Management of Cutaneous Squamous Cell Carcinoma of the Scalp in Kidney Transplant Recipients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
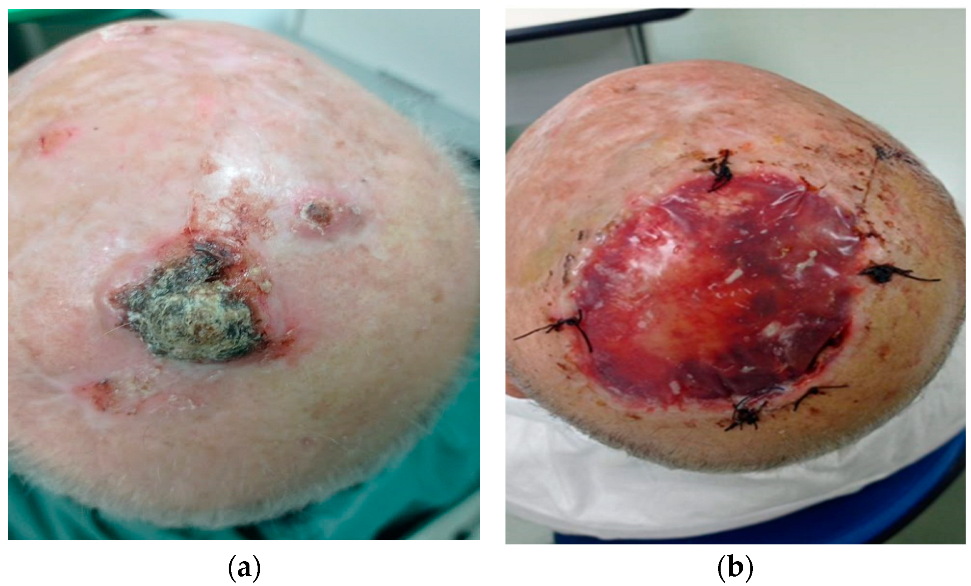
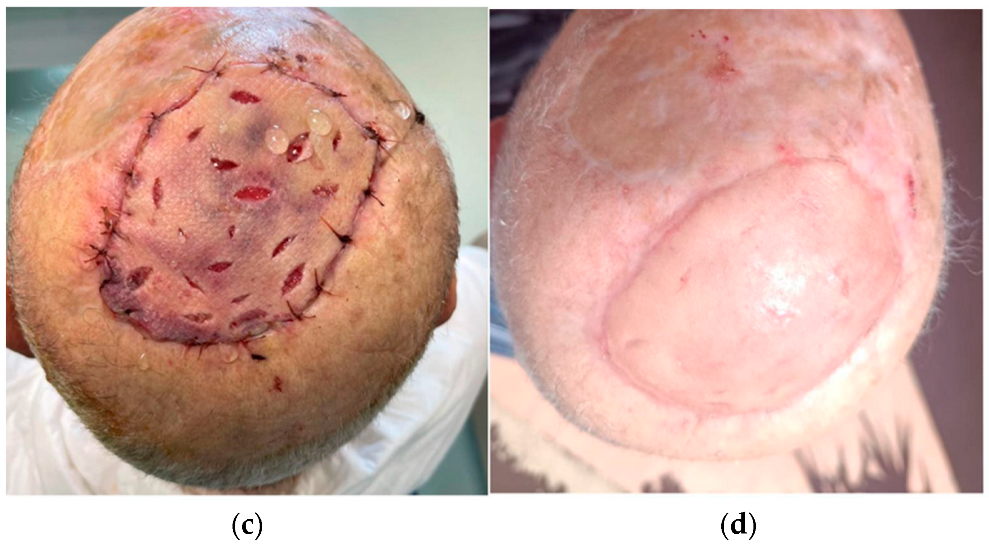
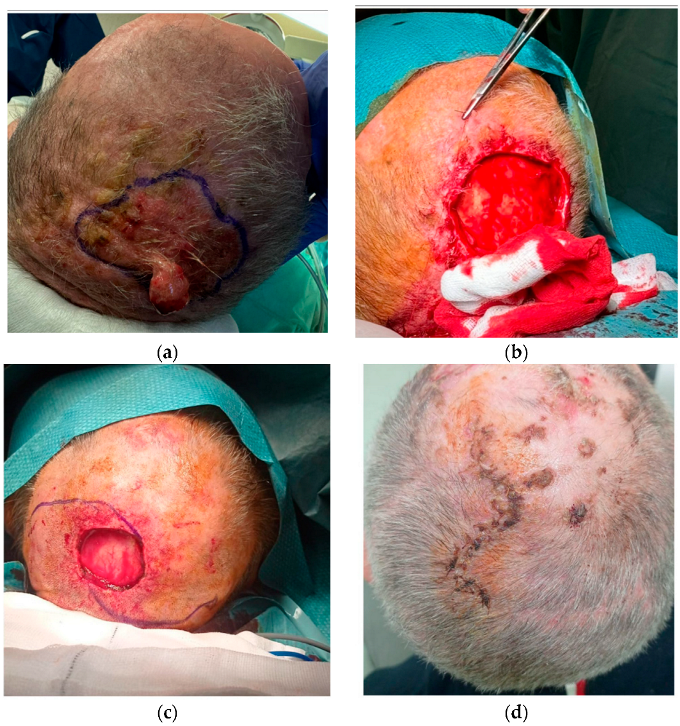
2.1. Surgical Techniques
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCC | Basal Cell Carcinoma |
| BWH | Brigham and women’s hospital |
| CNI | Calcineurin inhibitor |
| CTS | Computer Tomography Scan |
| cSCC | cutaneous Squamous Cell Carcinoma |
| ICIs | Immune Checkpoint Inhibitors |
| IS | Immunosuppressive |
| KTRs | Kidney Transplant Recipients |
| MMF | Mycophenolate Mofetil |
| mTORi | mammalian target of rapamycin inhibitor |
| NMSC | Non Melanoma skin cancer |
References
- Burke, M.T.; Isbel, N.; Barraclough, K.A.; Jung, J.W.; Wells, J.W.; Staatz, C.E. Genetics and nonmelanoma skin cancer in kidney transplant recipients. Pharmacogenomics 2015, 16, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.B.; Walker, R.; Tai, S.S.; Jiang, Q.; Russ, G.R. Randomized controlled trial of sirolimus for renal transplant recipients at high risk for nonmelanoma skin cancer. Am. J. Transplant. 2012, 12, 1146–1156. [Google Scholar] [PubMed]
- Thoms, K.M.; Kuschal, C.; Oetjen, E.; Mori, T.; Kobayashi, N.; Laspe, P.; Boeckmann, L.; Schön, M.P.; Emmert, S. Cyclosporin A, but not everolimus, inhibits DNA repair mediated by calcineurin: Implications for tumorigenesis under immunosuppression. Exp. Dermatol. 2011, 20, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.P.; Segundo, D.S.; Hollowood, K.; Marafioti, T.; Clark, T.G.; Harden, P.N.; Wood, K.J. Immune phenotype predicts risk for posttransplantation squamous cell carcinoma. J. Am. Soc. Nephrol. 2010, 21, 713–722. [Google Scholar] [CrossRef]
- Berg, D.; Otley, C.C. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J. Am. Acad. Dermatol. 2002, 47, 1–20. [Google Scholar] [PubMed]
- Hao, X.; Lai, W.; Xia, X.; Xu, J.; Wu, Y.; Lv, C.; Meng, Q.; Lv, K.; Huang, S.; Luo, Z.; et al. Skin cancer outcomes and risk factors in renal transplant recipients: Analysis of organ procurement and transplantation network data from 2000 to 2021. Front. Oncol. 2022, 12, 1017498. [Google Scholar]
- Martinez, J.C.; Otley, C.C. The management of melanoma and nonmelanoma skin cancer: A review for the primary care physician. Mayo. Clin. Proc. 2001, 76, 1253–1265. [Google Scholar]
- Ramsay, H.M.; Fryer, A.A.; Hawley, C.M.; Smith, A.G.; Harden, P.N. Non-melanoma skin cancer risk in the Queensland renal transplant population. Br. J. Dermatol. 2002, 147, 950–956. [Google Scholar] [CrossRef]
- Carucci, J.A. Management of squamous cell carcinoma in organ transplant recipients. Semin. Cutan. Med. Surg. 2003, 22, 177–186. [Google Scholar]
- Lott, D.G.; Manz, R.; Koch, C.; Lorenz, R.R. Aggressive behavior of nonmelanotic skin cancers in solid organ transplant recipients. Transplantation 2010, 90, 683–687. [Google Scholar]
- Kim, C.; Cheng, J.; Colegio, O.R. Cutaneous squamous cell carcinomas in solid organ transplant recipients: Emerging strategies for surveillance, staging, and treatment. Semin. Oncol. 2016, 43, 390–394. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly Zwald, F.; Brown, M. Skin cancer in solid organ transplant recipients: Advances in therapy and management: Part II. Management of skin cancer in solid organ transplant recipients. J. Am. Acad. Dermatol. 2011, 65, 263–279. [Google Scholar] [CrossRef]
- Brodland, D.G.; Zitelli, J.A. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 1992, 27, 241–248. [Google Scholar] [CrossRef]
- Ehrl, D.; Brueggemann, A.; Broer, P.N.; Koban, K.; Giunta, R.; Thon, N. Scalp Reconstruction after Malignant Tumor Resection: An Analysis and Algorithm. J. Neurol. Surg. B Skull. Base 2020, 81, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Rodio, M.; Tettamanzi, M.; Trignano, E.; Rampazzo, S.; Serra, P.L.; Grieco, F.; Boccaletti, R.; Veneziani Santonio, F.; Fadda, G.M.; Sanna, F.; et al. Multidisciplinary Management of Cutaneous Squamous Cell Carcinoma of the Scalp: An Algorithm for Reconstruction and Treatment. J. Clin. Med. 2024, 13, 1581. [Google Scholar] [CrossRef]
- Chaiyasate, K.; Oliver, L.N.; Kreitzberg, S.A.; Lyons, M.; Goldman, J.; Lu, S.M.; Bastiaans, T.; Lumley, C.; Sachanandani, N.S. Use of Pericranial Flaps with Dermal Substitute for Scalp Reconstruction: A Case Series. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3011. [Google Scholar] [CrossRef]
- The American Joint Committee on Cancer (AJCC). AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S., Greene, F., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; Springer International Publishing: Chicago, IL, USA, 2017. [Google Scholar]
- Union for International Cancer Control (UICC). TNM Classification of Malignant Tumours, 8th ed.; Brierley, J.D., Gospodarowicz, M.K., Wittekind, C., Eds.; John Wiley and Sons: Oxford, UK, 2017. [Google Scholar]
- Jambusaria-Pahlajani, A.; Kanetsky, P.A.; Karia, P.S.; Hwang, W.T.; Gelfand, J.M.; Whalen, F.M.; Elenitsas, R.; Xu, X.; Schmults, C.D. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013, 149, 402–410. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; van Akkooi, A.; Bataille, V.; Bastholt, L.; Dreno, B.; Dummer, R.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for invasive cutaneous squamous cell carcinoma. Part 1: Diagnostics and prevention-Update. Eur. J. Cancer 2023, 193, 113251. [Google Scholar] [PubMed]
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef]
- Bakker, D.; Bakker, W.J.; Bekkenk, M.W.; Luiten, R.M. Immunity against Non-Melanoma Skin Cancer and the Effect of Immunosuppressive Medication on Non-Melanoma Skin Cancer Risk in Solid Organ Transplant Recipients. Cells 2023, 12, 2441. [Google Scholar] [CrossRef]
- Willenbrink, T.J.; Jambusaria-Pahlajani, A.; Arron, S.; Seckin, D.; Harwood, C.A.; Proby, C.M. Treatment approaches in immunosuppressed patients with advanced cutaneous squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. 8), 57–60. [Google Scholar] [CrossRef]
- Ferrándiz-Pulido, C.; Leiter, U.; Harwood, C.; Proby, C.M.; Guthoff, M.; Scheel, C.H.; Westhoff, T.H.; Bouwes Bavinck, J.N.; Meyer, T.; Nägeli, M.C.; et al. Immune Checkpoint Inhibitors in Solid Organ Transplant Recipients with Advanced Skin Cancers-Emerging Strategies for Clinical Management. Transplantation 2023, 107, 1452–1462. [Google Scholar] [PubMed]
- Trignano, E.; Tettamanzi, M.; Rampazzo, S.; Trignano, C.; Boccaletti, R.; Fadda, G.M.; Sanna, F.; Bussu, F.; Cossu, A.; Rubino, C. Squamous cell carcinoma of the scalp: A combination of different therapeutic strategies. Case. Rep. Plast. Surg. Hand. Surg. 2023, 10, 2210670. [Google Scholar]
- Youl, P.H.; Janda, M.; Aitken, J.F.; Del Mar, C.B.; Whiteman, D.C.; Baade, P.D. Body-site distribution of skin cancer, pre-malignant and common benign pigmented lesions excised in general practice. Br. J. Dermatol. 2011, 165, 35–43. [Google Scholar] [CrossRef]
- Andrade, P.; Brites, M.M.; Vieira, R.; Mariano, A.; Reis, J.P.; Tellechea, O.; Figueiredo, A. Epidemiology of basal cell carcinomas and squamous cell carcinomas in a Department of Dermatology: A 5 year review. An. Bras. Dermatol. 2012, 87, 212–219. [Google Scholar] [PubMed]
- TerKonda, R.P.; Sykes, J.M. Concepts in scalp and forehead reconstruction. Otolaryngol. Clin. N. Am. 1997, 30, 519–539. [Google Scholar]
- Millard, D.R. The crane principle for the transport of subcutaneous tissue. Plast. Reconstr. Surg. 1969, 43, 451–462. [Google Scholar]
- Ship, A.G.; Porter, V. Split-thickness scalp flap for resurfacing full thickness forehead and temporal scalp defects. Br. J. Plast. Surg. 1971, 24, 351–356. [Google Scholar]
- Wolfe, S.A. The crane principle revisited: Application in the reconstruction of compound frontal defects. Ann. Plast. Surg. 1984, 13, 327–334. [Google Scholar]
- Romagnoli, J.; Tagliaferri, L.; Acampora, A.; Bianchi, V.; D’Ambrosio, V.; D’Aviero, A.; Esposito, I.; Hohaus, S.; Iezzi, R.; Lancellotta, V.; et al. Management of the kidney transplant patient with Cancer: Report from a Multidisciplinary Consensus Conference. Transplant. Rev. 2021, 35, 100636. [Google Scholar]
- Schena, F.P.; Pascoe, M.D.; Alberu, J.; del Carmen Rial, M.; Oberbauer, R.; Brennan, D.C.; Campistol, J.M.; Racusen, L.; Polinsky, M.S.; Goldberg-Alberts, R.; et al. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation 2009, 87, 233–242. [Google Scholar] [PubMed]
- Knoll, G.A.; Kokolo, M.B.; Mallick, R.; Beck, A.; Buenaventura, C.D.; Ducharme, R.; Barsoum, R.; Bernasconi, C.; Blydt-Hansen, T.D.; Ekberg, H.; et al. Effect of sirolimus on malignancy and survival after kidney transplantation: Systematic review and meta-analysis of individual patient data. BMJ 2014, 349, g6679. [Google Scholar] [PubMed]
- Rousseau, B.; Guillemin, A.; Duvoux, C.; Neuzillet, C.; Tlemsani, C.; Compagnon, P.; Azoulay, D.; Salloum, C.; Laurent, A.; de la Taille, A.; et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int. J. Cancer 2019, 144, 886–896. [Google Scholar]
- Nashan, B.; Citterio, F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: A critical review of the literature. Transplantation 2012, 94, 547–561. [Google Scholar] [CrossRef] [PubMed]
| Variable | Patients (N = 7) |
|---|---|
| Age, years, median (range) | 67.86 (59–75) |
| Male sex, n (%) | 6 (85.7) |
| Caucasian ethnicity | 7 (100) |
| Original Kidney disease, n (%) | |
| Diabetes mellitus type II | 1 (14.3) |
| Glomerulonephritis | 2 (28.6) |
| ADPKD | 1 (14.3) |
| Unknown disease | 3 (42.9) |
| Pre-transplant malignancies, n (%) | |
| Previous cSCC | 1 (14.3) |
| None | 6 (85.7) |
| Comorbidities, n (%) | |
| Diabetes mellitus type II | 1 (14.3) |
| Arterial hypertension | 7 (100) |
| Duration of dialysis treatment prior to transplantation, months, mean (range) | 68 (17–96) |
| Time since kidney transplant, years, median (range) | 11 (2–20) |
| Immunosuppressive (IS) regimen before enrollment, n (%) | |
| Tacrolimus | 7 (100) |
| Everolimus | 2 (28.6) |
| MMF | 5 (71.4) |
| Prednisone | 7 (100) |
| Induction immunosuppression, n (%) | |
| Methylprednisolone 500 mg and Basiliximab 20 mg e.v. | 7 (100) |
| Immunosuppressive (IS) regimen after surgery, n (%) | |
| Tacrolimus | 7 (100) |
| Everolimus | 7 (100) |
| MMF | 0 (0) |
| Prednisone | 7 (100) |
| Histological type, n (%) | |
| Cutaneous Squamous cell carcinoma | 7 (100) |
| Tumor stage 1, n (%) | |
| pT1 | 3 (42.9) |
| pT2 | 1 (14.3) |
| pT3 | 3 (42.9) |
| pT4 | 0 |
| Nodal stage 2, n (%) | |
| N0 | 7 (100%) |
| Metastatic disease 2, n (%) | |
| M0 | 7 (100%) |
| BWH tumor classification, n (%) | |
| T1 0 Hight-risk factors 4 | 1 (14.3%) |
| T2 a 1 Hight-risk factors | 5 (71.4%) |
| T2 b 2−3 Hight-risk factors | 1 (14.3%) |
| T3 4 Hight-risk factors or bone invasion | 0 |
| Stage 3, n (%) | |
| Stage I | 3 (42.86%) |
| Stage II | 1 (14.3%) |
| Stage III | 3 (42.86%) |
| Stage IVA-IVB | 0 |
| Relapse or new scalp tumours, n (%) | 3 (42.86%) |
| Adjuvant radiation, n (%) | 1 (14.3%) |
| Recurrence (n = 2) | No Recurrence (n = 5) | p-Value | |
|---|---|---|---|
| Age ≥ 67 years-old | 1.000 * | ||
| Yes | 1 (50) | 3 (60) | |
| No | 1 (50) | 2 (40) | |
| Male sex, n (%) | 2 (100) | 4 (80) | 1.000 * |
| Time since kidney transplant ≥ 12 years | 1.000 * | ||
| Yes | 1 (50) | 3 (60) | |
| No | 1 (50) | 2 (40) | |
| Tumor stage, n (%) | 0.600 * | ||
| pT1 | 0 | 2 (50) | |
| pT2 | 0 | 1 (25) | |
| pT3 | 2 (100) | 1 (25) | |
| Immunosuppressive regimen witheverolimus before enrollment | 1/1 | 1/4 | 1.000 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, L.; Caponio, C.; Vistoli, F.; Lupi, E.; Fargnoli, M.C.; Esposito, M.; Lancione, L.; Bellobono, M.; Hassan, T.; Iacobelli, E.; et al. Management of Cutaneous Squamous Cell Carcinoma of the Scalp in Kidney Transplant Recipients. Cancers 2025, 17, 1113. https://doi.org/10.3390/cancers17071113
Romano L, Caponio C, Vistoli F, Lupi E, Fargnoli MC, Esposito M, Lancione L, Bellobono M, Hassan T, Iacobelli E, et al. Management of Cutaneous Squamous Cell Carcinoma of the Scalp in Kidney Transplant Recipients. Cancers. 2025; 17(7):1113. https://doi.org/10.3390/cancers17071113
Chicago/Turabian StyleRomano, Lucia, Chiara Caponio, Fabio Vistoli, Ettore Lupi, Maria Concetta Fargnoli, Maria Esposito, Laura Lancione, Manuela Bellobono, Tarek Hassan, Elisabetta Iacobelli, and et al. 2025. "Management of Cutaneous Squamous Cell Carcinoma of the Scalp in Kidney Transplant Recipients" Cancers 17, no. 7: 1113. https://doi.org/10.3390/cancers17071113
APA StyleRomano, L., Caponio, C., Vistoli, F., Lupi, E., Fargnoli, M. C., Esposito, M., Lancione, L., Bellobono, M., Hassan, T., Iacobelli, E., Semproni, L., & Panarese, A. (2025). Management of Cutaneous Squamous Cell Carcinoma of the Scalp in Kidney Transplant Recipients. Cancers, 17(7), 1113. https://doi.org/10.3390/cancers17071113






