Fixed-Dose Versus Weight-Adapted Immune Checkpoint Inhibitor Therapy in Melanoma: A Retrospective Monocentric Analysis of Efficacy and Immune-Related Adverse Events
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
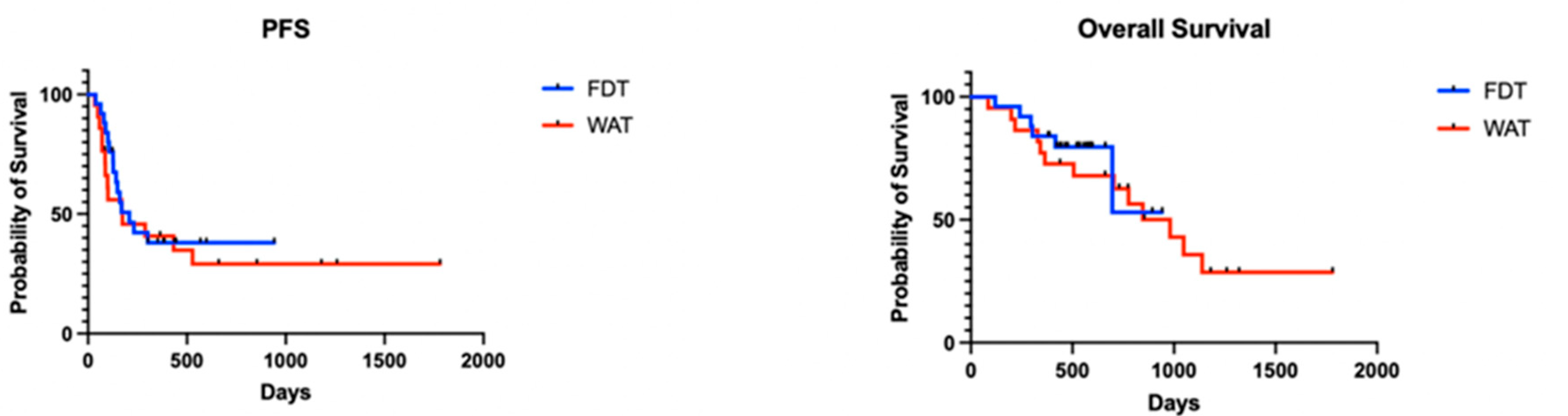
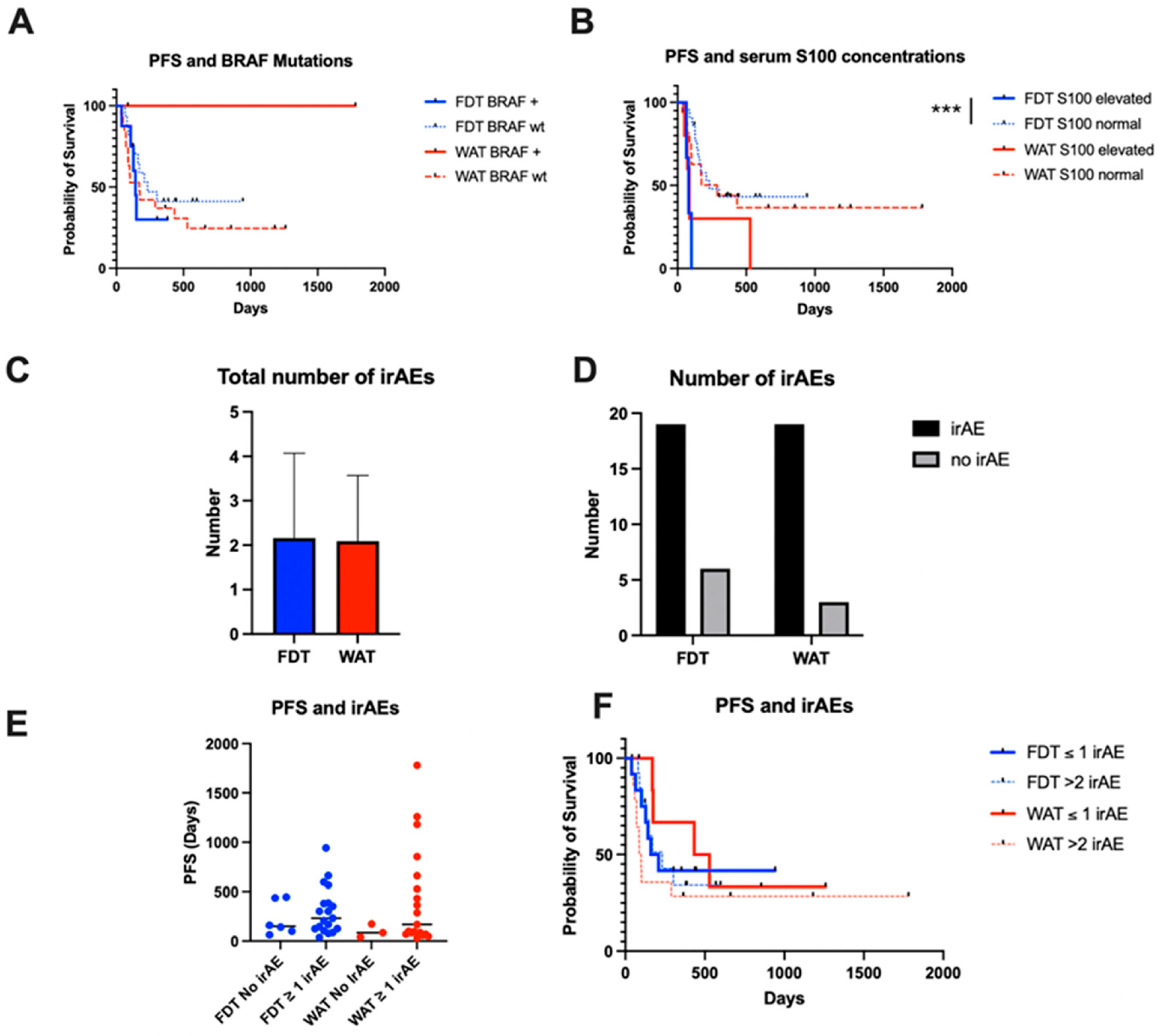
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terheyden, P.; Krackhardt, A.; Eigentler, T. The Systemic Treatment of Melanoma. Dtsch. Arztebl. Int. 2019, 116, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Rutkowski, P.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Queirolo, P.; Dummer, R.; Butler, M.O.; Hill, A.G.; et al. Final, 10-Year Outcomes with Nivolumab Plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2025, 392, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Willsmore, Z.N.; Coumbe, B.G.T.; Crescioli, S.; Reci, S.; Gupta, A.; Harris, R.J.; Chenoweth, A.; Chauhan, J.; Bax, H.J.; McCraw, A.; et al. Combined Anti-Pd-1 and Anti-Ctla-4 Checkpoint Blockade: Treatment of Melanoma and Immune Mechanisms of Action. Eur. J. Immunol. 2021, 51, 544–556. [Google Scholar] [CrossRef] [PubMed]
- van Zeijl, M.C.T.; van Breeschoten, J.; de Wreede, L.C.; Wouters, M.; Hilarius, D.L.; Blank, C.U.; Aarts, M.J.B.; van den Berkmortel, F.; de Groot, J.W.B.; Hospers, G.A.P.; et al. Real-world Outcomes of Ipilimumab Plus Nivolumab Combination Therapy in a Nation-Wide Cohort of Advanced Melanoma Patients in the Netherlands. J. Immunother. 2023, 46, 197–204. [Google Scholar] [CrossRef]
- Gide, T.N.; Wilmott, J.S.; Scolyer, R.A.; Long, G.V. Primary and Acquired Resistance to Immune Checkpoint Inhibitors in Metastatic Melanoma. Clin. Cancer Res. 2018, 24, 1260–1270. [Google Scholar] [CrossRef]
- Zaremba, A.; Eggermont, A.M.M.; Robert, C.; Dummer, R.; Ugurel, S.; Livingstone, E.; Ascierto, P.A.; Long, G.V.; Schadendorf, D.; Zimmer, L. The Concepts of Rechallenge and Retreatment with Immune Checkpoint Blockade in Melanoma Patients. Eur. J. Cancer 2021, 155, 268–280. [Google Scholar] [CrossRef]
- Kahler, K.C.; Kosova, K.; Bohne, A.S.; Schreiber, S.; Hauschild, A. Increased Risk of Immune Checkpoint Inhibitor-Induced Type 1 Diabetes Mellitus with the New Approved 6-Week Scheme of Pembrolizumab in Patients with Melanoma? Eur. J. Cancer 2020, 138, 169–171. [Google Scholar] [CrossRef]
- Freshwater, T.; Kondic, A.; Ahamadi, M.; Li, C.H.; De Greef, R.; De Alwis, D.; Stone, J.A. Evaluation of Dosing Strategy for Pembrolizumab for Oncology Indications. J. Immunother. Cancer 2017, 5, 43. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.-J.; Chiarion-Sileni, V.; et al. Adjuvant Nivolumab Versus Ipilimumab in Resected Stage IIIB-C and Stage IV Melanoma (CheckMate 238): 4-Year Results from a Multicentre, Double-Blind, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Ogungbenro, K.; Patel, A.; Duncombe, R.; Nuttall, R.; Clark, J.; Lorigan, P. Dose Rationalization of Pembrolizumab and Nivolumab Using Pharmacokinetic Modeling and Simulation and Cost Analysis. Clin. Pharmacol. Ther. 2018, 103, 582–590. [Google Scholar] [CrossRef]
- Elassaiss-Schaap, J.; Rossenu, S.; Lindauer, A.; Kang, S.P.; de Greef, R.; Sachs, J.R.; de Alwis, D.P. Using Model-Based “Learn and Confirm” to Reveal the Pharmacokinetics-Pharmacodynamics Relationship of Pembrolizumab in the Keynote-001 Trial. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 21–28. [Google Scholar] [CrossRef]
- Schadendorf, D.; Wolchok, J.D.; Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Chesney, J.; et al. Efficacy and Safety Outcomes in Patients with Advanced Melanoma Who Discontinued Treatment with Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. J. Clin. Oncol. 2017, 35, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of Body-Mass Index and Outcomes in Patients with Metastatic Melanoma Treated with Targeted Therapy, Immunotherapy, or Chemotherapy: A Retrospective, Multicohort Analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Cheun, H.; Kim, M.; Lee, H.; Oh, K.H.; Keam, B. Safety and Efficacy of Immune Checkpoint Inhibitors for End-Stage Renal Disease Patients Undergoing Dialysis: A Retrospective Case Series and Literature Review. Investig. New Drugs 2019, 37, 579–583. [Google Scholar] [CrossRef]
- Klee, G.; Hagelstein, V.; Kurzhals, J.K.; Zillikens, D.; Terheyden, P.; Langan, E.A. Dacarbazine in the Management of Metastatic Melanoma in the Era of Immune Checkpoint Therapy: A Valid Option or Obsolete? Melanoma Res. 2022, 32, 360–365. [Google Scholar] [CrossRef]
- Nebhan, C.A.; Cortellini, A.; Ma, W.; Ganta, T.; Song, H.; Ye, F.; Irlmeier, R.; Debnath, N.; Saeed, A.; Radford, M.; et al. Clinical Outcomes and Toxic Effects of Single-Agent Immune Checkpoint Inhibitors among Patients Aged 80 Years or Older with Cancer: A Multicenter International Cohort Study. JAMA Oncol. 2021, 7, 1856–1861. [Google Scholar] [CrossRef]
- Wong, S.K.; Blum, S.M.; Sun, X.; Da Silva, I.P.; Zubiri, L.; Ye, F.; Bai, K.; Zhang, K.; Ugurel, S.; Zimmer, L.; et al. Efficacy and Safety of Immune Checkpoint Inhibitors in Young Adults with Metastatic Melanoma. Eur. J. Cancer 2023, 181, 188–197. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer Immunotherapy Efficacy and Patients’ Sex: A Systematic Review and Meta-Analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Jang, S.R.; Nikita, N.; Banks, J.; Keith, S.W.; Johnson, J.M.; Wilson, M.; Lu-Yao, G. Association between Sex and Immune Checkpoint Inhibitor Outcomes for Patients with Melanoma. JAMA Netw. Open 2021, 4, e2136823. [Google Scholar] [CrossRef]
- Roccuzzo, G.; Moirano, G.; Fava, P.; Maule, M.; Ribero, S.; Quaglino, P. Obesity and Immune-Checkpoint Inhibitors in Advanced Melanoma: A Meta-Analysis of Survival Outcomes from Clinical Studies. Semin. Cancer Biol. 2023, 91, 27–34. [Google Scholar] [CrossRef]
- Yeung, C.; Kartolo, A.; Holstead, R.; Moffat, G.T.; Hanna, L.; Hopman, W.; Baetz, T. No Association between BMI and Immunotoxicity or Clinical Outcomes for Immune Checkpoint Inhibitors. Immunotherapy 2022, 14, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Yekeduz, E.; Koksoy, E.B.; Yazgan, S.C.; Karatas, G.; Senler, F.C.; Utkan, G.; Akbulut, H.; Demirkazik, A.; Urun, Y. Chronic Hyperglycemia Based on Diabetes Is Independently Associated with Decreased Survival in Patients with Advanced Cancer Treated with Immune Checkpoint Inhibitors. Anticancer. Drugs 2022, 33, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; D’Alessio, A.; Cleary, S.; Buti, S.; Bersanelli, M.; Bordi, P.; Tonini, G.; Vincenzi, B.; Tucci, M.; Russo, A.; et al. Type 2 Diabetes Mellitus and Efficacy Outcomes from Immune Checkpoint Blockade in Patients with Cancer. Clin. Cancer Res. 2023, 29, 2714–2724. [Google Scholar] [CrossRef] [PubMed]
- Agostinetto, E.; Ceppi, M.; Bruzzone, M.; Lambertini, M.; de Azambuja, E. Response to letter entitled: Re: Cardiotoxicity of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. Eur. J. Cancer 2021, 155, 303–306. [Google Scholar] [CrossRef]
- Agostinetto, E.; Eiger, D.; Lambertini, M.; Ceppi, M.; Bruzzone, M.; Ponde, N.; Plummer, C.; Awada, A.H.; Santoro, A.; Piccart-Gebhart, M.; et al. Cardiotoxicity of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. Eur. J. Cancer 2021, 148, 76–91. [Google Scholar] [CrossRef]
- Wagner, N.B.; Forschner, A.; Leiter, U.; Garbe, C.; Eigentler, T.K. S100B and LDH as Early Prognostic Markers for Response and Overall Survival in Melanoma Patients Treated with Anti-Pd-1 or Combined Anti-Pd-1 Plus Anti-Ctla-4 Antibodies. Br. J. Cancer 2018, 119, 339–346. [Google Scholar] [CrossRef]
- Heppt, M.V.; Heinzerling, L.; Kahler, K.C.; Forschner, A.; Kirchberger, M.C.; Loquai, C.; Meissner, M.; Meier, F.; Terheyden, P.; Schell, B.; et al. Prognostic Factors and Outcomes in Metastatic Uveal Melanoma Treated with Programmed Cell Death-1 or Combined Pd-1/Cytotoxic T-Lymphocyte Antigen-4 Inhibition. Eur. J. Cancer 2017, 82, 56–65. [Google Scholar] [CrossRef]
- van Not, O.J.; Blokx, W.A.M.; van den Eertwegh, A.J.M.; de Meza, M.M.; Haanen, J.B.; Blank, C.U.; Aarts, M.J.B.; van den Berkmortel, F.; de Groot, J.W.B.; Hospers, G.A.P.; et al. BRAF and NRAS Mutation Status and Response to Checkpoint Inhibition in Advanced Melanoma. JCO Precis. Oncol. 2022, 6, e2200018. [Google Scholar] [CrossRef]
- Murciano-Goroff, Y.R.; Pak, T.; Mondaca, S.; Flynn, J.R.; Montecalvo, J.; Rekhtman, N.; Halpenny, D.; Plodkowski, A.J.; Wu, S.L.; Kris, M.G.; et al. Immune Biomarkers and Response to Checkpoint Inhibition of Braf(V600) and Braf Non-V600 Altered Lung Cancers. Br. J. Cancer 2022, 126, 889–898. [Google Scholar] [CrossRef]
- Vasudevan, H.N.; Delley, C.; Chen, W.C.; Mirchia, K.; Pan, S.; Shukla, P.; Aabedi, A.A.; Nguyen, M.P.; Morshed, R.A.; Young, J.S.; et al. Molecular Features of Resected Melanoma Brain Metastases, Clinical Outcomes, and Responses to Immunotherapy. JAMA Netw. Open 2023, 6, e2329186. [Google Scholar] [CrossRef]
- Blum, S.M.; Rouhani, S.J.; Sullivan, R.J. Effects of Immune-Related Adverse Events (irAEs) and Their Treatment on Antitumor Immune Responses. Immunol. Rev. 2023, 318, 167–178. [Google Scholar] [CrossRef]
- Hussaini, S.; Chehade, R.; Boldt, R.G.; Raphael, J.; Blanchette, P.; Maleki Vareki, S.; Fernandes, R. Association between Immune-Related Side Effects and Efficacy and Benefit of Immune Checkpoint Inhibitors—A Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2021, 92, 102134. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xie, W.; Huang, H.; Wang, Y.; Li, G.; Geng, Y.; Hao, Y.; Zhang, Z. Association of Immune Related Adverse Events with Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Cancers: A Systemic Review and Meta-Analysis. Front. Oncol. 2021, 11, 633032. [Google Scholar] [CrossRef]
- Skudalski, L.; Waldman, R.; Kerr, P.E.; Grant-Kels, J.M. Melanoma: An Update on Systemic Therapies. J. Am. Acad. Dermatol. 2022, 86, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Suryawanshi, S.; Hruska, M.; Feng, Y.; Wang, X.; Shen, J.; Vezina, H.E.; McHenry, M.B.; Waxman, I.M.; Achanta, A.; et al. Assessment of Nivolumab Benefit-Risk Profile of a 240-Mg Flat Dose Relative to a 3-Mg/Kg Dosing Regimen in Patients with Advanced Tumors. Ann. Oncol. 2017, 28, 2002–2008. [Google Scholar] [CrossRef]
- Long, G.V.; Tykodi, S.S.; Schneider, J.G.; Garbe, C.; Gravis, G.; Rashford, M.; Agrawal, S.; Grigoryeva, E.; Bello, A.; Roy, A.; et al. Assessment of Nivolumab Exposure and Clinical Safety of 480 Mg Every 4 Weeks Flat-Dosing Schedule in Patients with Cancer. Ann. Oncol. 2018, 29, 2208–2213. [Google Scholar] [CrossRef]
- Bei, D.; Osawa, M.; Uemura, S.; Ohno, T.; Gobburu, J.; Roy, A.; Hasegawa, M. Benefit-Risk Assessment of Nivolumab 240 Mg Flat Dose Relative to 3 Mg/Kg Q2w Regimen in Japanese Patients with Advanced Cancers. Cancer Sci. 2020, 111, 528–535. [Google Scholar] [CrossRef]
- Lala, M.; Li, T.R.; de Alwis, D.P.; Sinha, V.; Mayawala, K.; Yamamoto, N.; Siu, L.L.; Chartash, E.; Aboshady, H.; Jain, L. A Six-Weekly Dosing Schedule for Pembrolizumab in Patients with Cancer Based on Evaluation Using Modelling and Simulation. Eur. J. Cancer 2020, 131, 68–75. [Google Scholar] [CrossRef]
- Hirsch, J.S.; Wanchoo, R.; Ng, J.H.; Khanin, Y.; Jhaveri, K.D. Use of Immune Checkpoint Inhibitors in End Stage Kidney Disease Patients, Single Center Experience and Review of the Literature. Kidney360 2020, 1, 399–402. [Google Scholar] [CrossRef]
- Kitchlu, A.; Jhaveri, K.D.; Sprangers, B.; Yanagita, M.; Wanchoo, R. Immune Checkpoint Inhibitor use in Patients with End-Stage Kidney Disease: An Analysis of Reported Cases and Literature Review. Clin. Kidney J. 2021, 14, 2012–2022. [Google Scholar] [CrossRef]
- Le Brun, I.C.; Dalle, S.; Mortier, L.; Dereure, O.; Rat, S.D.; Dutriaux, C.; Leccia, M.T.; Legoupil, D.; Montaudie, H.; De Quatrebarbes, J.; et al. Methods of Nivolumab Administration in Advanced Melanoma: A Comparison of Patients’ Clinical Outcomes Treated with Flat Dose or Weight-Adjusted Dose, a Multicenter Observational Study. Cancer 2025, 131, e35679. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.C.; Chen, G.M.; Wang, Y.; Yuan, S.Q.; Zhou, J.; Duan, J.L.; Liu, W.W.; Chen, S.; Cai, M.Y.; Li, Y.F. Association between Body Mass Index and Survival Outcomes in Patients Treated with Immune Checkpoint Inhibitors: Meta-Analyses of Individual Patient Data. J. Immunother. 2021, 44, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Kichenadasse, G.; Miners, J.O.; Mangoni, A.A.; Rowland, A.; Hopkins, A.M.; Sorich, M.J. Association between Body Mass Index and Overall Survival with Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2020, 6, 512–518. [Google Scholar] [CrossRef]
- Incorvaia, L.; Dimino, A.; Algeri, L.; Brando, C.; Magrin, L.; De Luca, I.; Pedone, E.; Perez, A.; Sciacchitano, R.; Bonasera, A.; et al. Body Mass Index and Baseline Platelet Count as Predictive Factors in Merkel Cell Carcinoma Patients Treated with Avelumab. Front. Oncol. 2023, 13, 1141500. [Google Scholar] [CrossRef]
- Bastacky, M.L.; Wang, H.; Fortman, D.; Rahman, Z.; Mascara, G.P.; Brenner, T.; Najjar, Y.G.; Luke, J.J.; Kirkwood, J.M.; Zarour, H.M.; et al. Immune-Related Adverse Events in Pd-1 Treated Melanoma and Impact Upon Anti-Tumor Efficacy: A Real World Analysis. Front. Oncol. 2021, 11, 749064. [Google Scholar] [CrossRef]
- McQuade, J.L.; Hammers, H.; Furberg, H.; Engert, A.; Andre, T.; Blumenschein, G., Jr.; Tannir, N.; Baron, A.; Larkin, J.; El-Khoueiry, A.; et al. Association of Body Mass Index with the Safety Profile of Nivolumab with or Without Ipilimumab. JAMA Oncol. 2023, 9, 102–111. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Santini, D.; Buti, S.; Tiseo, M.; Cannita, K.; Perrone, F.; Giusti, R.; De Tursi, M.; Zoratto, F.; et al. Another Side of the Association between Body Mass Index (BMI) and Clinical Outcomes of Cancer Patients Receiving Programmed Cell Death Protein-1 (Pd-1)/Programmed Cell Death-Ligand 1 (Pd-L1) Checkpoint Inhibitors: A Multicentre Analysis of Immune-Related Adverse Events. Eur. J. Cancer 2020, 128, 17–26. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, D.; Ahn, J.S.; Guallar, E.; Cho, J.; Lee, H.Y. Obesity Paradox in Patients with Non-Small Cell Lung Cancer Undergoing Immune Checkpoint Inhibitor Therapy. J. Cachexia Sarcopenia Muscle 2023, 14, 2898–2907. [Google Scholar] [CrossRef]
- Langan, E.A.; Gratz, V.; Billmann, F.; Zillikens, D.; Terheyden, P. Does the Gastrointestinal Microbiome Contribute to the ‘Obesity Paradox’ in Melanoma Survival? Br. J. Dermatol. 2018, 179, 225–226. [Google Scholar] [CrossRef]
- Hahn, A.W.; Venkatesh, N.; Msaouel, P.; McQuade, J.L. The Influence of Obesity on Outcomes with Immune Checkpoint Blockade: Clinical Evidence and Potential Biological Mechanisms. Cells 2023, 12, 2551. [Google Scholar] [CrossRef]
- Najjar, Y.G.; Menk, A.V.; Sander, C.; Rao, U.; Karunamurthy, A.; Bhatia, R.; Zhai, S.; Kirkwood, J.M.; Delgoffe, G.M. Tumor Cell Oxidative Metabolism as a Barrier to Pd-1 Blockade Immunotherapy in Melanoma. JCI Insight 2019, 4, 124989. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer Immunotherapy by Ctla-4 Blockade Relies on the Gut Microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Mengoni, M.; Braun, A.D.; Hinnerichs, M.S.; Tuting, T.; Surov, A. Subcutaneous Fat Abundance and Density Are Associated with an Enhanced Response to Immunotherapy in Metastatic Melanoma: A Retrospective Cohort Study. Acad. Radiol. 2023, 30 (Suppl. S1), S257–S267. [Google Scholar] [CrossRef]
- Lee, J.H.; Hyung, S.; Lee, J.; Choi, S.H. Visceral Adiposity and Systemic Inflammation in the Obesity Paradox in Patients with Unresectable or Metastatic Melanoma Undergoing Immune Checkpoint Inhibitor Therapy: A Retrospective Cohort Study. J. Immunother. Cancer 2022, 10, e005226. [Google Scholar] [CrossRef]
- Donnelly, D.; Bajaj, S.; Yu, J.; Hsu, M.; Balar, A.; Pavlick, A.; Weber, J.; Osman, I.; Zhong, J. The Complex Relationship between Body Mass Index and Response to Immune Checkpoint Inhibition in Metastatic Melanoma Patients. J. Immunother. Cancer 2019, 7, 222. [Google Scholar] [CrossRef]
- Naik, G.S.; Waikar, S.S.; Johnson, A.E.W.; Buchbinder, E.I.; Haq, R.; Hodi, F.S.; Schoenfeld, J.D.; Ott, P.A. Complex Inter-Relationship of Body Mass Index, Gender and Serum Creatinine on Survival: Exploring the Obesity Paradox in Melanoma Patients treated with checkpoint inhibition. J. Immunother. Cancer 2019, 7, 89. [Google Scholar] [CrossRef]
- Di Filippo, Y.; Dalle, S.; Mortier, L.; Dereure, O.; Dalac, S.; Dutriaux, C.; Leccia, M.T.; Legoupil, D.; Saiag, P.; Brunet-Possenti, F.; et al. Relevance of Body Mass Index as a Predictor of Systemic Therapy Outcomes in Metastatic Melanoma: Analysis of the Melbase French Cohort Data. Ann. Oncol. 2021, 32, 542–551. [Google Scholar] [CrossRef]
- Chatziioannou, E.; Leiter, U.; Thomas, I.; Keim, U.; Seeber, O.; Meiwes, A.; Boessenecker, I.; Gonzalez, S.S.; Torres, F.M.; Niessner, H.; et al. Features and Long-Term Outcomes of Stage IV Melanoma Patients Achieving Complete Response Under Anti-PD-1-Based Immunotherapy. Am. J. Clin. Dermatol. 2023, 24, 453–467. [Google Scholar] [CrossRef]
- Simetic, L.; Blazicevic, K.; Medugorac, K.; Golcic, M.; Herceg, D. Relative Change in S100 as a Biomarker of Survival in Patients With Metastatic Melanoma Treated with Pembrolizumab. Anticancer. Res. 2020, 40, 2157–2163. [Google Scholar] [CrossRef]
- Gebhardt, C.; Lichtenberger, R.; Utikal, J. Biomarker Value and Pitfalls of Serum S100b in the Follow-Up of High-Risk Melanoma Patients. J. Dtsch. Dermatol. Ges. 2016, 14, 158–164. [Google Scholar] [CrossRef]
- Burgermeister, S.; Gabrys, H.S.; Basler, L.; Hogan, S.A.; Pavic, M.; Bogowicz, M.; Martinez Gomez, J.M.; Vuong, D.; Tanadini-Lang, S.; Foerster, R.; et al. Improved Survival Prediction by Combining Radiological Imaging and S-100B Levels into a Multivariate Model in Metastatic Melanoma Patients Treated with Immune Checkpoint Inhibition. Front. Oncol. 2022, 12, 830627. [Google Scholar] [CrossRef]
- Kurzhals, J.K.; Klee, G.; Hagelstein, V.; Zillikens, D.; Terheyden, P.; Langan, E.A. Disease Recurrence During Adjuvant Immune Checkpoint Inhibitor Treatment in Metastatic Melanoma: Clinical, Laboratory, and Radiological Characteristics in Patients from a Single Tertiary Referral Center. Int. J. Mol. Sci. 2022, 23, 10723. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Mandala, M.; Ferrucci, P.F.; Guidoboni, M.; Rutkowski, P.; Ferraresi, V.; Arance, A.; Guida, M.; Maiello, E.; Gogas, H.; et al. Sequencing of Ipilimumab Plus Nivolumab and Encorafenib Plus Binimetinib for Untreated Braf-Mutated Metastatic Melanoma (SECOMBIT): A Randomized, Three-Arm, Open-Label Phase II Trial. J. Clin. Oncol. 2023, 41, 212–221. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.G.; Moon, H.H.; Davar, D.; O’Rourke, M.; Stephenson, J.J.; et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial-ECOG-ACRIN EA6134. J. Clin. Oncol. 2023, 41, 186–197. [Google Scholar] [CrossRef]
- Byrne, M.M.; Lucas, M.; Pai, L.; Breeze, J.; Parsons, S.K. Immune-Related Adverse Events in Cancer Patients Being Treated with Immune Checkpoint Inhibitors. Eur. J. Haematol. 2021, 107, 650–657. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Amoroso, V.; Gallo, F.; Alberti, A.; Paloschi, D.; Ferrari Bravo, W.; Esposito, A.; Cosentini, D.; Grisanti, S.; Pedersini, R.; Petrelli, F.; et al. Immune-Related Adverse Events as Potential Surrogates of Immune Checkpoint Inhibitors’ efficacy: A Systematic Review and Meta-Analysis of Randomized Studies. ESMO Open 2023, 8, 100787. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, K.; Wan, G.; Nguyen, N.; Lu, C.; Ugwu-Dike, P.; Raval, N.; Seo, J.; Alexander, N.A.; Jairath, R.; et al. Cutaneous Immune-Related Adverse Events Are Associated with Longer Overall Survival in Advanced Cancer Patients on Immune Checkpoint Inhibitors: A Multi-Institutional Cohort Study. J. Am. Acad. Dermatol. 2023, 88, 1024–1032. [Google Scholar] [CrossRef]
- Leroy, M.; Desmedt, E.; Deramoudt, L.; Vasseur, M.; Odou, P.; Behal, H.; Decaudin, B.; Mortier, L.; Simon, N. Retrospective Comparison of a Weight-Based Dose Every 2 Weeks with a Fixed Dose Every Month: A Real-Life Analysis of Nivolumab in the Treatment of Advanced Melanoma. Melanoma Res. 2024, 34, 258–264. [Google Scholar] [CrossRef]
- Ständer, H.F. A “real-world” retrospective analysis of efficacy and adverse events in weight-dependent versus fixed-dose immune checkpoint therapy in metastatic melanoma. J. Dtsch. Dermatol. Ges. 2023, 21, 1–139. [Google Scholar]


| Fixed Dose Therapy (FDT) | Weight Adapted Therapy (WAT) | |
|---|---|---|
| Total number of patients | ||
| Number | 25 | 22 |
| Sex | ||
| Male | 17 | 15 |
| Female | 8 | 7 |
| Age (Years) | ||
| <70 | 11 | 5 |
| ≥70 | 14 | 17 |
| Mutation status | ||
| BRAF | 8 | 2 |
| NRAS | 9 | 9 |
| cKit | 1 | 2 |
| ECOG Status | ||
| 0 | 14 | 19 |
| 1 | 10 | 3 |
| 2 | 1 | 0 |
| Melanoma Stage | ||
| IIc | 0 | 2 |
| III | 16 | 10 |
| IV | 9 | 10 |
| S100 elevated | ||
| Yes | 3 | 5 |
| no | 22 | 17 |
| Cardiovascular Disease | ||
| Yes | 10 | 10 |
| No | 15 | 12 |
| Diabetes | ||
| Yes | 7 | 6 |
| no | 18 | 16 |
| Boby Mass Index | ||
| <25 | 10 | 4 |
| ≥25 | 15 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staender, H.F.; Langan, E.A. Fixed-Dose Versus Weight-Adapted Immune Checkpoint Inhibitor Therapy in Melanoma: A Retrospective Monocentric Analysis of Efficacy and Immune-Related Adverse Events. Cancers 2025, 17, 1147. https://doi.org/10.3390/cancers17071147
Staender HF, Langan EA. Fixed-Dose Versus Weight-Adapted Immune Checkpoint Inhibitor Therapy in Melanoma: A Retrospective Monocentric Analysis of Efficacy and Immune-Related Adverse Events. Cancers. 2025; 17(7):1147. https://doi.org/10.3390/cancers17071147
Chicago/Turabian StyleStaender, Hans F., and Ewan Andrew Langan. 2025. "Fixed-Dose Versus Weight-Adapted Immune Checkpoint Inhibitor Therapy in Melanoma: A Retrospective Monocentric Analysis of Efficacy and Immune-Related Adverse Events" Cancers 17, no. 7: 1147. https://doi.org/10.3390/cancers17071147
APA StyleStaender, H. F., & Langan, E. A. (2025). Fixed-Dose Versus Weight-Adapted Immune Checkpoint Inhibitor Therapy in Melanoma: A Retrospective Monocentric Analysis of Efficacy and Immune-Related Adverse Events. Cancers, 17(7), 1147. https://doi.org/10.3390/cancers17071147





