1. Introduction
Oral squamous cell carcinoma (OSCC) is a prevalent and aggressive cancer type, which comprises about 90% of all malignancies in the oral cavity [
1]. Despite the progress made in treatment strategies, OSCC continues to be associated with poor survival outcomes and high recurrence rates, underscoring the necessity of dependable prognostic biomarkers to enhance patient management [
2,
3].
In contrast to primary tumors, recurrent OSCC (re-OSCC) presents a distinctive set of challenges. The mechanisms that drive recurrence are not completely understood and the biology of recurrent tumors can differ significantly from that of the primary tumor [
4]. The occurrence of lymph node metastases in the neck in oral cancer is associated with a worse prognosis, complicating the management of re-OSCC [
5,
6]. Higher tumor depth of invasion (DOI) is associated with a greater chance of lymph node metastasis, recurrence, and lower survival chances in early-stage oral squamous cell carcinoma [
7]. Patients with re-OSCC frequently encounter limited therapy alternatives and reduced survival rates, necessitating an understanding of the expression patterns of these markers in recurrent illness [
8]. As a result, the therapeutic strategies employed for re-OSCC are distinct from those employed for primary malignancies. Although surgical resection is the standard approach to initial OSCC management, recurrent cases frequently necessitate multimodal strategies, such as salvage surgery, re-irradiation, chemotherapy, or targeted therapies, that are tailored to the tumor’s changing biology and the patient’s previous treatments [
9,
10,
11].
Re-OSCC frequently exhibits distinct biological characteristics compared to primary tumors, including increased resistance to apoptosis, enhanced invasiveness and alterations in gene expression, which contribute to its more aggressive nature [
9,
12,
13]. Borsetto et al. examined key prognostic factors in the locoregional recurrence of OSCC, demonstrating that tumor size at initial diagnosis, the extent of recurrent disease, and positive surgical margins at primary surgery are strong predictors of poor overall survival [
14]. Their results underscore the clinical challenge of re-OSCC, as patients with extensive locoregional recurrence have a substantially shorter survival rate than those with isolated local recurrence [
15].
Cancer stem cells (CSCs) have emerged as key participants in tumor initiation, development, metastasis, and recurrence. These cells have self-renewal capacities and the ability to differentiate into various tumor types, making them important targets for therapeutic intervention [
16]. Abnormal expression of transcription factors SOX2 (SRY-related HMG-box gene 2), NANOG, and OCT4 is known to have different functions in tumor initiation and metastasis [
17,
18,
19,
20]. NANOG is critical for maintaining CSC self-renewal, regulating cell proliferation via cyclin D interactions, decreasing E-cadherin to increase migration and disrupting p53 to avoid apoptosis [
21]. SOX2, located on 3q26.3–q27, is identified as a major oncogene in OSCC [
13], associated with tumor proliferation, metastasis, therapeutic resistance, and the facilitation of epithelial–mesenchymal transition (EMT) [
17,
22,
23].
High expression of these markers is frequently related to higher tumor aggressiveness and poor clinical outcomes [
12,
24]. The significance of SOX2 in radioresistance is intricate, with research yielding conflicting results. Some study indicates that SOX2 amplifies the effects of irradiation, resulting in a better prognosis for patients receiving radiotherapy, whereas other evidence suggests that suppressing SOX2 may generate a radioresistant phenotype [
25]. Nanog signaling augments radioresistance in ALDH-positive breast cancer cells by increasing ALDH activity and promoting double-strand break (DSB) repair, possibly through the Notch1 and Akt pathways [
26]. While the involvement of SOX2 and NANOG in primary OSCC has been explored [
4,
11,
13,
27,
28,
29,
30], their prognostic significance in re-OSCC remains largely understudied.
The purpose of this study is to examine the expression patterns of SOX2 and NANOG in both primary and re-OSCC, with an emphasis on how adjuvant therapy affects their expression dynamics. Furthermore, we evaluate the prognostic importance of these markers by examining their expression in re-OSCC and their relationship with post-recurrence overall survival (prOS) and post-recurrence disease-free survival (prDFS) in a well-defined, prospectively collected single-center cohort. This study seeks to improve risk assessment and facilitate the development of customized treatment regimens for patients with re-OSCC by clarifying the roles of SOX2 and NANOG in OSCC progression and medication response.
2. Methods
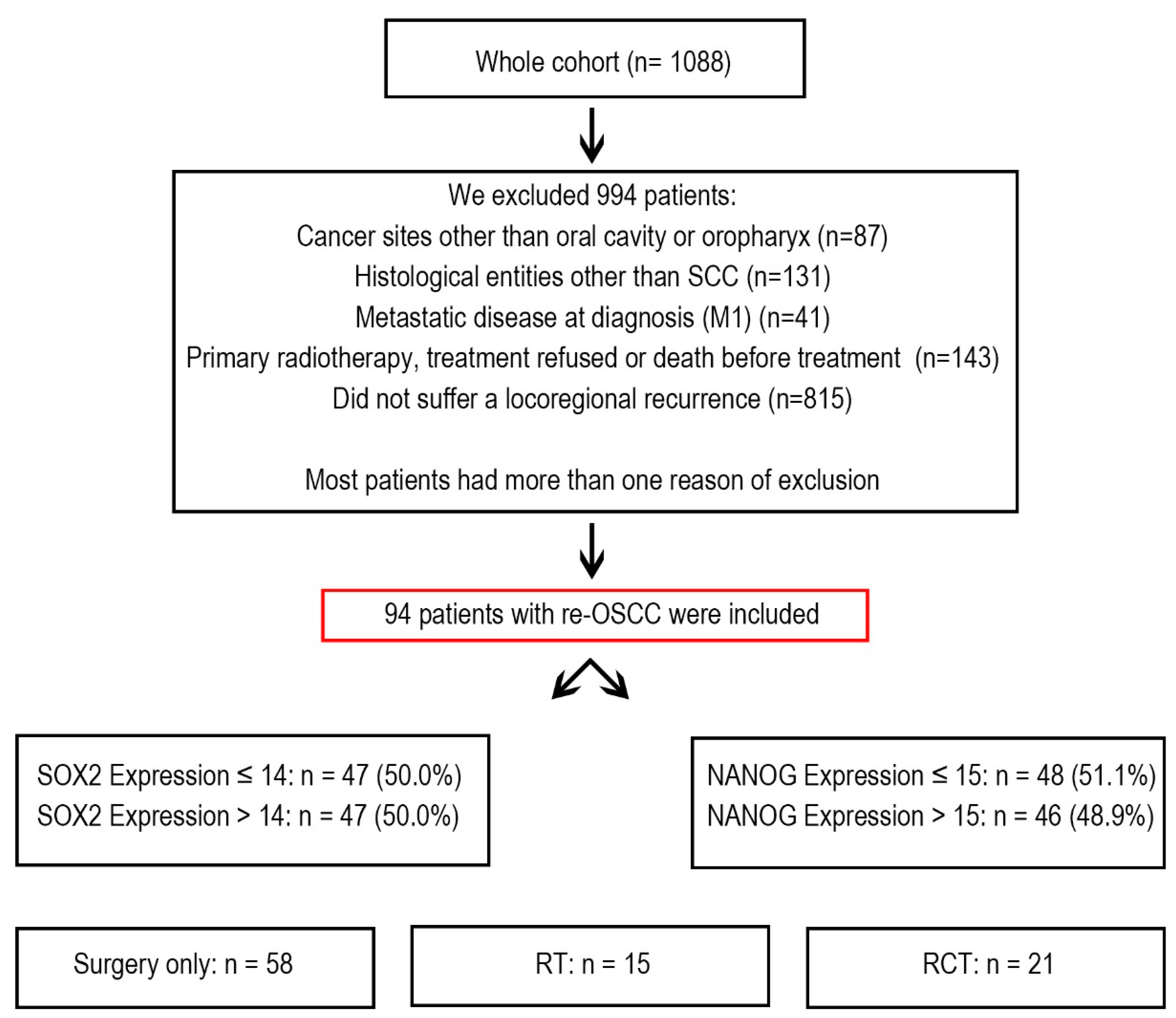
2.1. Patient Cohort
Between 1992 and 2019, the Department of Maxillofacial Surgery at the University Medical Centre of Lübeck in Germany treated 1088 cancer patients. From this group, we selected 94 patients who developed re-OSCC and underwent treatment with curative intent. These patients underwent multiple treatments, including surgery, radiotherapy (RT), chemoradiotherapy (RCT), or a combination of these (
Figure 1).
Patients were excluded if they had oropharyngeal carcinoma, a histological diagnosis other than squamous cell carcinoma, metastatic disease at the time of diagnosis, or if they declined treatment or passed away before therapy could begin. Additionally, patients who did not develop locoregional recurrence were excluded from the study.
All eligible patients were registered in a structured recall system to ensure continuous follow-up for five years after treatment. Follow-up appointments were scheduled every three months for the first two years, then every six months following. At each visit, demographic information, risk factors, clinical tumor features, and therapy details were carefully documented. Each patient’s general health state was assessed using the Charlson Comorbidity Index (CCI) [
31], incorporating tumor staging and additional prognostic variables into the cohort analysis [
32].
Tissue Microarray and Immunohistochemical Staining
Before analysis, each tissue punch was visually inspected for tissue folds, staining artifacts, low tissue quality, or insufficient tumor content. Any punches that did not meet quality standards were excluded.
Tissue microarrays (TMAs) were constructed using a manual Tissue Arrayer MTA-1 (AlphaMetrix Biotech, Rödermark, Germany). Tumor regions from formalin-fixed, paraffin-embedded (FFPE) tissue blocks were cut out using 1.2 mm punches and subsequently transferred to recipient blocks. To guarantee the precise representation of intratumoral intricacy, three punches from each tissue block were incorporated into the TMAs. The recipient blocks were sliced to a thickness of 4 μm using a Microm HM355S microtome (Thermo Scientific, Boston, MA, USA). The slices were subsequently positioned on slides, immersed in a 50 °C water bath and incubated in a 60 °C hot air oven for 1 h prior to additional processing. Immunohistochemical (IHC) staining protocols were modified from prior studies and refined with positive and negative controls to guarantee dependable outcomes [
32]. Antibodies used in the study are listed in
Table 1.
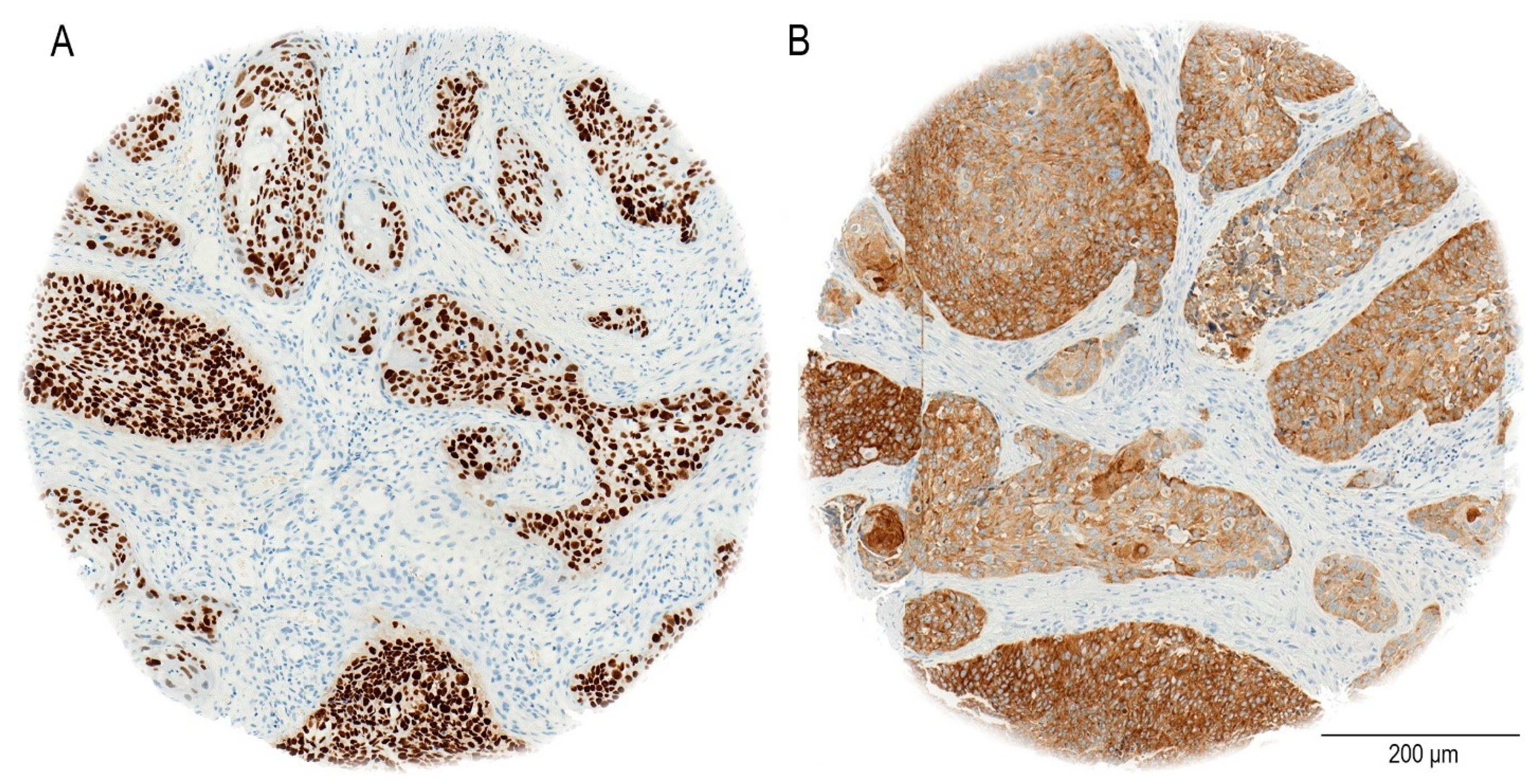
The staining procedure was entirely automated utilizing the Ventana BenchMark system (Roche, Basel, Switzerland). In our study, the immunohistochemical analysis demonstrates that SOX2 exhibits nuclear staining, as previously reported [
33], NANOG shows a cytoplasmic staining pattern in oral OSCC patients, as seen in our cohort (
Figure 2). In particular, NANOG has strong cytoplasmic staining in a considerable majority of OSCC cases, in contrast to the nuclear localization seen in germ cell malignancies [
34,
35].
Antigen retrieval for SOX2 staining was conducted using heat-mediated retrieval in sodium citrate buffer (pH 6.0) at 92 °C for 32 min. The sections were treated with anti-SOX2 antibody (dilution 1 µg/mL) at ambient temperature, and thereafter, detected with a biotin-conjugated secondary antibody and streptavidin–peroxidase solution. Counterstaining was conducted with hematoxylin.
Antigen retrieval for NANOG staining was performed using sodium citrate buffer (pH 6.0) at 92 °C for 32 min. The slides were treated with anti-NANOG antibody at a dilution of 1:1000 to 1:2500 at room temperature. After the primary antibody incubation, a biotin-conjugated secondary antibody was administered for 30 min, succeeded by streptavidin–peroxidase incubation. The staining was observed utilizing 3′,3-diaminobenzidine tetrahydrochloride (DAB) and counterstained with hematoxylin.
Positive controls for SOX2 included human glioblastoma tissue and NCCIT (human pluripotent embryonal carcinoma) cell line samples. Positive controls for NANOG staining included NCCIT cells and embryonic carcinoma tissue.
Negative controls were prepared by omitting the primary antibodies during the staining process to confirm specificity.
For digital analysis, slides were scanned using a Ventana iScan HT Scanner (Ventana, Tucson, AZ, USA). Image analysis was conducted using the open-source software QuPath (Version 0.2.3) [
36]. TMA cores were selected using the integrated TMA dearrayer function in QuPath software. Patient data were imported from prepared Excel spreadsheets to ensure that each core was correctly matched to the corresponding patient. Regions of interest, including tumor cells, immune cells, and stromal compartments, were manually annotated for analysis. Positive cell detection was performed using the Optical Density Sum (OD Sum) method to reduce nucleus fragmentation. Parameters such as requested pixel size, background radius, median filter radius, nucleus size thresholds, intensity threshold, and cell expansion were optimized to ensure accurate detection of cells. The smoothed measurement heat maps function was subsequently generated to visualize classification-relevant cellular features. A Random Forest classifier was trained based on the annotated regions to facilitate accurate automated classification of tumor cells. Finally, cellular measurements were exported via the TMA Data Viewer, allowing for the calculation and validation of the H-score.
We utilized QuPath for image processing and H-score quantification, leveraging its demonstrated reliability in several tumor biology studies [
22,
32,
37]. The cited research validates the reproducibility of QuPath for H-score assessments across different tumor contexts. Two independent pathologists supervised the process to minimize subjectivity.
Digitized image files were imported into QuPath as .tif files for further examination on a Windows 10-based system with a 12.3″ display (2736 × 1824 pixels resolution).
2.2. Statistical Analysis
Descriptive statistics were summarized using frequencies, percentages, medians, and ranges. Continuous data are presented as means (SD) and medians with an interquartile range (IQR) for a comprehensive representation of data distribution.
For survival analysis, the Kaplan–Meier method was applied to estimate prDFS and prOS probabilities.
prDFS was defined as the time from the first recurrence until a second recurrence, death, or last follow-up, whichever occurred first. During this period, the patient remained disease-free.
prOS was defined as the time from the first recurrence to death from any cause or the last follow-up date, whichever came first.
Group comparisons were conducted using univariate analysis with the log-rank test and Schoenfeld residuals were examined to verify the proportional hazards assumption.
To determine optimal cutoff points for marker expression, the “rolr” package was used, specifically its “rhier” function, which identifies the best two-group split based on survival outcomes. Cutoff points were selected based on median values where appropriate.
Kaplan–Meier plots were generated using a combination of “survival”, “survminer”, “ggplot2”, and “plotly” packages. Hazard ratios (HR), confidence intervals (CI), and p-values were estimated using the “coxph” function from the “survival” package.
All statistical tests were two-tailed, with significance set at
p < 0.05. Results were reported with 95% confidence intervals (CI). Statistical analyses were conducted using R Core Team (2023), R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria) (
https://www.R-project.org/ (accessed on 29 July 2024)).
This study is among the first to examine survival outcomes in recurrent OSCC by analyzing SOX2 and NANOG expression using semi-automated immunohistochemical marker analysis with QuPath software. The applied method assessed post-recurrence survival in high-risk groups by correlating standardized H-score-based IHC expression with clinical follow-up data.
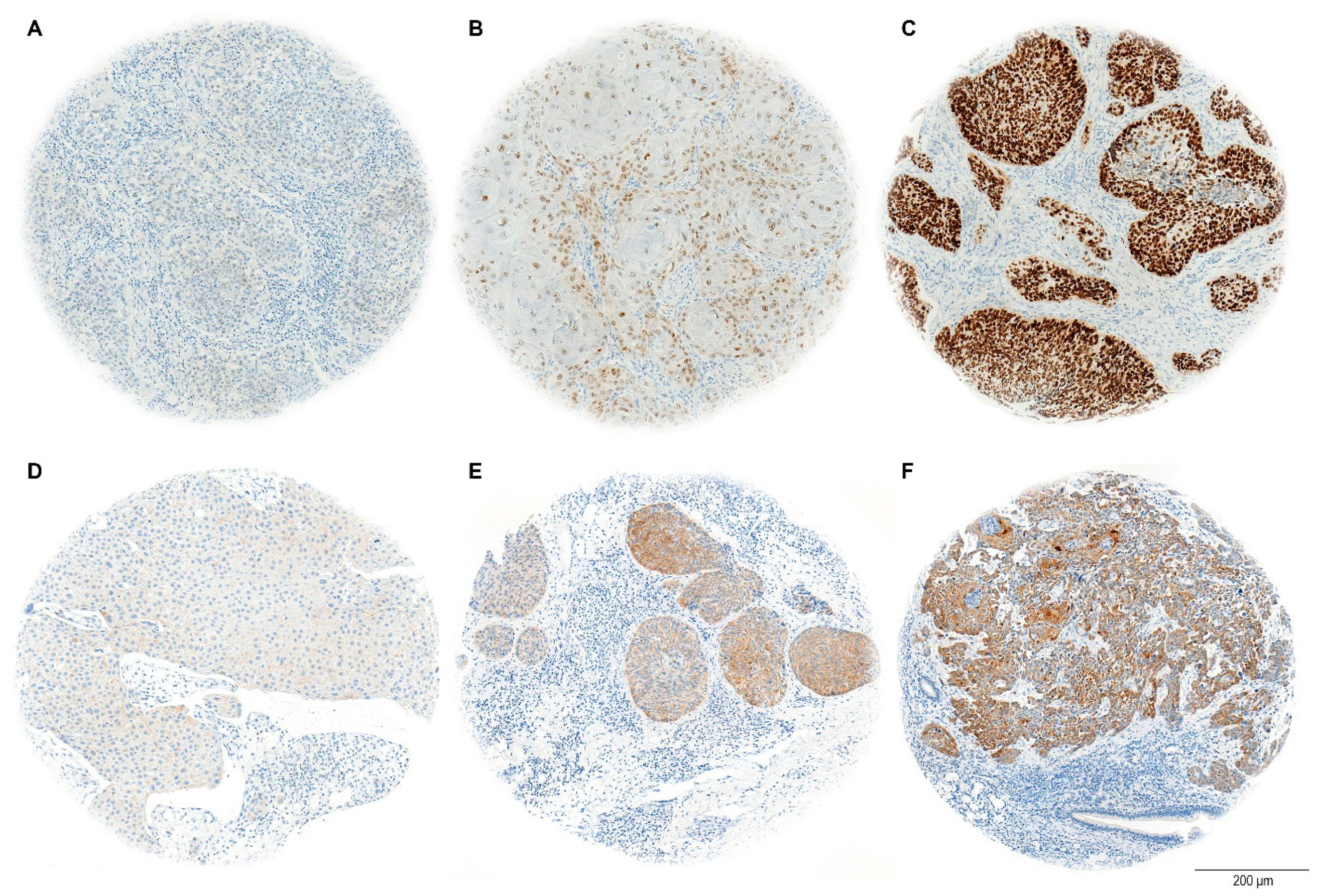
The H-score evaluation of immunostaining quantifies protein expression in tissue samples, with a maximum possible score of 300. This score is calculated by adding the weighted contributions of stained cells based on their intensity: strongly stained cells are weighted at 3, moderately stained cells at 2, and weakly stained cells at 1. Cells without expression are weighted at 0 (
Figure 3). The percentage of positively stained tumor cells was considered relative to the total tumor area [
38].
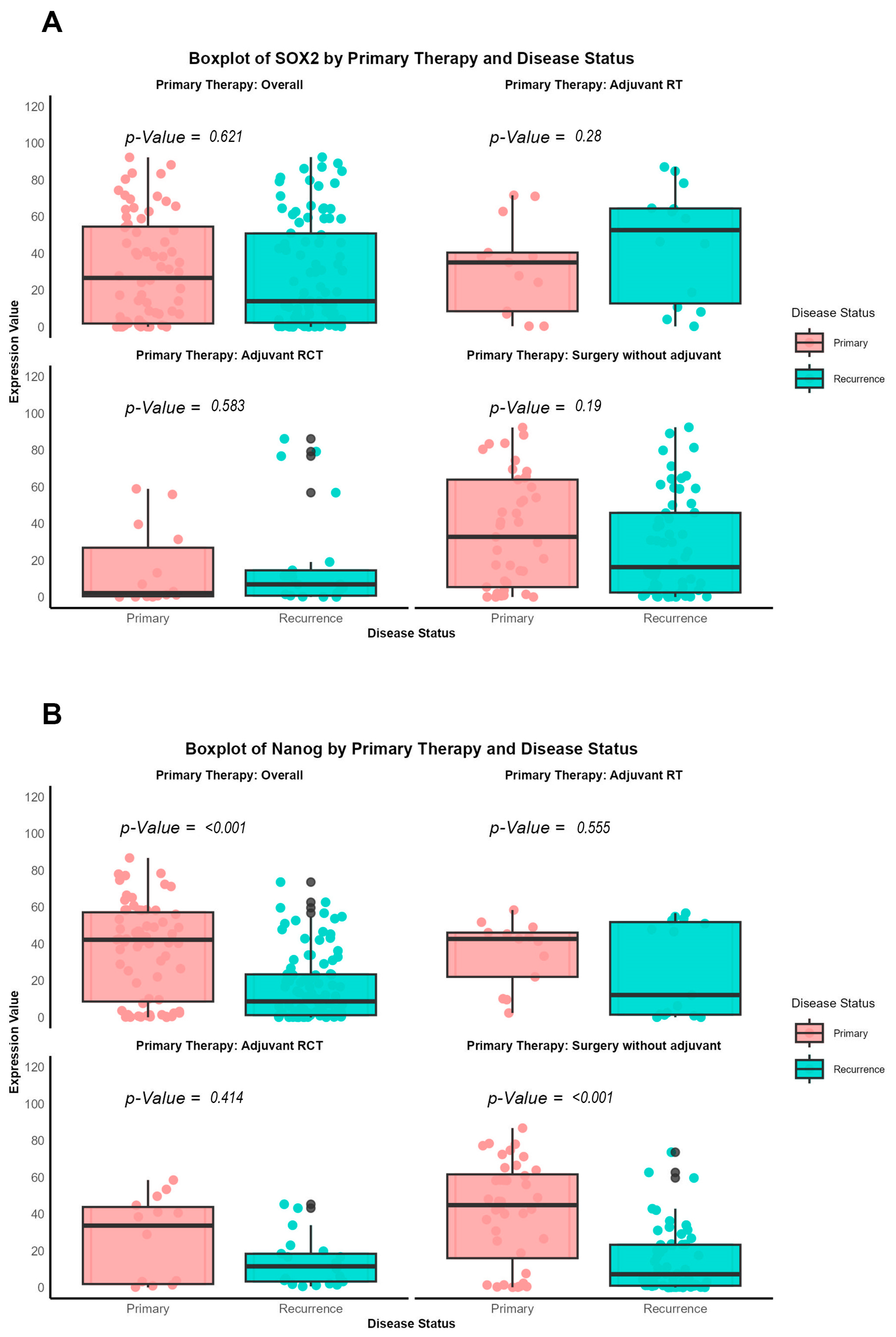
Additionally, a direct comparison of antibody expression between primary and recurrent tumors was performed, categorized according to the specific therapeutic approaches (surgery alone, adjuvant radiotherapy, or radiochemotherapy), using the nonparametric Wilcoxon signed-rank test, as the data were not normally distributed.
Ethics
Upon admission, all participants signed consent forms authorizing the collection and anonymous use of their data for academic research. The study (ID: 16-272A) was approved by the ethics review committee of the University of Lübeck in April 2017.
4. Discussion
A growing body of evidence highlights the complexity of re-OSCC, which frequently exhibits more aggressive biological behavior and poorer survival outcomes than primary tumors. The underlying mechanisms causing these differences are only partially understood. In this context, understanding the prognostic roles of pluripotency transcription factors, particularly SOX2 and NANOG, in re-OSCC is critical, given their established links to CSC biology and tumor progression.
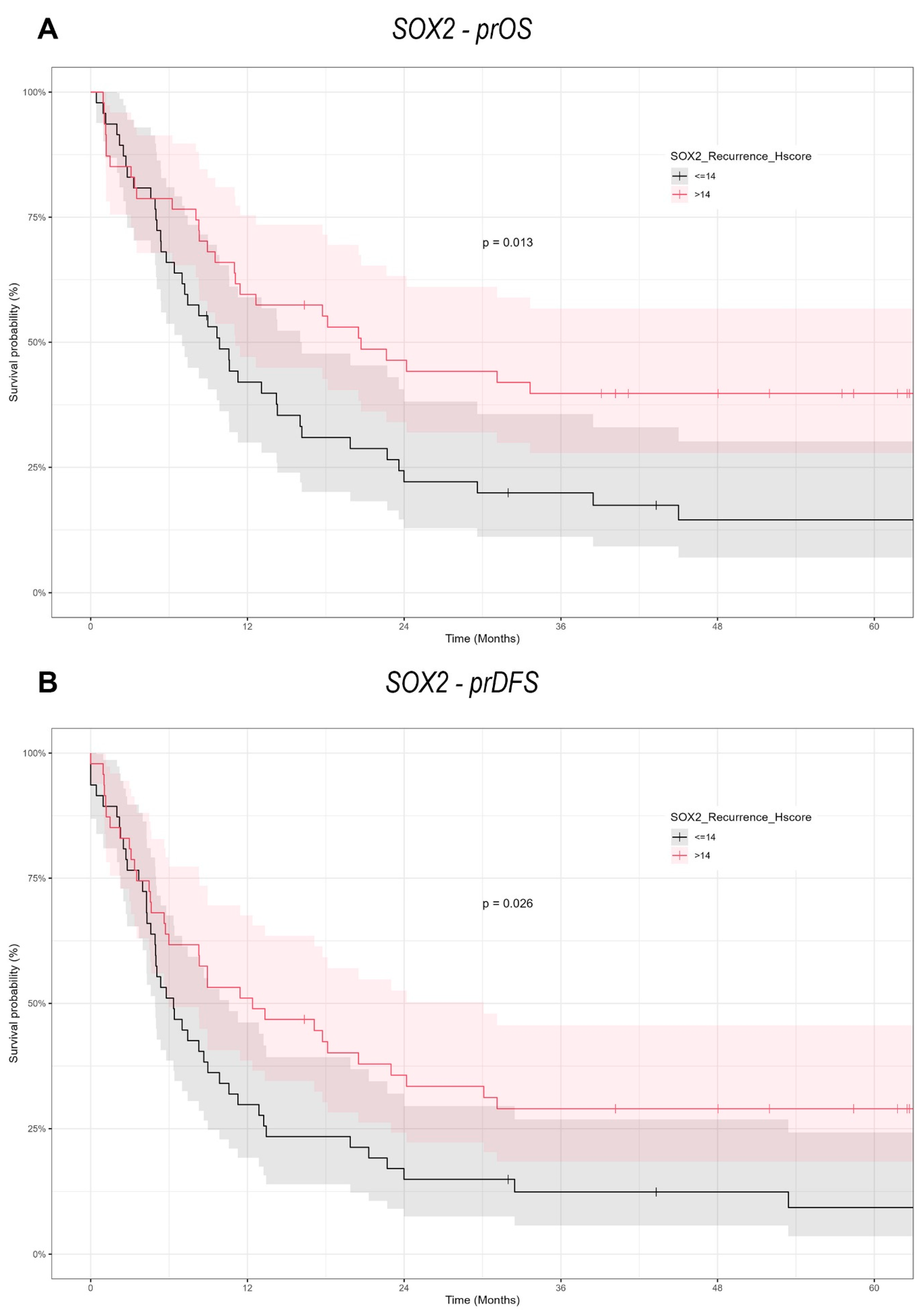
Our findings provide a notable contrast to the prevailing literature, which often associates high expression of pluripotency-associated transcription factors (NANOG and SOX2) with increased metastatic potential in tumor cells. In the context of re-OSCC, however, we observed that elevated SOX2 expression correlates with better prognosis, including improved prOS and prDFS. This discrepancy may stem from the distinct biological features of recurrent versus primary malignancies, suggesting that markers like SOX2 could behave differently depending on a tumor’s evolutionary history.
The involvement of SOX2 and NANOG in OSCC has been thoroughly examined in primary tumors. Our study is focused on recurrent oral cavity tumors. This distinction is crucial, as prior research frequently grouped HNSCC from different anatomical locations (e.g., oropharynx, larynx), which could suggest varying biological behaviors [
25,
39,
40,
41]. By refining our cohort to re-OSCC, we want to find out whether the expression patterns of these CSC markers vary at recurrence and how this may influence patient outcomes.
This study is especially important for CSC-marker research because it suggests that recurrence-specific tumor biology influences the prognostic implications of NANOG and SOX2. Although these factors are generally implicated in stemness and tumor progression, their roles may differ across clinical stages and disease contexts [
27].
Several investigations on SOX2 in OSCC have yielded mixed or conflicting prognostic results. Züllig et al. examined 120 patients with early-stage OSCC, investigated SOX2 by immunohistochemistry on TMA, assessed by an Intensity/Reactivity Score (IRS ≥ 8). Elevated SOX2 expression was associated with an absence of lymph node metastasis, indicating a potential protective function in original tumors. The present investigation on re-OSCC, utilizing the H-score for SOX2 assessment, indicates that overexpression correlates with enhanced post-recurrence survival, underscoring its prognostic relevance in primary versus recurrent disease and accentuating the necessity for context-specific biomarker evaluation [
13].
Freier et al. examined SOX2 changes in OSCC, revealing gene copy number amplification in 52% of 223 tumors and elevated SOX2 protein expression in 18% of 271 cases via immunohistochemistry. Their findings indicate that SOX2 functions as a proto-oncogene in primary OSCC [
42].
Sacco et al. examined 24 OSCC patients, assessing SOX2 expression using qRT-PCR and Western blot analysis. SOX2 was found to be elevated in both the tumor core and close margin, correlating with tumor size and lymph node involvement, indicating a potential role in local dissemination [
43]. However, our data reveals a distinct prognostic significance in re-OSCC, wherein SOX2 overexpression is associated with enhanced post-recurrence survival, indicating a change in its biological role between primary and recurrent disease.
Steen et al. examined 222 OSCC patients, including 75 matched lymph node metastases and 33 recurrent tumors, to measure SOX2 expression using immunohistochemistry on tissue microarrays and entire histological slices. The authors used QuPath for digital pathology scoring, with an H-score cutoff of 12 (low: 0–11, high: ≥12). Their findings revealed no significant relationship between SOX2 expression and overall survival, whereas SOX9 overexpression and the SOX2
lowSOX9
high subgroup were associated with a poor prognosis. Our study, using an H-score cutoff of 14 (low: ≤14, high: >14), demonstrated that SOX2 overexpression in re-OSCC was linked with improved survival. These disparities demonstrate how variations in cutoff criteria and tumor context may influence SOX2’s prognostic relevance, stressing the importance of standardizing scoring techniques in OSCC research [
22].
In other malignancies, such as colorectal cancer (CRC) and small cell lung cancer (SCLC), SOX2 often correlates with worse survival, chiefly through its roles in promoting stemness, chemoresistance, and EMT [
44,
45]. These differences further highlight the context-dependent roles of SOX2 and the necessity of examining its effects in specific clinical contexts, such as re-OSCC.
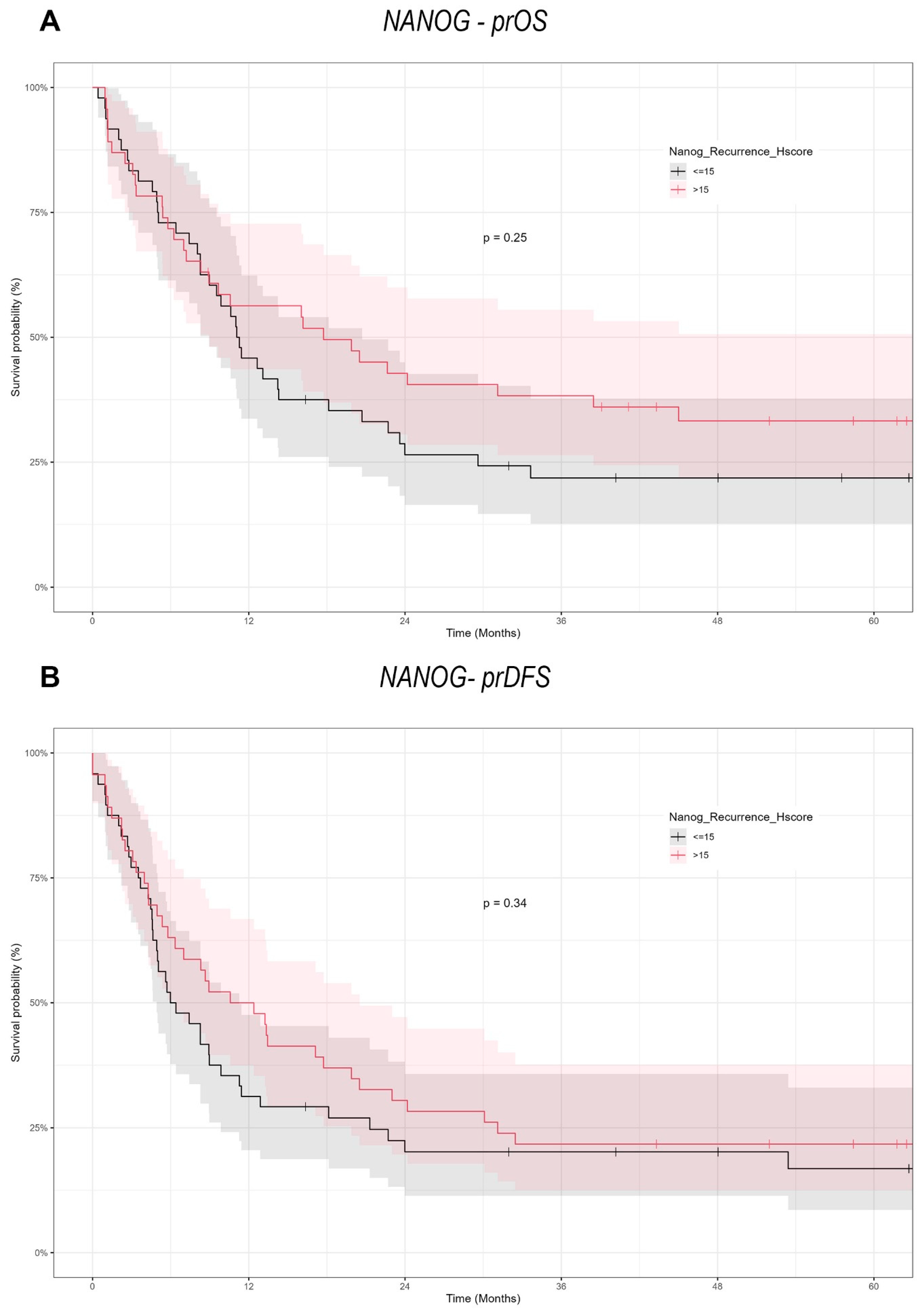
NANOG, another important stemness factor, is similarly involved in CSC maintenance and therapeutic resistance [
27,
30,
46]. However, its prognostic importance in OSCC continues to be a topic of debate. Some studies correlate NANOG overexpression with OSCC progression, lymph node metastases and cisplatin resistance [
30,
47]. Others claim more complex roles, such as potential tumor-suppressive effects or involvement primarily in the early phases [
27]. Our results did not reveal a significant association between NANOG expression and survival in recurrent OSCC, suggesting its impact may be more relevant in tumor initiation or primary disease settings than at recurrence. For instance, while one group found that NANOG and Midkine overexpression correlated with advanced stages and poor prognosis, we did not observe a similar relationship in re-OSCC [
48]. Similarly, Kashyap et al. identified NANOG as a driver of OSCC aggressiveness and cisplatin resistance, yet our data imply that in the recurrent context, NANOG expression does not predict survival [
47]. In addition, studies by Lee et al. highlight that the co-expression of NANOG and mutant p53 is particularly detrimental for survival in primary OSCC [
28]. However, such combined molecular interactions were not explored in our study of re-OSCC, pointing to additional complexities in NANOG-mediated pathways that might require further investigation.
While most evidence portrays SOX2 as an oncogenic driver in primary OSCC, our study suggests it may serve as a favorable prognostic biomarker in re-OSCC. One hypothesis is that elevated SOX2 expression at recurrence could reflect a more differentiated, therapy-responsive subpopulation, rather than one prone to unchecked aggression. Alternatively, SOX2 might activate pathways that specifically enhance sensitivity to second-line treatments or radiotherapy at recurrence.
In support of this, several meta-analyses have shown that SOX2 expression in non–small cell lung cancer (NSCLC) and esophageal squamous cell carcinoma (ESCC) correlates with better survival outcomes [
49,
50,
51]. A similar pattern may be emerging in re-OSCC. By contrast, NANOG seems more strongly implicated in primary tumor aggressiveness and therapy resistance, particularly in facilitating radioresistance and cisplatin resistance [
26,
52].
Our findings further indicate that SOX2 expression is higher in recurrent tumors of patients who received adjuvant radiotherapy, implying a potential link between SOX2 status and therapy responsiveness. Silencing SOX2 or suppressing related signals, such as β-catenin and hedgehog, may improve therapeutic efficacy and overcome resistance in re-OSCC [
41,
53,
54,
55].
SOX2 is well known for its relationship with a number of druggable pathways, the most notable being the Notch pathway. According to Hao Wu et al., Notch1 and SOX2 have a positive feedback regulation mechanism, showing a strong relationship between the two components. This link suggests that targeting the Notch pathway could be a possible therapeutic option in SOX2-related diseases like glioma [
56]. NANOG interacts with a variety of druggable pathways, including HA-CD44, STAT3, PKC, and metabolic pathways, which all contribute to drug resistance and tumor progression. Focusing on these interactions may enable novel treatment techniques to improve chemosensitivity and more effectively treat cancer [
57,
58,
59].
Despite the fact that this study offers valuable insights into the prognostic significance of SOX2 and NANOG in re-OSCC, it is important to recognize its drawbacks. Initially, the data were obtained from a single-center cohort, which could affect the overall validity of the results. In order to verify these findings, it is necessary to conduct multicenter studies with more diverse and extensive patient populations. Secondly, the study lacks to provide mechanistic insights into how SOX2 influences tumor biology or treatment response, despite the fact that it demonstrates an association between SOX2 expression and improved survival. In order to investigate the functional role of SOX2 in re-OSCC, future research should incorporate in vitro and in vivo models. Furthermore, the investigation mainly utilizes immunohistochemical analysis, neglecting molecular profiling methodologies such as gene expression analysis or RNA sequencing, which could offer a more thorough comprehension of the regulatory pathways that regulate SOX2 and NANOG expression.
A data-driven methodology employing survival-based optimization established the H-score threshold for SOX2 in our samples. This threshold successfully categorized patients into prognostically separate categories, although it may not be universally relevant to other cohorts. Distinct datasets may demonstrate divergent distributions of SOX2 expression, requiring validation in different cohorts to ascertain its prognostic relevance. The fact that the majority of re-OSCC tumors demonstrate diminished SOX2 expression (median H-score ~14) underscores the significance of context-specific cutoff determination in biomarker research. The same applies to NANOG. At present, there is no universal agreement on the ideal H-score cutoffs for both SOX2 and NANOG in re-OSCC. The survival-based cutoff selection guarantees statistical validity, although it may not accurately represent biological thresholds that have practical relevance. Future research should focus on standardizing cutoff definitions utilizing multi-institutional data.
In conclusion, our study demonstrates the context-dependent functions of SOX2 and NANOG in re-OSCC. In contrast to many primary OSCC reports, which tend to indicate a poor prognosis due to SOX2 overexpression, we observed a significant positive correlation between SOX2 expression and prOS/prDFS in re-OSCC cells. On the other hand, NANOG lacked prognostic significance in this recurrent setting, which means that it may be more critical during the early stages of tumorigenesis. These results reinforce the necessity of conducting larger, multicenter trials to independently confirm the recurrent disease-specific functions of these CSC markers and to determine whether targeted inhibition or modulation of SOX2 and NANOG can enhance outcomes in re-OSCC. It will be essential to learn about the process in which CSC-related factors transition between primary and recurrent disease in order to create more personalized, effective therapeutic strategies for OSCC.