Psychosocial Outcomes in Parents of Children with Acute Lymphoblastic Leukaemia in Australia and New Zealand Through and Beyond Treatment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. Statistical Analysis
2.4. Psychological Safeguards
3. Results
3.1. Demographics
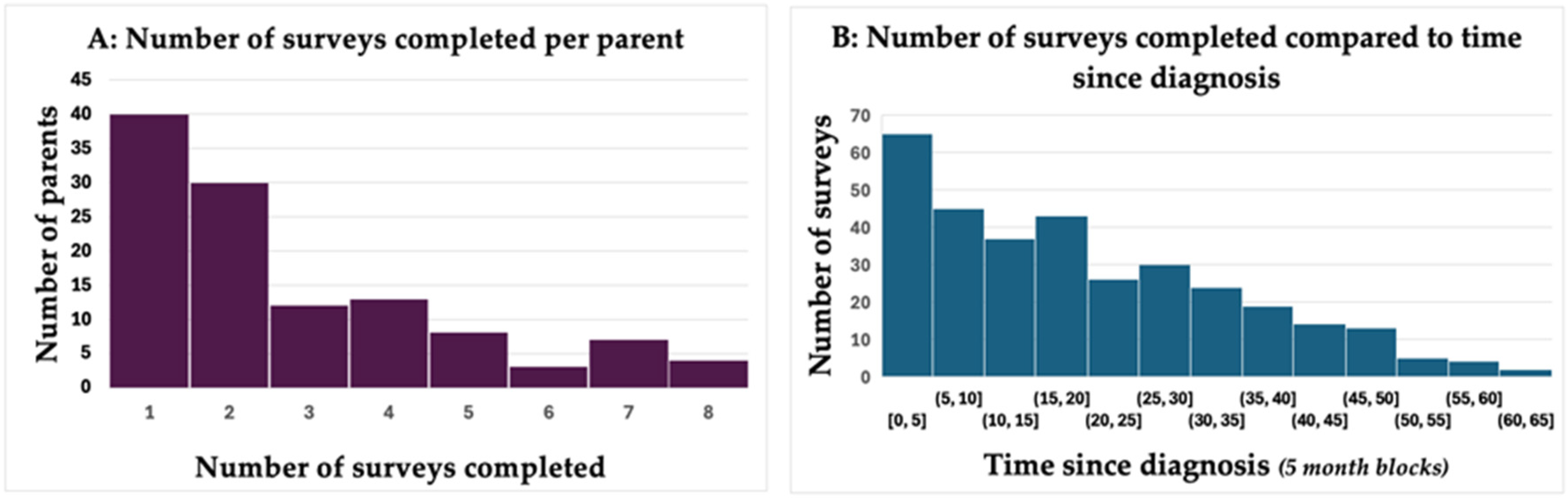
3.2. Trends over Time
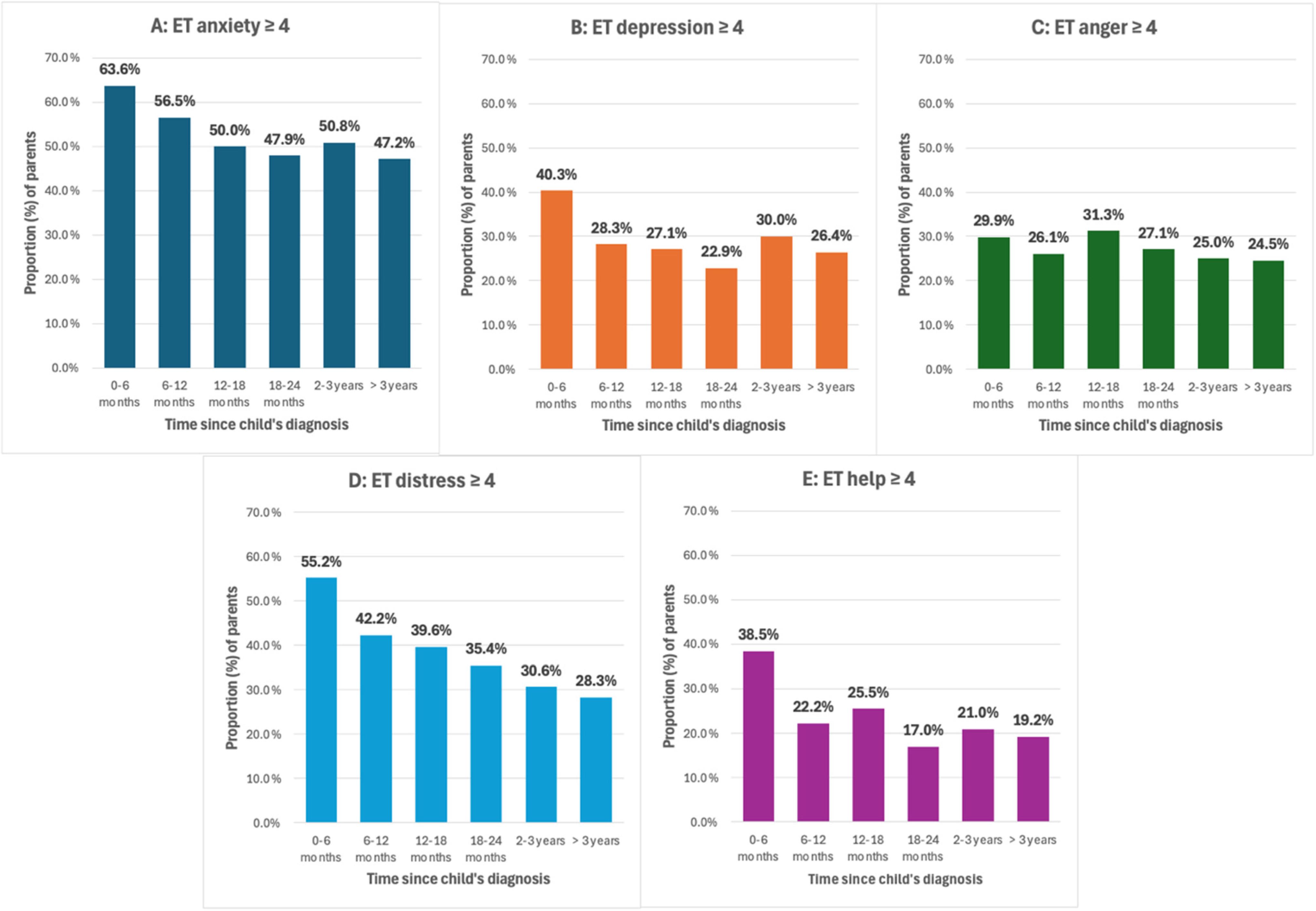
3.3. Emotion Thermometer Responses
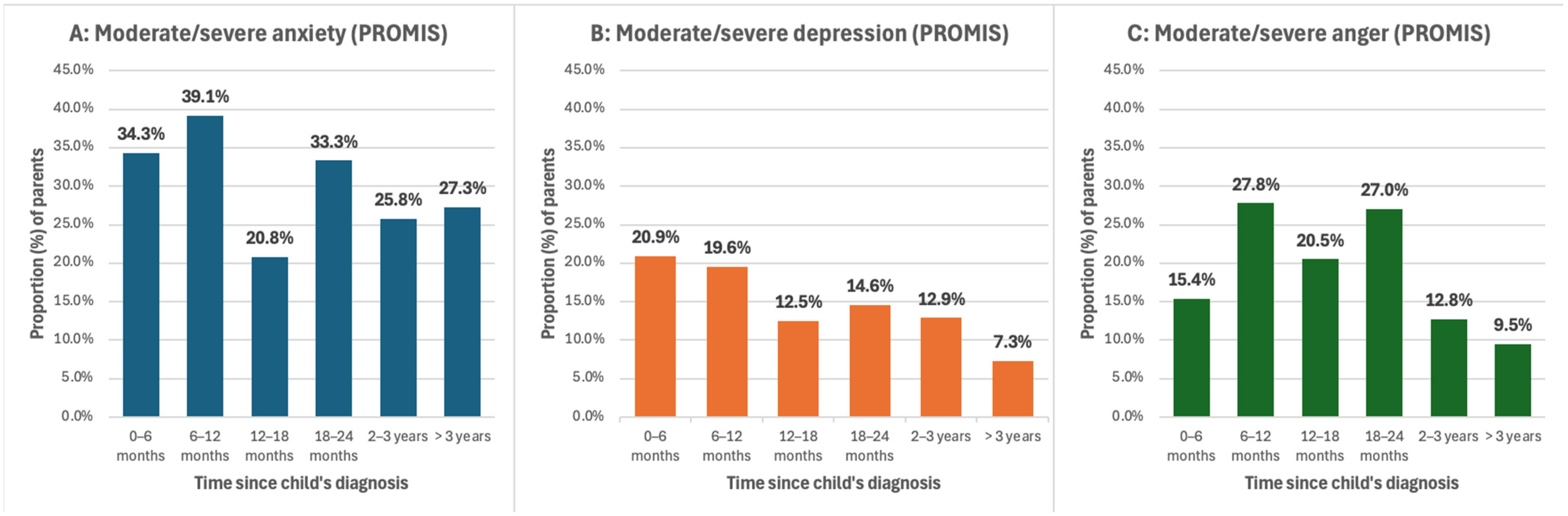
3.4. PROMIS Questionnaire Responses
3.5. Risk Factor Analysis
3.6. Correlation Between the Emotion Thermometer Tool and PROMIS Questionnaires
4. Discussion
4.1. Strengths and Limitations
4.2. Future Research Directions
4.3. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Emotion Thermometer (ET) Scores 2 | n | % |
|---|---|---|
| Median (IQR) ET distress | 2 (0–5) | |
| Above clinical cut-off | 126 | 38.5 |
| Missing response | 4 | 1.2 |
| Median (IQR) ET anxiety | 4 (1–7) | |
| Above clinical cut-off | 171 | 52.3 |
| Missing response | 5 | 1.5 |
| Median (IQR) ET depression | 1.5 (0–4) | |
| Above clinical cut-off | 96 | 29.4 |
| Missing response | 5 | 1.5 |
| Median (IQR) ET anger | 2 (0–4) | |
| Above clinical cut-off | 88 | 26.9 |
| Missing response | 5 | 1.5 |
| Median (IQR) ET need for help | 1 (0–3) | |
| Above clinical cut-off | 78 | 23.9 |
| Missing response | 9 | 2.8 |
| PROMIS scores 3 | n | % |
| Median (IQR) PROMIS anxiety T-score | 54.7 (46.2–61.5) | |
| Anxiety within normal limits | 164 | 50.2 |
| Mild anxiety | 64 | 19.6 |
| Moderate anxiety | 86 | 26.3 |
| Severe anxiety | 12 | 3.7 |
| Missing response | 1 | 0.3 |
| Median (IQR) PROMIS depression T-score | 51.3 (44.4–56.9) | |
| Depression within normal limits | 219 | 67.0 |
| Mild depression | 59 | 18.0 |
| Moderate depression | 46 | 14.1 |
| Severe depression | 2 | 0.6 |
| Missing response | 1 | 0.3 |
| Median (IQR) PROMIS anger T-score | 54.3 (44.7–58.8) | |
| Anger within normal limits | 132 | 57.1 |
| Mild anger | 55 | 23.8 |
| Moderate anger | 44 | 19.05 |
| Severe anger | 0 | 0 |
| Missing response | 1 | 0.4 |
References
- Pui, C.-H.; Yang, J.J.; Hunger, S.P.; Pieters, R.; Schrappe, M.; Biondi, A.; Vora, A.; Baruchel, A.; Silverman, L.B.; Schmiegelow, K.; et al. Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration. J. Clin. Oncol. 2015, 33, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.-H.; Evans, W.E. A 50-Year Journey to Cure Childhood Acute Lymphoblastic Leukemia. Semin. Hematol. 2013, 50, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Vrijmoet-Wiersma, C.M.J.; van Klink, J.M.M.; Kolk, A.M.; Koopman, H.M.; Ball, L.M.; Maarten Egeler, R. Assessment of parental psychological stress in pediatric cancer: A review. J. Pediatr. Psychol. 2008, 33, 694–706. [Google Scholar] [CrossRef]
- Boman, K.; Lindahl, A.; Björk, O. Disease-related distress in parents of children with cancer at various stages after the time of diagnosis. Acta Oncol. Stockh. Swed. 2003, 42, 137–146. [Google Scholar] [CrossRef]
- Fadhilla, A.; Nurhidayah, I.; Adistie, F. The correlation between family functioning and quality of life of caregiver of children with leukemia. Belitung Nurs. J. 2019, 5, 32–40. [Google Scholar] [CrossRef]
- Magni, G.; Silvestro, A.; Tamiello, M.; Zanesco, L.; Carli, M. An integrated approach to the assessment of family adjustment to acute lymphocytic leukemia in children. Acta Psychiatr. Scand. 1988, 78, 639–642. [Google Scholar] [CrossRef]
- Christian, B.J. Translational Research—The Value of Family-Centered Care for Improving the Quality of Care for Children and their Families. J. Pediatr. Nurs. 2016, 31, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, A.; Santos, M.; Pereira, M.G. Parental distress in childhood acute lymphoblastic leukemia: A systematic review of the literature. J. Fam. Psychol. 2024, 38, 149–160. [Google Scholar] [CrossRef]
- Magni, G.; Carli, M.; De Leo, D.; Tshilolo, M.; Zanesco, L. Longitudinal evaluations of psychological distress in parents of children with malignancies. Acta Paediatr. Scand. 1986, 75, 283–288. [Google Scholar] [CrossRef]
- Magni, G.; Messina, C.; De Leo, D.; Mosconi, A.; Carli, M. Psychological distress in parents of children with acute lymphatic leukemia. Acta Psychiatr. Scand. 1983, 68, 297–300. [Google Scholar] [CrossRef]
- Van Schoors, M.; De Paepe, A.L.; Norga, K.; Cosyns, V.; Morren, H.; Vercruysse, T.; Goubert, L.; Verhofstadt, L.L. Family Members Dealing With Childhood Cancer: A Study on the Role of Family Functioning and Cancer Appraisal. Front. Psychol. 2019, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Mostert, S.; Sitaresmi, M.N.; Gundy, C.M.; Sutaryo; Veerman, A.J.P. Parental experiences of childhood leukemia treatment in Indonesia. J. Pediatr. Hematol. Oncol. 2008, 30, 738–743. [Google Scholar] [CrossRef]
- Rocha-Garcia, A.; Alvarez Del Rio, A.; Hernandez-Pena, P.; Martinez-Garcia, M.D.C.; Marin-Palomares, T.; Lazcano-Ponce, E. The emotional response of families to children with leukemia at the lower socio-economic level in central Mexico: A preliminary report. Psychooncology 2003, 12, 78–90. [Google Scholar] [PubMed]
- Yamazaki, S.; Sokejima, S.; Mizoue, T.; Eboshida, A.; Fukuhara, S. Health-related quality of life of mothers of children with leukemia in Japan. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2005, 14, 1079–1085. [Google Scholar] [CrossRef]
- Baran, G.; Arda Surucu, H.; Hulya Uzel, V. Resilience, life satisfaction, care burden and social support of mothers with a child with acute lymphoblastic leukaemia: A comparative study. Scand. J. Caring Sci. 2020, 34, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Nurhidayah, I.; Hendrawati, S.; Hasriyadhi, D.P. Quality of life of family caregivers of children with leukemia: A descriptive quantitative study. Belitung Nurs. J. 2020, 6, 52–58. [Google Scholar] [CrossRef]
- Saritshasombat, P.; Sangchart, B. Mothers’ Lived Experiences in Caring for Adolescents with Leukemia in the Northeast of Thailand. Pak. J. Med. Health Sci. 2021, 15, 3455–3457. [Google Scholar] [CrossRef]
- Atout, M.; Almomani, E.M.; Alhusban, R.Y.; Al-Tarawneh, F.S.; Mohammad, S. Stress levels and coping strategies among Jordanian parents caring for newly diagnosed children with leukemia: A cross sectional descriptive correlational study. J. Psychosoc. Oncol. 2022, 40, 632–651. [Google Scholar] [CrossRef]
- Mogensen, N.; Saaranen, E.; Olsson, E.; Klug Albertsen, B.; Lahteenmaki, P.M.; Kreicbergs, U.; Heyman, M.; Harila-Saari, A. Quality of life in mothers and fathers of children treated for acute lymphoblastic leukaemia in Sweden, Finland and Denmark. Br. J. Haematol. 2022, 198, 1032–1040. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sarkar, I.; Roy, A.K.S.; Das, A. A cross-sectional survey on burden of caregivers of pediatric leukemia patients attending a tertiary care hospital in Kolkata, India. Natl. J. Physiol. Pharm. Pharmacol. 2023, 13, 726–731. [Google Scholar] [CrossRef]
- van Warmerdam, J.; Zabih, V.; Kurdyak, P.; Sutradhar, R.; Nathan, P.C.; Gupta, S. Prevalence of anxiety, depression, and posttraumatic stress disorder in parents of children with cancer: A meta-analysis. Pediatr. Blood Cancer 2019, 66, e27677. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, M.; Antoniou, G.; Toogood, I.; Rice, M. Childhood cancer: A two-year prospective study of the psychological adjustment of children and parents. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 1736–1743. [Google Scholar] [PubMed]
- McGrath, P. Findings on the impact of treatment for childhood acute lymphoblastic leukaemia on family relationships. Child Fam. Soc. Work 2001, 6, 229–237. [Google Scholar] [CrossRef]
- Wang, J.; Shen, N.; Zhang, X.; Shen, M.; Xie, A.; Howell, D.; Yuan, C. Care burden and its predictive factors in parents of newly diagnosed children with acute lymphoblastic leukemia in academic hospitals in China. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2017, 25, 3703–3713. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Mo, L.; Shen, X.; Zhong, D. The demands of caregivers of children with acute lymphoblastic leukaemia in different therapy stages and the exploration of possible interventions: A longitudinal investigation survey at a Tertiary Medical Institution. Nurs. Open 2023, 10, 2273–2281. [Google Scholar] [CrossRef]
- Magni, G.; Silvestro, A.; Carli, M.; de Leo, D. Social support and psychological distress of parents of children with acute lymphocytic leukaemia. Br. J. Med. Psychol. 1986, 59, 383–385. [Google Scholar] [CrossRef]
- Rensen, N.; Steur, L.M.H.; Grootenhuis, M.A.; Van Eijkelenburg, N.K.A.; Van Der Sluis, I.M.; Dors, N.; Van Den Bos, C.; Tissing, W.J.E.; Kaspers, G.J.L.; Van Litsenburg, R.R.L. Parental functioning during maintenance treatment for childhood acute lymphoblastic leukemia: Effects of treatment intensity and dexamethasone pulses. Pediatr. Blood Cancer 2020, 67, e28697. [Google Scholar] [CrossRef]
- Kohlsdorf, M.; Costa Junior, A.L. Coping strategies and caregiver’s anxiety in pediatric oncohematology. Psicol. Reflexao E Crit. 2011, 24, 272–280. [Google Scholar]
- Kazak, A.E.; Abrams, A.N.; Banks, J.; Christofferson, J.; DiDonato, S.; Grootenhuis, M.A.; Kabour, M.; Madan-Swain, A.; Patel, S.K.; Zadeh, S.; et al. Psychosocial Assessment as a Standard of Care in Pediatric Cancer. Pediatr. Blood Cancer 2015, 62, S426–S459. [Google Scholar] [CrossRef]
- Hinds, P.S.; Wang, J.; Cheng, Y.I.; Stern, E.; Waldron, M.; Gross, H.; DeWalt, D.A.; Jacobs, S.S. PROMIS pediatric measures validated in a longitudinal study design in pediatric oncology. Pediatr. Blood Cancer 2019, 66, e27606. [Google Scholar] [CrossRef]
- Schilstra, C.E.; McCleary, K.; Fardell, J.E.; Donoghoe, M.W.; McCormack, E.; Kotecha, R.S.; Lourenco, R.D.A.; Ramachandran, S.; Cockcroft, R.; Conyers, R.; et al. Prospective longitudinal evaluation of treatment-related toxicity and health-related quality of life during the first year of treatment for pediatric acute lymphoblastic leukemia. BMC Cancer 2022, 22, 985. [Google Scholar] [CrossRef] [PubMed]
- National Statement on Ethical Conduct in Human Research | NHMRC. Available online: https://www.nhmrc.gov.au/research-policy/ethics/national-statement-ethical-conduct-human-research (accessed on 18 January 2025).
- Roth, A.J.; Kornblith, A.B.; Batel-Copel, L.; Peabody, E.; Scher, H.I.; Holland, J.C. Rapid screening for psychologic distress in men with prostate carcinoma: A pilot study. Cancer 1998, 82, 1904–1908. [Google Scholar] [CrossRef]
- Jacobsen, P.B.; Donovan, K.A.; Trask, P.C.; Fleishman, S.B.; Zabora, J.; Baker, F.; Holland, J.C. Screening for psychologic distress in ambulatory cancer patients: A multicenter evaluation of the Distress Thermometer. Cancer 2005, 103, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A. Emotion Thermometers Tool: A Rapid Modular Screening Tool for Detection and Monitoring of Emotional Disorders in Clinical Practice [Internet]. Available online: http://www.psycho-oncology.info/ET.htm (accessed on 21 March 2025).
- Mitchell, A.J. Pooled results from 38 analyses of the accuracy of distress thermometer and other ultra-short methods of detecting cancer-related mood disorders. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 4670–4681. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Steginga, S.K.; Ferguson, M.; Beeden, A.; Walls, M.; Cairns, W.; Dunn, J. Measuring distress in cancer patients: The Distress Thermometer in an Australian sample. Prog. Palliat. Care 2009, 17, 61–68. [Google Scholar] [CrossRef]
- Rothrock, N.E.; Amtmann, D.; Cook, K.F. Development and validation of an interpretive guide for PROMIS scores. J. Patient-Rep. Outcomes 2020, 4, 16. [Google Scholar] [CrossRef]
- What is PROMIS? [Internet]. PROMIS Health Organization. Available online: https://www.promishealth.org/57461-2/ (accessed on 21 March 2025).
- Sunderland, M.; Batterham, P.; Calear, A.; Carragher, N. Validity of the PROMIS depression and anxiety common metrics in an online sample of Australian adults. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2018, 27, 2453–2458. [Google Scholar] [CrossRef]
- Hinz, A.; Mitchell, A.J.; Dégi, C.L.; Mehnert-Theuerkauf, A. Normative values for the distress thermometer (DT) and the emotion thermometers (ET), derived from a German general population sample. Qual. Life Res. 2019, 28, 277–282. [Google Scholar] [CrossRef]
- Haverman, L.; van Oers, H.A.; Limperg, P.F.; Houtzager, B.A.; Huisman, J.; Darlington, A.-S.; Maurice-Stam, H.; Grootenhuis, M.A. Development and validation of the distress thermometer for parents of a chronically ill child. J. Pediatr. 2013, 163, 1140–1146.e2. [Google Scholar] [CrossRef]
- Zwahlen, D.; Hagenbuch, N.; Carley, M.I.; Recklitis, C.J.; Buchi, S. Screening cancer patients’ families with the distress thermometer (DT): A validation study. Psychooncology. 2008, 17, 959–966. [Google Scholar] [CrossRef]
- Cella, D.; Riley, W.; Stone, A.; Rothrock, N.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.M.; Schanberg, L.E.; Wang, M.; Von Scheven, E.; Lucas, N.; Hernandez, A.; Ringold, S.; Reeve, B.B. Identifying clinically meaningful severity categories for PROMIS pediatric measures of anxiety, mobility, fatigue, and depressive symptoms in juvenile idiopathic arthritis and childhood-onset systemic lupus erythematosus. Qual. Life Res. 2020, 29, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Accessibility/Remoteness Index of Australia (ARIA+) [Internet]. The Australian Centre for Housing Research | University of Adelaide. Available online: https://able.adelaide.edu.au/housing-research/data-gateway/aria (accessed on 18 January 2025).
- Socio-Economic Indexes for Areas (SEIFA), Australia, 2021 | Australian Bureau of Statistics. Available online: https://www.abs.gov.au/statistics/people/people-and-communities/socio-economic-indexes-areas-seifa-australia/latest-release (accessed on 18 January 2025).
- Profile of Australia’s Population [Internet]. Australian Institute of Health and Welfare. 2024. Available online: https://www.aihw.gov.au/reports/australias-health/profile-of-australias-population (accessed on 18 January 2025).
- New Zealand Government StatsNZ: Estimated Resident Population (2018-base): At 30 June 2018. Available online: https://www.stats.govt.nz/information-releases/estimated-resident-population-2018-base-at-30-june-2018 (accessed on 24 November 2024).
- National Study of Mental Health and Wellbeing, 2020–2022 | Australian Bureau of Statistics. Available online: https://www.abs.gov.au/statistics/health/mental-health/national-study-mental-health-and-wellbeing/latest-release (accessed on 18 January 2025).
- Wilson, A.; Nicolson, M. Mental Health in Aotearoa: Results from the 2018 Mental Health Monitor and the 2018/19 New Zealand Health Survey [Internet]. Health New Zealand. Available online: https://healthnz.figshare.com/articles/report/Mental_Health_in_Aotearoa_Results_from_the_2018_Mental_Health_Monitor_and_the_2018_19_New_Zealand_Health_Survey/26525755/1 (accessed on 18 January 2025).
- Pettitt, N.J.; Petrella, A.R.; Neilson, S.; Topping, A.; Taylor, R.M. Psychosocial and Support Needs of the Main Caregiver for Adolescents and Young Adults Undergoing Treatment for Cancer. Cancer Nurs. 2024. [Google Scholar] [CrossRef]
- Piersol, L.W.; Johnson, A.; Wetsel, A.; Holtzer, K.; Walker, C. Decreasing psychological distress during the diagnosis and treatment of pediatric leukemia. J. Pediatr. Oncol. Nurs. 2008, 25, 323–330. [Google Scholar] [CrossRef]
- Peterson, R.K.; Chung, J.; Barrera, M. Emotional symptoms and family functioning in caregivers of children with newly diagnosed leukemia/lymphomas and solid tumors: Short-term changes and related demographic factors. Pediatr. Blood Cancer 2020, 67, e28059. [Google Scholar] [CrossRef]
- Wakefield, C.E.; Butow, P.; Fleming, C.A.K.; Daniel, G.; Cohn, R.J. Family information needs at childhood cancer treatment completion. Pediatr. Blood Cancer 2012, 58, 621–626. [Google Scholar] [CrossRef]
- Keller, M.C.; Needham, A.; Holden, E.; Engelke, K.; Foy, K.; Hart, L.; Hinderer, K. We Are Done! Now What? Exploring End of Treatment Needs of Childhood Cancer Survivors and Their Parents. J. Pediatr. Hematol. Nurs. 2024, 41, 96–106. [Google Scholar] [CrossRef]
- Sawyer, M.G.; Antoniou, G.; Toogood, I.; Rice, M.; Baghurst, P.A. A prospective study of the psychological adjustment of parents and families of children with cancer. J. Paediatr. Child Health 1993, 29, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, M.J.; Phipps, S.; Fairclough, D.L.; Sahler, O.J.Z.; Askins, M.; Noll, R.B.; Butler, R.W.; Varni, J.W.; Katz, E.R. Trajectories of adjustment in mothers of children with newly diagnosed cancer: A natural history investigation. J. Pediatr. Psychol. 2007, 32, 771–782. [Google Scholar] [CrossRef]
- Best, M.; Streisand, R.; Catania, L.; Kazak, A.E. Parental distress during pediatric leukemia and posttraumatic stress symptoms (PTSS) after treatment ends. J. Pediatr. Psychol. 2001, 26, 299–307. [Google Scholar] [CrossRef]
- Alderfer, M.A.; Cnaan, A.; Annunziato, R.A.; Kazak, A.E. Patterns of posttraumatic stress symptoms in parents of childhood cancer survivors. J. Fam. Psychol. JFP J. Div. Fam. Psychol. Am. Psychol. Assoc. Div. 43 2005, 19, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, M.; Antoniou, G.; Toogood, I.; Rice, M.; Baghurst, P. Childhood cancer: A 4-year prospective study of the psychological adjustment of children and parents. J. Pediatr. Hematol. Oncol. 2000, 22, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Harju, E.; Roser, K.; Eisenreich, B.; Scheinemann, K.; Michel, G. Psychological distress in survivors of childhood cancer and their parents before and after a follow-up appointment: The need for screening and validation of the emotion thermometer. J. Psychosoc. Oncol. Res. Pract. 2023, 5. Available online: https://journals.lww.com/jporp/fulltext/2023/04000/psychological_distress_in_survivors_of_childhood.1.aspx# (accessed on 19 January 2025). [CrossRef]
- Vetsch, J.; Wakefield, C.E.; Robertson, E.G.; Trahair, T.N.; Mateos, M.K.; Grootenhuis, M.; Marshall, G.M.; Cohn, R.J.; Fardell, J.E. Health-related quality of life of survivors of childhood acute lymphoblastic leukemia: A systematic review. Qual. Life Res. 2018, 27, 1431–1443. [Google Scholar] [CrossRef] [PubMed]

| Patient Information | n | % |
|---|---|---|
| Median age in years at diagnosis (range) | 7 (0.5–18) | |
| Age < 10 | 85 | 72.6 |
| Age ≥ 10 | 32 | 27.4 |
| Male | 71 | 60.7 |
| Female | 46 | 39.3 |
| Metropolitan * | 90 | 76.9 |
| Inner regional * | 22 | 18.8 |
| Outer regional or remote * | 5 | 4.3 |
| SEIFA # rank <20th centile | 7 | 6.0 |
| SEIFA rank 20–40th centile | 19 | 16.2 |
| SEIFA rank 40–60th centile | 21 | 17.9 |
| SEIFA rank 60–80th centile | 27 | 23.1 |
| SEIFA rank >80th centile | 32 | 27.4 |
| Unknown (New Zealand) | 11 | 9.4 |
| Treated on COG protocols | 75 | 64.1 |
| Treated on BFM protocols | 42 | 35.9 |
| Low/standard risk ALL | 41 | 35.0 |
| Medium risk ALL | 35 | 29.9 |
| High risk ALL | 41 | 35.0 |
| Presence or development of treatment-related toxicity | 35 | 29.9 |
| Child relapsed during study | 4 | 3.4 |
| Child died during study | 2 | 1.7 |
| Time Since Child’s Diagnosis | Total Number of Survey Responses | Number of Clinically Elevated Measures in Parent Responses = n (%) | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ≥4 | ||
| 0–6 months | 67 | 9 (13.4) | 11 (16.4) | 10 (14.9) | 10 (14.9) | 27 (40.3) |
| 6–12 months | 46 | 15 (32.6) | 6 (13.0) | 2 (4.3) | 6 (13.0) | 17 (37.0) |
| 12–18 months | 48 | 21 (43.8) | 6 (12.5) | 3 (6.3) | 2 (4.2) | 16 (33.3) |
| 18–24 months | 48 | 17 (35.4) | 9 (18.8) | 4 (8.3) | 3 (6.3) | 15 (31.3) |
| 2–3 years | 63 | 27 (42.9) | 8 (12.7) | 1 (1.6) | 8 (12.7) | 19 (30.2) |
| >3 years | 55 | 21 (38.2) | 9 (16.4) | 6 (10.9) | 6 (10.9) | 13 (23.6) |
| All time points | 327 | 110 (33.6) | 49 (15.0) | 26 (8.0) | 35 (10.7) | 107 (32.7) |
| Single Variable Analysis | Multiple Variable Analysis | ||||
|---|---|---|---|---|---|
| Estimate (95% CI) | p-Value | Estimate (95% CI) | p-Value | ||
| ET Distress | Time since diagnosis | −0.04 (−0.06 to −0.01) | 0.001 * | −0.07 (−0.13 to −0.02) | 0.013 * |
| Child’s age at diagnosis | 0.02 (−0.08 to 0.13) | 0.67 | 0.10 (−0.02 to 0.36) | 0.49 | |
| Child’s sex | 0.40 (−0.43 to 1.23) | 0.34 | 0.06 (−1.47 to 1.59) | 0.94 | |
| SEIFA index | −0.01 (−0.03 to 0.004) | 0.13 | −0.01 (−0.04 to 0.02) | 0.38 | |
| ALL study group (COG/BFM) | −0.35 (−1.19 to 0.49) | 0.41 | −0.02 (−1.75 to 1.35) | 0.80 | |
| Risk stratification | −0.33 (−1.35 to 0.70) | 0.53 | 0.73 (−1.29 to 2.74) | 0.48 | |
| Presence of toxicity | 0.29 (−0.65 to 1.23) | 0.54 | 0.22 (−1.26 to 1.70) | 0.77 | |
| ET Anxiety | Time since diagnosis | −0.02 (−0.05 to 0.001) | 0.06 | −0.06 (−0.12 to 0.01) | 0.11 |
| Child’s age at diagnosis | −0.03 (−0.14 to 0.09) | 0.63 | 0.04 (−0.29 to 0.38) | 0.80 | |
| Child’s sex | 0.44 (−0.45 to 1.33) | 0.33 | 0.68 (−1.21 to 2.56) | 0.48 | |
| SEIFA index | −0.01 (−0.03 to 0.004) | 0.15 | −0.02 (−0.05 to 0.02) | 0.38 | |
| ALL study group (COG/BFM) | 0.09 (−0.91 to 0.99) | 0.85 | 0.003 (−1.90 to 1.90) | 1.00 | |
| Risk stratification | −0.38 (−1.46 to 0.70) | 0.49 | −1.05 (−3.18 to 1.08) | 0.33 | |
| Presence of toxicity | 0.32 (−0.68 to 1.33) | 0.53 | 0.28 (−1.51 to 2.06) | 0.76 | |
| ET Depression | Time since diagnosis | −0.01 (−0.03 to 0.01) | 0.33 | −0.02 (−0.06 to 0.01) | 0.21 |
| Child’s age at diagnosis | 0.02 (−0.08 to 0.12) | 0.74 | 0.03 (−0.12 to 0.18) | 0.69 | |
| Child’s sex | 0.40 (−0.41 to 1.21) | 0.33 | 0.22 (−0.89 to 1.32) | 0.70 | |
| SEIFA index | −0.01 (−0.02 to 0.01) | 0.42 | −0.001 (−0.02 to 0.02) | 0.95 | |
| ALL study group (COG/BFM) | −0.36 (−1.18 to 0.46) | 0.39 | −0.04 (−1.21 to 1.12) | 0.94 | |
| Risk stratification | −0.36 (−1.34 to 0.62) | 0.47 | −0.39 (−1.79 to 1.01) | 0.59 | |
| Presence of toxicity | 0.13 (−0.79 to 1.05) | 0.78 | 0.50 (−0.76 to 1.76) | 0.44 | |
| ET Anger | Time since diagnosis | −0.01 (−0.03 to 0.01) | 0.53 | −0.006 (−0.03 to 0.02) | 0.62 |
| Child’s age at diagnosis | −0.01 (−0.11 to 0.09) | 0.84 | −0.05 (−0.16 to 0.07) | 0.42 | |
| Child’s sex | 0.38 (−0.35 to 1.11) | 0.31 | 0.30 (−0.47 to 1.07) | 0.45 | |
| SEIFA index | −0.02 (−0.03 to −0.004) | 0.013 * | −0.02 (−0.03 to −0.001) | 0.038 * | |
| ALL study group (COG/BFM) | −0.76 (−1.49 to −0.04) | 0.040 * | −0.48 (−1.27 to 0.32) | 0.24 | |
| Risk stratification | 0.21 (0.68 to 1.10) | 0.64 | 0.15 (−0.79 to 1.09) | 0.76 | |
| Presence of toxicity | 0.39 (−0.42 to 1.20) | 0.34 | 0.14 (−0.73 to 1.02) | 0.75 | |
| ET Need for Help | Time since diagnosis | −0.02 (−0.04 to 0.00) | 0.045 * | −0.04 (−0.08 to 0.01) | 0.11 |
| Child’s age at diagnosis | −0.06 (−0.16 to 0.03) | 0.19 | 0.07 (−0.23 to 0.38) | 0.63 | |
| Child’s sex | 0.090 (−0.68 to 0.86) | 0.82 | 0.23 (−1.47 to 1.92) | 0.79 | |
| SEIFA index | −0.01 (−0.03 to 0.003) | 0.11 | −0.01 (−0.04 to 0.02) | 0.40 | |
| ALL study group (COG/BFM) | 0.12 (−0.65 to 0.89) | 0.75 | 0.15 (−1.54 to 1.84) | 0.86 | |
| Risk stratification | 0.11 (−0.81 to 1.04) | 0.81 | −0.68 (−2.56 to 1.20) | 0.48 | |
| Presence of toxicity | 0.10 (−0.75 to 0.95) | 0.81 | 0.15 (−1.34 to 1.64) | 0.84 | |
| PROMIS Anxiety | Time since diagnosis | −0.19 (−0.28 to −0.10) | <0.001 * | −0.18 (−0.28 to −0.09) | <0.001 * |
| Child’s age at diagnosis | −0.29 (−0.75 to 0.16) | 0.21 | 0.14 (−0.38 to 0.66) | 0.60 | |
| Child’s sex | 2.83 (−0.70 to 6.36) | 0.11 | 2.17 (−1.59 to 5.92) | 0.26 | |
| SEIFA index | 0.001 (−0.07 to 0.07) | 0.97 | −0.002 (−0.08 to 0.07) | 0.95 | |
| ALL study group (COG/BFM) | 2.20 (−1.38 to 5.78) | 0.23 | 3.30 (−0.62 to 7.22) | 0.10 | |
| Risk stratification | 0.69 (−3.62 to 4.99) | 0.75 | 0.54 (−4.17 to 5.26) | 0.82 | |
| Presence of toxicity | −2.60 (−6.42 to 1.21) | 0.18 | −2.57 (−6.79 to 1.65) | 0.23 | |
| PROMIS Depression | Time since diagnosis | −0.12 (−0.21 to −0.04) | 0.004 * | −0.13 (−0.22 to −0.04) | 0.006 * |
| Child’s age at diagnosis | −0.15 (−0.58 to 0.27) | 0.48 | 0.28 (−0.21 to 0.77) | 0.26 | |
| Child’s sex | 1.48 (−1.71 to 4.67) | 0.36 | 0.41 (−3.04 to 3.85) | 0.82 | |
| SEIFA index | −0.007 (−0.07 to 0.06) | 0.82 | 0.003 (−0.06 to 0.07) | 0.92 | |
| ALL study group (COG/BFM) | −0.23 (−3.47 to 3.01) | 0.89 | 0.30 (−3.29 to 3.89) | 0.87 | |
| Risk stratification | −0.85 (−4.73 to 3.04) | 0.67 | −1.60 (−5.85 to 2.66) | 0.46 | |
| Presence of toxicity | −2.28 (−5.56 to 1.00) | 0.17 | −2.53 (−6.18 to 1.13) | 0.18 | |
| PROMIS Anger | Time since diagnosis | −0.024 (−0.14 to 0.09) | 0.68 | −0.03 (−0.16 to 0.10) | 0.63 |
| Child’s age at diagnosis | −0.17 (−0.67 to 0.32) | 0.50 | −0.02 (−0.60 to 0.56) | 0.94 | |
| Child’s sex | 1.64 (−2.44 to 5.72) | 0.43 | 2.05 (−2.39 to 6.49) | 0.36 | |
| SEIFA index | 0.004 (−0.07 to 0.08) | 0.92 | −0.02 (−0.10 to 0.07) | 0.67 | |
| ALL study group (COG/BFM) | 1.38 (−2.69 to 5.45) | 0.50 | 1.37 (−3.16 to 5.90) | 0.55 | |
| Risk stratification | 0.11 (−4.69 to 4.91) | 0.96 | −0.89 (−6.28 to 4.51) | 0.75 | |
| Presence of toxicity | −3.28 (−7.33 to 0.78) | 0.11 | −4.24 (−8.81 to 0.34) | 0.07 | |
| Pearson Correlation (95% CI) | p-Value | |
|---|---|---|
| Anxiety | 0.44 (0.35–0.52) | <0.001 * |
| Depression | 0.34 (0.24–0.43) | <0.001 * |
| Anger | 0.17 (0.05–0.30) | 0.008 * |
| Kappa Statistic | p-Value | |
|---|---|---|
| Anxiety | 0.14 | <0.001 * |
| Depression | 0.16 | <0.001 * |
| Anger | 0.09 | 0.060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, C.; Schilstra, C.E.; McCleary, K.; Martin, M.; Trahair, T.N.; Kotecha, R.S.; Ramachandran, S.; Cockcroft, R.; Conyers, R.; Cross, S.; et al. Psychosocial Outcomes in Parents of Children with Acute Lymphoblastic Leukaemia in Australia and New Zealand Through and Beyond Treatment. Cancers 2025, 17, 1238. https://doi.org/10.3390/cancers17071238
Parker C, Schilstra CE, McCleary K, Martin M, Trahair TN, Kotecha RS, Ramachandran S, Cockcroft R, Conyers R, Cross S, et al. Psychosocial Outcomes in Parents of Children with Acute Lymphoblastic Leukaemia in Australia and New Zealand Through and Beyond Treatment. Cancers. 2025; 17(7):1238. https://doi.org/10.3390/cancers17071238
Chicago/Turabian StyleParker, Clare, Clarissa E. Schilstra, Karen McCleary, Michelle Martin, Toby N. Trahair, Rishi S. Kotecha, Shanti Ramachandran, Ruellyn Cockcroft, Rachel Conyers, Siobhan Cross, and et al. 2025. "Psychosocial Outcomes in Parents of Children with Acute Lymphoblastic Leukaemia in Australia and New Zealand Through and Beyond Treatment" Cancers 17, no. 7: 1238. https://doi.org/10.3390/cancers17071238
APA StyleParker, C., Schilstra, C. E., McCleary, K., Martin, M., Trahair, T. N., Kotecha, R. S., Ramachandran, S., Cockcroft, R., Conyers, R., Cross, S., Dalla-Pozza, L., Downie, P., Revesz, T., Osborn, M., Marshall, G. M., Wakefield, C. E., Mateos, M. K., & Fardell, J. E. (2025). Psychosocial Outcomes in Parents of Children with Acute Lymphoblastic Leukaemia in Australia and New Zealand Through and Beyond Treatment. Cancers, 17(7), 1238. https://doi.org/10.3390/cancers17071238






