Simple Summary
The tumor suppressor phosphatase and tensin homologue (PTEN) is the most frequently mutated or deleted gene in endometrioid endometrial carcinoma (i.e., usually Type I endometrial cancer). The conditional mutagenesis of Pten alleles in the murine female reproductive tract recapitulates the development of endometrial hyperplasia and cancer with a similar consistency found in women. Identifying targets to reduce the incidence and severity of Pten loss-of-function-induced endometrial hyperplasia and cancer is critical for developing better treatment options for this disease. Using a murine model, the ablation of progesterone receptor membrane component 2 (Pgrmc2) was shown to attenuate the timing, aggression, and lethality of Pten loss-of-function-induced endometrial hyperplasia and cancer.
Abstract
The expression of members of the progesterone receptor membrane component (PGRMC) family, particularly PGRMC1, is elevated in diverse types of cancers, particularly those of the female reproductive system. While xenograft tumor studies using human transformed cell lines in immunocompromised mice have suggested that PGRMC1 enhances tumor growth and chemoresistance, the exact role of members of the PGRMC family in cancer development in vivo remains unclear. In this study, we examined the effect of deleting Pgrmc2 on the development of endometrial hyperplasia and cancer using a murine phosphatase and tensin homologue (Pten) conditional loss-of-function model. We previously established that PGRMCs are cell survival factors that are required for normal estrogen-induced uterine epithelial cell proliferation and normal female fertility. The deletion of Pgrmc2 reduced the incidence and severity of endometrial hyperplasia and cancer in mice with conditional Pten-heterozygous uteri and increased lifespan in mice with conditional Pten-knockout uteri. Mechanistically, the deletion of Pgrmc2 decreased uterine glandular epithelial cell proliferation. Pten loss-of-function-induced endometrial hyperplasia and cancer elevated uterine inflammation, but this was not impacted by PGRMC2 deficiency. This study identifies PGRMC2 as a potential therapeutic target to be inhibited in the treatment of endometrial hyperplasia and cancer, particularly where PTEN activity is reduced due to gene mutation or loss.
1. Introduction
Endometrial cancer is the fourth most common cancer in women in the United States, and the most common gynecologic cancer. Based on estimates from the American Cancer Society, 67,880 women were diagnosed with endometrial cancer in 2024 in the U.S., and an estimated 13,250 died from the disease [1]. While hysterectomy is the leading treatment option, better therapies and earlier detection methods are needed to increase survival rates, particularly in younger women hoping to have children. In endometrioid endometrial carcinoma (EEC), 17-β-estradiol (E2) drives epithelial cell proliferation while progesterone (P4) inhibits E2-induced epithelial cell proliferation. Thus, the unopposed actions of E2 can lead to endometrial carcinoma, and P4 analogs are often used as a treatment option [2]. E2 signaling indirectly activates the AKT pathway of cell survival and proliferation. A negative regulator of AKT signaling is the phosphatase and tensin homologue (PTEN). The loss of PTEN activity via gene mutation leads to elevated AKT activity and increased endometrial epithelial cell proliferation [3]. Inflammation likely plays a prominent role in the development and progression of endometrial cancer [4]. Notably, PTEN is mutated in 83% of type I endometrial carcinomas [5]. In mice, the deletion of both alleles of Pten in the uterus leads to endometrial cancer in 100% by one month of age, and Pten heterozygosity results in 100% hyperplasia and 22% endometrial cancer [6,7,8]. Pten heterozygosity in mice is often used as a model to study endometrial oncogenesis in an effort to assess the contributions of other genes thought to be involved in endometrial neoplasm formation or those that prevent the development and progression of the disease (e.g., [9,10,11]).
Progesterone receptor membrane component (PGRMC) family members PGRMC1 and PGRMC2 were first cloned from porcine vascular smooth muscle cells [12] and human cells [13]. Whereas PGRMC1 expression is highest in the liver and kidney, PGRMC2 expression is most abundant in placenta [13]. Both proteins are expressed in most tissues. Initial characterization studies using porcine liver fractions demonstrated that PGRMC1 binds steroid hormones with affinities in the low- to mid-nanomolar range [14]. PGRMC1 was found to have the highest affinity for P4 (i.e., 11 nM), but it also bound corticosterone, cortisol, testosterone, and cholesterol. PGRMC1 has a poor affinity for 17-β estradiol and aldosterone [14]. Despite being labelled as a P4 receptor, PGRMC2 has not yet been shown to bind and become activated by P4. PGRMC proteins have pleiotropic functions in female reproduction and P4 signaling [15,16,17], mitosis and meiosis [18], energy metabolism and Warburg glycolysis [19], angiogenesis [20], receptor trafficking to the plasma membrane [21], drug metabolism [22], heme transport [23], and neural development [24]. Gene mutations and aberrant expression profiles in PGRMC1 and PGRMC2 are associated with a host of reproductive diseases [15,25,26]. Through association-based studies in humans, as well as cell culture and xenograft studies in immunocompromised mice, PGRMC1 is thought to contribute to the development and progression of solid tumors in many different organs [26,27,28,29,30,31,32]. Similarly, much of what is known about PGRMC2 stems from descriptive studies in cell lines and reproductive tissues [15,25,26,27]. PGRMC2 expression correlates with the development of endometriosis in a non-human primate model of the disease (reviewed in [26]). The conditional ablation of Pgrmc2 in mice results in subfertility that progresses to premature reproductive senescence [16,26,27]. PGRMC2 was shown to function in adipocytes as an essential intracellular heme chaperone required for normal function [23], as well as a pressure–volume regulator for accommodating stress responses in cardiomyocytes [33]. PGRMC2 plays an important role in human placental extravillous trophoblast invasion during early pregnancy where it also helps regulate immune homeostasis at the maternal–fetal interface [34,35]. Considerably less is known about a role for PGRMC2 in cancer. As with PGRMC1, PGRMC2 is elevated in ovarian cancer and a large number of ovarian cancer cell lines [27]. However, to date, we are unaware of any studies that assess the function of PGRMC2 in the context of the development or progression of cancer in vivo.
Given that PGRMC proteins are generally overexpressed in many tumor types, we hypothesize that selectively deleting a PGRMC family member from the endometrium with the heterozygous or complete loss of Pten would prevent the development of or reduce the severity of Pten loss-of-function-induced hyperplasia and cancer. Because of the general lack of information about PGRMC2 in the context of cancer, Pgrmc2 was specifically evaluated in this context.
2. Materials and Methods
2.1. Animals
All animal protocols were approved by the Institutional Animal Care and Use Committees at Washington State University or the University of Wyoming. The floxed Pgrmc2 mice (Pgrmc2fl) and their use with Pgrcre/+ mice were described previously [16]. Floxed Pten (Ptenfl/fl) mice were obtained from Jackson laboratories. Mice of 10 different genotypes were generated for this study by crossing Pgrcre/+ mice with Ptenfl/fl and/or Pgrmc2fl/fl mice. Tissues were collected from mice at 6–12 weeks of age or at 9 months of age at the completion of the study. In some cases, such as with Pgrcre/+; Ptenfl/fl (Ptend/d) mice, animals were euthanized at 4–9 months of age due to rapid development of cancer. Animals aged 4–9 months were first ovariectomized and tissues were collected 1 week later for fixation in 4% paraformaldehyde (PFA) and paraffin embedded or snap frozen in liquid nitrogen and stored at −80 °C until use. For assessing the endometrial epithelial mitotic response to 17β-estradiol (E2), young (6–12 weeks) Pten+/fl; Pgrmc2fl/fl, Pten+/d, and Pten+/d; Pgrmc2d/d mice were ovariectomized, allowed to rest for 1–2 weeks, and then subjected to sesame oil vehicle treatment that mirrored treatment with E2 (100 ng E2 for 2 days, 5 days without treatment, 50 ng E2, and tissue collection 18 h later). Tissues were again collected for paraffin embedding and some were snap frozen.
2.2. Histology and Immunohistochemistry
All human and mouse tissues were fixed in 10% buffered formalin or PFA, then stored in 70% ethanol until paraffin embedding. Tissues were processed through a graded series of ethanol (70–100%) and xylenes, embedded in paraffin, and sectioned at 5 µm by microtome. Human tissues were collected deidentified, and contributed to a Northwestern University tissue repository as part of NCATS/UH3TR00120. Proliferative and secretory specimens were obtained from women with benign gynecological conditions such as fibroids. For general histological analyses, tissue sections were deparaffinized with xylenes, rehydrated, and stained with hematoxylin and eosin. Immunohistochemistry (IHC) was completed as previously described [16,17,31,32]. Antibodies used for IHC included anti-phosphohistone H3 (phH3) primary antibody (Ser10, Millipore 06-570, 1:750, Burlington, MA, USA), anti-PGRMC2 primary antibody (Sigma, 1:100, St. Louis, MO, USA), and biotinylated anti-rabbit IgG secondary antibody (Vector BA-1000, 1:750, Burlingame, CA, USA). From young ovariectomized Pten+/fl; Pgrmc2fl/fl, Pten+/d, and Pten+/d; Pgrmc2d/d mice treated with vehicle or E2 and aged ovariectomized Pten+/fl; Pgrmc2fl/fl, Pgrmc2d/d, Pten+/d, and Pten+/d; Pgrmc2d/d mice, mitotic (phH3+ cells) counts were made from three different tissue sections for each biological replicate. From 9-month-old Pten+/fl; Pgrmc2fl/fl, Pten+/d, and Pten+/d; Pgrmc2d/d mice ovariectomized one week prior to euthanasia, the number of granulated and degranulated mast cells was counted on a per section basis. Following deparaffinization and rehydration, 5 µm sections were stained with toluidine blue working solution (1 mg/mL) and counter-stained with hematoxylin. Mast cells were quantified by counting dark blue cells and establishing a mean value from three non-adjacent sections for each biological replicate.
2.3. RNA Isolation and qPCR
For assessing mRNA levels of the classical estrogen receptor (Esr1) and progesterone receptor (Pgr) from uterine tissues collected from ovarictomized and sesame oil vehicle treated Pten+/fl; Pgrmc2fl/fl, Pten+/d, and Pten+/d; Pgrmc2d/d mice, total cellular RNA was isolated using TRI-Reagent (Sigma, St. Louis, MO, USA). RNA samples were subjected to DNAse I digestion (Promega, Madison, WI, USA), and complementary DNA was generated using iScript Reverse-Transcription Supermix (BioRad, Hong Kong, China). qPCR was performed to compare expression of Esr1 and Pgr in complementary DNA samples. Rpl13a was included for normalization. A negative control (no reverse transcription) was included to confirm the absence of genomic DNA. Table 1 provides primer information for qPCR, as well as for primers used for genotyping animals.

Table 1.
Genotyping and qPCR primers.
2.4. Data Analyses
Animals with the same genotype were randomly assigned to the various treatment groups. All data are presented as the mean ± SEM for n = 3–28 samples. Individual animals represented a single biological replicate. Differences between genotypes were assessed by Student’s t-test, where the mean values of two groups were compared. Differences between treatment groups or genotypes with more than two groups were analyzed by one-way analysis of variance followed by Tukey’s post hoc test. A chi-square analysis was used for incidence studies and a Kaplan–Meier survival curve was generated for lifespan analyses. For semi-quantitatively comparing PGRMC2 protein levels in human endometrial tissues, a composite h-score was established by multiplying the staining intensity [scored as 1 (low) or 2 (high)] and percentage of positive cells [scored as 0 (no staining), 1 (1–10% staining), 2 (11–50% staining), or 3 (>50% staining)] within each tissue specimen (n=10 each). Contingency graphs were then generated to show the relationship between staining intensity and the percentage of positive cells by showing the frequencies of different combinations of these variables. All data were analyzed using GraphPad 5.0 software (San Diego, CA, USA) where p ≤ 0.05 was considered statistically significant and p > 0.05 ≤ 0.10 was considered a trend.
3. Results
3.1. PGRMC2 Expression in Human Endometrium and Endometrial Carcinoma
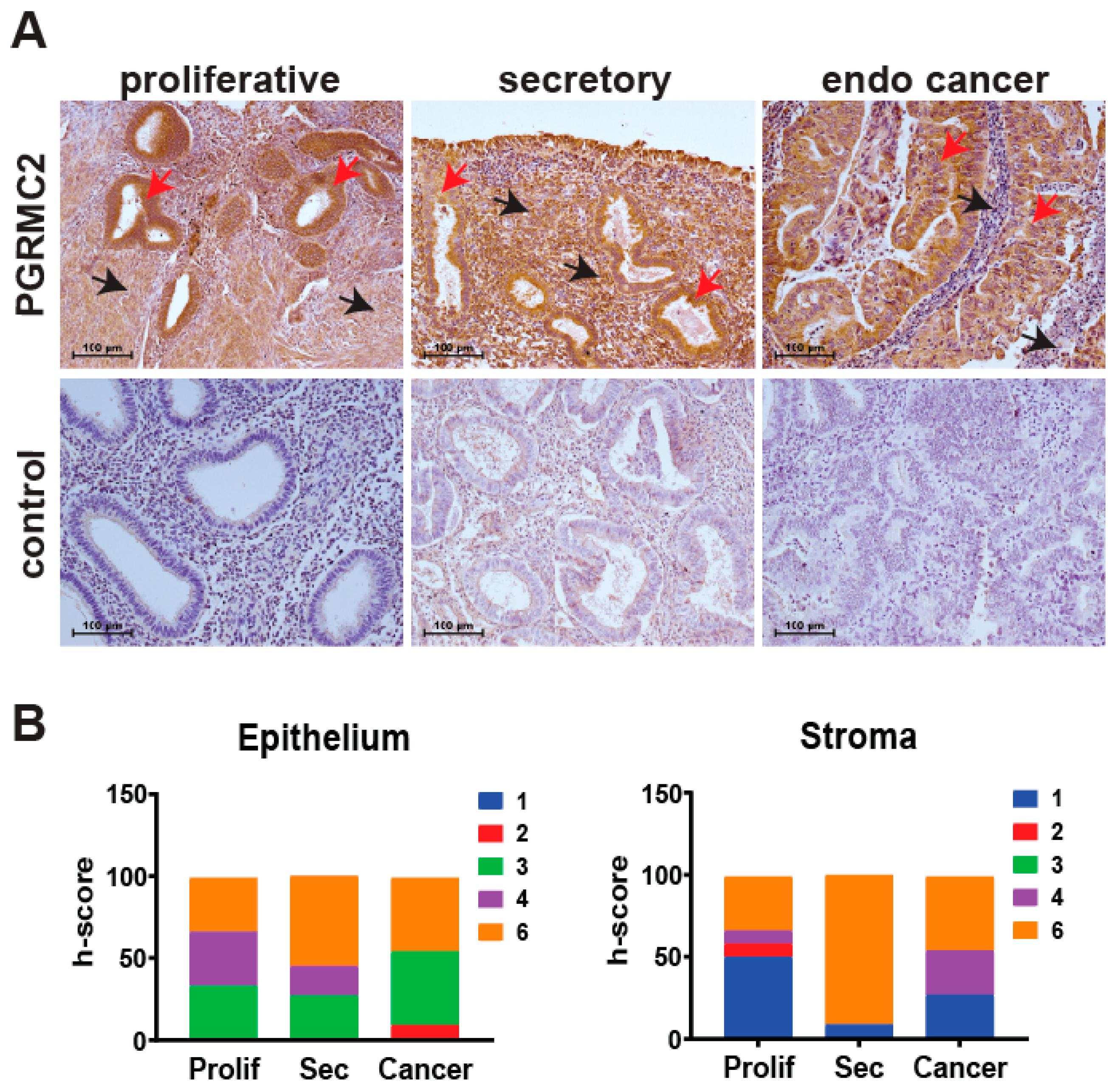
The level of PGRMC2 protein was evaluated by immunohistochemistry in human endometrial samples during the proliferative and secretory phases of the menstrual cycle, as well as in endometrial cancer specimens (Figure 1). Semi-quantitative h-scoring was used to show that PGRMC2 protein levels did not change in epithelial or stromal tissues across the different groups. During the proliferative phase, PGRMC2 was abundant in both the glandular and luminal epithelia and, to a lesser extent, in the stroma. PGRMC2 remained abundant in epithelia during the secretory phase, and appeared to be elevated in the stromal compartment compared with stromal tissue from the proliferative phase. However, a difference in PGRMC2 between epithelial and stromal tissues was not found to be statistically different during the menstrual cycle or endometrial cancer. PGRMC2 seemed more abundant in the neoplastic epithelium of endometrial cancer tissues when compared to adjacent stromal tissue, but this may likely be due to the presence of stratified epithelium in this neoplastic tissue.
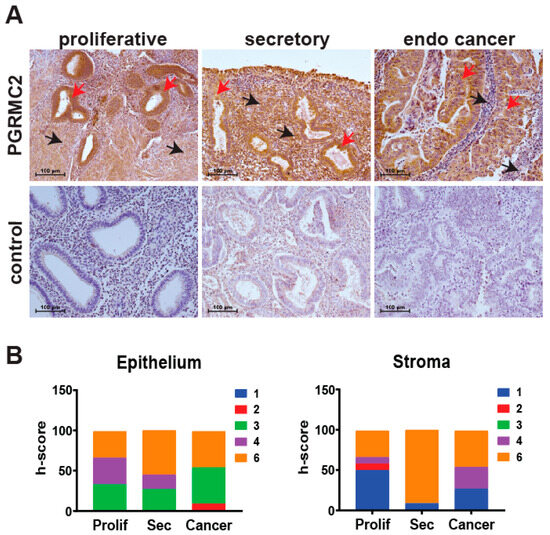
Figure 1.
Human endometrial PGRMC2 protein levels during the menstrual cycle and in endometrial cancer. (A) Localization of PGRMC2 (brown stain) in human endometrial stroma (black arrows) and epithelia (red arrows) collected during the proliferative and secretory phases of the menstrual cycle and endometrial (endo) cancer detected by immunohistochemistry. Lower panels show sections stained without primary antibody as a negative control. (B) Contingency plots comparing semi-quantitative h-score values for PGRMC2 in endometrial epithelial and stromal tissues. The h-score index ranges from low (blue) to high (orange). N = 10, scale bar = 100 µm.
3.2. Pgrmc2 Deficiency Reduces the Incidence and Severity of Pten Loss-of-Function-Induced Endometrial Hyperplasia and Carcinoma
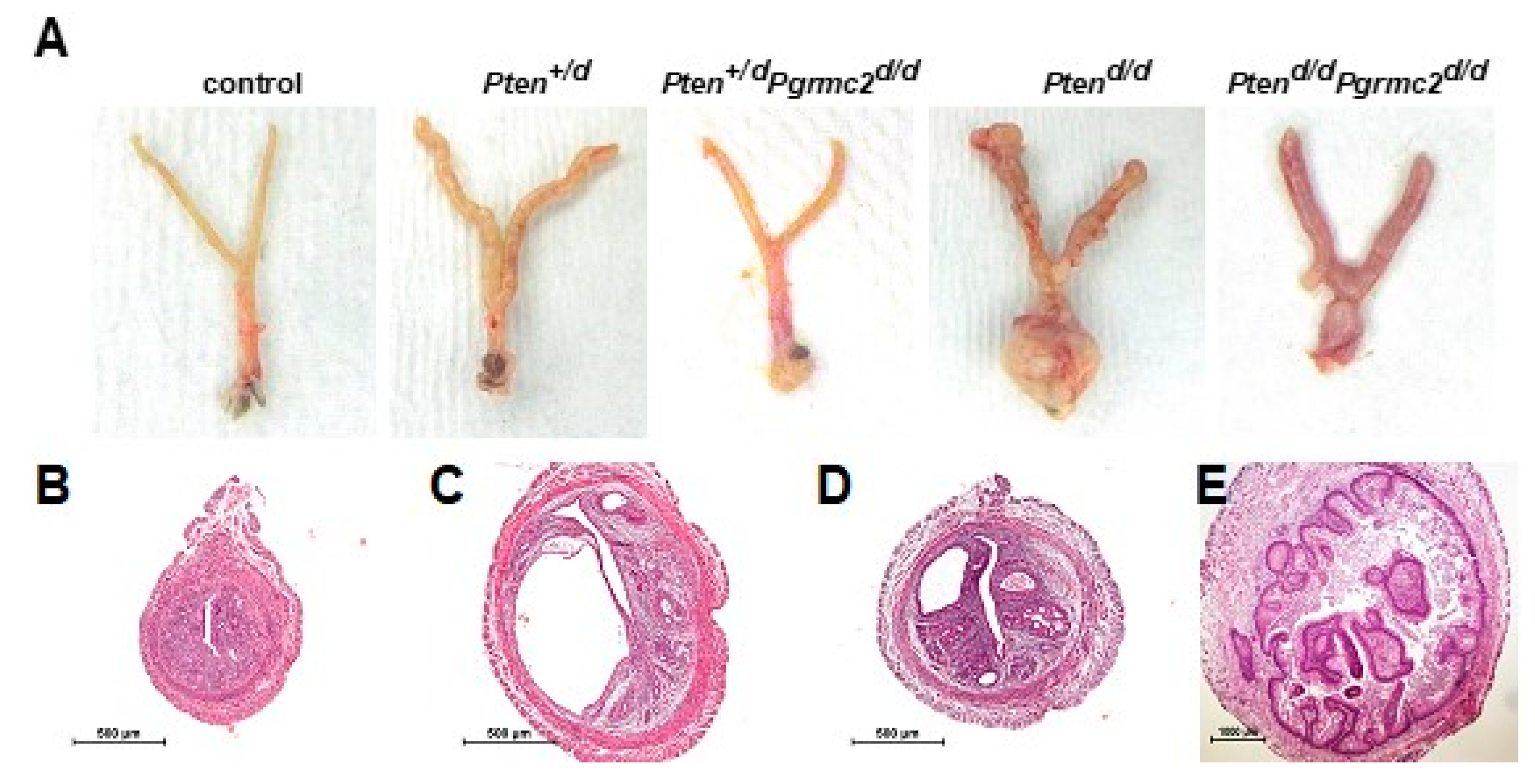
The most commonly mutated gene in human endometrioid endometrial carcinoma is PTEN [36]. The deletion of a single Pten allele in mice results in a condition similar to Cowden’s syndrome in women. This phenotype is accompanied by endometrial hyperplasia in 100% of mice and endometrial carcinoma in 22% of mice by six months of age [37]. The conditional ablation of both alleles causes endometrial carcinoma in 100% of mice by around one month of age [6]. Conditional mutagenesis in mice in which one or both Pten alleles are ablated is commonly used as a model to study Pten-based endometrial carcinoma to understand how other gene mutations contribute to endometrial neoplasms [6,7,8,10,11,38]. Given the lack of in vivo information about a role for PGRMC2 in oncogenesis, we sought to determine if endometrial Pgrmc2 ablation would impact the development and progression of endometrial neoplasia in a well-established model of endometrial cancer. In this study, the PgrCre/+ mouse line was used in which Cre recombinase is expressed in cells of the female reproductive tract under the control of the Pgr promoter [39]. PgrCre/+ mice were crossed with Ptenfl/fl and/or Pgrmc2fl/fl mice to conditionally ablate one or both copies of these genes in Pgr-expressing cells. Figure 2A shows the gross morphology of female reproductive tracts isolated from control (Pten+/fl; Pgrmc2fl/fl), Pten+/d, Pten+/d; Pgrmc2d/d, Ptend/d, and Ptend/d; Pgrmc2d/d mice one week after ovariectomy at nine months of age. The exterior surface of tracts from Pten+/d and Ptend/d were rough compared to the smooth surface of tracts isolated from Pten+/d; Pgrmc2d/d and Ptend/d; Pgrmc2d/d mice. Overt signs of carcinoma were clearly present in Ptend/d mice, particularly at the distal end of the uterus near the oviduct and the cervix. Images of the H&E-stained uterine cross-sections are shown in Figure 2. No signs of hyperplasia or carcinoma were found in control mice (Figure 2B). As previously described [16], non-hyperplastic cystic glands formed in uteri from Pgrmc2d/d mice, consistent with a premature aging phenotype (Figure 2C). Also shown are representative images of atypical endometrial hyperplasia (Figure 2D) and carcinoma (Figure 2E) commonly observed in uteri isolated from Ptend/d and Ptend/d; Pgrmc2d/d mice.
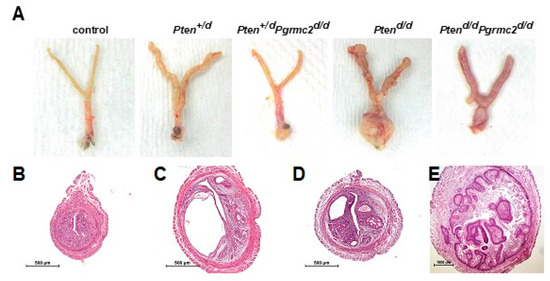
Figure 2.
Gross anatomy of female reproductive tracts and uterine histology. (A) Gross reproductive tract anatomy from Pten+/fl; Pgrmc2fl/fl (control), Pten+/d, Pten+/d; Pgrmc2d/d, Ptend/d, Ptend/d, and Ptend/d; Pgrmc2d/d mice at one week after ovariectomy at 9 months of age. H&E-stained uterine cross-sections from (B) Pten+/fl; Pgrmc2fl/fl (control), (C) Pgrmc2d/d, (D) Pten+/d; Pgrmc2d/d, and (E) Ptend/d mice. N = 3–28. scale bar = 100 µm.
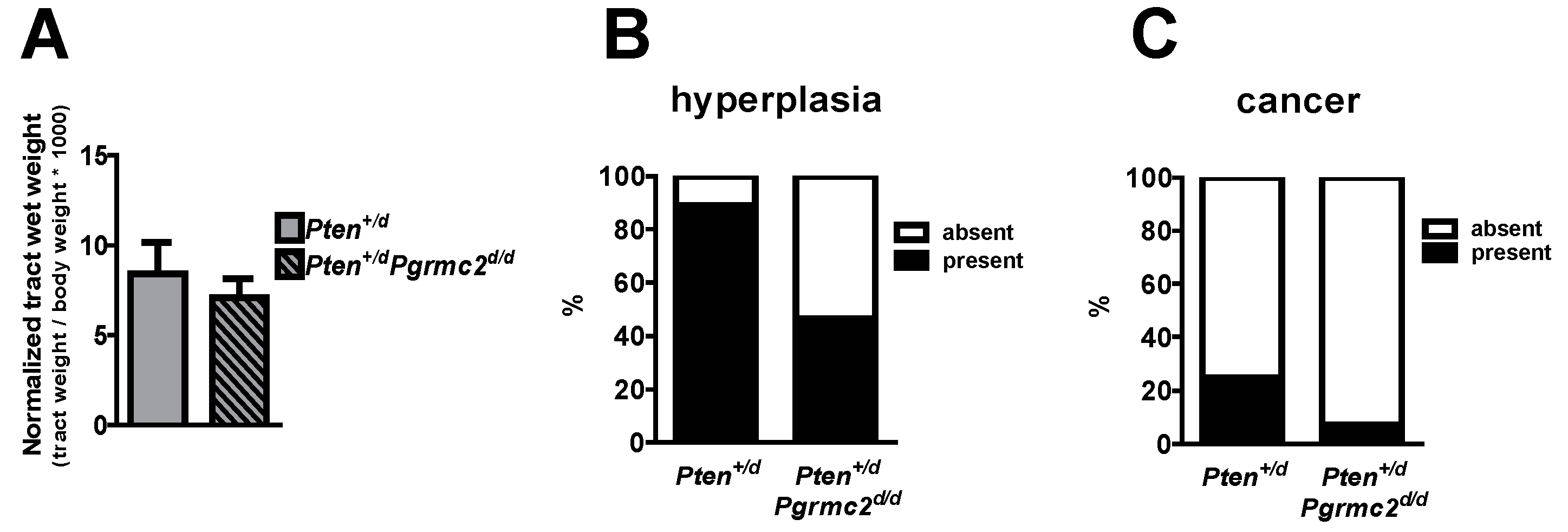
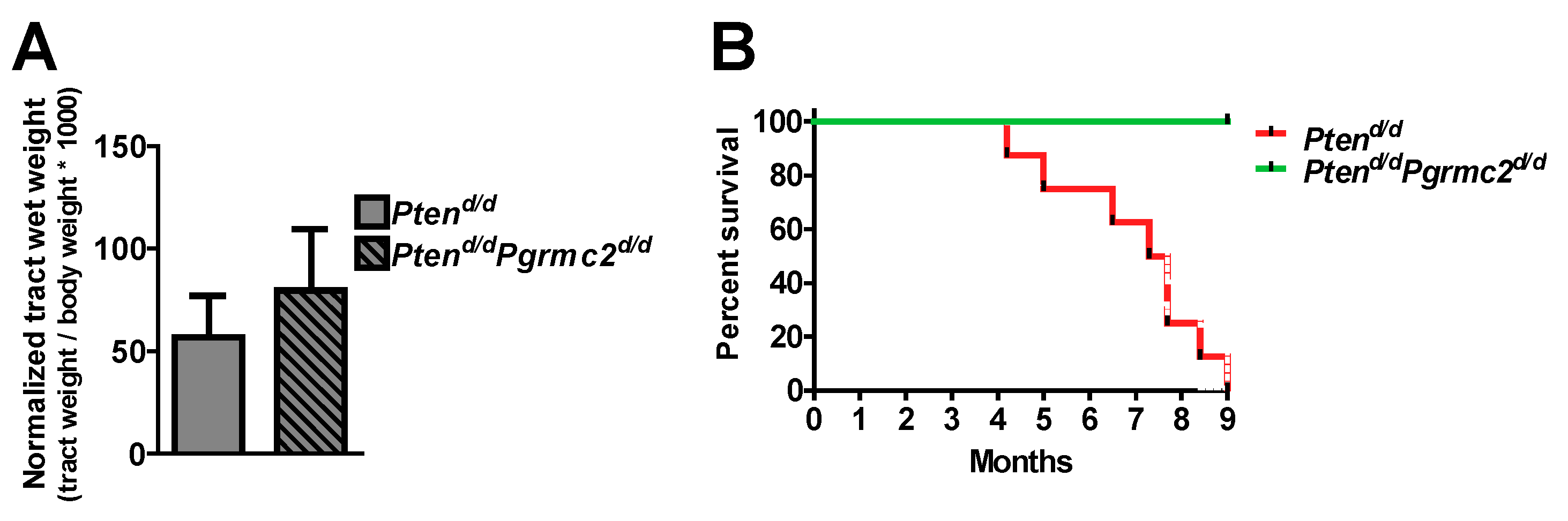
The incidence of endometrial hyperplasia and cancer was determined in mice with 10 different Pten and Pgrmc2 genotypes as outlined in Table 2. Reproductive tracts were collected for analyses at nine months of age, except for Ptend/d mice which were collected at 4–9 months of age due to the lethality of this phenotype. While the tract wet weight was not different between Pten+/d and Pten+/d; Pgrmc2d/d mice, hyperplasia was reduced from 88.9% in Pten+/d mice to 46.4% in Pten+/d; Pgrmc2d/d mice (Figure 3A,B). The ablation of Pgrmc2 on a Pten+/d background also decreased the incidence of endometrial cancer to 7.1% compared to the 22.2% in Pten+/d mice (Figure 3C). Similarly, the tract weight did not differ between Ptend/d, and Ptend/d; Pgrmc2d/d mice (Figure 4). The ablation of Pgrmc2 (i.e., Ptend/d; Pgrmc2d/d) did not reduce the 100% incidence of hyperplasia or cancer observed in Ptend/d mice (Table 2). However, whereas all Ptend/d mice had to be euthanized prior to the end of the study at nine months, 100% of Ptend/d; Pgrmc2d/d mice survived to nine months of age, suggesting that the ablation of Pgrmc2 attenuated the progression of endometrial cancer (Figure 4B).

Table 2.
Endometrial hyperplasia and cancer incidence in PgrCre/+-driven Pten and/or Pgrmc2 conditional mutant mice.
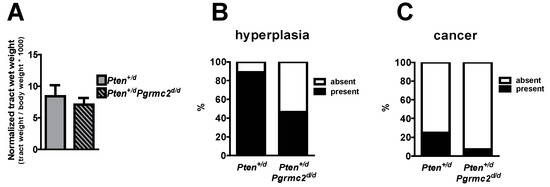
Figure 3.
Endometrial hyperplasia and cancer in Pten+/d and Pten+/d; Pgrmc2d/d mice. Uterine wet weight (A), incidence of hyperplasia (B), and incidence of endometrial cancer (C) are compared between Pten+/d and Pten+/d; Pgrmc2d/d mice. n = 9 or 28.
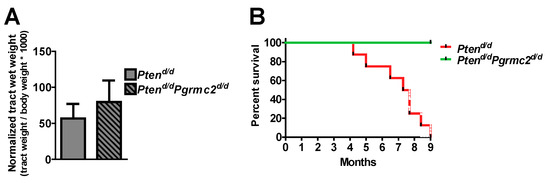
Figure 4.
Impact of Pgrmc2 ablation on Pten loss-of-function-induced cancer and lethality. (A) Comparison of normalized reproductive tract weight from Ptend/d and Ptend/dPgrmc2d/d mice (p > 0.05). (B) Kaplan–Meier survival analysis comparing lethality of endometrial carcinoma in Ptend/d and Ptend/dPgrmc2d/d mice. p ≤ 0.0001, n = 8–9.
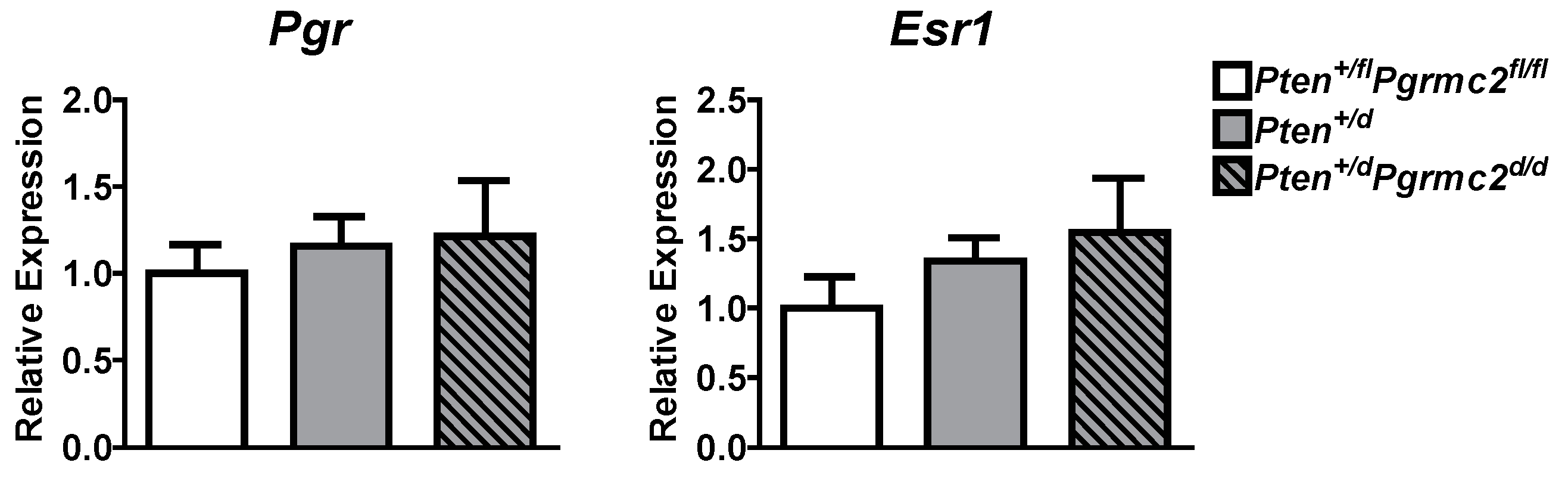
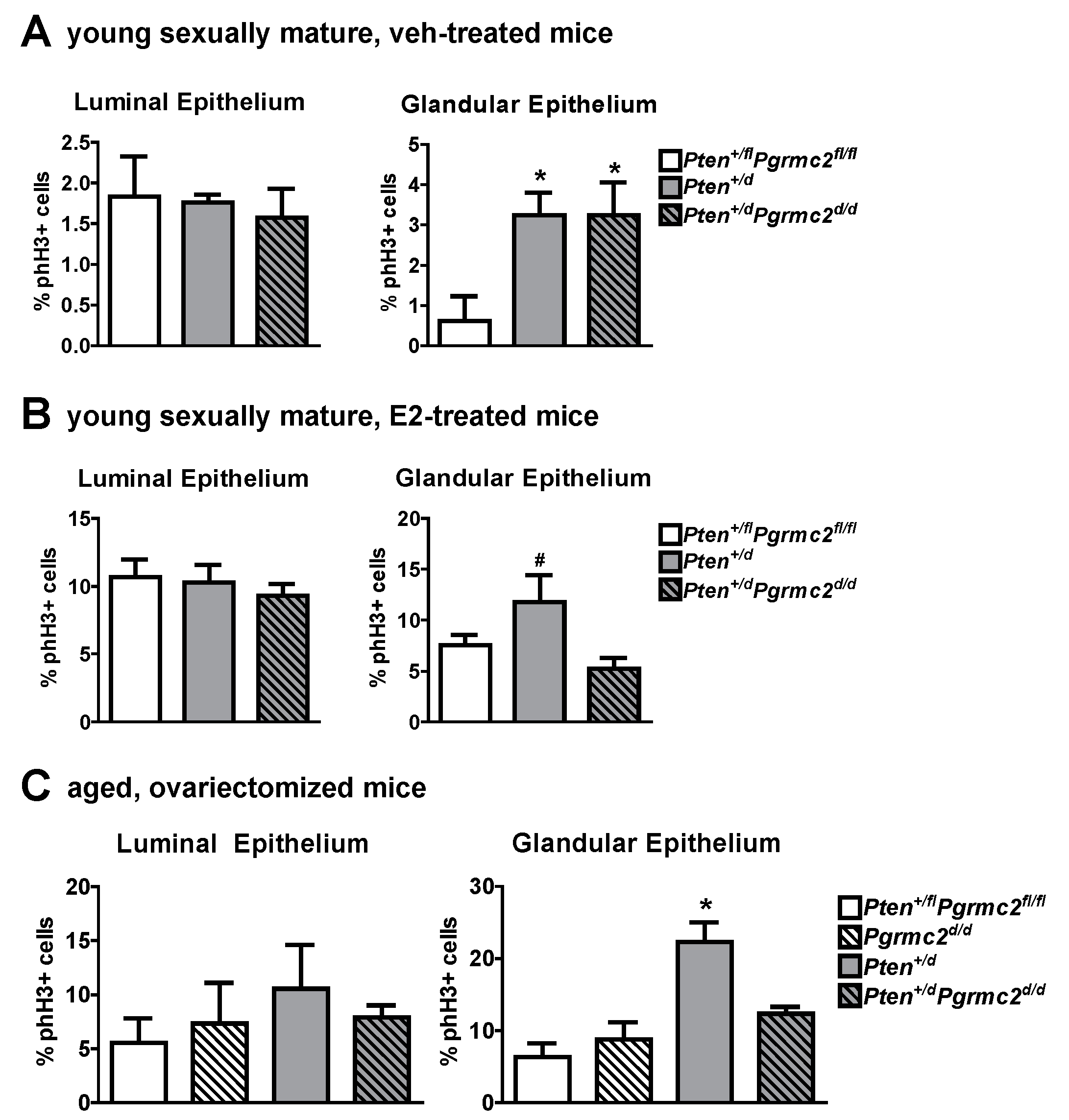
The mRNA levels of Pgr and Esr1 were evaluated in uterine tissues from Pten+/fl; Pgrmc2fl/fl (control), Pten+/d, and Pten+/d; Pgrmc2d/d mice and found not to be different between the groups (Figure 5). Given that PGRMC proteins regulate proliferation in granulosa cells and xenograft tumors [15,18,25,26,27,30,32,40], we next evaluated endometrial luminal and glandular epithelial cell mitosis by phospho-histone H3 immunohistochemsitry in young sexually mature ovariectomized Pten+/fl; Pgrmc2fl/fl, Pten+/d, and Pten+/d; Pgrmc2d/d mice treated with either vehicle or E2. While there was no difference in basal luminal epithelial cell mitosis across genotypes, basal glandular epithelial mitosis was approximately six times higher in uterine tissue from Pten+/d and Pten+/d; Pgrmc2d/d mice than from Pten+/fl; Pgrmc2fl/fl mice. The ablation of Pgrmc2 had no effect on the elevated basal mitosis observed in young Pten+/d mice (Figure 6A). The treatment of Pten+/fl; Pgrmc2fl/fl (control), Pten+/d, and Pten+/d; Pgrmc2d/d mice with E2 equitably elevated luminal epithelial cell proliferation to approximately 10%. The ablation of a single copy of the Pten gene tended (p = 0.07) to increase E2-induced glandular epithelial mitosis by about 40% over glandular epithelium from Pten+/fl; Pgrmc2fl/fl mice. Interestingly, the elevated mitosis observed in Pten+/d mice was lost upon the ablation of Pgrmc2 in Pten+/d; Pgrmc2d/d mice (Figure 6B). In aged ovariectomized mice, basal endometrial luminal epithelial cell mitosis was not different in Pten+/fl; Pgrmc2fl/fl, Pgrmc2d/d, Pten+/d, and Pten+/d; Pgrmc2d/d mice. In contrast, glandular epithelial cell mitosis was significantly higher in Pten+/d mice than in all other groups, including Pten+/d; Pgrmc2d/d mice (Figure 6C).
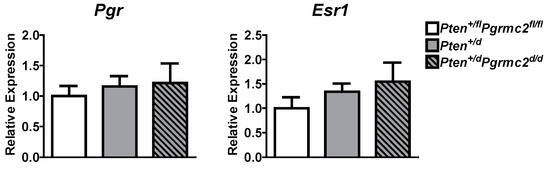
Figure 5.
Messenger RNA levels of the classical progesterone receptor (Pgr) and estrogen receptor alpha (Esr1) were measured by qPCR in Pten+/fl; Pgrmc2fl/fl, Pten+/d, and Pten+/d; Pgrmc2d/d mice. n = 5.
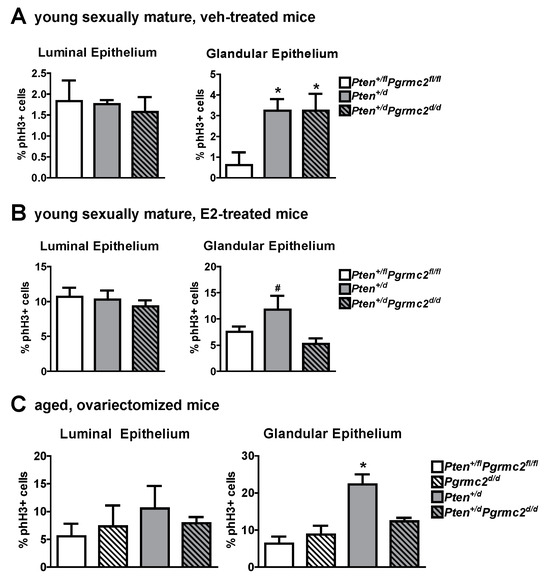
Figure 6.
Effect of Pten heterozygosity and Pgrmc2 ablation on endometrial epithelial cell mitosis in young and aged Pten+/fl; Pgrmc2fl/fl, Pten+/d, and Pten+/d; Pgrmc2d/d mice. The marker phospho-histone H3 (phH3) was used to quantify mitosis in endometrial luminal and glandular epithelia in young ovariectomized sexually mature Pten+/fl; Pgrmc2fl/fl, Pten+/d, and Pten+/d; Pgrmc2d/d mice treated with vehicle (veh, sesame oil) (A) or E2 (100 ng E2 for 2 days, 5 days without treatment, 50 ng E2, and tissue collection 18 h later) (B). (C) Quantification of basal mitosis in ovariectomized aged (i.e., 9 months) Pten+/fl; Pgrmc2fl/fl, Pgrmc2d/d, Pten+/d, and Pten+/d; Pgrmc2d/d mice. N = 3–4 per group, * p ≤ 0.05, # p = 0.07.
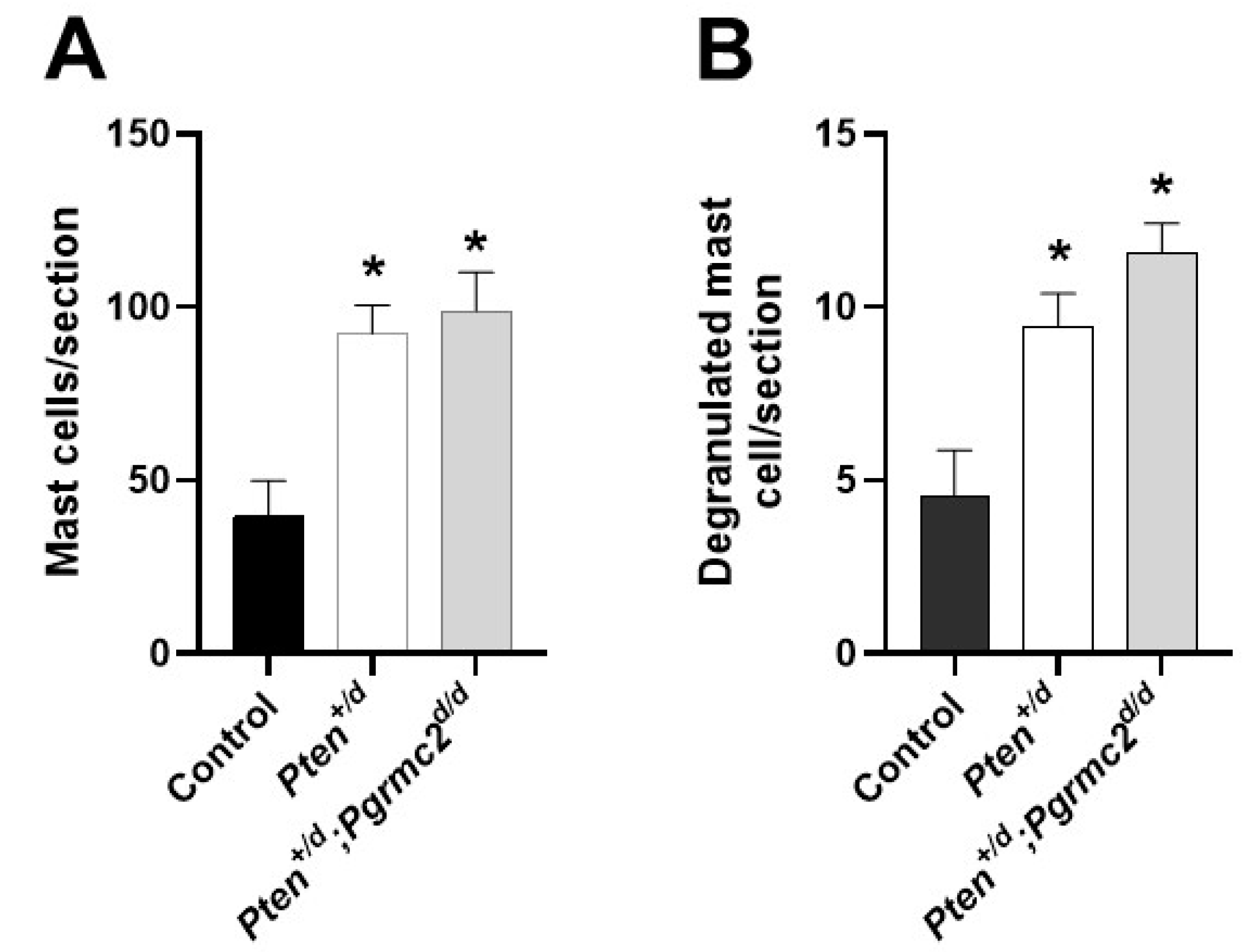
Conditional Pten heterozygosity or deficiency in the uterus causes inflammation [6]. In the final study, we quantified the uterine infiltration of mast cells and their degranulation in Pten+/fl; Pgrmc2fl/fl, Pten+/d, and Pten+/d; Pgrmc2d/d mice. As shown in Figure 7, an approximate 2.5-fold increase in mast cells infiltrated occurred in uterine tissue from Pten+/d mice compared with Pten+/fl; Pgrmc2flfl control mice. The conditional ablation of Pgrmc2 in Pten+/d mice failed to reduce mast cell infiltration. Approximately 10% of the total mast cells were degranulated in all tissues examined. As with total mast cells, the number of degranulated mast cells was about 2.5-fold higher in uterine tissues isolated from Pten+/d and Pten+/d; Pgrmc2d/d mice compared with uterine tissue from Pten+/fl; Pgrmc2fl/fl mice (Figure 7B).
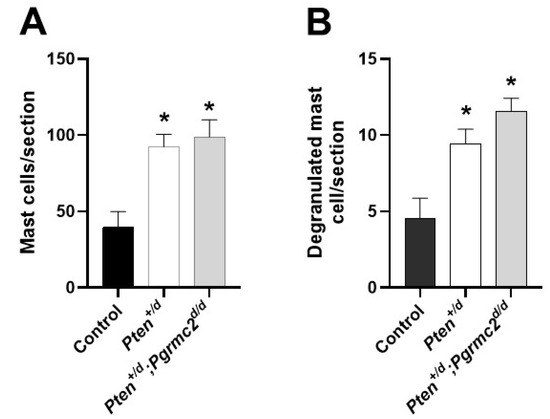
Figure 7.
Uterine mast cell infiltration and degranulation in Pten+/fl; Pgrmc2fl/fl, Pten+/d, and Pten+/d; Pgrmc2d/d mice. The total number of toluidine-stained mast cells (A) and degranulated mast cells (B) was determined in uterine histological sections from 9-month-old ovariectomized Pten+/fl; Pgrmc2fl/fl, Pten+/d, and Pten+/d; Pgrmc2d/d mice. * p ≤ 0.05, n = 3.
4. Discussion
Coupled with supporting mutations in the cell cycle, adhesion, and/or apoptosis genes, mutations of tumor suppressor genes are causally responsible for neoplastic transformation in various organs. Endometrioid endometrial carcinoma, which generally exists as type 1 endometrial cancer, is the most common gynecological cancer. Mutations in the tumor suppressor gene PTEN occur in over 80% of type 1 endometrial cancer cases [5]. PTEN is a phosphatase that attenuates the activity of the AKT, a kinase activated by pro-survival and pro-growth ligand:receptor-initiated phosphorylation cascades. The loss of even one PTEN allele reduces the overall cellular PTEN phosphatase activity. This haploinsufficiency results in accelerated proliferation and neoplastic transformation in epithelial tissues of several organs due, in part, to its role in maintaining the chromatin structure and the integrity of the genome [3,41]. Because many tumor suppressor genes play vital roles in diverse normal cellular functions, the global ablation or mutation of these genes generally causes embryonic lethality. To circumvent this limitation, the loxP-Cre system allows for the ablation, mutation, or overexpression of genes in a cell-specific fashion [42]. The objective of this study was to conditionally ablate the Pten gene from endometrial cells and evaluate the consequences of concomitant Pgrmc2 ablation. This was accomplished by using the Pgr-Cre mouse, which expresses Cre recombinase in cells that normally express the classical Pgr. Of note, an endometrial-specific Cre mouse line does not exist, so the Pgr-Cre mouse is routinely used to ablate, mutate, or overexpress genes in the endometrium despite having Cre recombinase expression in other organs of the female reproductive tract, peri-ovulatory follicles, mammary gland, and gonadotropes [39]. This approach has been used extensively to determine causal relationships between PTEN and other genes. For instance, deleting the glucose-regulated protein-78 (Grp78) gene prevented the development of endometrial carcinoma in Pten-ablated mice [43]. Similarly, because Pten deletion deregulated lipid metabolism [7], the overexpression of an mfat-1 transgene in mice reinstated lipid homeostasis and prevented Pten-heterozygous mice from developing hyperplasia and cancer [44]. The overexpression of the tumor suppressor mitogen-inducible gene-6 (Mig-6) abrogated the development of Pten-loss-of function-induced endometrial carcinoma [45], whereas the ablation of Mig-6 accelerated the timeline of developing endometrial carcinoma and its severity [46]. The deletion of epithelial cadherin (Cdh1) or the tumor suppressor liver kinase B1 (Lkb1) along with Pten increases the severity of endometrial cancer invasiveness in mice [9,47]. The use of this genetic approach should facilitate the identification of gene targets for the treatment of Pten-related endometrial cancer. By example, the pharmacological inhibition of mammalian target of rapamycin (mTOR) signaling was shown to substantially inhibit the growth and progression of Pten/Lkb1-deficient endometrial cancer. Given that E2 enhances and P4 attenuates type 1 endometrial cancer, the Pten loss-of-function model of endometrial cancer has utility in understanding the endocrine actions of these female sex steroids [2,48,49,50].
PGRMC2 expression was not different between endometrial cancer tissue compared with endometrial tissue obtained during the proliferative and secretory phase of the menstrual cycle (Figure 1). The ablation of Pgrmc2 reduced the percentage of Pten+/d mice developing hyperplasia by about 50% and those developing endometrial cancer by 66% (Table 2 and Figure 3). More striking was the survival data when both Pten alleles were ablated. Here, 100% of Ptend/d mice developed endometrial cancer and were euthanized prior to the end of the nine-month trial. In contrast, 100% of Ptend/d; Pgrmc2d/d mice survived to nine months despite all developing endometrial cancer suggesting the Pgrmc2 not only attenuates tumor growth, but also the likely metastatic spread.
The actions of PGRMC2 are consistent with prior studies in which PGRMC1 knockdown in endometrial cancer cells dramatically reduced xenograft tumor growth in immunocompromised mice while also elevating chemosensitivity [31]. Similar findings were observed in PGRMC1-deplete ovarian and breast cancer xenograft tumors [30,32]. Our prior evaluation of Pgrmc1d/d and/or Pgrmc2d/d endometrial tissue demonstrated that the ESR1 and PGR levels do not differ from the corresponding control tissues in which Pgrmc genes remain intact [16,17]. Consistent with these findings, Esr1 and Pgr mRNA levels did not change following ablation with Pten and/or Pgrmc2 in the present study (Figure 5). Additional studies are needed to determine if Pgrmc2 fits the generally accepted definition of a proto-oncogene, a normal gene that produces proteins which helps cells grow and proliferate, or to simply help cells survive. The ablation of Pgrmc2 clearly attenuated the incidence/development, progression, and aggressiveness of Pten loss-of-function-induced endometrial cancer by inhibiting endometrial glandular epithelial cell proliferation (Figure 6). A great deal of excitement surrounds the notion that inflammation initiates neoplastic transformation and/or promotes the tumor progression of solid tumors [51,52]. The conditional ablation of Pten using Pgr-Cre mice elevates endometrial inflammation [6]. We assessed the recruitment of mast cells in Pten+/fl; Pgrmc2fl/fl, Pten+/d, and Pten+/d; Pgrmc2d/d mice and observed an increase in endometrial mast cells in Pten+/d mice compared with Pten+/fl; Pgrmc2fl/fl control mice (Figure 7). The ablation of Pgrmc2 had no effect on mast cell recruitment, indicating that the ability of a Pgrmc2 deficiency to slow the progression of endometrial cancer on a Pten haploinsufficient background does not stem from changes in mast cell recruitment or degranulation.
5. Conclusions
Through the use of a well-established mouse model of Pten loss-of-function-induced endometrioid endometrial carcinoma, this study demonstrates that the loss of Pgrmc2 attenuates endometrial hyperplasia and cancer incidence and severity, in part, by inhibiting endometrial glandular epithelial cell proliferation. These findings on a member of the PGRMC family conducted using a mouse model of endometrial cancer advance our prior studies demonstrating that the knockdown of PGRMC1 in a human endometrial xenograft tumor attenuated tumor development, growth, and progression in vivo [31], a finding also supported by ovarian and breast cancer xenograft studies [30,32].
Author Contributions
The study was conceptualized by N.C.K., C.A.P., J.J.P. and J.K.P., supervised by J.J.K., J.J.P. and J.K.P., written by N.C.K. and J.K.P., and a draft manuscript edited to final version by N.C.K., C.A.P., S.P., J.P.L., J.J.K., J.J.P. and J.K.P. Studies were conducted and analyzed by N.C.K., C.A.P., S.P., J.J.K., J.J.P. and J.K.P. Reagents were provided by J.P.L., J.J.K., J.J.P. and J.K.P. Funding was procured by N.C.K., J.J.K., J.J.P. and J.K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NIH grants OD016564 (J.J.P. and J.K.P.), HD102386 and HD086402 (J.K.P.), R01CA155513 (J.J.K.), and NCATS/UH3TR00120 (J.J.K.), as well as the NSF grant GRFP1347973 (N.C.K.), the USDA (WYO-657-24 and WYO-632-22; J.K.P.), and the Curtis and Marian Rochelle Endowment (J.K.P.).
Institutional Review Board Statement
All animal protocols were approved by the Institutional Animal Care and Use Committee at Washington State University or the University of Wyoming. Our animal protocol number is 2022-0148 and the most recent approval data is 1 October 2025.
Informed Consent Statement
Approval and informed consent were obtained from all subjects involved in the study [53].
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors have no conflicts of interest.
References
- Available online: https://www.cancer.org/cancer/types/endometrial-cancer/about/key-statistics.html (accessed on 1 January 2025).
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [PubMed]
- Hollander, M.C.; Blumenthal, G.M.; Dennis, P.A. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat. Rev. Cancer 2011, 11, 289–301. [Google Scholar] [PubMed]
- Wallace, A.E.; Gibson, D.A.; Saunders, P.T.K.; Jabbour, H.N. Inflammatory events in endometrial adenocarcinoma. J. Endocrinol. 2010, 206, 141–157. [Google Scholar] [PubMed]
- Kong, D.; Suzuki, A.; Zou, T.T.; Sakurada, A.; Kemp, L.W.; Wakatsuki, S.; Yokoyama, T.; Yamakawa, H.; Furukawa, T.; Sato, M.; et al. PTEN1 is frequently mutated in primary endometrial carcinomas. Nat. Genet. 1997, 17, 143–144. [Google Scholar]
- Daikoku, T.; Hirota, Y.; Tranguch, S.; Joshi, A.R.; DeMayo, F.J.; Lydon, J.P.; Ellenson, L.H.; Dey, S.K. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008, 68, 5619–5627. [Google Scholar]
- Daikoku, T.; Jackson, L.; Besnard, V.; Whitsett, J.; Ellenson, L.H.; Dey, S.K. Cell-specific conditional deletion of Pten in the uterus results in differential phenotypes. Gynecol. Oncol. 2011, 122, 424–429. [Google Scholar]
- Podsypanina, K.; Ellenson, L.H.; Nemes, A.; Gu, J.; Tamura, M.; Yamada, K.M.; Cordon-Cardo, C.; Catoretti, G.; Fisher, P.E.; Parsons, R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl. Acad. Sci. USA 1999, 96, 1563–1568. [Google Scholar]
- Cheng, H.; Liu, P.; Zhang, F.; Xu, E.; Symonds, L.; Ohlson, C.E.; Bronson, R.T.; Maira, S.M.; Di Tomaso, E.; Li, J.; et al. A genetic mouse model of invasive endometrial cancer driven by concurrent loss of Pten and Lkb1 is highly responsive to mTOR inhibition. Cancer Res. 2014, 74, 15–23. [Google Scholar]
- Wang, X.; Wendel, R.H.; Emerson, R.E.; Broaddus, R.R.; Creighton, C.J.; Rusch, D.B.; Buechlein, A.; DeMayo, F.J.; Lydon, J.P.; Hawkins, S.M. Pten and Dicer1 loss in the mouse uterus causes poorly differentiated endometrial adenocarcinoma. Oncogene 2020, 39, 6286–6299. [Google Scholar]
- Terakawa, J.; Serna, V.A.; Taketo, M.M.; Daikoko, T.; Suarez, A.A.; Kurita, T. Ovarian insufficiency and CTNNB1 mutations drive malignant transformation of endometrial hyperplasia with altered PTEN/PI3K activities. Proc. Natl. Acad. Sci. USA 2019, 116, 4528–4537. [Google Scholar]
- Falkenstein, E.; Meyer, C.; Eisen, C.; Scriba, P.C.; Wehling, M. Full-length cDNA sequence of progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem. Biophys. Res. Comm. 1996, 229, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, D.; Wehling, M.; Leube, B.; Falkenstein, E. Cloning and tissue expression of two putative steroid membrane receptors. Biol. Chem. 1998, 379, 907–911. [Google Scholar] [PubMed]
- Meyer, C.; Schmid, R.; Scriba, P.C.; Wehling, M. Purification and partial sequencing of high-affinity progesterone-binding sites(s) from porcine liver membranes. Eur. J. Biochem. 1996, 239, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Peluso, J.J.; Pru, J.K. Non-canonical progesterone signaling in granulosa cells function. Reproduction 2014, 147, R169–R178. [Google Scholar] [CrossRef]
- Clark, N.C.; Pru, C.A.; Yee, S.P.; Lydon, J.P.; Peluso, J.J.; Pru, J.K. Conditional ablation of progesterone receptor membrane component 2 causes female premature reproductive senescence. Endocrinology 2016, 158, 640–651. [Google Scholar] [CrossRef]
- McCallum, M.L.; Pru, C.A.; Niikura, Y.; Yee, S.P.; Lydon, J.P.; Peluso, J.J.; Pru, J.K. Conditional ablation of progesterone receptor membrane component 1 results in subfertility in the female and development of endometrial cysts. Endocrinology 2016, 157, 3309–3319. [Google Scholar] [CrossRef]
- Lodde, V.; Barros, R.G.; Terzaghi, L.; Franciosi, F.; Luciano, A.M. Insights on the role of PGRMC1 in mitotic and meiotic cell division. Cancers 2022, 14, 5755. [Google Scholar] [CrossRef]
- Sabbir, M.G. Progesterone induced Warburg effect in HEK293 cells is associated with post-translational modifications and proteasomal degradation of progesterone receptor membrane component 1. J. Steroid Biochem. Mol. Biol. 2019, 191, 105376. [Google Scholar] [CrossRef]
- Xu, X.; Ruan, X.; Wang, Z.; Yang, Y.; Cheng, J.; Gu, M.; Mueck, A.O. Progesterone receptor membrane component-1 may promote survival of human brain microvascular endothelial cells in Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Demen 2022, 37, 15333175221109749. [Google Scholar] [CrossRef]
- Ahmed, I.S.; Rohe, H.J.; Twist, K.E.; Craven, R.J. Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity. J. Biol. Chem. 2010, 285, 24775–24782. [Google Scholar] [CrossRef]
- Rohe, H.J.; Ahmed, I.S.; Twist, K.E.; Craven, R.J. PGRMC1 (progesterone receptor membrane component 1): A targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacol. Ther. 2009, 121, 14–19. [Google Scholar] [PubMed]
- Galmozzi, A.; Kok, B.P.; Kim, A.S.; Montenegro-Burke, J.; Lee, J.Y.; Spreafico, R.; Mosure, R.; Albert, V.; Cintron-Colon, R.; Godio, C.; et al. PGRMC2 is an intracellular haem chaperone critical for adipocyte function. Nature 2019, 576, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Runko, E.; Kaprielian, Z. Caenorhabditis elegans VEM-1, a novel membrane protein, regulates the guidance of ventral nerve cord-associated axons. J. Neurosci. 2004, 24, 9015–9026. [Google Scholar] [PubMed]
- Pru, J.K.; Clark, N.C. PGRMC1 and PGRMC2 in uterine physiology and disease. Front. Neurosci. 2013, 19, 168. [Google Scholar]
- Pru, J.K. Pleiotropic actions of PGRMC proteins in cancer. Endocrinology 2022, 163, bpac078. [Google Scholar]
- Peluso, J.J.; Pru, J.K. Progesterone receptor membrane component (PGRMC) 1 and PGRMC2 and their roles in ovarian and endometrial cancer. Cancers 2021, 13, 5953. [Google Scholar] [CrossRef]
- Crudden, G.; Loesel, R.; Craven, R.J. Overexpression of the cytochrome P450 activator hpr6(heme-1 domain protein/human progestrone receptor) in tumors. Tumour Biol. 2005, 26, 142–146. [Google Scholar]
- Peluso, J.J.; Liu, X.; Saunders, M.M.; Claffey, K.P.; Phoenix, K. Regulation of ovarian cancer cell viability and sensitivity to cisplatin by progesterone receptor membrae component-1. J. Clin. Endocrinol. Metab 2008, 93, 1592–1599. [Google Scholar] [CrossRef]
- Peluso, J.J.; Gawkowska, A.; Liu, X.; Shioda, T.; Pru, J.K. Progesterone receptor membrane component-1 regulates the development of cisplatin sensitivity of human ovarian tumors in athymic nude mice. Endocrinology 2009, 150, 4846–4854. [Google Scholar]
- Friel, A.M.; Zhang, L.; Pru, C.A.; Clark, N.C.; McCallum, M.L.; Blok, L.J.; Shioda, T.; Peluso, J.J.; Rueda, B.R.; Pru, J.K. Progesterone receptor membrane component 1 deficiency attenuates growth while promoting chemosensitivity of human endometrial xenograft tumors. Cancer Lett. 2015, 356, 434–443. [Google Scholar]
- Clark, N.C.; Friel, A.M.; Pru, C.A.; Zhang, L.; Shioda, T.; Rueda, B.R.; Peluso, J.J.; Pru, J.K. Progesterone receptor membrane component 1 promotes survival of human breast cancer cells and the growth of xenograft tumors. Cancer Biol. Ther. 2016, 17, 262–271. [Google Scholar] [PubMed]
- Amirrad, F.; La, V.; Ohadi, S.; Albotaif, M.; Webster, S.; Pru, J.K.; Shamloo, K.; Mohiedlin, A.M.; Nauli, S.M. PGRMC2 is a pressure-volume regulator critical for myocardial responses to stress in mice. Nat. Commun. 2025, 16, 2422. [Google Scholar] [PubMed]
- Yokouchi-Konishi, T.; Liu, Y.; Feng, L. Progesterone receptor membrane component 2 is critical for human placental extravillous trophoblast invasion. Biol. Reprod 2023, 109, 759–771. [Google Scholar] [PubMed]
- Lintao, R.C.V.; Richardson, L.S.; Kammala, A.K.; Chapa, J.; Yunque-Yap, D.A.; Khanipov, K.; Golovko, G.; Dalmacio, L.M.M.; Menon, R. PGRMC2 and HLA-G regulate immune homeostasis in a microphysiological model of human maternal-fetal membrane interface. Commun. Biol. 2024, 7, 1041. [Google Scholar]
- De Cristofano, A.; Ellenson, L.H. Endometrial carcinoma. Annu. Rev. Pathol. 2007, 2, 57–85. [Google Scholar]
- Stambolic, V.; Tsao, M.S.; Macpherson, D.; Suzuki, A.; Chapman, W.B.; Mak, T.W. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/- mice. Cancer Res. 2000, 60, 3605–3611. [Google Scholar]
- Lesche, R.; Groszer, M.; Gao, J.; Wang, Y.; Messing, A.; Sun, H.; Liu, X.; Wu, H. Cre/loxP-mediated inactivation of the murine Pten gene. Genesis 2002, 32, 148–159. [Google Scholar]
- Soyal, S.M.; Mukherjee, A.; Lee, K.Y.; Li, J.; Li, H.; DeMayo, F.J.; Lydon, J.P. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005, 41, 58–66. [Google Scholar]
- Peluso, J.J.; Pru, C.A.; Liu, X.; Kelp, N.C.; Pru, J.K. Progesterone receptor membrane component 1 and 2 regulate granulosa cell mitosis and survival through a NFKB-dependent mechanism. Biol. Reprod 2019, 100, 1571–1580. [Google Scholar]
- Fan, X.; Kraynak, J.; Kinsely, J.P.S.; Formenti, S.C.; Shen, W.H. PTEN as a guardian of the genome: Pathways and targets. Cold Spring Harb Perspect. Med. 2020, 10, a036194. [Google Scholar]
- Brault, V.; Besson, V.; Magnol, L.; Duchon, A.; Herault, Y. Cre/loxP-mediated chromosome engineering of the mouse genome. Handb. Exp. Pharmacol. 2007, 178, 29–48. [Google Scholar]
- Lin, Y.G.; Shen, J.; Yoo, E.; Liu, R.; Yen, H.Y.; Mehta, A.; Rajaei, A.; Yang, W.; Mhawech-Fauceglia, P.; DeMayo, F.J.; et al. Targeting the glucose-regulated protein-78 abrogates Pten-null driven AKT activation and endometrioid tumorigenesis. Oncogene 2015, 34, 5418–5426. [Google Scholar] [PubMed]
- Pan, J.; Cheng, L.; Bi, X.; Zhang, X.; Liu, S.; Bai, X.; Li, F.; Zhao, A.Z. Elevation of omega-3 polyunsaturated fatty acids attenuates PTEN-deficiency induced endometrial cancer development through regulation of COX-2 and PGE2 production. Sci. Rep. 2015, 5, 14958. [Google Scholar]
- Kim, T.H.; Yoo, J.Y.; Kim, H.I.; Gilbert, J.; Ku, B.J.; Li, J.; Mills, G.B.; Broaddus, R.R.; Lydon, J.P.; Lim, J.M.; et al. Mig-6 suppresses endometrial cancer associated with Pten deficiency and ERK activation. Cancer Res. 2014, 74, 7371–7382. [Google Scholar]
- Kim, T.H.; Franco, H.L.; Jung, S.Y.; Qin, J.; Broddus, R.R.; Lydon, J.P.; Jeong, J.W. The synergistic effect of Mig-6 on Pten ablation on endometrial cancer development and progression. Oncogene 2010, 29, 3770–3780. [Google Scholar]
- Lindberg, M.E.; Stodden, G.R.; King, M.L.; MacLean, J.A., 2nd; Mann, J.L.; DeMayo, F.J.; Lydon, J.P.; Hayashi, K. Loss of CDH1 and Pten accelerates cellular invasiveness and angiogenesis in the mouse uterus. Biol. Reprod. 2013, 89, 8. [Google Scholar]
- Ito, K.; Utsunomiya, H.; Yaegashi, N.; Sasano, H. Biological roles of estrogen and progesterone in human endometrial carcinoma--new developments in potential endocrine therapy for endometrial cancer. Endocr. J. 2007, 54, 667–679. [Google Scholar]
- Yang, C.H.; Almomen, A.; Wee, Y.S.; Jarboe, E.A.; Peterson, C.M.; Janat-Amsbury, M.M. An estrogen-induced endometrial hyperplasia mouse model recapitulating human disease progression and genetic aberrations. Cancer Med. 2015, 4, 1039–1050. [Google Scholar]
- Yang, S.; Thiel, K.W.; Leslie, K.K. Progesterone: The ultimate endometrial tumor suppressor. Trends Endocrinol. Metab 2011, 22, 145–152. [Google Scholar]
- Segura-Villablobos, D.; Ramirez-Moreno, I.; Martinex-Aguilar, M.; Ibarra-Sanchez, A.; Munoz-Bello, J.O.; Anaya-Rubio, I.; Padilla, A.; Macias-Silvia, M.; Lizano, M.; Gonzalez-Espinosa, C. Mast cell-tumor interactions: Molecular mechanisms of recruitment, intratumoral communication and potential therapeutic targets for tumor growth. Cells 2022, 11, 249. [Google Scholar] [CrossRef]
- Ma, Y.; Kroemer, G. The cancer-immune dialogue in the context of stress. Nat. Rev. Immunol. 2024, 24, 264–281. [Google Scholar] [CrossRef]
- Peevey, J.F.; Seagle, B.L.L.; Manair, K.P.; Kim, J.J. Association of body mass index with ER, PR, and 14-3-3σ expression in tumor and stroma of type I and type II endometrial carcinoma. Oncotarget 2017, 8, 42548–42559. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).