Evaluating the Usefulness of the Blood Apolipoprotein A2 Isoform Index for Pancreatic Cancer Diagnosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Outcomes and Definitions
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
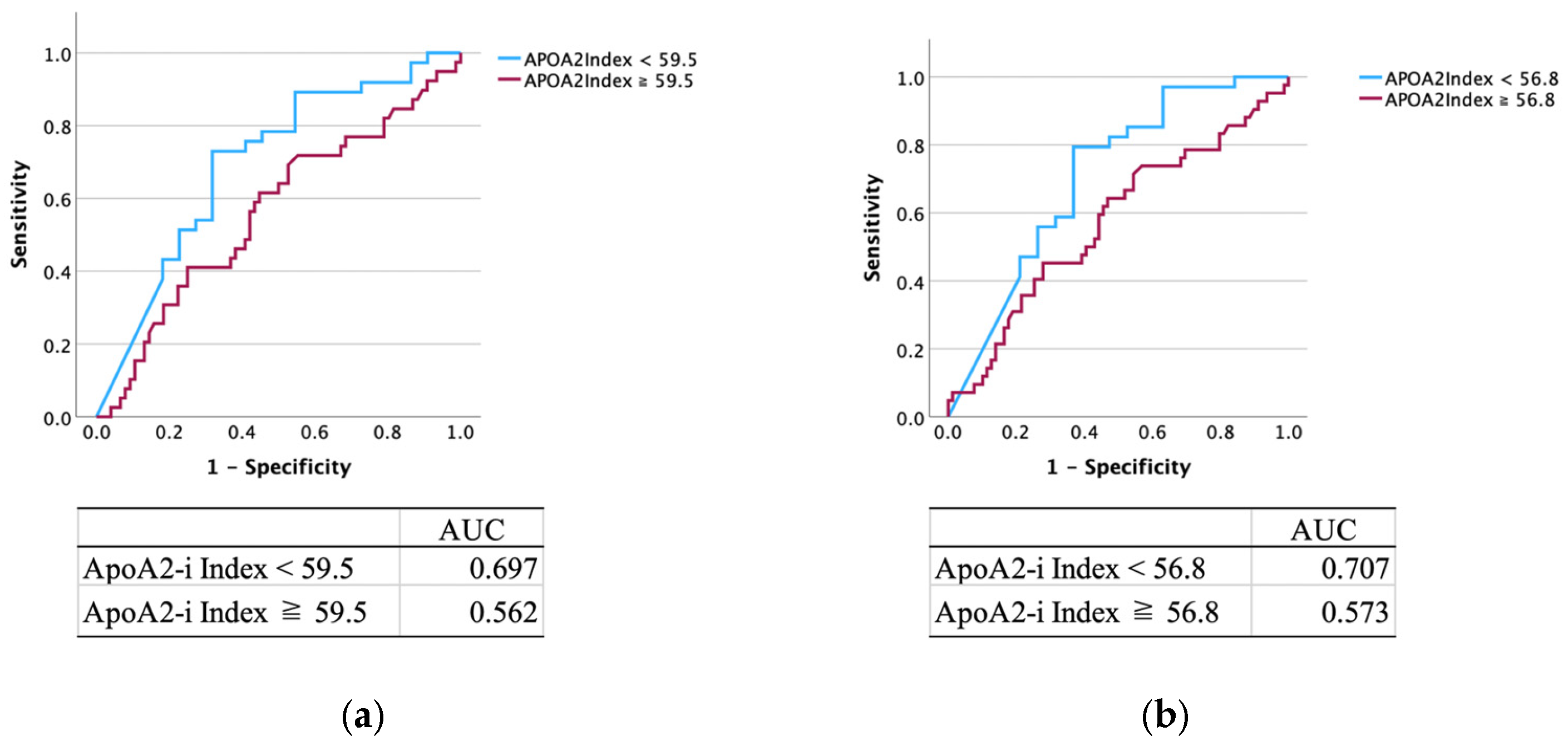
3.2. Diagnostic Performance of APOA2-i Index
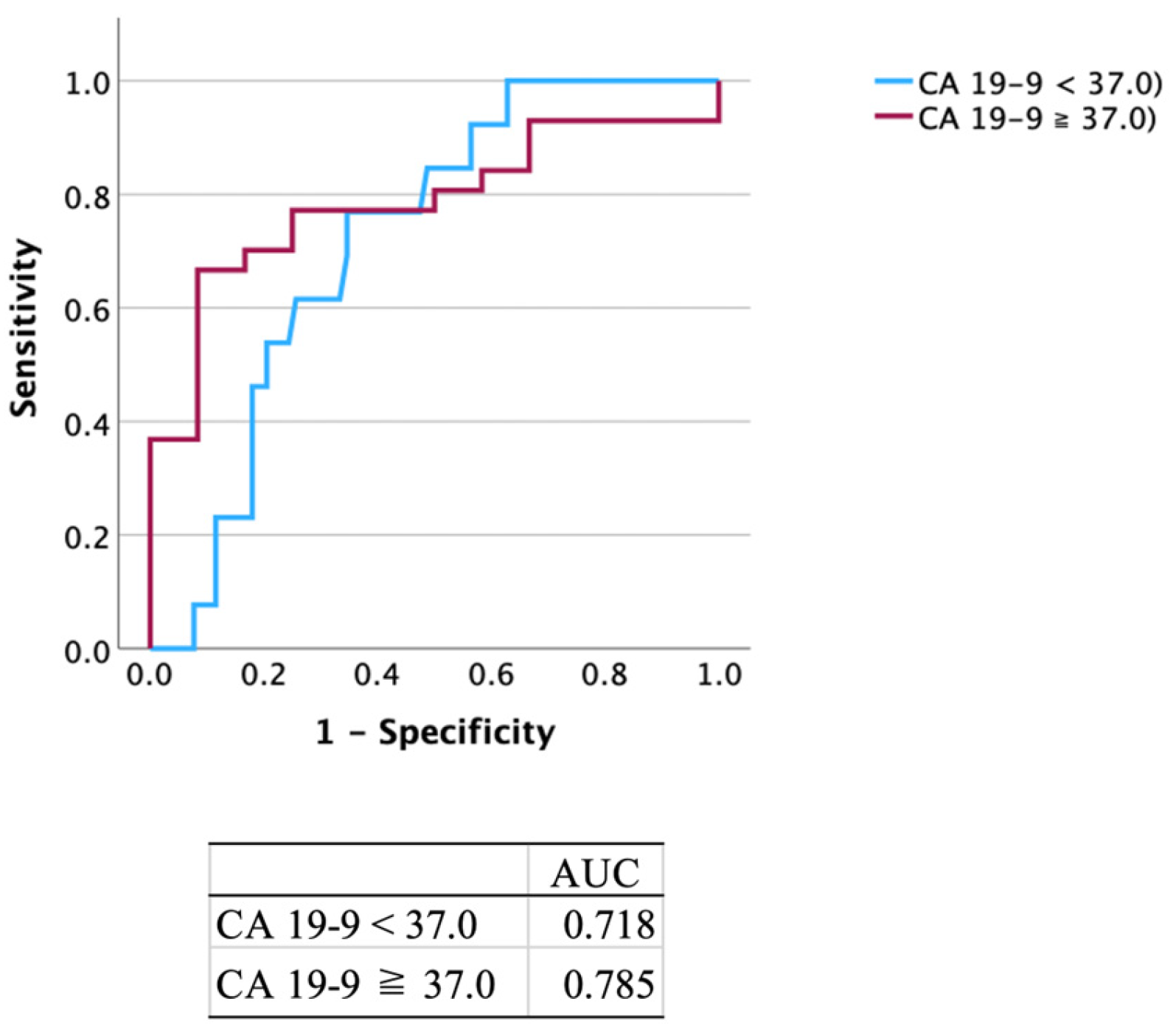
3.3. Diagnostic Performance of CA 19-9
3.4. Diagnostic Performance of Combination of APOA2-i Index with CA 19-9
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APOA2 | Apolipoprotein A2 |
| CA 19-9 | Carbohydrate antigen 19-9 |
| APOA2-i | Apolipoprotein A2-isoform |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
| OR | Odds ratio |
| PC | Pancreatic cancer |
| CI | Confidence interval |
| ROC | Receiver operating characteristic |
| EUS | Endoscopic ultrasound |
| IPMN | Intraductal papillary mucinous neoplasm |
| CP | Chronic pancreatitis |
| AUC | Area under the curve |
| CT | Computed tomography |
| MPD | Main pancreatic duct |
References
- Cai, J.; Chen, H.; Lu, M.; Zhang, Y.; Lu, B.; You, L.; Zhang, T.; Dai, M.; Zhao, Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021, 520, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Shi, J.; Amundadottir, L.T. Artificial intelligence and improved early detection for pancreatic cancer. Innovation 2023, 4, 100457. [Google Scholar] [CrossRef]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar]
- Koprowski, H.; Brockhaus, M.; Blaszczyk, M.; Magnani, J.; Steplewski, Z.; Ginsburg, V. Lewis blood-type may affect the incidence of gastrointestinal cancer. Lancet 1982, 1, 1332–1333. [Google Scholar] [CrossRef]
- Ritts, R.E.; Pitt, H.A. CA 19-9 in pancreatic cancer. Surg. Oncol. Clin. N. Am. 1998, 7, 93–101. [Google Scholar] [CrossRef]
- Goonetilleke, K.S.; Siriwardena, A.K. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. 2007, 33, 266–270. [Google Scholar] [CrossRef]
- Poruk, K.E.; Gay, D.Z.; Brown, K.; Mulvihill, J.D.; Boucher, K.M.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: Diagnostic and prognostic updates. Curr. Mol. Med. 2013, 13, 340–351. [Google Scholar] [CrossRef]
- Capello, M.; Bantis, L.E.; Scelo, G.; Zhao, Y.; Li, P.; Dhillon, D.S.; Patel, N.J.; Kundnani, D.L.; Wang, H.; Abbruzzese, J.L.; et al. Sequential validation of blood-based protein biomarker candidates for early-stage pancreatic cancer. J. Natl. Cancer Inst. 2017, 109, djw266. [Google Scholar] [CrossRef]
- Scarà, S.; Bottoni, P.; Scatena, R. CA 19-9: Biochemical and clinical aspects. Adv. Exp. Med. Biol. 2015, 867, 247–260. [Google Scholar] [CrossRef]
- Parra-Robert, M.; Santos, V.M.; Canis, S.M.; Pla, X.F.; Fradera, J.M.A.; Porto, R.M. Relationship between CA 19.9 and the Lewis phenotype: Options to improve diagnostic efficiency. Anticancer Res. 2018, 38, 5883–5888. [Google Scholar] [CrossRef] [PubMed]
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Honda, K. Risk stratification of pancreatic cancer by a blood test for apolipoprotein A2-isoforms. Cancer Biomark. 2022, 33, 503–512. [Google Scholar] [CrossRef]
- Kashiro, A.; Kobayashi, M.; Oh, T.; Miyamoto, M.; Atsumi, J.; Nagashima, K.; Takeuchi, K.; Nara, S.; Hijioka, S.; Morizane, C.; et al. Clinical development of a blood biomarker using apolipoprotein A2 isoforms for early detection of pancreatic cancer. J. Gastroenterol. 2024, 59, 263–278. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017. [Google Scholar]
- Berry, W.; Lundy, J.; Croagh, D.; Jenkins, B.J. Reviewing the utility of EUS FNA to advance precision medicine in pancreatic cancer. Cancer 2018, 10, 35. [Google Scholar] [CrossRef]
- Takahashi, K.; Yasuda, I.; Hanaoka, T.; Hayashi, Y.; Motoo, I.; Kajiura, S.; Ando, T.; Fujinami, H.; Tajiri, K.; Imura, J.; et al. Comparison of histological sample volumes among various endoscopic ultrasound-guided biopsy needles. J. Clin. Med. 2021, 10, 3560. [Google Scholar] [CrossRef]
- Versteijne, E.; van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: Long-term results of the Dutch randomized PREOPANC trial. J. Clin. Oncol. 2022, 40, 1220–1230. [Google Scholar] [CrossRef]
- Catalano, C.; Laghi, A.; Fraioli, F.; Pediconi, F.; Napoli, A.; Danti, M.; Reitano, I.; Passariello, R. Pancreatic carcinoma: The role of high-resolution multislice spiral CT in the diagnosis and assessment of resectability. Eur. Radiol. 2003, 13, 149–156. [Google Scholar] [CrossRef]
- Freeny, P.C.; Traverso, L.W.; Ryan, J.A. Diagnosis and staging of pancreatic adenocarcinoma with dynamic computed tomography. Am. J. Surg. 1993, 165, 600–606. [Google Scholar] [CrossRef]
- Gangi, S.; Fletcher, J.G.; Nathan, M.A.; Christensen, J.A.; Harmsen, W.S.; Crownhart, B.S.; Chari, S.T. Time interval between abnormalitiesseen on CT and the clinical diagnosis of pancreatic cancer: Retrospective review of CT scans obtained before diagnosis. AJR Am. J. Roentgenol. 2004, 182, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Kusumoto, S.; Nishikawa, K.; Hoshi, H.; Jinnouchi, S.; Kodama, T.; Koga, K.; Wakuta, Y.; Nakayama, S.; Kakitsubata, Y. Limitation of CT in diagnosis of pancreatic cancer. Radiat. Med. 1991, 9, 61–67. [Google Scholar]
- Laghi, A.; Iannaccone, R.; Catalano, C.; Carbone, I.; Sansoni, I.; Mangiapane, F.; Passariello, R. Multislice spiral computed tomography in diagnosis and staging of pancreatic carcinoma: Preliminary experience. Dig. Liver Dis. 2002, 34, 732–738. [Google Scholar] [CrossRef]
- Yamada, Y.; Mori, H.; Matsumoto, S.; Kiyosue, H.; Hori, Y.; Hongo, N. Pancreatic adenocarcinoma versus chronic pancreatitis: Differentiation with triple-phase helical CT. Abdom. Imaging 2010, 35, 163–171. [Google Scholar] [CrossRef]
- Bronstein, Y.L.; Loyer, E.M.; Kaur, H.; Choi, H.; David, C.; DuBrow, R.A.; Broemeling, L.D.; Cleary, K.R.; Charnsangavej, C. Detection of small pancreatic tumors with multiphasic helical CT. AJR Am. J. Roentgenol. 2004, 182, 619–623. [Google Scholar] [CrossRef]
- Shionoya, K.; Koizumi, K.; Masuda, S.; Makazu, M.; Kubota, J.; Jinushi, R.; Kimura, K. Difficulty in the diagnosis of pancreatic cancer based on the initial CT report: A retrospective study. Medicine 2024, 103, e36224. [Google Scholar] [CrossRef]
- Akahoshi, K.; Chijiiwa, Y.; Nakano, I.; Nawata, H.; Ogawa, Y.; Tanaka, M.; Nagai, E.; Tsuneyoshi, M. Diagnosis and staging of pancreatic cancer by endoscopic ultrasound. Br. J. Radiol. 1998, 71, 492–496. [Google Scholar] [CrossRef]
- DeWitt, J.; Devereaux, B.; Chriswell, M.; McGreevy, K.; Howard, T.; Imperiale, T.F.; Ciaccia, D.; Lane, K.A.; Maglinte, D.; Kopecky, K.; et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann. Intern. Med. 2004, 141, 753–763. [Google Scholar] [CrossRef]
- Kitano, M.; Kudo, M.; Maekawa, K.; Suetomi, Y.; Sakamoto, H.; Fukuta, N.; Nakaoka, R.; Kawasaki, T. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut 2004, 53, 854–859. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med. Biol. 2008, 34, 525–532. [Google Scholar] [CrossRef]
- Jemaa, Y.; Houissa, F.; Trabelsi, S.; Moussa, A.; Belhouchet, H.; Mouelhi, L.; Bouraoui, M.; Bouzaidi, S.; Debbeche, R.; Ben Yedder, J.; et al. Endoscopic ultrasonography versus helical CT in diagnosis and staging of pancreatic cancer. Tunis. Med. 2008, 86, 346–349. [Google Scholar] [PubMed]
- Matsubara, H.; Itoh, A.; Kawashima, H.; Kasugai, T.; Ohno, E.; Ishikawa, T.; Itoh, Y.; Nakamura, Y.; Hiramatsu, T.; Nakamura, M.; et al. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas 2011, 40, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Nakao, M.; Ioka, T.; Takakura, R.; Takano, Y.; Tsukuma, H.; Uehara, H.; Suzuki, R.; Fukuda, J. Slight dilatation of the main pancreaticduct and presence of pancreatic cysts as predictive signs of pancreatic cancer: A prospective study. Radiology 2010, 254, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Jun, E.; Okugawa, Y.; Toiyama, Y.; Borazanci, E.; Bolton, J.; Taketomi, A.; Kim, S.C.; Shang, D.; Von Hoff, D.; et al. A Circulating Panel of circRNA Biomarkers for the Noninvasive and Early Detection of Pancreatic Ductal Adenocarcinoma. Gastroenterology 2024, 166, 178–190.e16. [Google Scholar] [CrossRef]
- Asai, Y.; Itoi, T.; Sugimoto, M.; Sofuni, A.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Mukai, S.; Fujita, M.; et al. Elevated polyamines in saliva of pancreatic cancer. Cancers 2018, 10, 43. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Verbeke, C.; Salvia, R.; Klöppel, G.; Werner, J.; McKay, C.; Friess, H.; Manfredi, R.; Van Cutsem, E.; Löhr, M.; et al. European experts consensus statement on cystic tumours of the pancreas. Dig. Liver Dis. 2013, 45, 703–711. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Fernandez-Del Castillo, C.; Furukawa, T.; Hijioka, S.; Jang, J.Y.; Lennon, A.M.; Miyasaka, Y.; Ohno, E.; Salvia, R.; Wolfgang, C.L.; et al. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology 2024, 24, 255–270. [Google Scholar] [CrossRef]
| Age (Year) | Median (Year) [Range] | 70.5 [25–94] |
|---|---|---|
| Sex | Male | 99 |
| Female | 75 | |
| Diseases | PC | 76 |
| Intraductal papillary mucinous neoplasm | 36 | |
| Chronic pancreatitis | 33 | |
| Auto immune pancreatitis | 9 | |
| Pancreatic neuroendocrine neoplasm | 8 | |
| Others | 12 | |
| PC stages | Stage 0 | 5 |
| Stage I | 4 | |
| Stage II | 15 | |
| Stage III | 19 | |
| Stage IV | 33 | |
| Biomarker | ApoA2-I Index positive | 58 |
| CA 19-9 positive | 69 | |
| Either positive | 92 |
| ApoA2-i Index | CA 19-9 | Either Positive | ||
|---|---|---|---|---|
| Overall results | 33.3% (58/174) | 33.3% (58/174) | 39.7% (69/174) | 52.9% (92/174) |
| Age | 65 year > | 28.8% (19/66) | 34.8% (23/66) | 51.5% (34/66) |
| 65 year ≦ | 36.1% (39/108) | 39.8% (43/108) | 53.7% (58/108) | |
| p-value | 0.32 | 0.96 | 0.78 | |
| Sex | Male | 37.4% (37/99) | 35.4% (35/99) | 49.5% (49/99) |
| Female | 28% (21/75) | 45.3% (34/75) | 60% (45/75) | |
| p-value | 0.18 | 0.20 | 0.31 | |
| PC | PC cases | 48.7% (37/76) | 76.3% (58/76) | 84.2% (64/76) |
| non PC cases | 21.4% (21/98) | 11.2% (11/98) | 28.6% (28/98) | |
| p-value | <0.001 | <0.001 | <0.001 | |
| CP | CP cases | 24.2% (8/33) | 12.1% (4/33) | 30.3% (10/33) |
| non CP cases | 35.5% (50/141) | 46.1% (65/141) | 58.2% (82/141) | |
| p-value | 0.22 | <0.001 | 0.004 | |
| PC stage | Stage 0 or I | 33.3% (3/9) | 22.2% (2/9) | 44.4% (4/9) |
| Others | 33.3% (55/165) | 40.6% (67/165) | 53.3% (88/165) | |
| p-value | N/A | 0.27 | 0.60 | |
| Stage II to IV | 50.7% (34/67) | 83.6% (56/67) | 89.6% (60/67) | |
| Others | 22.4% (24/107) | 12.1% (13/107) | 29.9% (32/107) | |
| p-value | <0.001 | <0.001 | <0.001 |
| Sensitivity | Specificity | PPV | NPV | ||
|---|---|---|---|---|---|
| ApoA2-i Index | PC | 48.7% (37/76) | 78.6% (77/98) | 63.8% (37/58) | 66.4% (77/116) |
| Stage 0 or I | 33.3% (3/9) | 66.7% (110/165) | 5.2% (3/58) | 94.8% (110/116) | |
| Stage II to IV | 50.7% (34/67) | 77.6% (83/107) | 58.6% (34/58) | 71.6% (83/116) | |
| CA 19-9 | PC | 76.3% (58/76) | 88.8% (87/98) | 84.1% (58/69) | 82.9% (87/105) |
| Stage 0 or I | 22.2% (2/9) | 59.4% (98/165) | 2.9% (2/69) | 93.3% (98/105) | |
| Stage II to IV | 83.6% (56/67) | 87.9% (94/107) | 81.2% (56/69) | 89.5% (94/105) | |
| Either positive | PC | 84.2% (62/76) | 71.4% (70/98) | 69.6% (64/92) | 85.4% (70/82) |
| Stage 0 or I | 44.4% (4/9) | 46.7% (77/165) | 4.3% (4/92) | 93.9% (77/82) | |
| Stage II to IV | 89.6% (60/67) | 70.1% (75/107) | 65.2% (60/92) | 91.5% (75/82) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| ApoA2-i Index | p-Value | OR | 95% CI | p-Value |
| PC cases | <0.001 | 3.48 | 1.80–6.73 | <0.001 |
| Age | 0.50 | |||
| Sex | 0.17 | |||
| CP cases | 0.44 | |||
| CA 19-9 | ||||
| PC cases | <0.001 | 25.5 | 11.2–57.9 | <0.001 |
| Age | 0.33 | |||
| Sex | 0.088 | |||
| CP cases | 0.84 | |||
| Either positive | ||||
| PC cases | <0.001 | 13.3 | 6.26–28.4 | <0.001 |
| Age | 0.64 | |||
| Sex | 0.24 | |||
| CP cases | 0.79 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shionoya, K.; Sofuni, A.; Mukai, S.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Yamamoto, K.; Nagai, K.; Matsunami, Y.; Kojima, H.; et al. Evaluating the Usefulness of the Blood Apolipoprotein A2 Isoform Index for Pancreatic Cancer Diagnosis. Cancers 2025, 17, 1071. https://doi.org/10.3390/cancers17071071
Shionoya K, Sofuni A, Mukai S, Tsuchiya T, Tanaka R, Tonozuka R, Yamamoto K, Nagai K, Matsunami Y, Kojima H, et al. Evaluating the Usefulness of the Blood Apolipoprotein A2 Isoform Index for Pancreatic Cancer Diagnosis. Cancers. 2025; 17(7):1071. https://doi.org/10.3390/cancers17071071
Chicago/Turabian StyleShionoya, Kento, Atsushi Sofuni, Shuntaro Mukai, Takayoshi Tsuchiya, Reina Tanaka, Ryosuke Tonozuka, Kenjiro Yamamoto, Kazumasa Nagai, Yukitoshi Matsunami, Hiroyuki Kojima, and et al. 2025. "Evaluating the Usefulness of the Blood Apolipoprotein A2 Isoform Index for Pancreatic Cancer Diagnosis" Cancers 17, no. 7: 1071. https://doi.org/10.3390/cancers17071071
APA StyleShionoya, K., Sofuni, A., Mukai, S., Tsuchiya, T., Tanaka, R., Tonozuka, R., Yamamoto, K., Nagai, K., Matsunami, Y., Kojima, H., Minami, H., Hirakawa, N., Asano, K., Yamaguchi, Y., Hama, K., & Itoi, T. (2025). Evaluating the Usefulness of the Blood Apolipoprotein A2 Isoform Index for Pancreatic Cancer Diagnosis. Cancers, 17(7), 1071. https://doi.org/10.3390/cancers17071071







