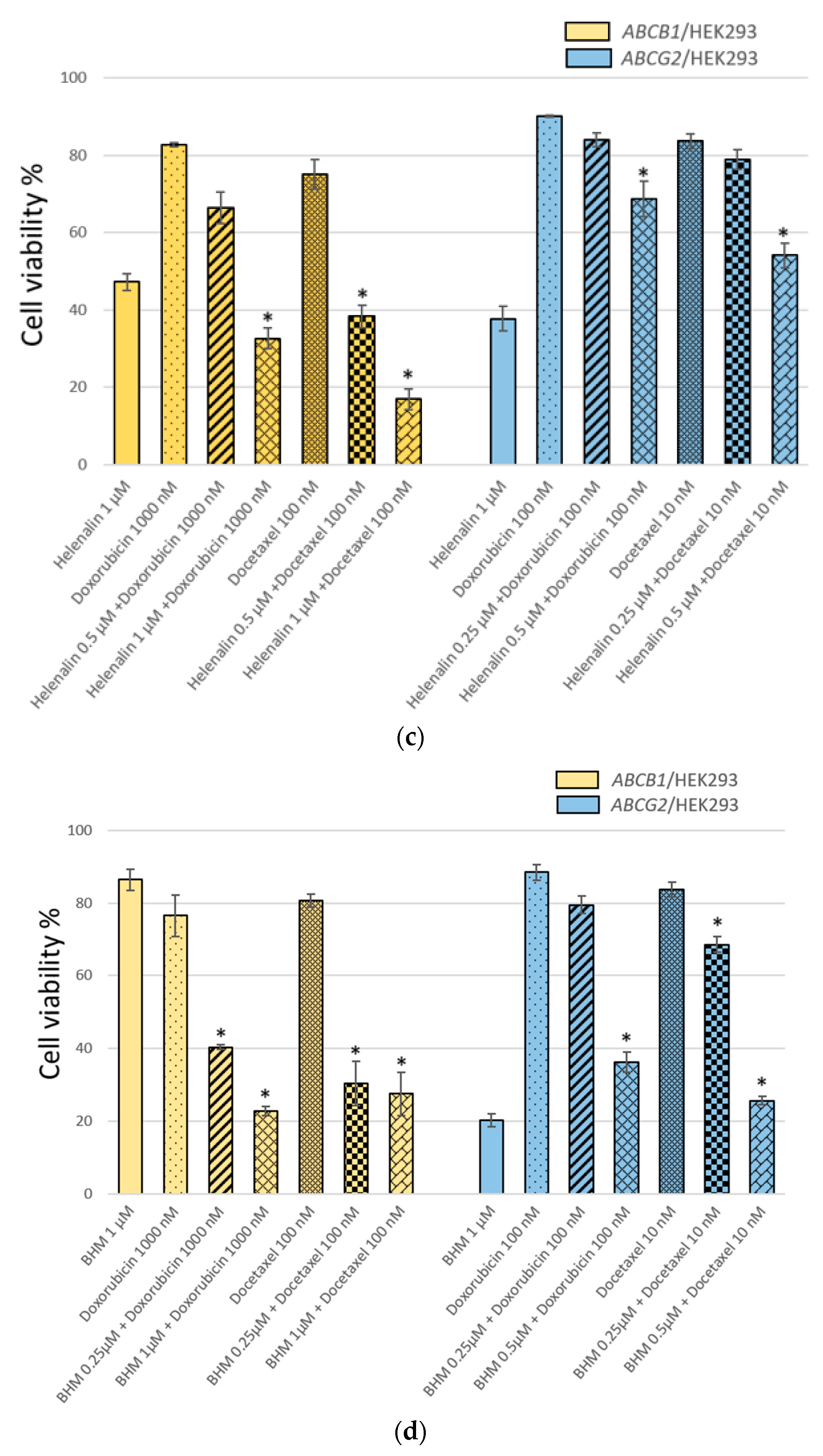
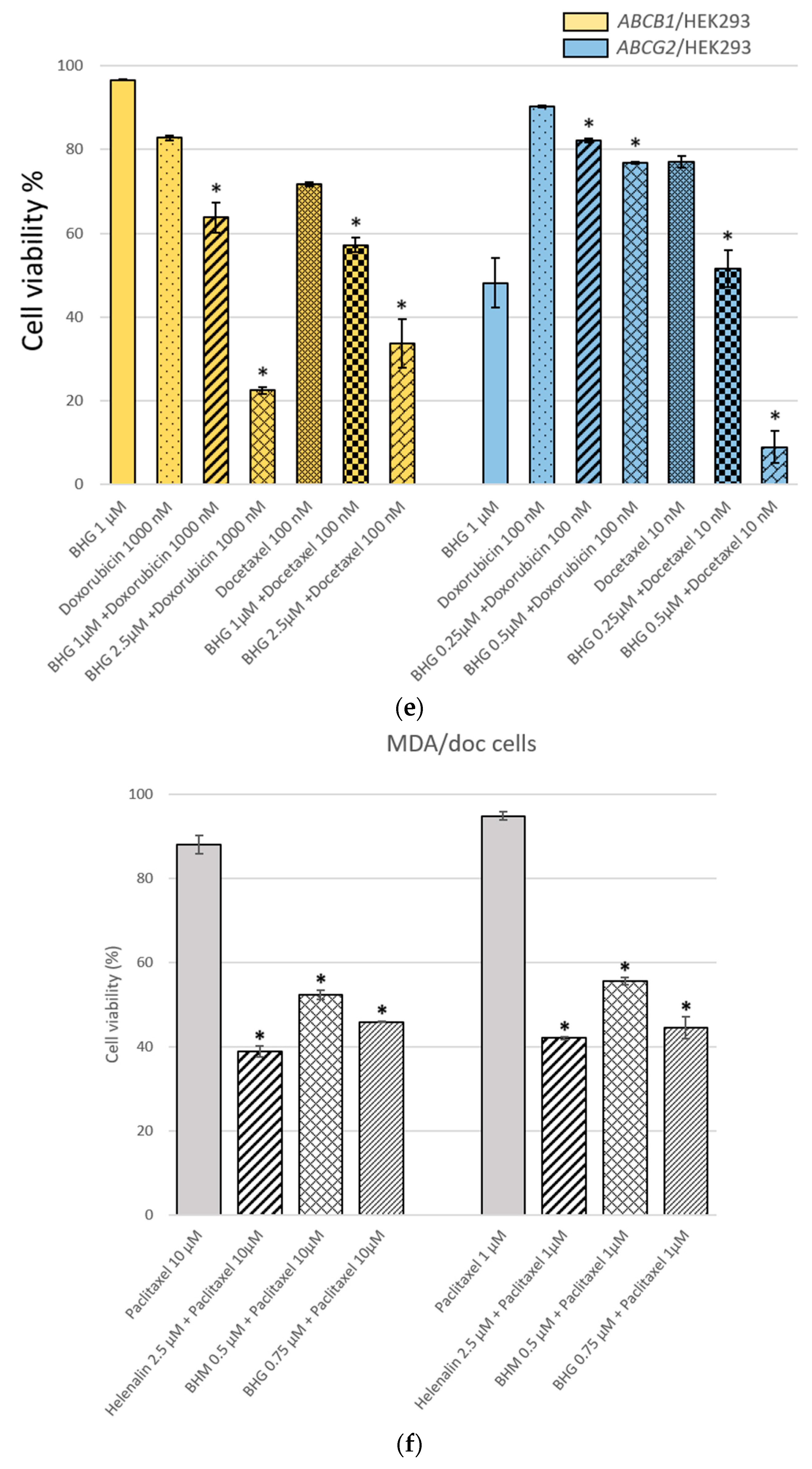
2.11. Compound–Drug Combination Assay
2.12. Synergism Analysis
2.13. Genomic Data Analysis from Breast Cancer Dataset
2.14. Zebrafish Xenograft Assay
2.15. Statistical and Data Analysis
3. Results
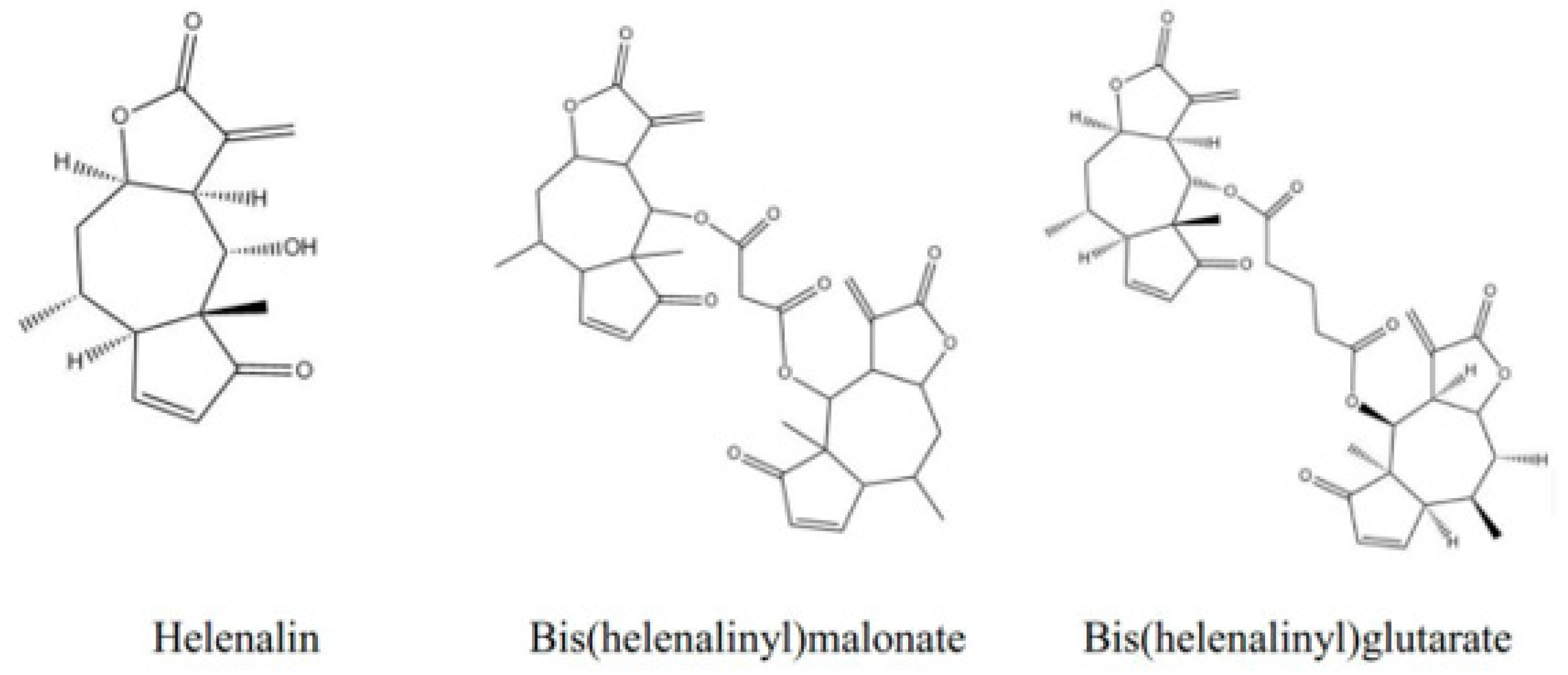
3.1. The MDR Prevention Effect of Phyto-Sesquiterpene Lactones Through Inhibiting ABC Transporters
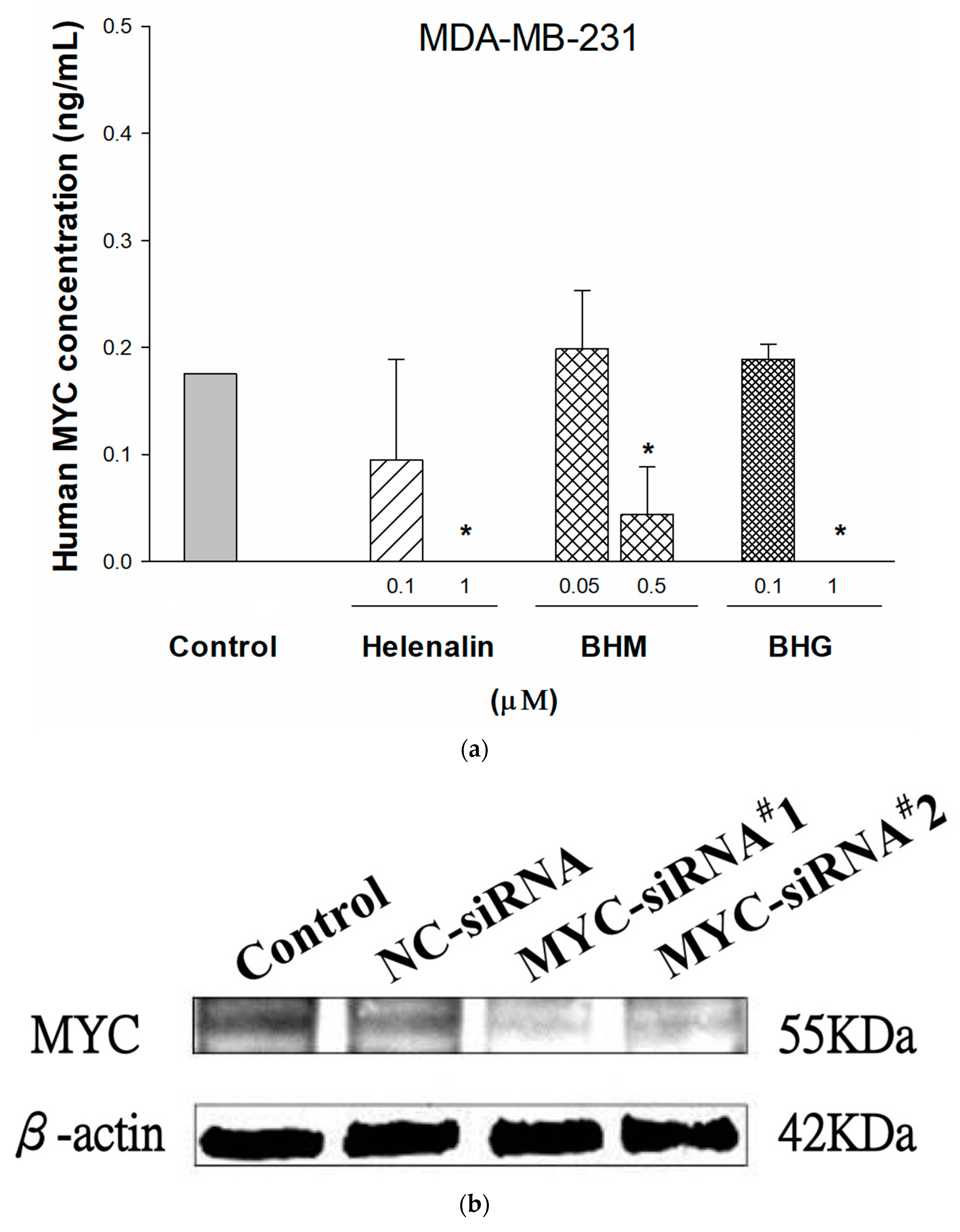
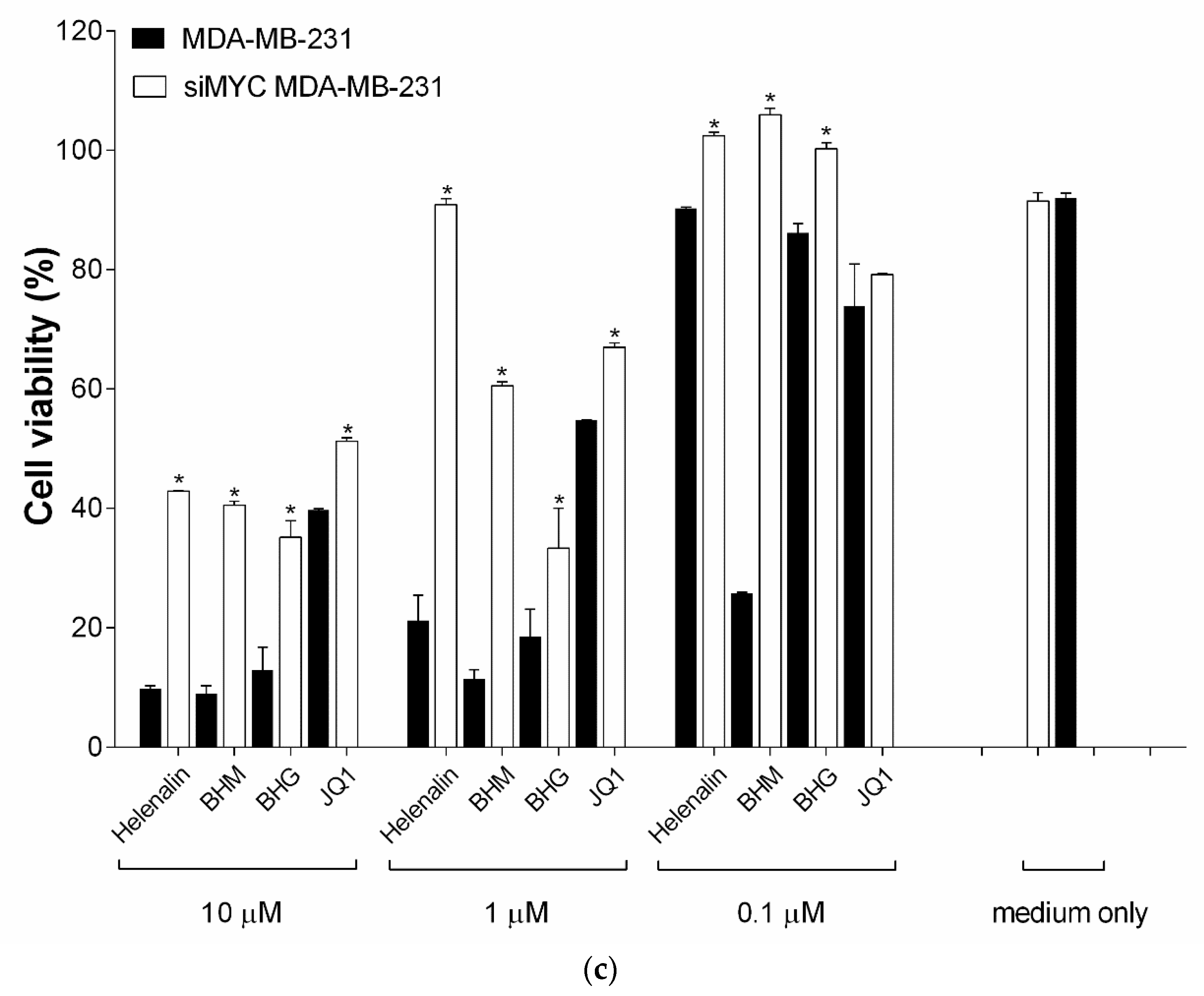
3.2. MYC Suppressing Effect of Phyto-Sesquiterpene Lactones on TNBC Cells
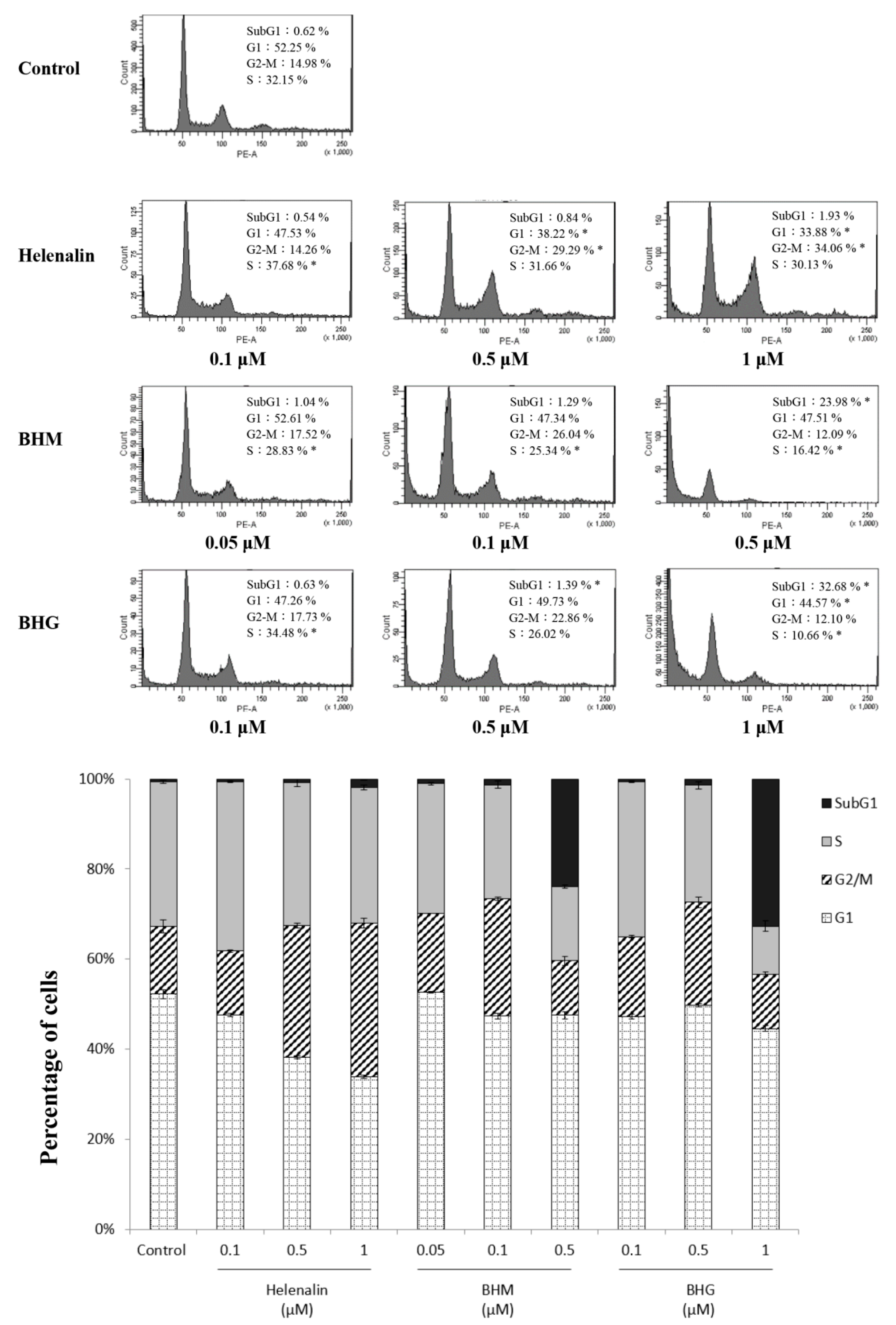
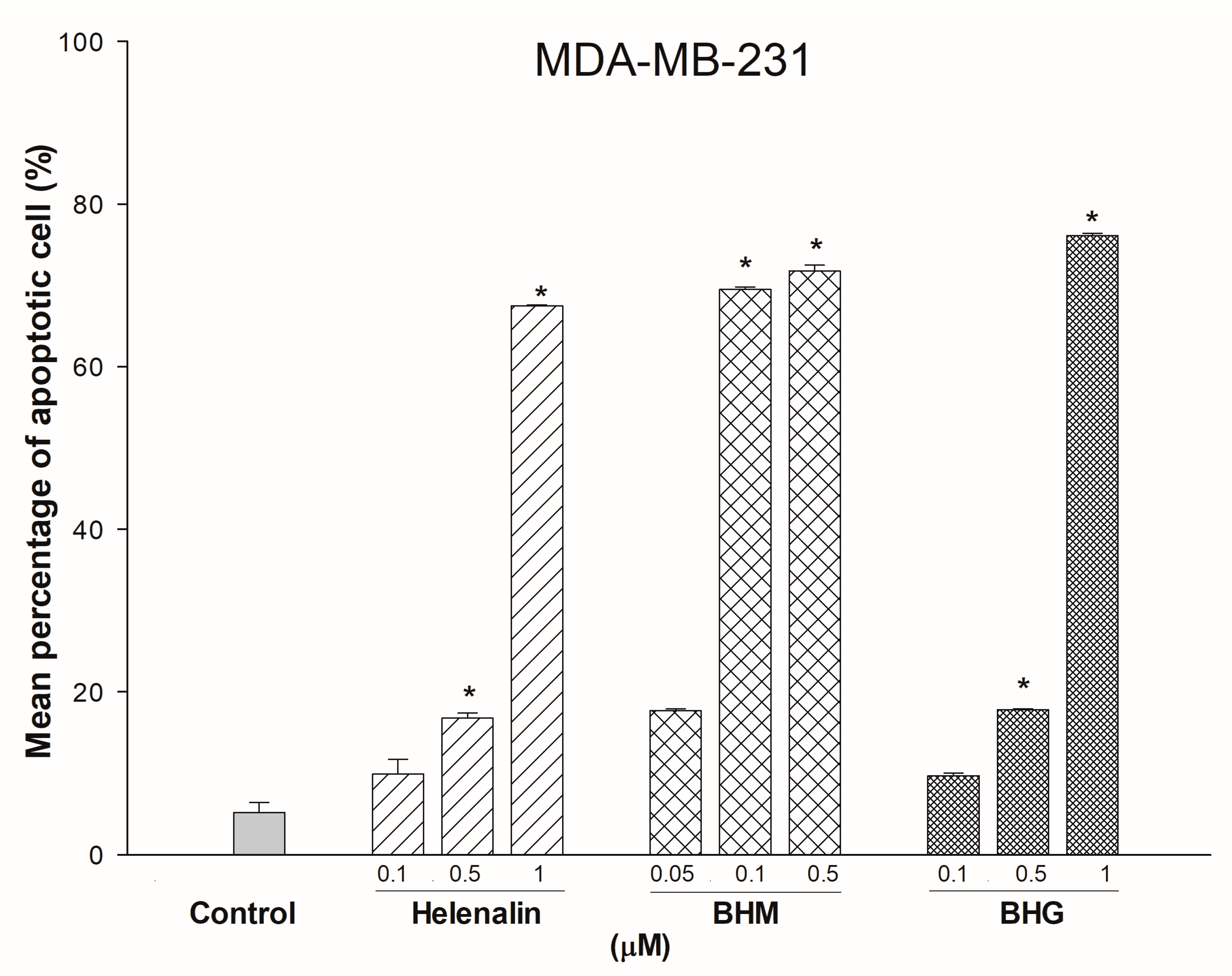
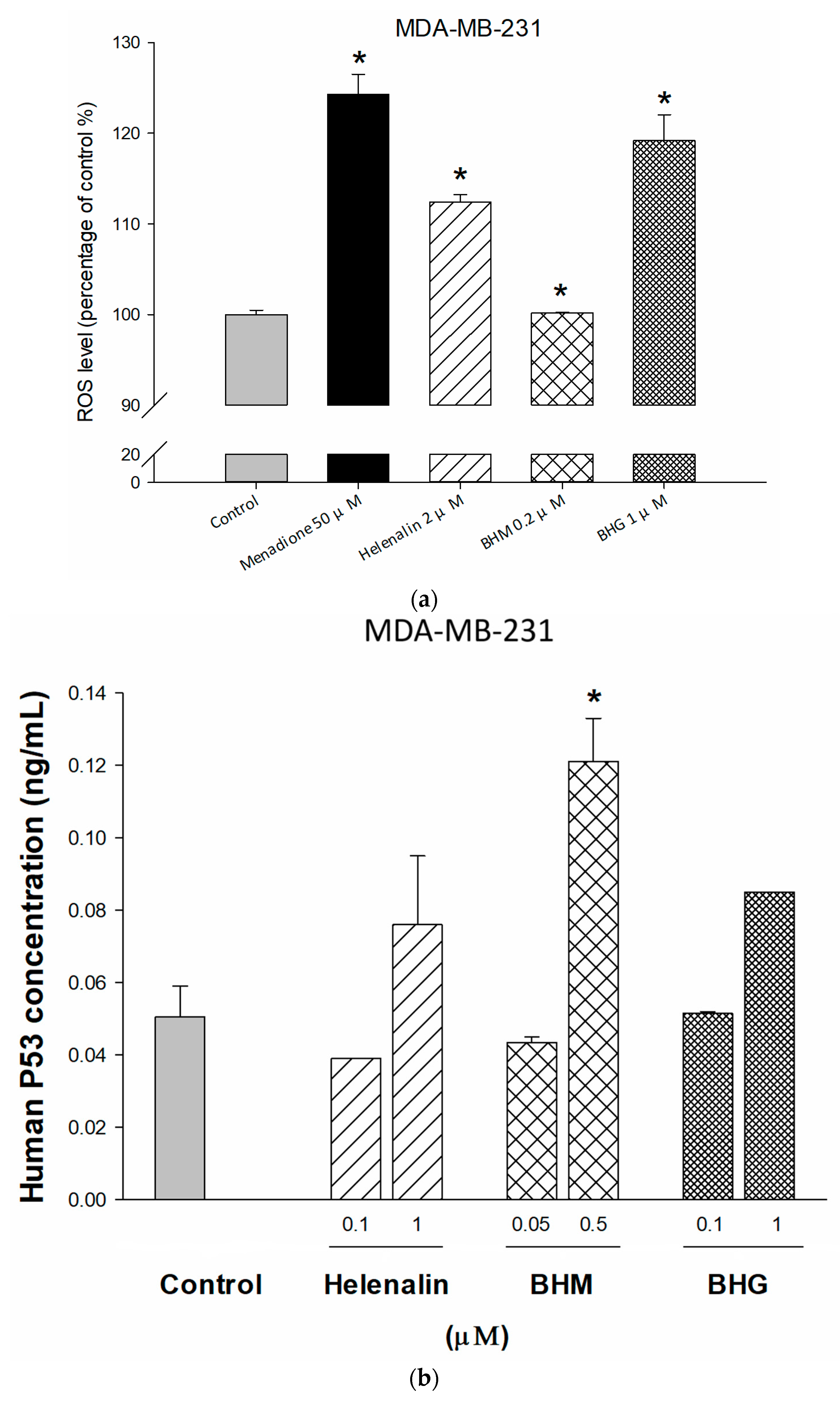
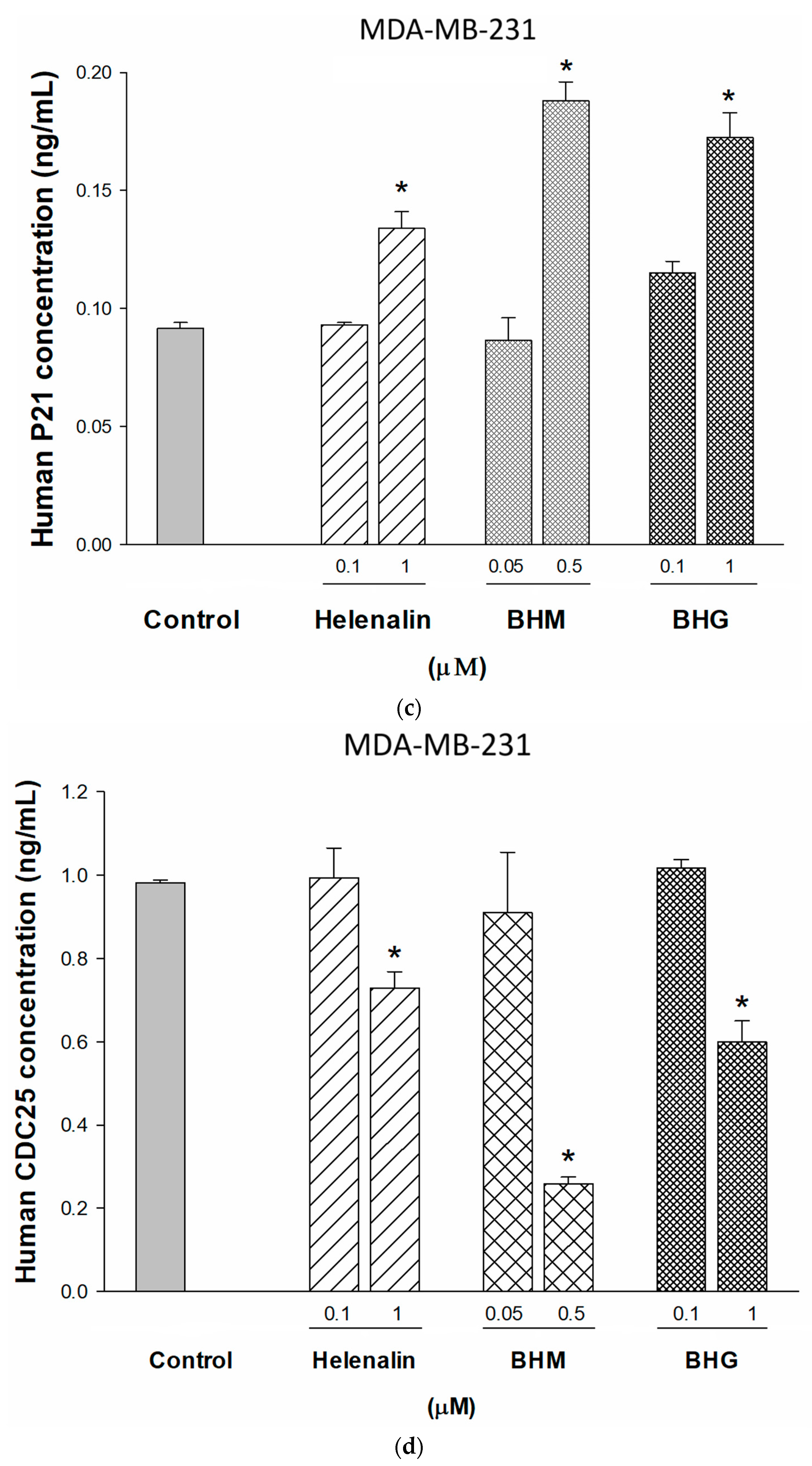
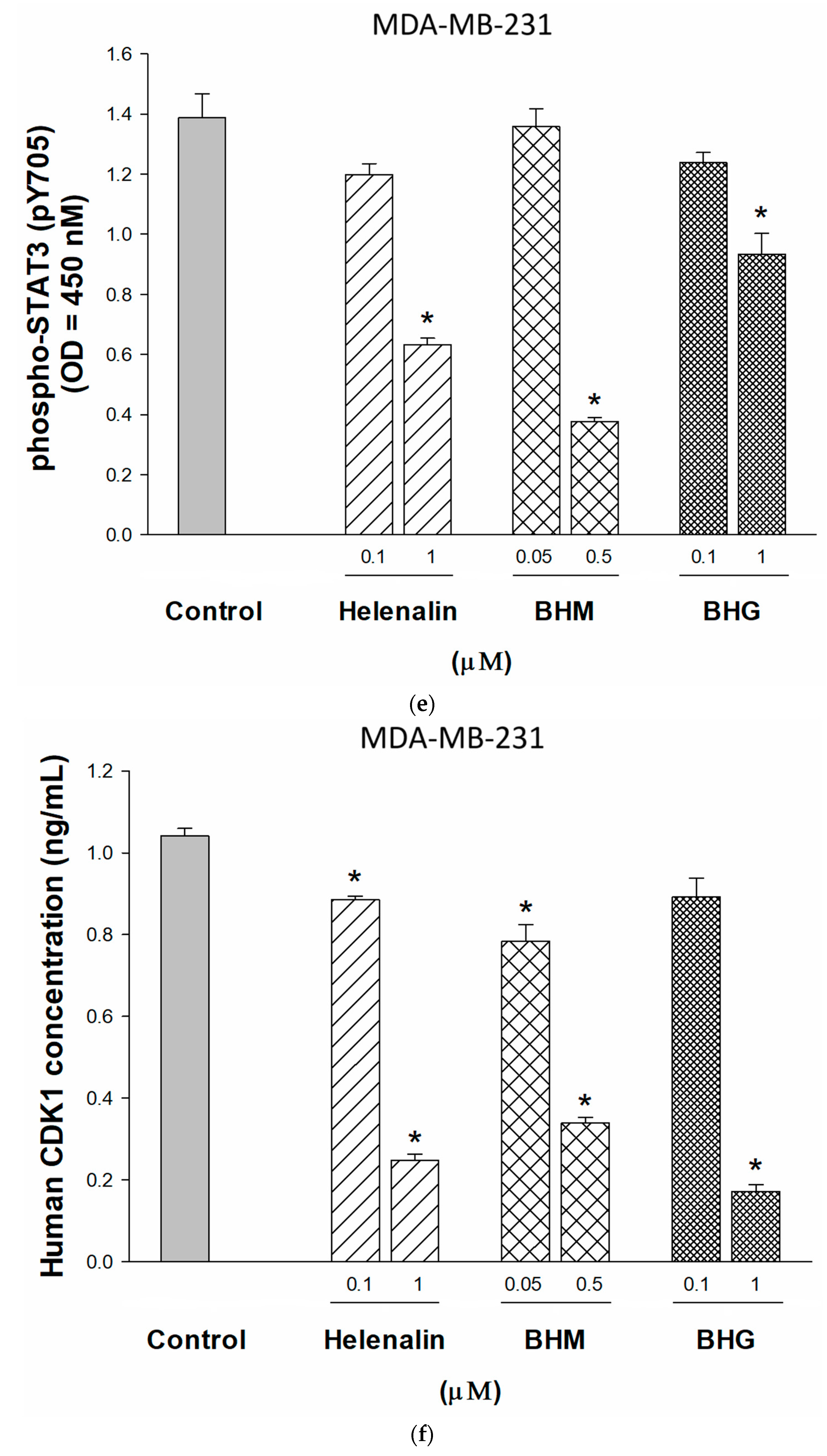
3.3. Phyto-Sesquiterpene Lactones Induced Cell-Cycle Arrest or Apoptosis Through MYC Related Pathway in TNBC Cells
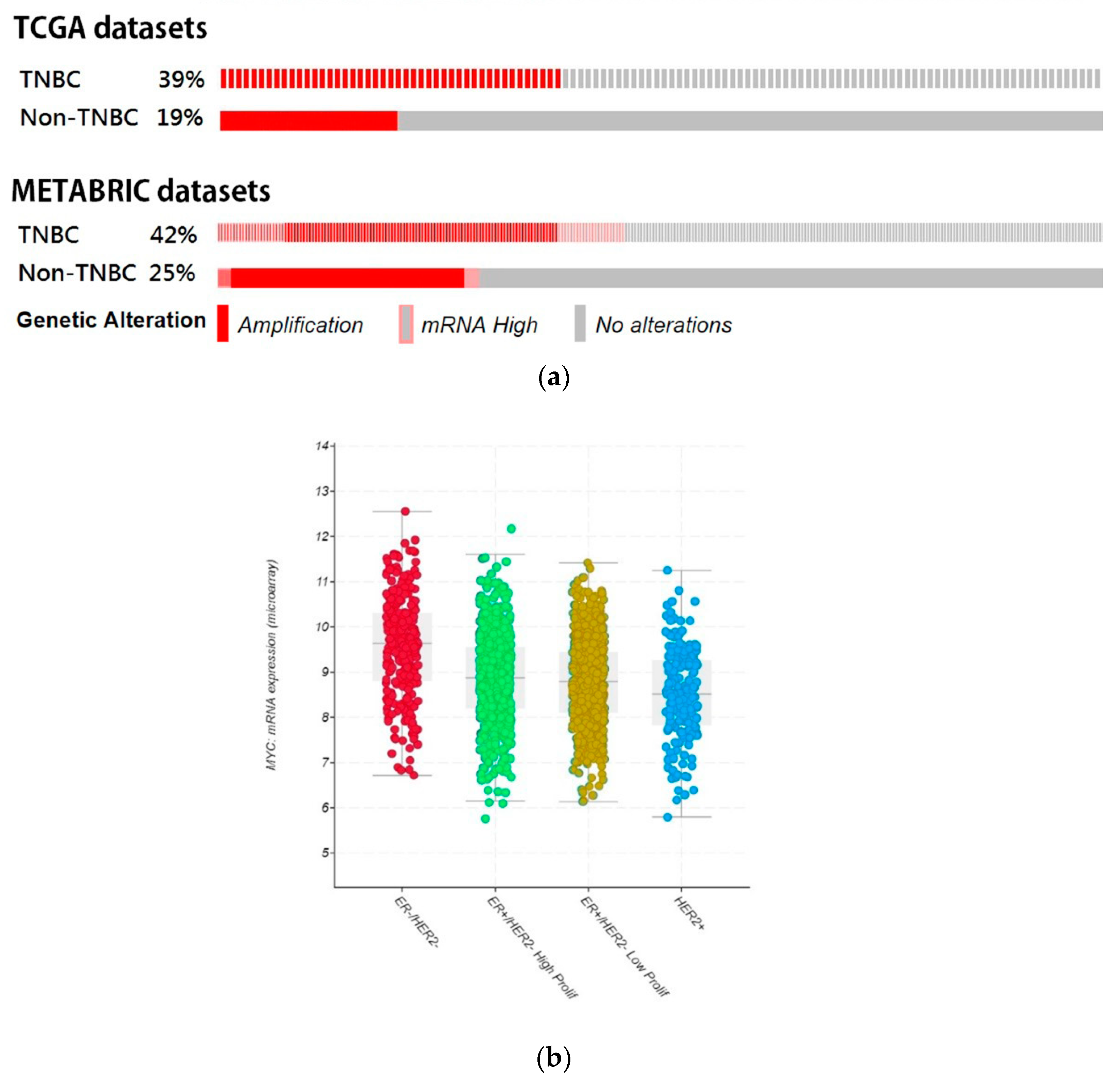
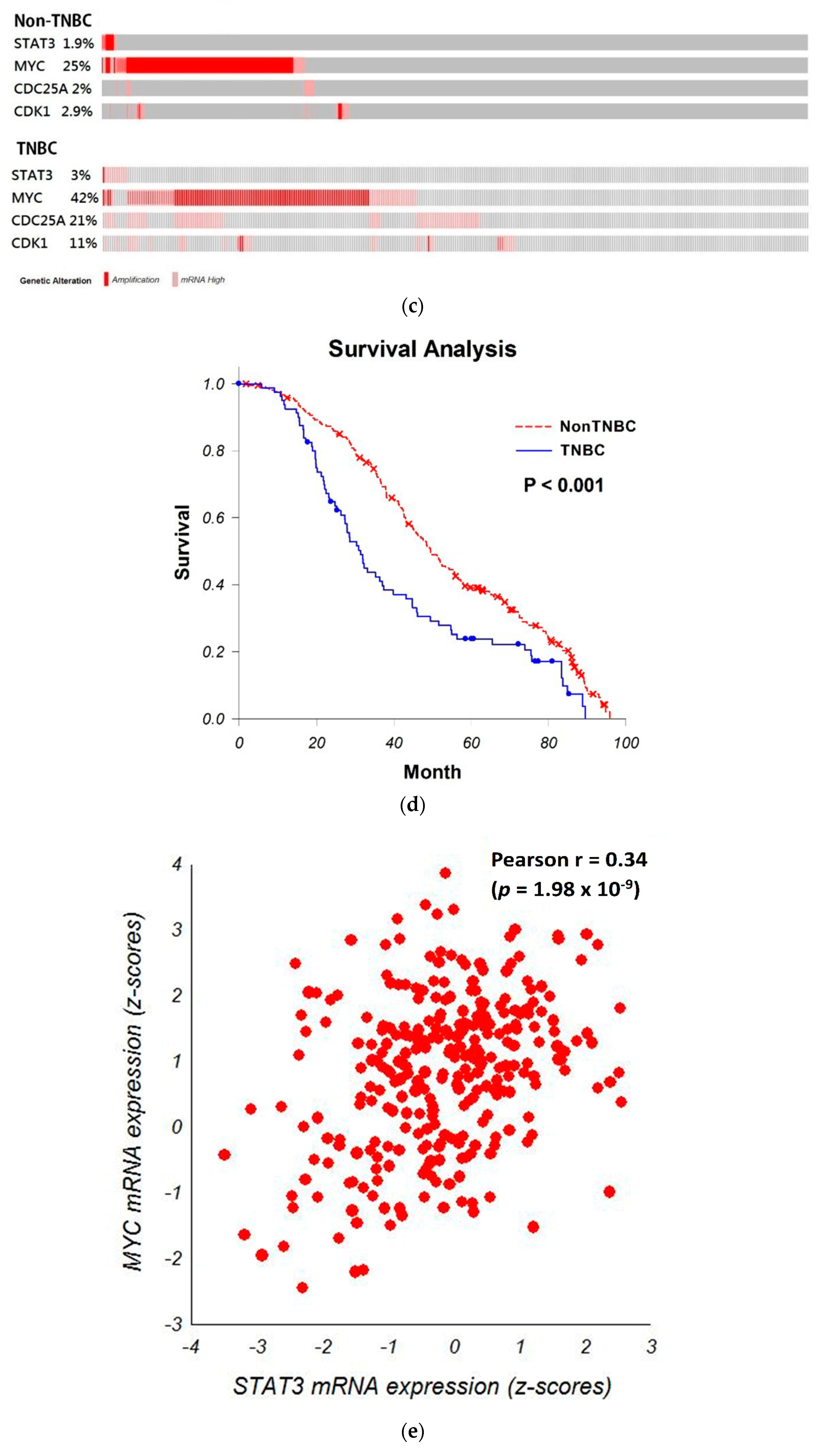
3.4. Bioinformatic Analyze the Role of STAT3-MYC Pathway on TNBC
3.5. Phyto-Sesquiterpene Lactones Inhibits the Growth of TNBC Cells In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| BCRP | Breast cancer resistance protein |
| BHG | Bis(helenalinyl)glutarate |
| BHM | Bis(helenalinyl)malonate |
| CDC25 | Cell division cycle protein 25 |
| CDK1 | Cyclin-dependent kinase 1 |
| ELISA | Enzyme-linked immunosorbent assay |
| ER | Estrogen receptor |
| FTC | Fumitremorgin C |
| HER2 | Human epidermal growth factor receptor type 2 |
| MDR | Multidrug resistance |
| MRP1 | Multidrug resistance protein 1 |
| PARP | Poly (ADP-ribose) polymerase |
| PBS | Phosphate-buffered saline |
| P-gp | P-glycoprotein |
| PI | Propidium iodide |
| PR | Progesterone receptor |
| ROS | Reactive oxygen species |
| SRB | Sulforhodamine B |
| STAT3 | Signal transducer and activator of transcription 3 |
| TCA | Trichloroacetic acid |
| TNBC | Triple-negative breast cancer |
References
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- Narod, S.; Booth, C.M.; Foulkes, W.D. Olaparib for Metastatic Germline BRCA-Mutated Breast Cancer. N. Engl. J. Med. 2017, 377, 1792. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat. Med. 2013, 19, 1381–1388. [Google Scholar] [CrossRef]
- Kathawala, R.J.; Gupta, P.; Ashby, C.R., Jr.; Chen, Z.S. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2015, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.; Eilers, M. Transcriptional regulation and transformation by Myc proteins. Nat. Rev. Mol. Cell Biol. 2005, 6, 635–645. [Google Scholar] [CrossRef]
- Felsher, D.W.; Bishop, J.M. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell 1999, 4, 199–207. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Olopade, O.I. MYC and Breast Cancer. Genes Cancer 2010, 1, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Alles, M.C.; Gardiner-Garden, M.; Nott, D.J.; Wang, Y.; Foekens, J.A.; Sutherland, R.L.; Musgrove, E.A.; Ormandy, C.J. Meta-analysis and gene set enrichment relative to er status reveal elevated activity of MYC and E2F in the “basal” breast cancer subgroup. PLoS ONE 2009, 4, e4710. [Google Scholar] [CrossRef]
- Horiuchi, D.; Kusdra, L.; Huskey, N.E.; Chandriani, S.; Lenburg, M.E.; Gonzalez-Angulo, A.M.; Creasman, K.J.; Bazarov, A.V.; Smyth, J.W.; Davis, S.E.; et al. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. J. Exp. Med. 2012, 209, 679–696. [Google Scholar] [CrossRef]
- Cheng, Y.D.; Lu, C.C.; Hsu, Y.M.; Tsai, F.J.; Bau, D.T.; Tsai, S.C.; Cheng, C.C.; Lin, J.J.; Huang, Y.Y.; Juan, Y.N.; et al. In Silico and In Vitro studies of taiwan chingguan yihau (NRICM101) on TNF-α/IL-1β-induced human lung cells. Biomedicine 2022, 12, 56–71. [Google Scholar] [CrossRef]
- Qin, J.J.; Yan, L.; Zhang, J.; Zhang, W.D. STAT3 as a potential therapeutic target in triple negative breast cancer: A systematic review. J. Exp. Clin. Cancer Res. CR 2019, 38, 195. [Google Scholar] [CrossRef]
- Bowman, T.; Broome, M.A.; Sinibaldi, D.; Wharton, W.; Pledger, W.J.; Sedivy, J.M.; Irby, R.; Yeatman, T.; Courtneidge, S.A.; Jove, R. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 7319–7324. [Google Scholar] [CrossRef] [PubMed]
- Lage, H. An overview of cancer multidrug resistance: A still unsolved problem. Cell. Mol. Life Sci. CMLS 2008, 65, 3145–3167. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Weber, T.K.; Morse, B.; Arceci, R.; Staniunas, R.; Steele, G., Jr.; Summerhayes, I.C. Constitutive expression of multidrug resistance in human colorectal tumours and cell lines. Br. J. Cancer 1993, 67, 959–968. [Google Scholar] [CrossRef]
- Zhang, L.H.; Yang, A.J.; Wang, M.; Liu, W.; Wang, C.Y.; Xie, X.F.; Chen, X.; Dong, J.F.; Li, M. Enhanced autophagy reveals vulnerability of P-gp mediated epirubicin resistance in triple negative breast cancer cells. Apoptosis 2016, 21, 473–488. [Google Scholar] [CrossRef]
- Guestini, F.; Ono, K.; Miyashita, M.; Ishida, T.; Ohuchi, N.; Nakagawa, S.; Hirakawa, H.; Tamaki, K.; Ohi, Y.; Rai, Y.; et al. Impact of Topoisomerase IIalpha, PTEN, ABCC1/MRP1, and KI67 on triple-negative breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2018, 173, 275–288. [Google Scholar] [CrossRef]
- Collina, F.; Di Bonito, M.; Li Bergolis, V.; De Laurentiis, M.; Vitagliano, C.; Cerrone, M.; Nuzzo, F.; Cantile, M.; Botti, G. Prognostic Value of Cancer Stem Cells Markers in Triple-Negative Breast Cancer. BioMed Res. Int. 2015, 2015, 158682. [Google Scholar] [CrossRef]
- Lee, K.H.; Hall, I.H.; Mar, E.C.; Starnes, C.O.; ElGebaly, S.A.; Waddell, T.G.; Hadgraft, R.I.; Ruffner, C.G.; Weidner, I. Sesquiterpene antitumor agents: Inhibitors of cellular metabolism. Science 1977, 196, 533–536. [Google Scholar] [CrossRef]
- Hall, I.H.; Lee, K.H.; Starnes, C.O.; Muraoka, O.; Sumida, Y.; Waddell, T.G. Antihyperlipidemic activity of sesquiterpene lactones and related compounds. J. Pharm. Sci. 1980, 69, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Li, R.J.; Cheng, M.S.; Kim, Y.S. Alantolactone selectively suppresses STAT3 activation and exhibits potent anticancer activity in MDA-MB-231 cells. Cancer Lett. 2015, 357, 393–403. [Google Scholar] [CrossRef]
- Nakagawa-Goto, K.; Chen, J.Y.; Cheng, Y.T.; Lee, W.L.; Takeya, M.; Saito, Y.; Lee, K.H.; Shyur, L.F. Novel sesquiterpene lactone analogues as potent anti-breast cancer agents. Mol. Oncol. 2016, 10, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Chen, Y.; Yang, B.; Lou, C.; Zhu, R.; Zhao, Y.; Zhao, H. F1012-2 inhibits the growth of triple negative breast cancer through induction of cell cycle arrest, apoptosis, and autophagy. Phytother. Res. 2018, 32, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Shen, J.W.; Zhou, D.H.; Zhao, Y.P.; Wang, W.Q.; Zhu, Y.; Zhao, H.J. Precise discovery of a STAT3 inhibitor from Eupatorium lindleyanum and evaluation of its activity of anti-triple-negative breast cancer. Nat. Prod. Res. 2019, 33, 477–485. [Google Scholar] [CrossRef]
- Lee, K.H.; Huang, E.S.; Piantadosi, C.; Pagano, J.S.; Geissman, T.A. Cytotoxicity of sesquiterpene lactones. Cancer Res. 1971, 31, 1649–1654. [Google Scholar]
- Williams, W.L., Jr.; Hall, I.H.; Grippo, A.A.; Oswald, C.B.; Lee, K.H.; Holbrook, D.J.; Chaney, S.G. Inhibition of nucleic acid synthesis in P-388 lymphocytic leukemia tumor cells by helenalin and bis(helenalinyl)malonate in vivo. J. Pharm. Sci. 1988, 77, 178–184. [Google Scholar] [CrossRef]
- Lee, K.H.; Ibuka, T.; Sims, D.; Muraoka, O.; Kiyokawa, H.; Hall, I.H.; Kim, H.L. Antitumor agents. 44. Bis(helenalinyl) esters and related derivatives as novel potent antileukemic agents. J. Med. Chem. 1981, 24, 924–927. [Google Scholar] [CrossRef]
- Jang, J.H.; Iqbal, T.; Min, K.J.; Kim, S.; Park, J.W.; Son, E.I.; Lee, T.J.; Kwon, T.K. Helenalin-induced apoptosis is dependent on production of reactive oxygen species and independent of induction of endoplasmic reticulum stress in renal cell carcinoma. Toxicol. In Vitro 2013, 27, 588–596. [Google Scholar] [CrossRef]
- Teng, Y.-N.; Chang, C.-S.; Lee, T.-E.; Hung, C.-C. Cordycepin re-sensitizes multidrug resistance cancer cells to chemotherapeutic agents through modulating P-glycoprotein expression and ATPase function. J. Funct. Foods 2016, 26, 681–690. [Google Scholar] [CrossRef]
- Jhou, A.J.; Chang, H.C.; Hung, C.C.; Lin, H.C.; Lee, Y.C.; Liu, W.T.; Han, K.F.; Lai, Y.W.; Lin, M.Y.; Lee, C.H. Chlorpromazine, an antipsychotic agent, induces G2/M phase arrest and apoptosis via regulation of the PI3K/AKT/mTOR-mediated autophagy pathways in human oral cancer. Biochem. Pharmacol. 2021, 184, 114403. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.S.; Ma, W.; Mao, D.Y.; Benchimol, S. p53-Dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol. Cell. Biol. 2005, 25, 7423–7431. [Google Scholar] [CrossRef]
- Yang, Y.; Xue, K.; Li, Z.; Zheng, W.; Dong, W.; Song, J.; Sun, S.; Ma, T.; Li, W. c-Myc regulates the CDK1/cyclin B1 dependentG2/M cell cycle progression by histone H4 acetylation in Raji cells. Int. J. Mol. Med. 2018, 41, 3366–3378. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Breast Cancer (Version 1.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 14 March 2019).
- Khan, F.; Ricks-Santi, L.J.; Zafar, R.; Kanaan, Y.; Naab, T. Expression of p27 and c-Myc by immunohistochemistry in breast ductal cancers in African American women. Ann. Diagn. Pathol. 2018, 34, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Qin, S.; Schulte, B.A.; Ethier, S.P.; Tew, K.D.; Wang, G.Y. MYC Inhibition Depletes Cancer Stem-like Cells in Triple-Negative Breast Cancer. Cancer Res. 2017, 77, 6641–6650. [Google Scholar] [CrossRef]
- Liou, Y.F.; Hall, I.H.; Lee, K.H.; Williams, W.L., Jr.; Chaney, S.G. Investigation of sesquiterpene lactones as protein synthesis inhibitors of P-388 lymphocytic leukemia cells. Biochim. Biophys. Acta 1983, 739, 190–196. [Google Scholar] [CrossRef]
- Chen, C.H.; Yang, L.M.; Lee, T.T.; Shen, Y.C.; Zhang, D.C.; Pan, D.J.; McPhail, A.T.; McPhail, D.R.; Liu, S.Y.; Li, D.H.; et al. Antitumor agents—CLI. Bis(helenalinyl)glutarate and bis(isoalantodiol-B)glutarate, potent inhibitors of human DNA topoisomerase II. Bioorganic Med. Chem. 1994, 2, 137–145. [Google Scholar] [CrossRef]
- Beer, M.F.; Bivona, A.E.; Sanchez Alberti, A.; Cerny, N.; Reta, G.F.; Martin, V.S.; Padron, J.M.; Malchiodi, E.L.; Sulsen, V.P.; Donadel, O.J. Preparation of Sesquiterpene Lactone Derivatives: Cytotoxic Activity and Selectivity of Action. Molecules 2019, 24, 1113. [Google Scholar] [CrossRef]
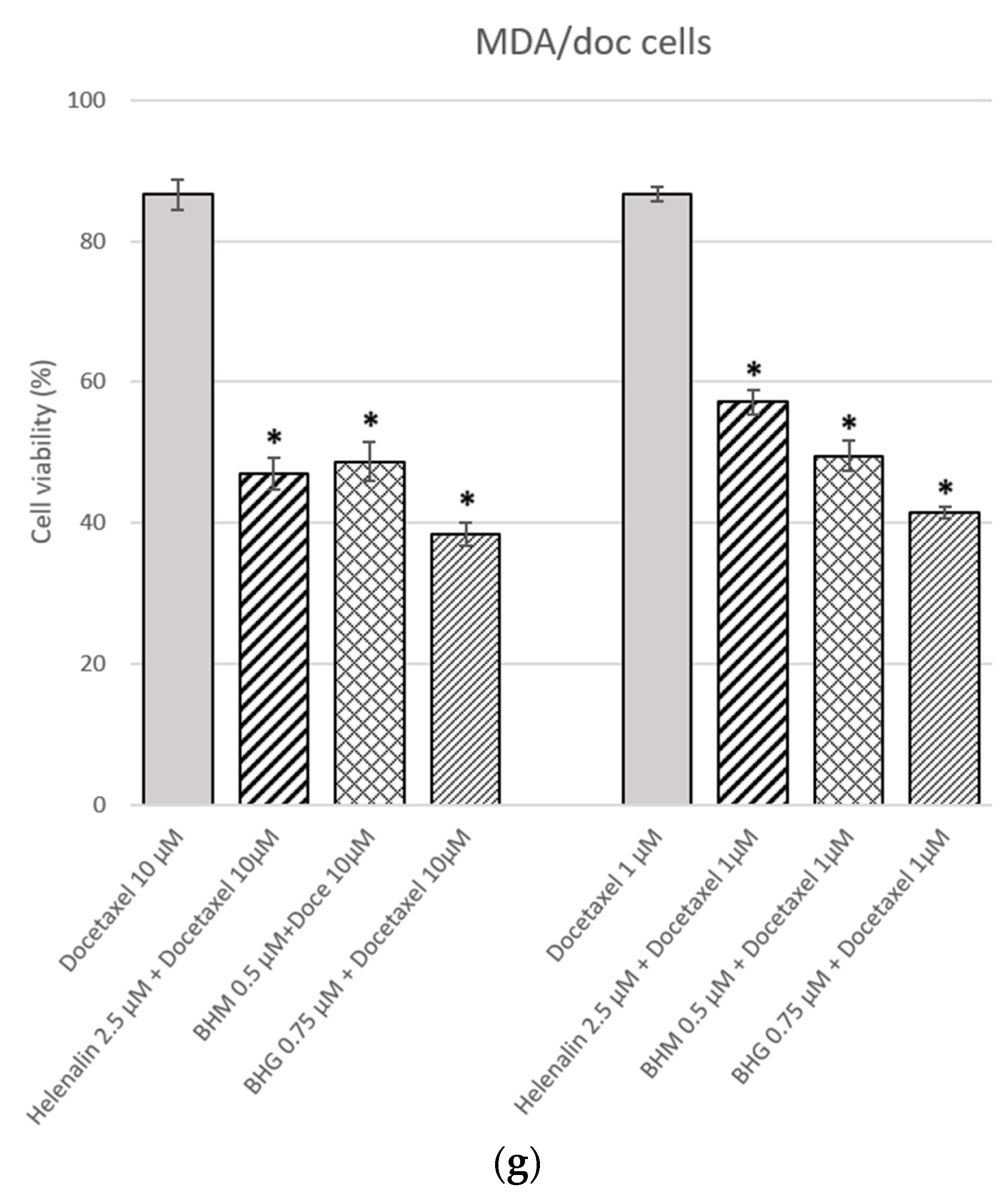
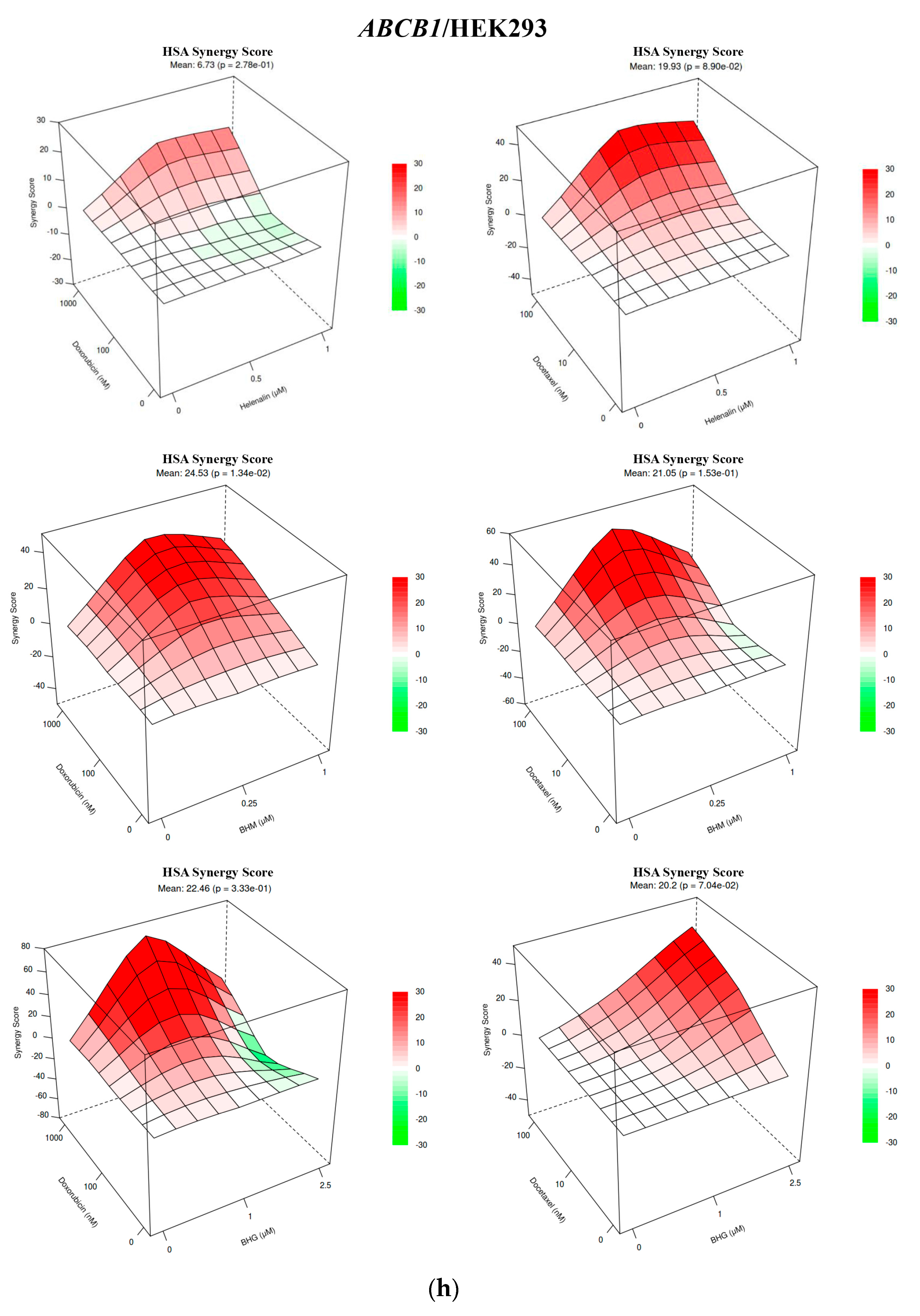
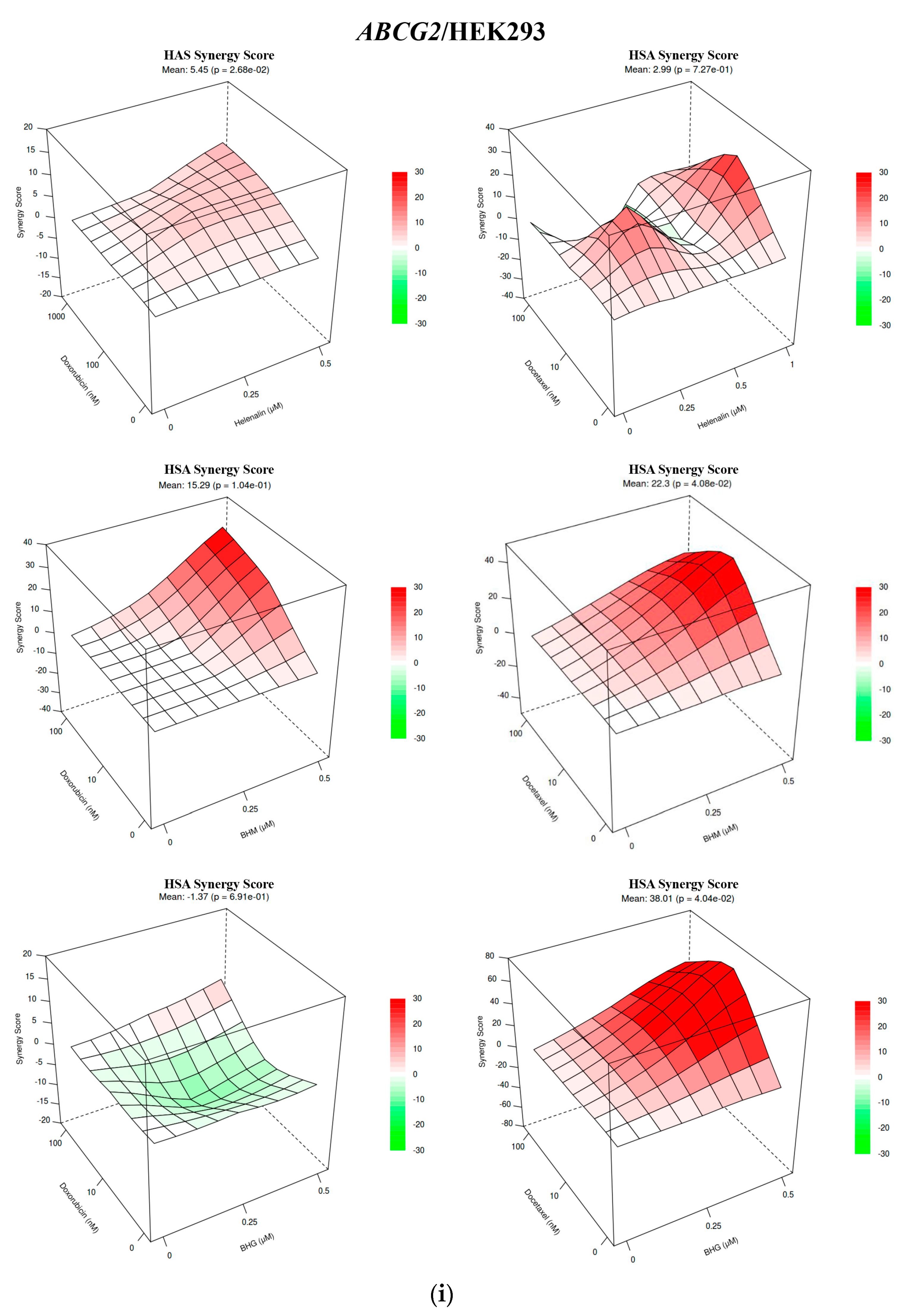
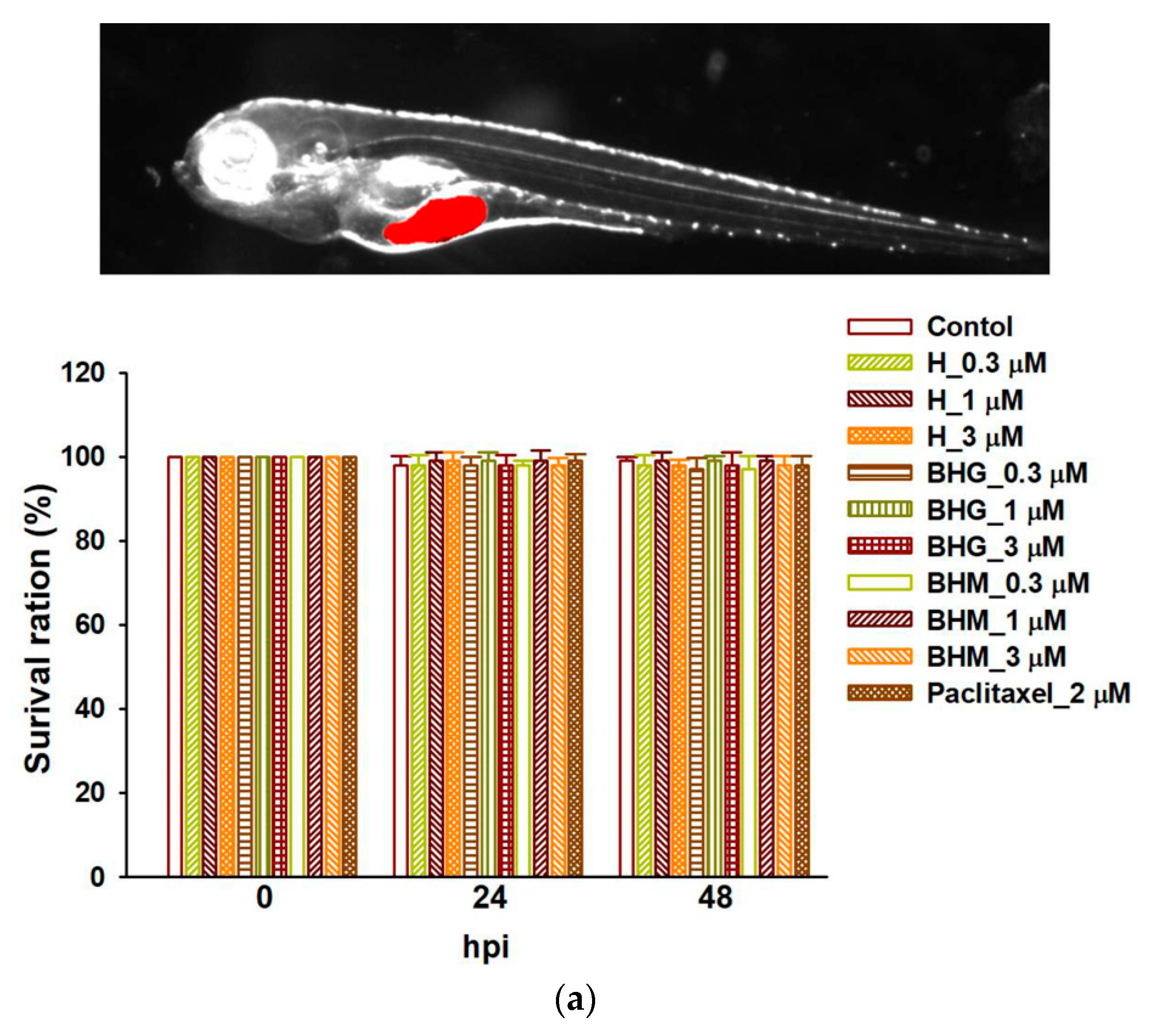
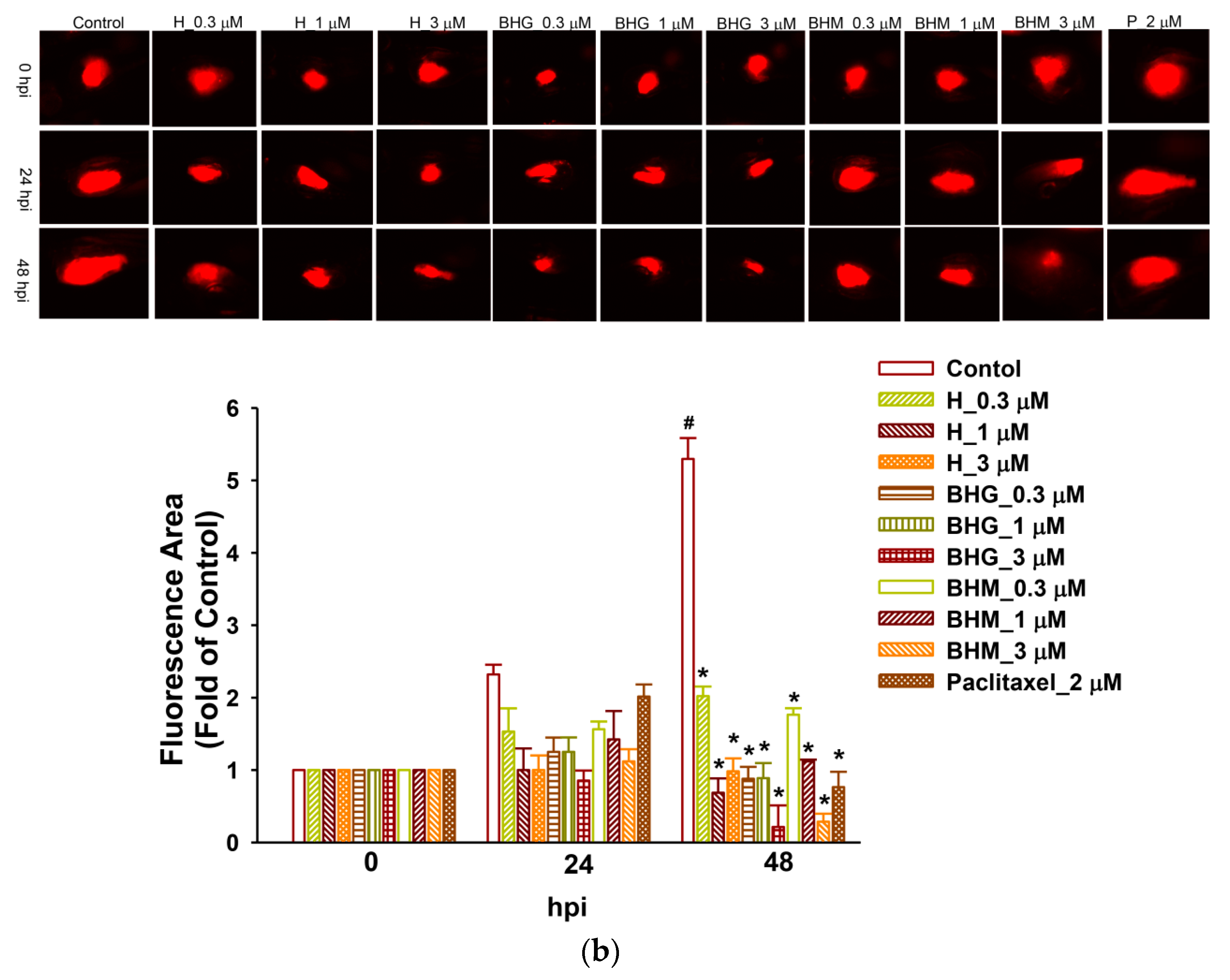
| Compound | MDA-MB-231 | MDA-MB-453 | MDA-MB-361 | MCF7 | HepG2 | NCI-H460 | MDA/doc |
|---|---|---|---|---|---|---|---|
| IC50 (μM) | |||||||
| Helenalin | 0.63 ± 0.03 | 5.71 ± 0.67 * | 7.63 ± 0.20 * | 1.75 ± 0.12 * | 2.80 ± 0.07 * | 4.52 ± 0.07 * | 7.69 ± 0.19 * |
| BHM | 0.07 ± 0.001 | 3.24 ± 0.27 * | 5.34 ± 3.34 | 0.58 ± 0.03 * | 0.69 ± 0.002 * | 0.99 ± 0.16 * | 0.73 ± 0.03 * |
| BHG | 0.55 ± 0.003 | 4.86 ± 0.73 * | 16.33 ± 0.39 * | 2.50 ± 0.12 * | 3.60 ± 0.004 * | 4.29 ± 0.09 * | 0.86 ± 0.06 * |
| Paclitaxel | 0.06 ± 0.01 | N/A | N/A | N/A | N/A | N/A | 90.56 ± 3.92 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-T.; Wu, I.-T.; Lee, C.-H.; Hung, C.-C. Phyto-Sesquiterpene Lactones Prevent the Development of Multidrug Resistance in TNBC via ABC Transporters Inhibition and STAT3/MYC Signaling. Cancers 2025, 17, 1321. https://doi.org/10.3390/cancers17081321
Chang Y-T, Wu I-T, Lee C-H, Hung C-C. Phyto-Sesquiterpene Lactones Prevent the Development of Multidrug Resistance in TNBC via ABC Transporters Inhibition and STAT3/MYC Signaling. Cancers. 2025; 17(8):1321. https://doi.org/10.3390/cancers17081321
Chicago/Turabian StyleChang, Ying-Tzu, I-Ting Wu, Chien-Hsing Lee, and Chin-Chuan Hung. 2025. "Phyto-Sesquiterpene Lactones Prevent the Development of Multidrug Resistance in TNBC via ABC Transporters Inhibition and STAT3/MYC Signaling" Cancers 17, no. 8: 1321. https://doi.org/10.3390/cancers17081321
APA StyleChang, Y.-T., Wu, I.-T., Lee, C.-H., & Hung, C.-C. (2025). Phyto-Sesquiterpene Lactones Prevent the Development of Multidrug Resistance in TNBC via ABC Transporters Inhibition and STAT3/MYC Signaling. Cancers, 17(8), 1321. https://doi.org/10.3390/cancers17081321








