Machine Learning for Thyroid Cancer Detection, Presence of Metastasis, and Recurrence Predictions—A Scoping Review
Simple Summary
Abstract
1. Introduction
- Follicular cell-derived neoplasms—Benign tumors, low-risk neoplasms, and malignant neoplasms. The malignant forms include the following:
- Follicular thyroid carcinoma;
- Invasive encapsulated follicular variant papillary thyroid carcinoma;
- Papillary thyroid carcinoma;
- Oncocytic carcinoma of the thyroid;
- Follicular-derived carcinomas, high-grade:
- –
- Poorly differentiated thyroid carcinoma;
- –
- Differentiated high-grade thyroid carcinoma;
- Anaplastic follicular cell-derived thyroid carcinoma.
- Thyroid C-cell-derived carcinoma—Medullary thyroid carcinoma.
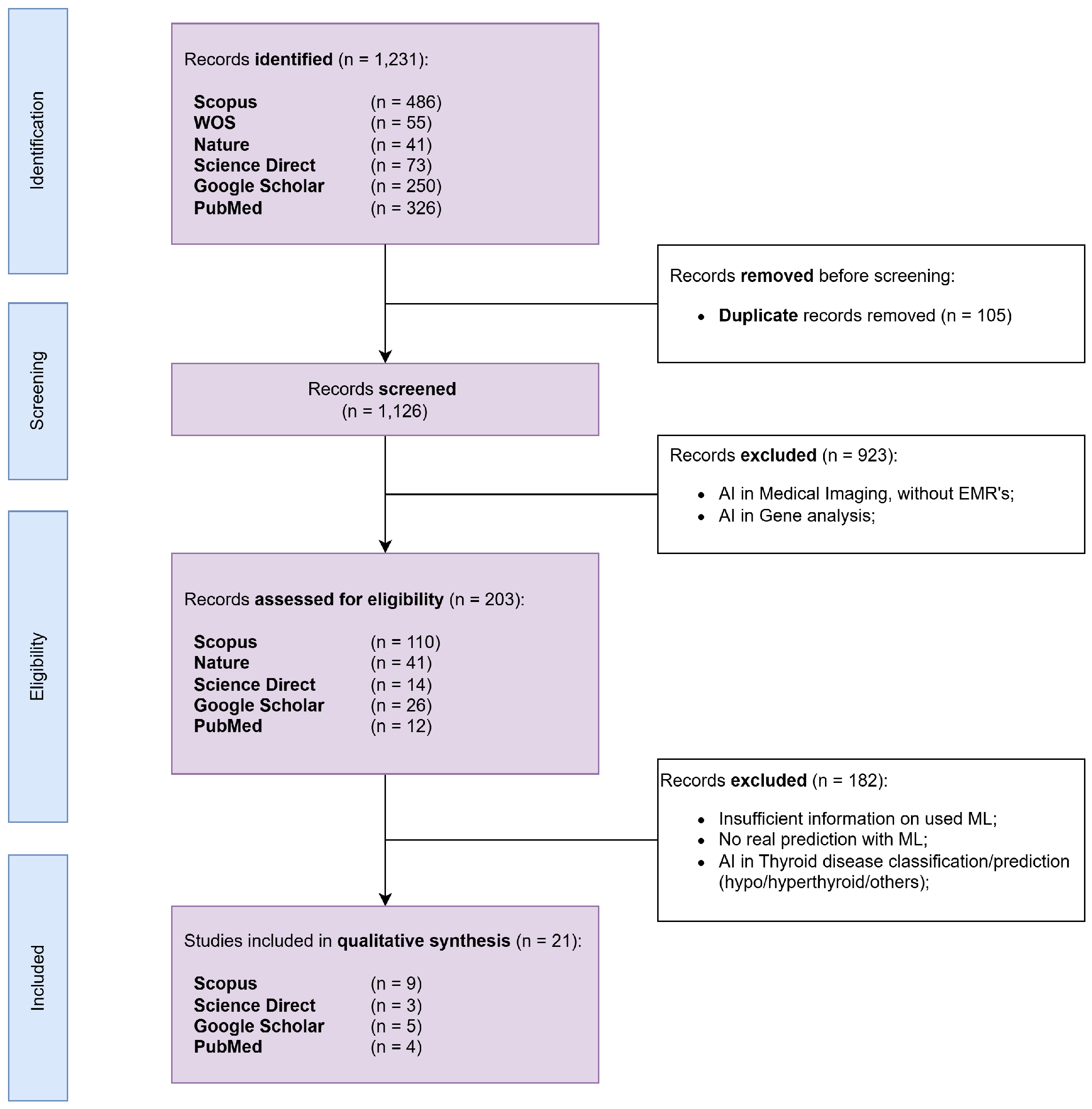
2. Materials and Methods
3. Results
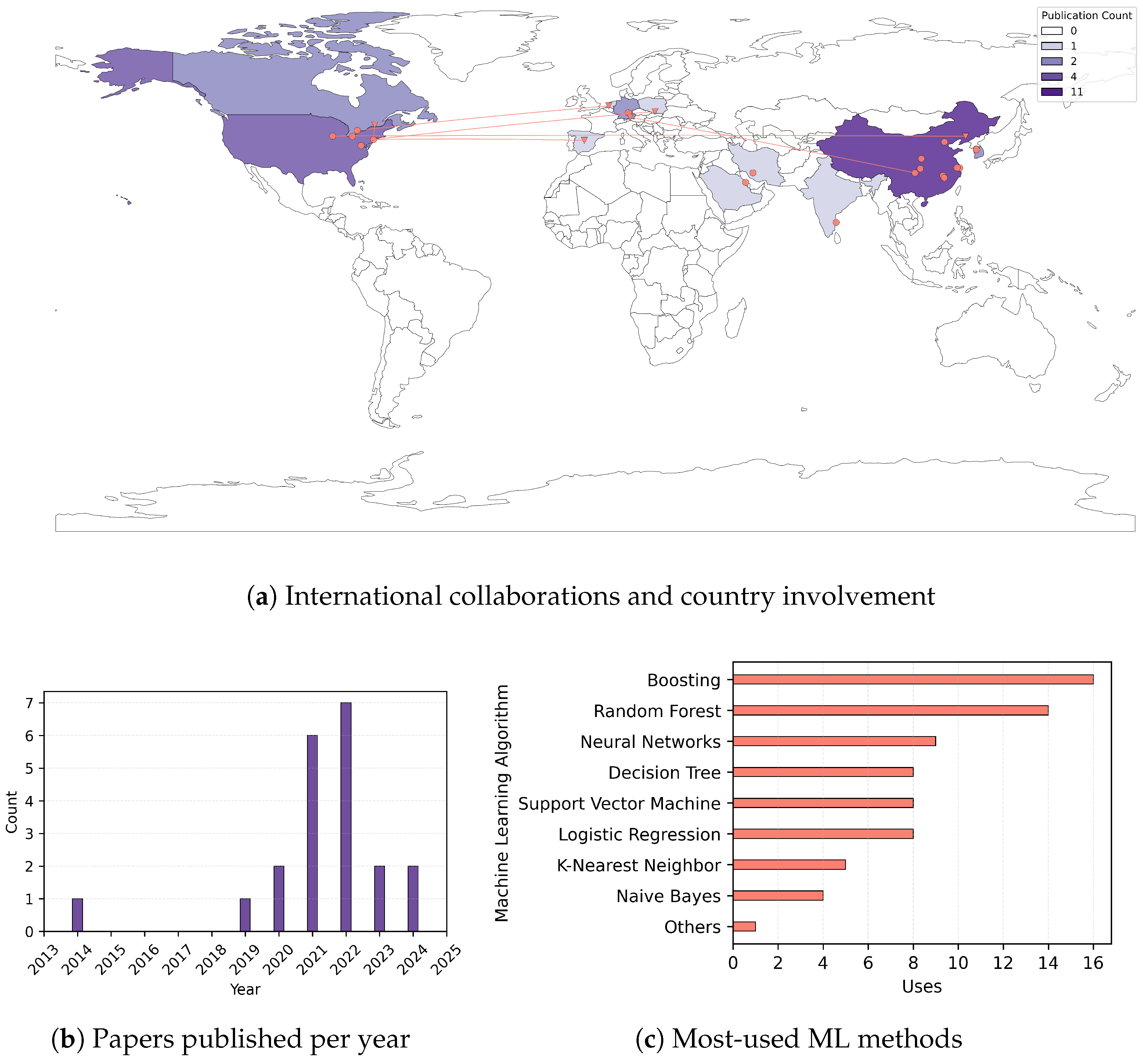
3.1. Descriptives of Selected Studies
3.2. Analysis of Selected Studies
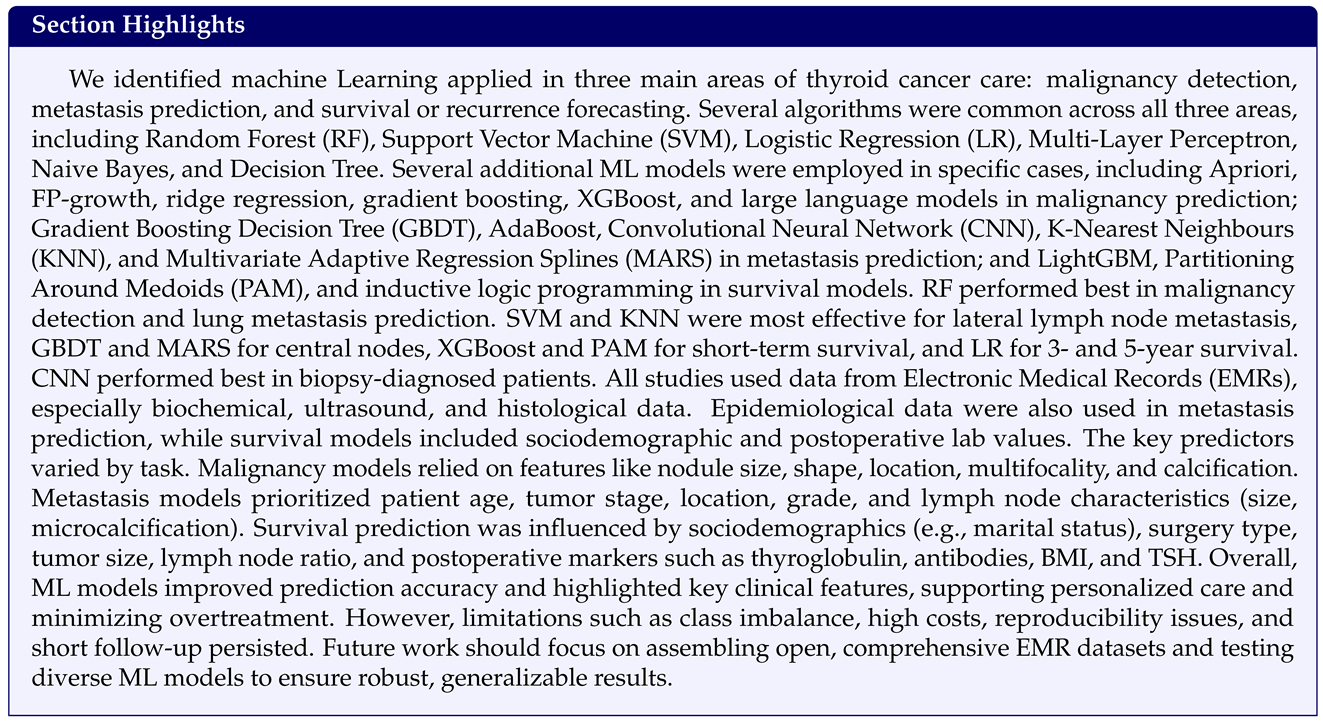
3.2.1. Improving Thyroid Cancer Diagnosis Through Malignancy Prediction and Metastatic Nodule Classification
3.2.2. Identifying Secondary Metastases Arising from Thyroid Cancer
3.2.3. Predicting Recurrence and Survival in Thyroid Cancer Patients
4. Discussion
4.1. Evidence Synthesis
- (a)
- Dataset acquisition—sourcing and collecting relevant data;
- (b)
- Data preprocessing and dimensionality reduction—cleaning data, exclusion sets, normalization, standardization;
- (c)
- ML model training and disease prediction—also implying feature selection, grouping methods, applied ML techniques;
- (d)
- Model evaluation—assessing model performance using predefined and well-documented metrics.
4.2. Principal Challenges and Limitations
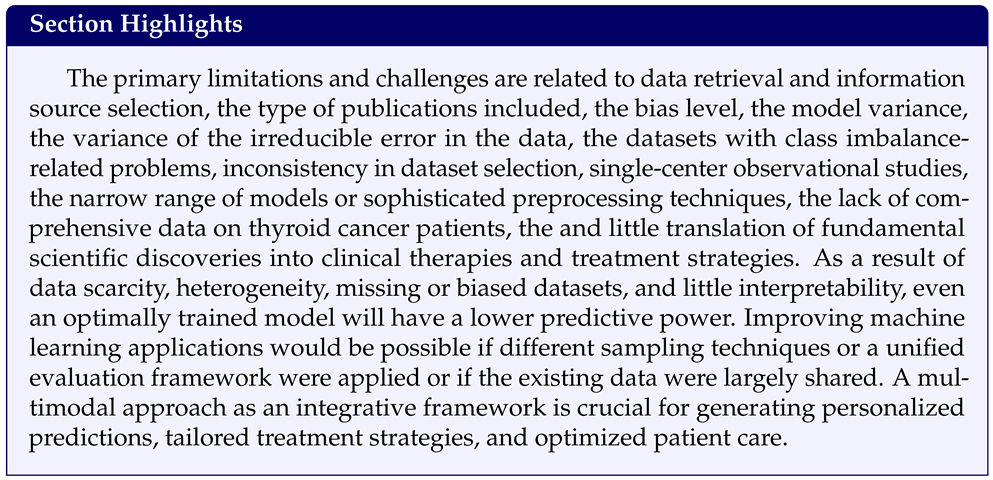
4.3. Perspectives and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AJCC | American Joint Committee on Cancer |
| ATC | Anaplastic Thyroid Carcinoma |
| BMI | Body Mass Index |
| CLNM | Central Lymph Node Metastasis |
| CNN | Convolutional Neural Network |
| DLNM | Delphian Lymph Node Metastasis |
| DT | Decision Tree |
| EMR | Electronic Medical Record |
| GBDT | Gradient Boosting Decision Tree |
| FTC | Follicular Thyroid Carcinoma |
| ILP | Inductive Logic Programming |
| LLM | Large Language Model |
| LLNM | Lateral Lymph Node Metastasis |
| ML | Machine Learning |
| MLP | Multi-Layer Perceptron |
| MTC | Medullary Thyroid Carcinoma |
| PTC | Papillary Thyroid Carcinoma |
| PAM | Partitioning Around Medoids |
| QoL | Quality of Life |
| RF | Random Forest |
| SEER | The Surveillance, Epidemiology and End Results |
| SMOTE | Synthetic Minority Over-sampling Technique |
| SVM | Support Vector Machine |
| TC | Thyroid Cancer |
| TI-RADS | Thyroid Imaging Reporting and Data System |
| TNM | Tumor-Nodule-Metastasis |
| XGBoost | eXtreme Gradient Boosting |
Appendix A. Additional Information on the Identified ML Methods
- Decision Tree—Makes predictions by recursively splitting the training data into branches based on feature values. It follows a tree-like structure, where each internal node represents a decision rule, and each leaf node represents a final prediction. After training, it predicts using the learned decision rules.
- Ensemble learning—Aggregates the predictions of multiple models to improve performance and accuracy.
- Boosting—An ensemble learning technique that combines multiple weak learners (typically decision trees) to create a stronger predictive model. It works by sequentially training models, where each new model focuses on correcting the errors of the previous one. The final prediction is a weighted combination of all models.
- Gradient Boosting—A variant of boosting that is trained by minimizing a loss function using gradient descent, an optimization algorithm that works by computing the gradient (derivative) of the loss function with respect to the model’s parameters and updating them in small steps controlled by a learning rate. Popular implementations of gradient boosting include XGBoost and LightGBM.
- Bagging (Bootstrap Aggregating)—An ensemble learning technique that improves model stability and accuracy by training multiple models on different subsets of data and averaging their predictions.
- Random Forest—An implementation of the bagging technique where the models used are decision trees.
- Logistic Regression—Computes a weighted sum of input features and applies the sigmoid function to map the output to a probability between 0 and 1. A decision threshold (commonly 0.5) is then used to classify the input.
- Support Vector Machine—Finds the optimal hyperplane that best separates data points into different classes while maximizing the margin between them. The data points closest to this hyperplane (i.e., support vectors) define the decision boundary. For non-linear data, SVM can use a kernel function to map data into higher dimensions where linear separation is possible.
- Naive Bayes—Based on Bayes’ theorem, it computes the probability of a class given certain features while assuming that all features are independent.
- K-Nearest Neighbors—It finds the K closest data points (neighbors) to a given input based on a distance metric (e.g., Euclidean distance) and makes predictions based on majority voting (for classification) or averaging (for regression). It requires no prior training but can be computationally expensive for large datasets since it needs to compute distances for all points during prediction.
- Multi-Layer Perceptron—One of the most common architectures of artificial neural networks composed of multiple layers of neurons, including an input layer, one or more hidden layers, and an output layer. Each neuron applies a weighted sum of inputs followed by a non-linear activation function (such as ReLU or Sigmoid) to learn complex patterns. It is trained using backpropagation and gradient descent to adjust weights and minimize errors.
- SHapley Additive exPlanations—A method for interpreting the predictions of ML models by attributing contributions to each feature. Based on Shapley values from cooperative game theory, SHAP quantifies how much each feature contributes to a model’s output for a given prediction.
- Association Rule Mining—A data mining technique used to discover relationships or patterns between variables in large datasets. It identifies frequent itemsets and generates if–then rules that describe how items or features are associated. Common algorithms for association rule mining include Apriori and FP-growth.
References
- Voichiţa, M.; Simona, M. Clasic si modern in cancerul tiroidian diferenţiat. Jurnalul Chir. 2010, 6, 97–103. [Google Scholar]
- Boucai, L.; Zafereo, M.; Cabanillas, M.E. Thyroid cancer: A review. Jama 2024, 331, 425–435. [Google Scholar] [CrossRef]
- Demeulemeester, R.; Grosclaude, P.; Grunenwald, S.; Saint-Pierre, P.; Savy, N.; Costa, N. Identification and Economic Evaluation of Differentiated Thyroid Cancer Care Consumption Patterns Using Sequence Analysis. Int. J. Public Health 2024, 69, 1606664. [Google Scholar] [CrossRef]
- Ţîbîrnă, A.; Ţîbîrnă, G.; Mereuţă, I. Cancerul glandei tiroide, conform stadializării noi. Bul. Acad. Ştiinţe Moldovei Ştiinţe Medicale 2019, 64, 108–134. [Google Scholar]
- Cree, I.A. (Ed.) Endocrine and Neuroendocrine Tumours; WHO Classification of Tumours Series, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2025; Volume 10. [Google Scholar]
- Nguyen, M.; He, G.; Lam, A.K.Y. Clinicopathological and molecular features of secondary cancer (metastasis) to the thyroid and advances in management. Int. J. Mol. Sci. 2022, 23, 3242. [Google Scholar] [CrossRef]
- Bach, K.; Ansari, P.; Ansari, H.; Mott, N.M.; Elfenbein, D.M.; Underwood, H.; Pitt, S.C. Health-related quality of life in patients with low-risk differentiated thyroid cancer: A systematic review examining the extent of thyroidectomy. Thyroid 2024, 34, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Maleki, Z.; Hassanzadeh, J.; Ghaem, H. Correlation between socioeconomic indices and epidemiological indices of thyroid cancer from 1990 to 2019 year: A global ecologic study. BMC Cancer 2024, 24, 467. [Google Scholar] [CrossRef]
- PRISMA GROUP. PRISMA Extension for Scoping Reviews (PRISMA-ScR). 2020. Available online: https://www.prisma-statement.org/prisma-2020-flow-diagram (accessed on 30 November 2024).
- Xi, N.M.; Wang, L.; Yang, C. Improving the diagnosis of thyroid cancer by machine learning and clinical data. Sci. Rep. 2022, 12, 11143. [Google Scholar] [CrossRef]
- Park, Y.M.; Lee, B.J. Machine learning-based prediction model using clinico-pathologic factors for papillary thyroid carcinoma recurrence. Sci. Rep. 2021, 11, 4948. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Ye, Z.; Xu, P.; Xia, X.; Guo, M. Prediction of lung metastases in thyroid cancer using machine learning based on SEER database. Cancer Med. 2022, 11, 2503–2515. [Google Scholar] [CrossRef]
- Mourad, M.; Moubayed, S.; Dezube, A.; Mourad, Y.; Park, K.; Torreblanca-Zanca, A.; Torrecilla, J.S.; Cancilla, J.C.; Wang, J. Machine learning and feature selection applied to SEER data to reliably assess thyroid cancer prognosis. Sci. Rep. 2020, 10, 5176. [Google Scholar] [CrossRef]
- Jajroudi, M.; Baniasadi, T.; Kamkar, L.; Arbabi, F.; Sanei, M.; Ahmadzade, M. Prediction of survival in thyroid cancer using data mining technique. Technol. Cancer Res. Treat. 2014, 13, 353–359. [Google Scholar] [CrossRef]
- Liu, W.C.; Li, Z.Q.; Luo, Z.W.; Liao, W.J.; Liu, Z.L.; Liu, J.M. Machine learning for the prediction of bone metastasis in patients with newly diagnosed thyroid cancer. Cancer Med. 2021, 10, 2802–2811. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zheng, J.; Li, L.; Huang, R.; Ren, H.; Wang, D.; Dai, Z.; Su, X. Application of machine learning algorithms to predict central lymph node metastasis in T1-T2, non-invasive, and clinically node negative papillary thyroid carcinoma. Front. Med. 2021, 8, 635771. [Google Scholar] [CrossRef]
- Wu, Y.; Rao, K.; Liu, J.; Han, C.; Gong, L.; Chong, Y.; Liu, Z.; Xu, X. Machine learning algorithms for the prediction of central lymph node metastasis in patients with papillary thyroid cancer. Front. Endocrinol. 2020, 11, 577537. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Sathyalakshmi, S. A Study on the Explainability of Thyroid Cancer Prediction: SHAP Values and Association-Rule Based Feature Integration Framework. Comput. Mater. Contin. 2024, 79, 3111–3138. [Google Scholar] [CrossRef]
- Olatunji, S.O.; Alotaibi, S.; Almutairi, E.; Alrabae, Z.; Almajid, Y.; Altabee, R.; Altassan, M.; Ahmed, M.I.B.; Farooqui, M.; Alhiyafi, J. Early diagnosis of thyroid cancer diseases using computational intelligence techniques: A case study of a Saudi Arabian dataset. Comput. Biol. Med. 2021, 131, 104267. [Google Scholar] [CrossRef]
- Sievert, M.; Conrad, O.; Mueller, S.K.; Rupp, R.; Balk, M.; Richter, D.; Mantsopoulos, K.; Iro, H.; Koch, M. Risk stratification of thyroid nodules: Assessing the suitability of ChatGPT for text-based analysis. Am. J. Otolaryngol. 2024, 45, 104144. [Google Scholar] [CrossRef]
- Lai, S.w.; Fan, Y.l.; Zhu, Y.h.; Zhang, F.; Guo, Z.; Wang, B.; Wan, Z.; Liu, P.l.; Yu, N.; Qin, H.d. Machine learning-based dynamic prediction of lateral lymph node metastasis in patients with papillary thyroid cancer. Front. Endocrinol. 2022, 13, 1019037. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, Y.I.; Kim, H.J.; Chang, H.; Kim, S.M.; Lee, Y.S.; Kwon, S.S.; Shin, H.; Chang, H.S.; Park, C.S. New approach of prediction of recurrence in thyroid cancer patients using machine learning. Medicine 2021, 100, e27493. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, Z.; Li, M.; Wang, Y.; Yan, C.; Fan, J.; Xu, F.; Meng, H.; Kong, J.; Li, S.; et al. Model development to predict central lymph node metastasis in cN0 papillary thyroid microcarcinoma by machine learning. Ann. Transl. Med. 2022, 10, 892. [Google Scholar] [CrossRef]
- Radebe, L.; van der Kaay, D.C.; Wasserman, J.D.; Goldenberg, A. Predicting malignancy in pediatric thyroid nodules: Early experience with machine learning for clinical decision support. J. Clin. Endocrinol. Metab. 2021, 106, e5236–e5246. [Google Scholar] [CrossRef] [PubMed]
- Luong, G.; Idarraga, A.J.; Hsiao, V.; Schneider, D.F. Risk stratifying indeterminate thyroid nodules with machine learning. J. Surg. Res. 2022, 270, 214–220. [Google Scholar] [CrossRef]
- Yang, C.Q.; Gardiner, L.; Wang, H.; Hueman, M.T.; Chen, D. Creating prognostic systems for well-differentiated thyroid cancer using machine learning. Front. Endocrinol. 2019, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Huang, Y.; Xu, L.; Liang, J.; Lin, W.; Huang, H.; Li, L.; Wen, J.; Chen, G. Surgical methods and social factors are associated with long-term survival in follicular thyroid carcinoma: Construction and validation of a prognostic model based on machine learning algorithms. Front. Oncol. 2022, 12, 816427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qu, L.; Chen, Q.; Zhou, Y.; Duan, H.; Li, B.; Weng, Y.; Su, J.; Yi, W. Deep learning-based multifeature integration robustly predicts central lymph node metastasis in papillary thyroid cancer. BMC Cancer 2023, 23, 128. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Q.; Zheng, D.; Zhu, L.; Zhou, Z.; Xu, S.; Shi, B.; Jin, C.; Zheng, G.; Cai, Y. A novel and effective model to predict skip metastasis in papillary thyroid carcinoma based on a support vector machine. Front. Endocrinol. 2022, 13, 916121. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, C.; Yang, B.; Zhu, Z.; Huang, Y.; Shen, B.; Dong, X.; Xu, X.; Liu, G. Preoperative US integrated random forest model for predicting Delphian lymph node metastasis in patients with papillary thyroid cancer. Curr. Med. Imaging Rev. 2023, 19, 1031–1040. [Google Scholar] [CrossRef]
- Ho, T.K. Random decision forests. In Proceedings of the 3rd International Conference on Document Analysis and Recognition, Montreal, QC, Canada, 14–16 August 1995; Volume 1, pp. 278–282. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Zhang, H. The optimality of naive Bayes. AAAI 2004, 1, 3. [Google Scholar]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Cox, D.R. The regression analysis of binary sequences. J. R. Stat. Soc. Ser. Methodol. 1958, 20, 215–232. [Google Scholar] [CrossRef]
- Agrawal, R.; Srikant, R. Fast Algorithms for Mining Association Rules in Large Databases. In Proceedings of the VLDB ’94 20th International Conference on Very Large Data Bases, San Francisco, CA, USA, 12–15 September 1994; pp. 487–499. [Google Scholar]
- Han, J.; Pei, J. Mining frequent patterns by pattern-growth: Methodology and implications. ACM SIGKDD Explor. Newsl. 2000, 2, 14–20. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the KDD ’16 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, New York, NY, USA, 13–17 August 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Breiman, L. Classification and Regression Trees; Routledge: London, UK, 2017. [Google Scholar]
- Lundberg, S.M.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. In Advances in Neural Information Processing Systems 30; Guyon, I., Luxburg, U.V., Bengio, S., Wallach, H., Fergus, R., Vishwanathan, S., Garnett, R., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2017; pp. 4765–4774. [Google Scholar]
- Hoerl, A.E.; Kennard, R.W. Ridge regression: Biased estimation for nonorthogonal problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Deng, H. Interpreting tree ensembles with intrees. Int. J. Data Sci. Anal. 2019, 7, 277–287. [Google Scholar] [CrossRef]
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program; National Cancer Institute: Bethesda, MD, USA, 2022.
- Cover, T.; Hart, P. Nearest neighbor pattern classification. IEEE Trans. Inf. Theory 1967, 13, 21–27. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Int. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Schapire, R.E. Explaining adaboost. In Empirical Inference; Springer: Berlin/Heidelberg, Germany, 2013; pp. 37–52. [Google Scholar]
- Friedman, J.H. Multivariate adaptive regression splines. Ann. Stat. 1991, 19, 1–67. [Google Scholar] [CrossRef]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.Y. LightGBM: A highly efficient gradient boosting decision tree. In Proceedings of the NIPS’17 31st International Conference on Neural Information Processing Systems, Red Hook, NY, USA, 4–9 December 2017; pp. 3149–3157. [Google Scholar]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Cox, D.R. Regression models and life-tables. J. R. Stat. Soc. Ser. B Methodol. 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Systèmes, D. DELMIA—Process Rules Discovery (RDY). Available online: https://www.3ds.com/fr/produits-et-services/catia/produits/catia-v5/portefeuille/domain/Intelligence/product/RDY/ (accessed on 22 October 2021).
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, L.; Rousseeuw, P.J. Finding Groups in Data: An Introduction to Cluster Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Wang, S.; Li, D.; Song, X.; Wei, Y.; Li, H. A feature selection method based on improved fisher’s discriminant ratio for text sentiment classification. Expert Syst. Appl. 2011, 38, 8696–8702. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Wu, B.; Chen, C.; Kechadi, T.M.; Sun, L. A comparative evaluation of filter-based feature selection methods for hyper-spectral band selection. Int. J. Remote Sens. 2013, 34, 7974–7990. [Google Scholar] [CrossRef]
- Guo, M.; Sun, Y.; Wei, Y.; Xu, J.; Zhang, C. Advances in targeted therapy and biomarker research in thyroid cancer. Front. Endocrinol. 2024, 15, 1372553. [Google Scholar] [CrossRef]
- Habchi, Y.; Himeur, Y.; Kheddar, H.; Boukabou, A.; Atalla, S.; Chouchane, A.; Ouamane, A.; Mansoor, W. AI in Thyroid Cancer Diagnosis: Techniques, Trends, and Future Directions. Systems 2023, 11, 519. [Google Scholar] [CrossRef]
- The Royal College of Pathologists. Cancer Datasets and Tissue Pathways. 2024. Available online: https://www.rcpath.org/profession/guidelines/cancer-datasets-and-tissue-pathways.html (accessed on 10 February 2025).
- Mehdi, D. DDTI Thyroid Ultrasound Images Dataset. 2024. Available online: https://www.kaggle.com/datasets/dasmehdixtr/ddti-thyroid-ultrasound-images (accessed on 2 February 2025).
- National Center for Biotechnology Information. Gene Expression Omnibus (GEO). 2024. Available online: https://www.ncbi.nlm.nih.gov/geo (accessed on 2 February 2025).
- National Cancer Institute. The Cancer Genome Atlas (TCGA) Program. 2024. Available online: https://www.cancer.gov/ccg/research/genome-sequencing/tcga (accessed on 2 February 2025).
- Vaswani, A. Attention is all you need. In Advances in Neural Information Processing Systems; MIT Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Zhang, Z.; Yao, L.; Wang, W.; Jiang, B.; Xia, F.; Li, X. A bibliometric analysis of 34,692 publications on thyroid cancer by machine learning: How much has been done in the past three decades? Front. Oncol. 2021, 11, 673733. [Google Scholar] [CrossRef]
- Alagoz, O.; Zhang, Y.; Arroyo, N.; Fernandes-Taylor, S.; Yang, D.Y.; Krebsbach, C.; Venkatesh, M.; Hsiao, V.; Davies, L.; Francis, D.O. Modeling Thyroid Cancer Epidemiology in the United States Using Papillary Thyroid Carcinoma Microsimulation Model. Value Health 2024, 27, 367–375. [Google Scholar] [CrossRef]
- Liu, Y.H.; Jin, J.; Liu, Y.J. Machine learning–based random forest for predicting decreased quality of life in thyroid cancer patients after thyroidectomy. Support. Care Cancer 2022, 30, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, R.; Zhong, J.; Chen, W.; Shuai, J.; Chen, M.; Deng, F.; Wei, T.; Tang, H.; Li, Z.; et al. A multicenter cohort study of thyroidectomy-related decision regret in patients with low-risk papillary thyroid microcarcinoma. Nat. Commun. 2025, 16, 2317. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Steinhubl, S.R. Digital medicine in thyroidology: A new era of managing thyroid disease. Endocrinol. Metab. 2019, 34, 124–131. [Google Scholar] [CrossRef] [PubMed]

| Inclusion | Exclusion |
|---|---|
| Research articles and conference papers written in English that are fully available online | Review articles, study protocols, book chapters, notes, brief reports, letters, editorials, or case studies written in any language |
| Published in Scopus, Web of Science, Nature, Science Direct, Google Scholar, or PubMed between 2014 and 2024 with more than 10 citations between 2014–2022 and at least one between 2023 and 2024 | Articles focusing on thyroid biomarkers, gene expressions, hypothyroidism, or hyperthyroidism |
| Participants: Adults and children with current thyroid cancer diagnosis or just suspicions | Articles using ultrasound images or radiomics generated from images |
| Concept: Patients undergoing blood analyses, testing, and other imaging tests features in hospitals or input data from repositories for detecting disease progression | |
| Objective: Machine learning applied to medical data to make predictions and prognostics for thyroid cancer | |
| Context: Feature thyroid cancer management integrating ML tools into routine clinical workflows |
| Ref | Type | Input Data | Objective | BPC |
|---|---|---|---|---|
| [10] | blood test results, TNM stage, ultrasound features, surgical methods | predicting thyroid nodule malignancy | RF | |
| [11] | PTC | TNM stage, histological type, surgical methods | predict PTC recurrence | DT |
| [12] | PTC, FTC, MTC, ATC | TNM stage, histological type | predict lung metastasis in TC | RF |
| [13] | PTC, FTC | TNM stage, histological type, regional nodes examined, survived months | survival rate (for over 10 years since diagnosis) | MLP |
| [14] | TNM stage, histological type, type of treatment | survival prediction in TC patients | MLP | |
| [15] | PTC, FTC, MTC, ATC | TNM stage, histological type | predict bone metastasis in people with TC | RF |
| [16] | PTC | TNM stage, histological type, ultrasound features, surgical methods | predict CLNM | XGBoost |
| [17] | PTC | blood test results, ultrasound features, surgical methods | predict CLNM | GBDT |
| [18] | ultrasound features | TC prediction models, malignancy prediction | DT | |
| [19] | blood test results | detect TC at very early stages | RF | |
| [20] | ultrasound features | classify sonographic patterns in accordance with TI-RADS | LLM | |
| [21] | PTC | blood test results, TNM stage, and ultrasound features | predict LLNM in PTC patients | RF |
| [22] | Well-Differentiated TC | pathological and genetic information | predicting disease recurrence | ILP |
| [23] | PTC (≤1 cm) | family history of cancer, blood test results, pathological features, ultrasound features | predict the risk of CLNM | RF |
| [24] | PTC | TNM stage, histological type, ultrasound features | predict malignant nodules in PTC | RF |
| [25] | TNM stage, pathology and cytology data, ultrasound features | predict malignancy in indeterminate thyroid nodules | RF | |
| [26] | Well-Differentiated TC | TNM stage, histological type, follow up vital status | prognostic systems for well-differentiated TC | PAM |
| [27] | FTC | TNM Stage, histological type, surgical methods | predict the prognosis of FTC | XGBoost |
| [28] | PTC | TNM stage, histological type, ultrasound features, genetic information, surgical methods | predict CLNM in PTC | CNN |
| [29] | PTC | TNM stage, histological type | predict LLNM of PTC without central lymph node metastasis | SVM |
| [30] | PTC | blood test results, TNM Stage, ultrasound features | predict DLNM in PTC patients | RF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lixandru-Petre, I.-O.; Dima, A.; Musat, M.; Dascalu, M.; Gradisteanu Pircalabioru, G.; Iliescu, F.S.; Iliescu, C. Machine Learning for Thyroid Cancer Detection, Presence of Metastasis, and Recurrence Predictions—A Scoping Review. Cancers 2025, 17, 1308. https://doi.org/10.3390/cancers17081308
Lixandru-Petre I-O, Dima A, Musat M, Dascalu M, Gradisteanu Pircalabioru G, Iliescu FS, Iliescu C. Machine Learning for Thyroid Cancer Detection, Presence of Metastasis, and Recurrence Predictions—A Scoping Review. Cancers. 2025; 17(8):1308. https://doi.org/10.3390/cancers17081308
Chicago/Turabian StyleLixandru-Petre, Irina-Oana, Alexandru Dima, Madalina Musat, Mihai Dascalu, Gratiela Gradisteanu Pircalabioru, Florina Silvia Iliescu, and Ciprian Iliescu. 2025. "Machine Learning for Thyroid Cancer Detection, Presence of Metastasis, and Recurrence Predictions—A Scoping Review" Cancers 17, no. 8: 1308. https://doi.org/10.3390/cancers17081308
APA StyleLixandru-Petre, I.-O., Dima, A., Musat, M., Dascalu, M., Gradisteanu Pircalabioru, G., Iliescu, F. S., & Iliescu, C. (2025). Machine Learning for Thyroid Cancer Detection, Presence of Metastasis, and Recurrence Predictions—A Scoping Review. Cancers, 17(8), 1308. https://doi.org/10.3390/cancers17081308







