Innovative qPCR Algorithm Using Platelet-Derived RNA for High-Specificity and Cost-Effective Ovarian Cancer Detection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Blood Sample Collection
2.2. Platelet RNA Extraction
2.3. RNA Sequencing
2.4. Data Processing and Quantification
2.5. Quantitative Real-Time Polymerase Chain Reaction
2.6. Junction-Level RNA Sequencing Analysis and Marker Selection
2.7. Cutoff and Score for the Algorithm
2.8. Quantification and Statistical Analysis
3. Results
3.1. Study Population and Classification
3.2. Assessment of Batch Effects
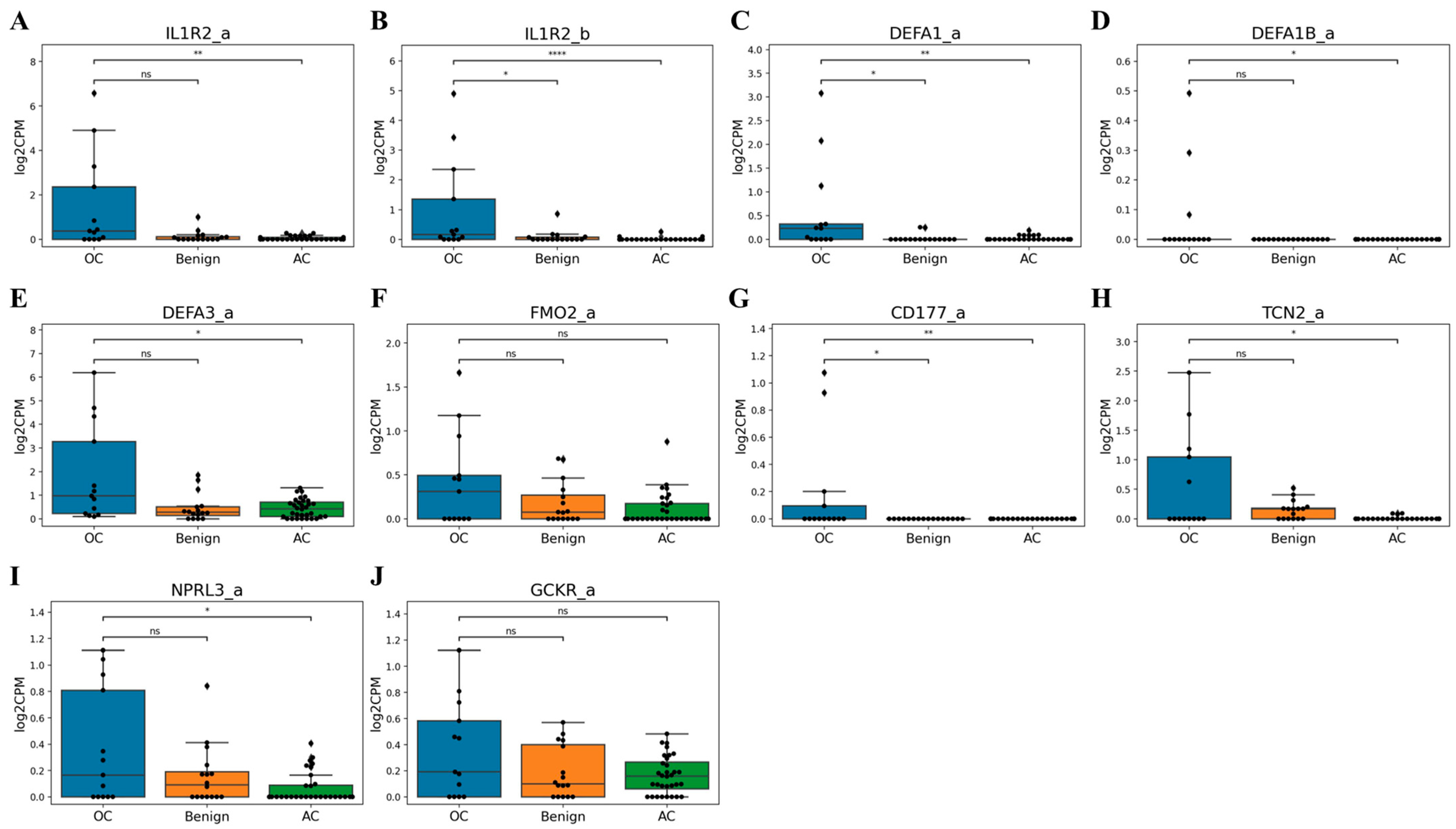
3.3. Dataset Composition and Marker Selection
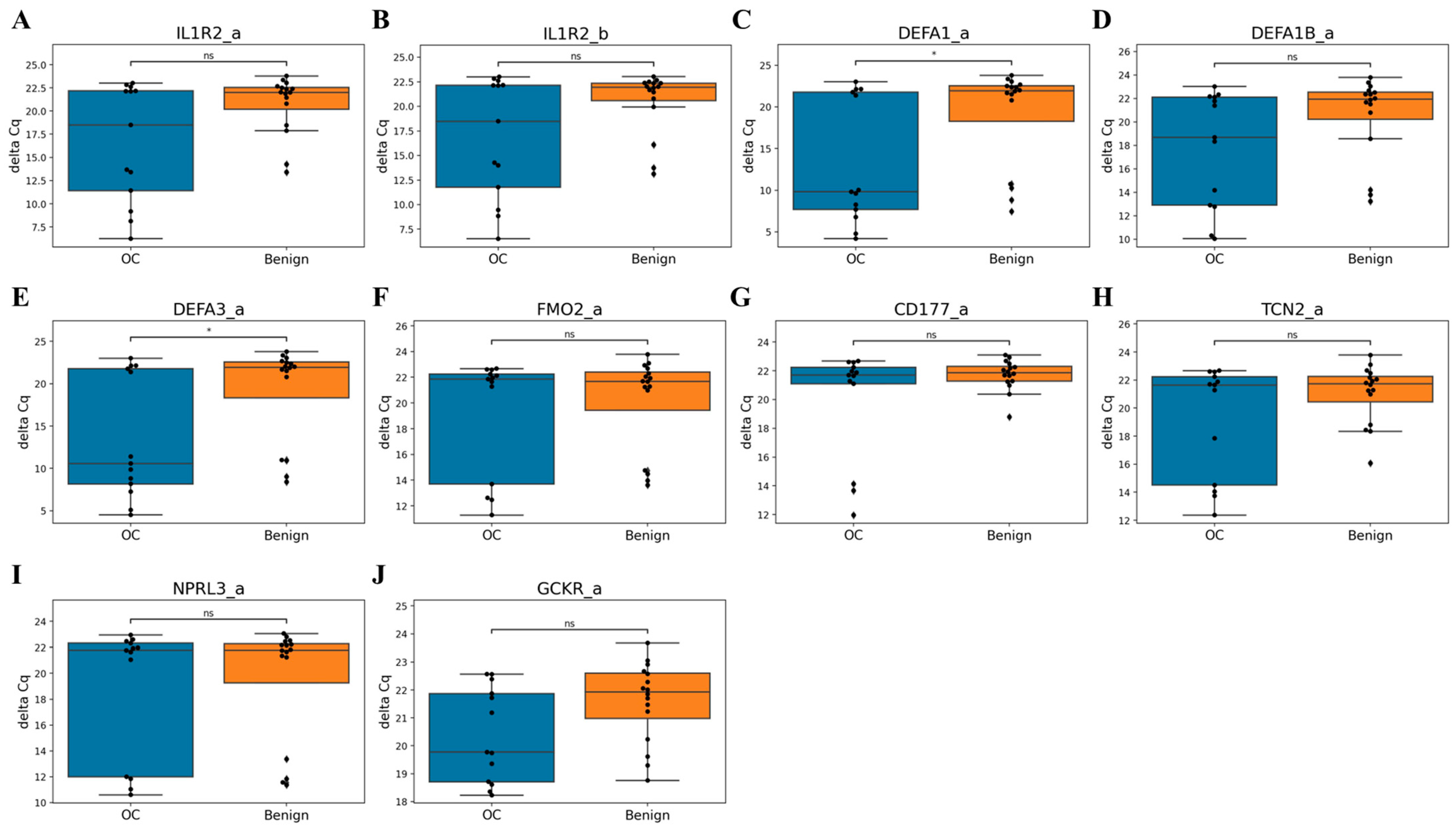
3.4. qPCR Validation of Candidate Markers
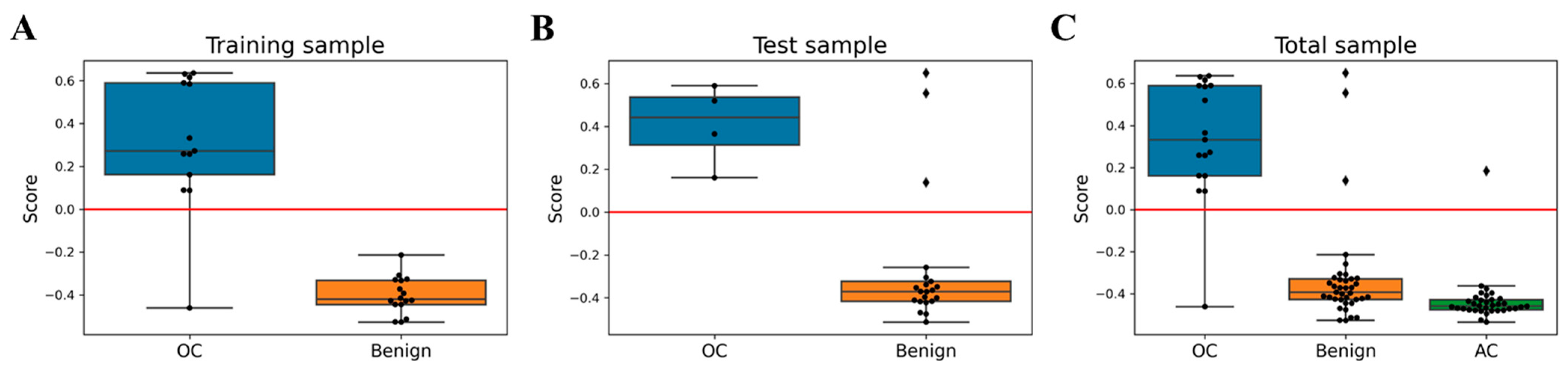
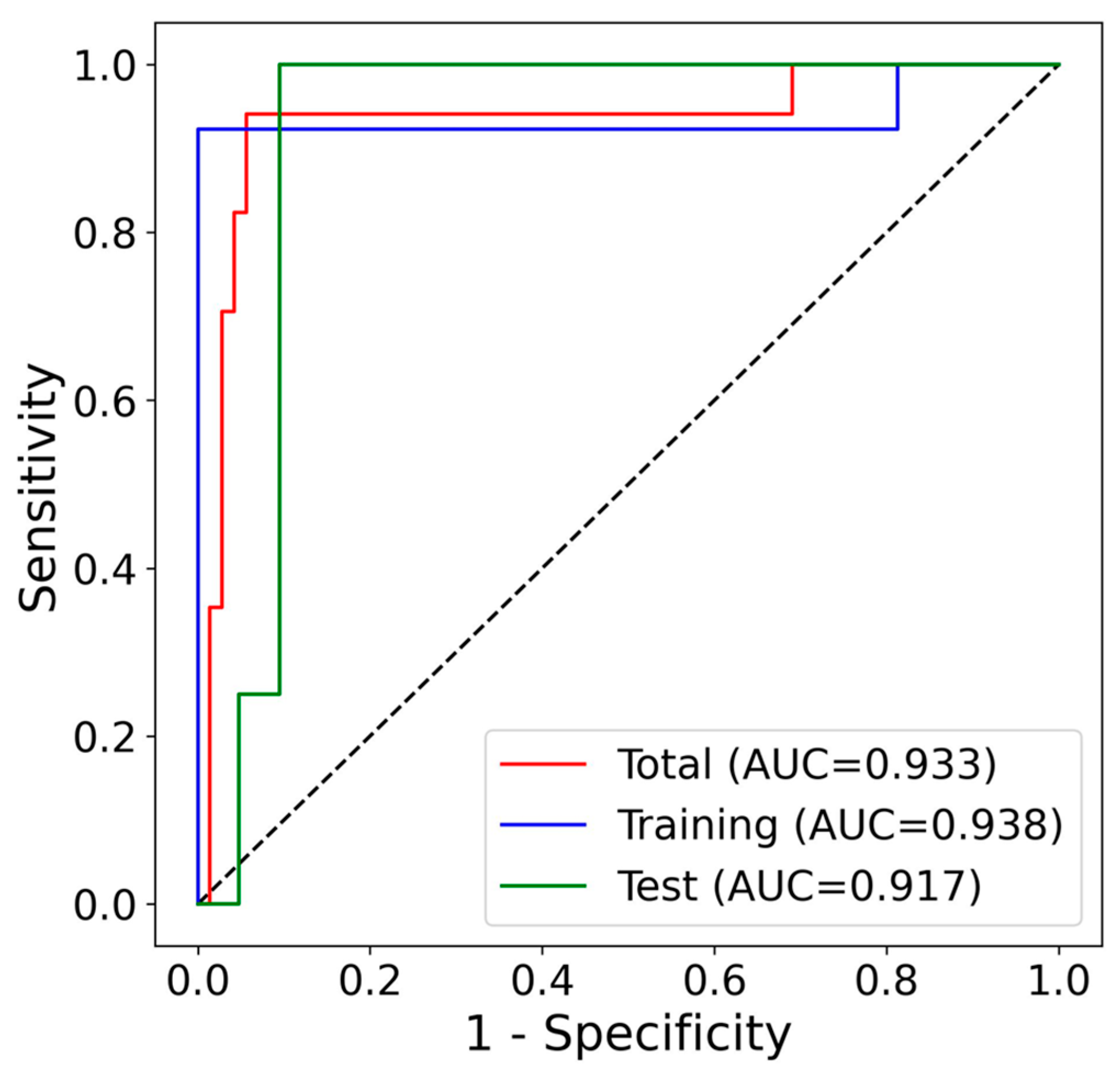
3.5. Algorithm Development and Performance Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. Wiley 2018, 68, 284–296. [Google Scholar]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K.; Amso, N.N.; Apostolidou, S.; Benjamin, E.; Cruickshank, D.; et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2016, 387, 945–956. [Google Scholar]
- Han, C.Y.; Lu, K.H.; Corrigan, G.; Perez, A.; Kohring, S.D.; Celestino, J.; Bedi, D.; Bedia, E.; Bevers, T.; Boruta, D.; et al. Normal Risk Ovarian Screening Study: 21-Year Update. J. Clin. Oncol. 2024, 42, 1102–1109. [Google Scholar]
- Barrett, J.E.; Jones, A.; Evans, I.; Reisel, D.; Herzog, C.; Chindera, K.; Kristiansen, M.; Leavy, O.C.; Manchanda, R.; Bjørge, L.; et al. The DNA methylome of cervical cells can predict the presence of ovarian cancer. Nat. Commun. 2022, 13, 448. [Google Scholar]
- Jia, D.; Nagaoka, Y.; Katsumata, M.; Orsulic, S. Inflammation is a key contributor to ovarian cancer cell seeding. Sci. Rep. 2018, 8, 12394. [Google Scholar]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [PubMed]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2012, 2, 98. [Google Scholar]
- Sánchez-Prieto, M.; Sánchez-Borrego, R.; Lubián-López, D.M.; Pérez-López, F.R. Etiopathogenesis of ovarian cancer. An inflamm-aging entity? Gynecol. Oncol. Rep. 2022, 42, 101018. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar]
- Price, J.; Lord, J.M.; Harrison, P. Inflammaging and platelet hyperreactivity: A new therapeutic target? J. Thromb. Haemost. 2020, 18, 3–5. [Google Scholar] [PubMed]
- In ’t Veld, S.G.J.G.; Wurdinger, T. Tumor-educated platelets. Blood 2019, 133, 2359–2364. [Google Scholar]
- Best, M.G.; Sol, N.; Kooi, I.; Tannous, J.; Westerman, B.A.; Rustenburg, F.; Schellen, P.; Verschueren, H.; Post, E.; Koster, J.; et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell 2015, 28, 666–676. [Google Scholar] [PubMed]
- Gao, Y.; Liu, C.J.; Li, H.Y.; Xiong, X.M.; Li, G.L.; In ’t Veld, S.G.J.G.; Cai, G.Y.; Xie, G.Y.; Zeng, S.Q.; Wu, Y.; et al. Platelet RNA enables accurate detection of ovarian cancer: An intercontinental, biomarker identification study. Protein Cell 2023, 14, 433–447. [Google Scholar]
- Oncul, S.; Cho, M.S. Interactions between Platelets and Tumor Microenvironment Components in Ovarian Cancer and Their Implications for Treatment and Clinical Outcomes. Cancers 2023, 15, 1282. [Google Scholar] [CrossRef]
- Eo, W.K.; Kim, K.H.; Park, E.J.; Kim, H.Y.; Kim, H.; Koh, S.B.; Namkung, J. Diagnostic accuracy of inflammatory markers for distinguishing malignant and benign ovarian masses. J. Cancer 2018, 9, 1165–1172. [Google Scholar] [PubMed]
- Kim, M.C.; Borcherding, N.; Ahmed, K.K.; Voigt, A.P.; Vishwakarma, A.; Kolb, R.; Kluz, P.N.; Pandey, G.; De, U.; Drashansky, T.; et al. CD177 modulates the function and homeostasis of tumor-infiltrating regulatory T cells. Nat. Commun. 2021, 12, 5764. [Google Scholar] [CrossRef]
- Hazlett, L.; Wu, M. Defensins in innate immunity. Cell Tissue Res. 2011, 343, 75–88. [Google Scholar]
- Chen, L.; Huang, H.; Zheng, X.; Li, Y.; Chen, J.; Tan, B.; Liu, Y.; Sun, R.; Xu, B.; Yang, M.; et al. IL1R2 increases regulatory T cell population in the tumor microenvironment by enhancing MHC-II expression on cancer-associated fibroblasts. J. Immunother. Cancer 2022, 10, e004585. [Google Scholar]
- Xia, J.; Zhang, L.; Peng, X.; Tu, J.; Li, S.; He, X.; Li, F.; Qiang, J.; Dong, H.; Deng, Q.; et al. IL1R2 Blockade Alleviates Immunosuppression and Potentiates Anti-PD-1 Efficacy in Triple-Negative Breast Cancer. Cancer Res. 2024, 84, 2282–2296. [Google Scholar]
- Yu, S.; Yang, R.i.; Xu, T.; Wu, S.; Zhang, J. Cancer-associated fibroblasts-derived FMO2 as a biomarker of macrophage infiltration and prognosis in epithelial ovarian cancer. Gynecol. Oncol. 2022, 167, 342–353. [Google Scholar] [PubMed]
- Bar-Peled, L.; Chantranupong, L.; Cherniack, A.D.; Chen, W.W.; Ottina, K.A.; Grabiner, B.C.; Spear, E.D.; Carter, S.L.; Meyerson, M.; Sabatini, D.M. A tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 2013, 340, 1100–1106. [Google Scholar] [CrossRef]
- Castro, R.; Barroso, M.; Rocha, M.; Esse, R.; Ramos, R.; Ravasco, P.; Rivera, I.; de Almeida, I.T. The TCN2 776C > G polymorphism correlates with vitamin B12 cellular delivery in healthy adult populations. Clin. Biochem. 2010, 43, 645–649. [Google Scholar]


| Group | Stage | n | Age | Weight | Height | BMI |
|---|---|---|---|---|---|---|
| Asymptomatic control | N.A. * | 34 | 25.5 [22.2, 33.0], n = 34 | 56.0 [52.3, 60.0], n = 34 | 160.6 [159.5, 163.0], n = 34 | 21.7 [20.5, 22.6], n = 34 |
| Benign | N.A. | 37 | 46.0 [38.2, 52.0], n = 36 | 65.8 [59.4, 73.5], n = 16 | 162.1 [159.0, 163.8], n = 16 | 25.3 [22.5, 27.8], n = 16 |
| Ovarian cancer (+Borderline ovarian tumors) | Total | 19 | 53.5 [50.5, 61.8], n = 18 | 59.2 [54.9, 67.6], n = 14 | 159.8 [154.9, 160.9], n = 14 | 23.6 [22.2, 26.4], n = 14 |
| Ovarian cancer | Early (I, II) | 6 | 52.5 [50.5, 53.0], n = 6 | 68.2 [61.1, 69.2], n = 5 | 160.2 [160.1, 161.5], n = 5 | 26.6 [23.4, 27.0], n = 5 |
| Late (III, IV) | 11 | 61.0 [52.5, 61.8], n = 10 | 55.3 [52.6, 62.8], n = 8 | 156.3 [154.5, 160.7], n = 8 | 22.6 [21.4, 24.3], n = 8 | |
| Borderline ovarian tumors | N.A. | 2 | 53.0 [43.0, 63.0], n = 2 | 57.2 [57.2, 57.2], n = 1 | 149.0 [149.0, 149.0], n = 1 | 25.8 [25.8, 25.8], n = 1 |
| Asymptomatic control and Benign vs. Ovarian cancer (+Borderline ovarian tumors) (p-value) | - | - | 0.0001 | 0.690588 | 0.103928 | 0.208145 |
| Actual | Total | |||
|---|---|---|---|---|
| OC | Benign | |||
| Predict | OC | 12 | 0 | 12 |
| Non-OC | 1 | 16 | 17 | |
| Total | 13 | 16 | 29 | |
| Actual | Total | |||
|---|---|---|---|---|
| OC | Benign | |||
| Predict | OC | 4 | 3 | 7 |
| Non-OC | 0 | 18 | 18 | |
| Total | 4 | 21 | 25 | |
| Actual | Total | ||||
|---|---|---|---|---|---|
| OC | Benign | AC | |||
| Predict | OC | 16 | 3 | 1 | 20 |
| Non-OC | 1 | 34 | 33 | 68 | |
| Total | 17 | 37 | 34 | 88 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, E.; Kim, S.I.; Park, S.; Kim, S.; Kim, H.; Lee, H.; Kim, H.; Song, E.J.; Ahn, T.; Song, Y.-S. Innovative qPCR Algorithm Using Platelet-Derived RNA for High-Specificity and Cost-Effective Ovarian Cancer Detection. Cancers 2025, 17, 1251. https://doi.org/10.3390/cancers17071251
Ahn E, Kim SI, Park S, Kim S, Kim H, Lee H, Kim H, Song EJ, Ahn T, Song Y-S. Innovative qPCR Algorithm Using Platelet-Derived RNA for High-Specificity and Cost-Effective Ovarian Cancer Detection. Cancers. 2025; 17(7):1251. https://doi.org/10.3390/cancers17071251
Chicago/Turabian StyleAhn, Eunyong, Se Ik Kim, Sungmin Park, Sarah Kim, Hyunjung Kim, Hyejin Lee, Heeyeon Kim, Eun Ji Song, TaeJin Ahn, and Yong-Sang Song. 2025. "Innovative qPCR Algorithm Using Platelet-Derived RNA for High-Specificity and Cost-Effective Ovarian Cancer Detection" Cancers 17, no. 7: 1251. https://doi.org/10.3390/cancers17071251
APA StyleAhn, E., Kim, S. I., Park, S., Kim, S., Kim, H., Lee, H., Kim, H., Song, E. J., Ahn, T., & Song, Y.-S. (2025). Innovative qPCR Algorithm Using Platelet-Derived RNA for High-Specificity and Cost-Effective Ovarian Cancer Detection. Cancers, 17(7), 1251. https://doi.org/10.3390/cancers17071251







