Exploratory Study to Evaluate the Impact of Interim PET/CT Assessment in First-Line Follicular Lymphoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Analysis of PET/CT Imaging
2.3. Statistics
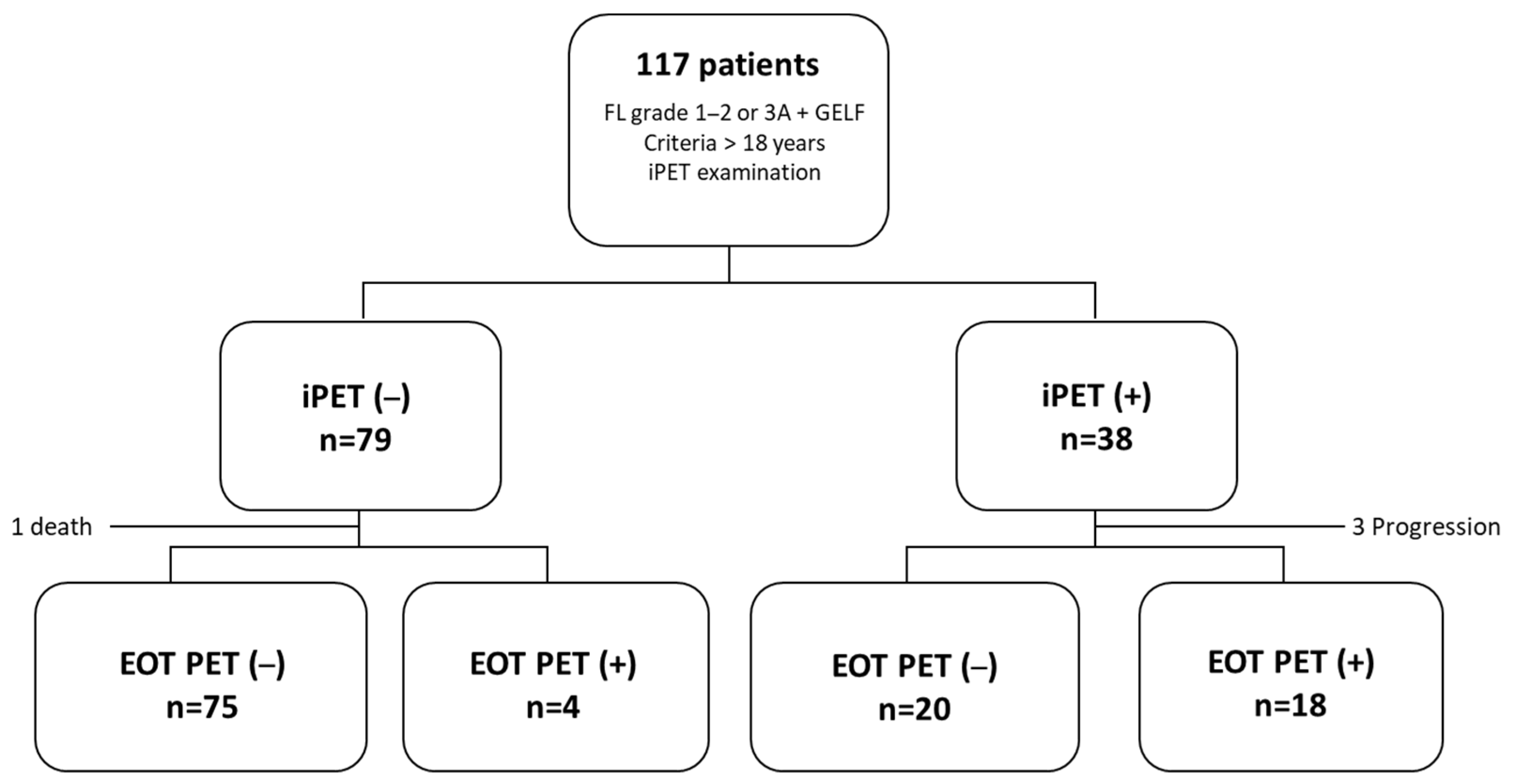
3. Results
3.1. Patient Characteristics
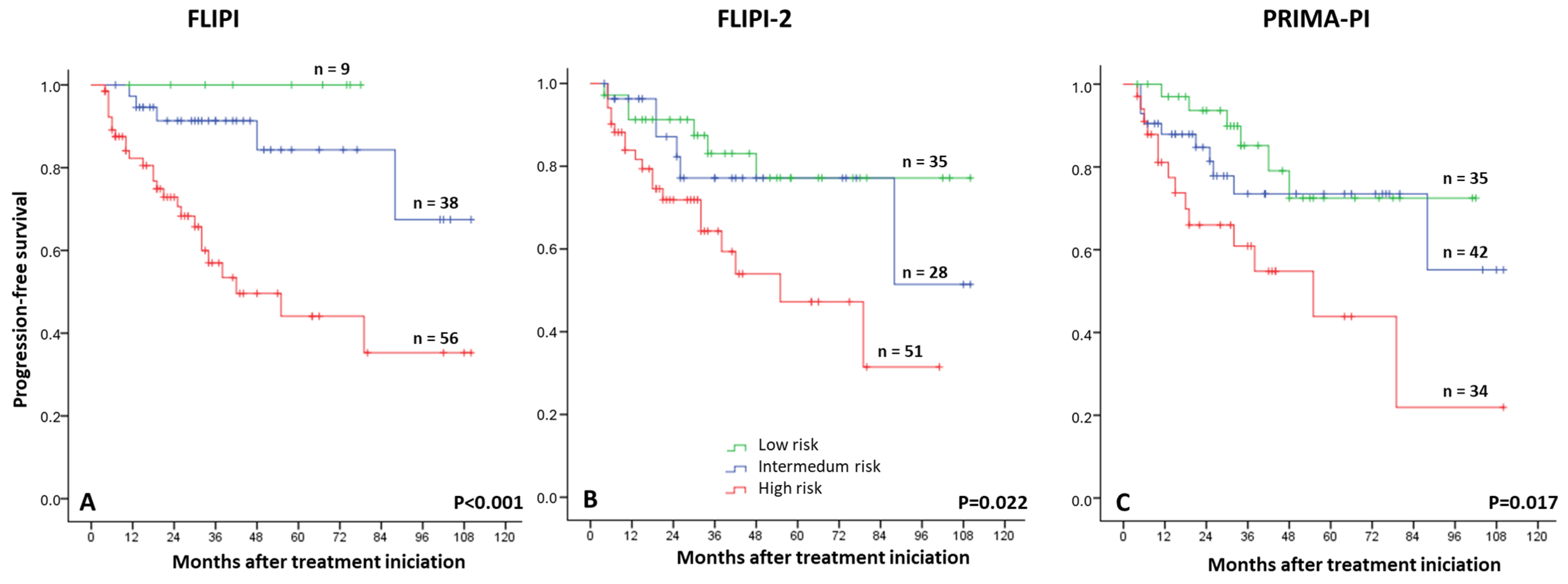
3.2. Risk Factors for Progression-Free Survival Analysis
3.3. Prognostic Value of iPET
3.4. Prognostic Value of EOT PET
3.5. Dynamics of PET Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheah, C.Y.; Chihara, D.; Horowitz, S.; Sevin, A.; Oki, Y.; Zhou, S.; Fowler, N.H.; Romaguera, J.E.; Turturro, F.; Hagemeister, F.B.; et al. Factors influencing outcome in advanced stage, low-grade follicular lymphoma treated at MD Anderson Cancer Center in the rituximab era. Ann. Oncol. 2016, 27, 895–901. [Google Scholar] [PubMed]
- Jiménez-Ubieto, A.; Grande, C.; Caballero, D.; Yáñez, L.; Novelli, S.; Hernández-Garcia, M.T.; Manzanares, M.; Arranz, R.; Ferreiro, J.J.; Bobillo, S.; et al. Autologous stem cell transplantation for follicular lymphoma: Favorable long-term survival irrespective of pretransplantation rituximab exposure. Biol. Blood Marrow Transplant. 2017, 23, 1631–1640. [Google Scholar]
- Anderson, J.R.; Armitage, J.O.; Weisenburger, D.D. Epidemiology of the non-Hodgkin’s lymphomas: Distributions of the major subtypes differ by geographic locations. Ann. Oncol. 1998, 9, 717–720. [Google Scholar]
- Bastos-Oreiro, M.; Muntañola, A.; Panizo, C.; Gonzalez-Barca, E.; de Villambrosia, S.G.; Córdoba, R.; López, J.L.B.; González-Sierra, P.; Terol, M.J.; Gutierrez, A.; et al. RELINF: Prospective epidemiological registry of lymphoid neoplasms in Spain. A project from the GELTAMO group. Ann. Hematol. 2020, 99, 799–808. [Google Scholar]
- Flinn, I.W.; van der Jagt, R.; Kahl, B.S.; Wood, P.; Hawkins, T.E.; Macdonald, D.; Hertzberg, M.; Kwan, Y.L.; Simpson, D.; Craig, M.; et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: The BRIGHT study. Blood 2014, 123, 2944–2952. [Google Scholar]
- Hiddemann, W.; Kneba, M.; Dreyling, M.; Schmitz, N.; Lengfelder, E.; Schmits, R.; Reiser, M.; Metzner, B.; Harder, H.; Hegewisch-Becker, S.; et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005, 106, 3725–3732. [Google Scholar] [PubMed]
- Morschhauser, F.; Fowler, N.H.; Feugier, P.; Bouabdallah, R.; Tilly, H.; Palomba, M.L.; Fruchart, C.; Libby, E.N.; Casasnovas, R.O.; Flinn, I.W.; et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N. Engl. J. Med. 2018, 379, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.; Davies, A.; Ando, K.; Klapper, W.; Opat, S.; Owen, C.; Phillips, E.; Sangha, R.; Schlag, R.; Seymour, J.F.; et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N. Engl. J. Med. 2017, 377, 1331–1344. [Google Scholar]
- Bachy, E.; Seymour, J.F.; Feugier, P.; Offner, F.; López-Guillermo, A.; Belada, D.; Xerri, L.; Catalano, J.V.; Brice, P.; Lemonnier, F.; et al. Sustained Progression-Free Survival Benefit of Rituximab Maintenance in Patients With Follicular Lymphoma: Long-Term Results of the PRIMA Study. J. Clin. Oncol. 2019, 37, 2815–2824. [Google Scholar]
- Salles, G.; Seymour, J.F.; Offner, F.; López-Guillermo, A.; Belada, D.; Xerri, L.; Feugier, P.; Bouabdallah, R.; Catalano, J.V.; Brice, P.; et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab pluschemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet 2011, 377, 42–51. [Google Scholar]
- Jiménez-Ubieto, A.; Grande, C.; Caballero, D.; Yáñez, L.; Novelli, S.; Hernández, M.T.; Manzanares, M.; Arranz, R.; Ferreiro, J.J.; Bobillo, S.; et al. Progression-free survival at 2 years post-autologous transplant: A surrogate endpoint for overall survival in follicular lymphoma. Cancer Med. 2017, 6, 2766–2774. [Google Scholar] [PubMed]
- Casulo, C.; Dixon, J.G.; Le-Rademacher, J.; Hoster, E.; Hochster, H.S.; Hiddemann, W.; Marcus, R.; Kimby, E.; Herold, M.; Sebban, C. Validation of POD24 as a robust early clinical endpoint of poor survival in FL from 5225 patients on 13 clinical trials. Blood 2022, 139, 1684–1693. [Google Scholar] [PubMed]
- Murakami, S.; Kato, H.; Higuchi, Y.; Yamamoto, K.; Yamamoto, H.; Saito, T.; Taji, H.; Yatabe, Y.; Nakamura, S.; Kinoshita, T. Prediction of high risk for death in patients with follicular lymphoma receiving rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in first-line chemotherapy. Ann. Hematol. 2016, 95, 1259–1269. [Google Scholar]
- Zhang, L.; Ghielmini, M.; Cheson, B.D.; Ujjani, C. Pros and cons of rituximab maintenance in follicular lymphoma. Cancer Treat. Rev. 2017, 58, 34–40. [Google Scholar] [PubMed]
- Federico, M.; Bellei, M.; Marcheselli, L.; Luminari, S.; Lopez-Guillermo, A.; Vitolo, U.; Pro, B.; Pileri, S.; Pulsoni, A.; Soubeyran, P.; et al. Follicular Lymphoma International Prognostic Index 2: A New Prognostic Index for Follicular Lymphoma Developed by the International Follicular Lymphoma Prognostic Factor Project. J. Clin. Oncol. 2009, 27, 4555–4562. [Google Scholar] [CrossRef]
- Solal-Céligny, P.; Roy, P.; Colombat, P.; White, J.; Armitage, J.O.; Arranz-Saez, R.; Au, W.Y.; Bellei, M.; Brice, P.; Caballero, D.; et al. Follicular Lymphoma International Prognostic Index. Blood 2004, 104, 1258–1265. [Google Scholar]
- Bachy, E.; Maurer, M.J.; Habermann, T.M.; Gelas-Dore, B.; Maucort-Boulch, D.; Estell, J.A.; Van den Neste, E.; Bouabdallah, R.; Gyan, E.; Feldman, A.L.; et al. A simplified scoring system in de novo follicular lymphoma treated initially with immunochemotherapy. Blood 2018, 132, 49–58. [Google Scholar]
- Mondello, P.; Fama, A.; Larson, M.C.; Feldman, A.L.; Villasboas, J.C.; Yang, Z.Z.; Galkin, I.; Svelolkin, V.; Postovalova, E.; Bagaev, A.; et al. Lack of intrafollicular memory CD4 + T cells is predictive of early clinical failure in newly diagnosed follicular lymphoma. Blood Cancer 2021, 11, 130. [Google Scholar]
- Mir, F.; Mattiello, F.; Grigg, A.; Herold, M.; Hiddemann, W.; Marcus, R.; Seymour, J.F.; Bolen, C.R.; Knapp, A.; Nielsen, T.; et al. Follicular Lymphoma Evaluation Index (FLEX): A new clinical prognostic model that is superior to existing risk scores for predicting progression-free survival and early treatment failure after frontline immunochemotherapy. Am. J. Hematol. 2020, 95, 1503–1510. [Google Scholar]
- Pastore, A.; Jurinovic, V.; Kridel, R.; Hoster, E.; Staiger, A.M.; Szczepanowski, M.; Pott, C.; Kopp, N.; Murakami, M.; Horn, H.; et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: A retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015, 16, 1111–1122. [Google Scholar]
- Jurinovic, V.; Kridel, R.; Staiger, A.M.; Szczepanowski, M.; Horn, H.; Dreyling, M.H.; Rosenwald, A.; Ott, G.; Klapper, W.; Zelenetz, A.D. Clinicogenetic risk models predict early progression of follicular lymphoma after first-line immunochemotherapy. Blood 2016, 128, 1112–1120. [Google Scholar]
- Huet, S.; Tesson, B.; Jais, J.P.; Feldman, A.L.; Magnano, L.; Thomas, E.; Traverse-Glehen, A.; Albaud, B.; Carrère, M.; Xerri, L.; et al. A gene expression profiling score for prediction of outcome in patients with follicular lymphoma: A retrospective training and validation analysis in three international cohorts. Lancet Oncol. 2018, 19, 549–561. [Google Scholar]
- Meignan, M.; Cottereau, A.S.; Versari, A.; Chartier, L.; Dupuis, J.; Boussetta, S.; Grassi, I.; Casasnovas, R.O.; Haioun, C.; Tilly, H.; et al. Baseline Metabolic Tumor Volume Predicts Outcome in High–Tumor-Burden Follicular Lymphoma: A Pooled Analysis of Three Multicenter Studies. J. Clin. Oncol. 2016, 34, 3618–3626. [Google Scholar] [CrossRef]
- Trotman, J.; Barrington, S.F.; Belada, D.; Meignan, M.; MacEwan, R.; Owen, C.; Ptáčník, V.; Rosta, A.; Fingerle-Rowson, G.R.; Zhu, J.; et al. Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (GALLIUM): Secondary analysis of a randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1530–1542. [Google Scholar]
- Dupuis, J.; Berriolo-Riedinger, A.; Julian, A.; Brice, P.; Tychyj-Pinel, C.; Tilly, H.; Mounier, N.; Gallamini, A.; Feugier, P.; Soubeyran, P.; et al. Impact of [18F]fluorodeoxyglucose positron emission tomography response evaluation in patients with high-tumor burden follicular lymphoma treated with immunochemotherapy: A prospective study from the Groupe d’Etudes des Lymphomes de l’Adulte and GOELAMS. J. Clin. Oncol. 2012, 30, 4317–4322. [Google Scholar]
- Galimberti, S.; Luminari, S.; Ciabatti, E.; Grassi, S.; Guerrini, F.; Dondi, A.; Marcheselli, L.; Ladetto, M.; Piccaluga, P.P.; Gazzola, A.; et al. Minimal residual disease after conventional treatment significantly impacts on progression-free survival of patients with follicular lymphoma: The FIL FOLL05 trial. Clin. Cancer Res. 2014, 20, 6398–6405. [Google Scholar]
- Levavi, H.; Lancman, G.; Gabrilove, J. Impact of rituximab on COVID-19 outcomes. Ann. Hematol. 2021, 100, 2805–2812. [Google Scholar] [PubMed]
- Trotman, J.; Presgrave, P.; Carradice, D.P.; Lenton, D.S.; Gandhi, M.K.; Cochrane, T.; Badoux, X.; Carlson, J.; Nkhoma, G.; Butcher, B.; et al. Lenalidomide Consolidation Added to Rituximab Maintenance Therapy in Patients Remaining PET Positive After Treatment for Relapsed Follicular Lymphoma: A Phase 2 Australasian Leukaemia & Lymphoma Group NHL26 Study. Hemasphere 2023, 7, e836. [Google Scholar]
- Johnson, P.; Federico, M.; Kirkwood, A.; Fosså, A.; Berkahn, L.; Carella, A.; d’Amore, F.; Enblad, G.; Franceschetto, A.; Fulham, M.; et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N. Engl. J. Med. 2016, 374, 2419–2429. [Google Scholar] [PubMed]
- Borchmann, P.; Goergen, H.; Kobe, C.; Lohri, A.; Greil, R.; Eichenauer, D.A.; Zijlstra, J.M.; Markova, J.; Meissner, J.; Feuring-Buske, M.; et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): Final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 2017, 390, 2790–2802. [Google Scholar] [PubMed]
- Casasnovas, R.O.; Bouabdallah, R.; Brice, P.; Lazarovici, J.; Ghesquieres, H.; Stamatoullas, A.; Dupuis, J.; Gac, A.C.; Gastinne, T.; Joly, B.; et al. PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): A randomised, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2019, 20, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Dührsen, U.; Müller, S.; Hertenstein, B.; Thomssen, H.; Kotzerke, J.; Mesters, R.; Berdel, W.E.; Franzius, C.; Kroschinsky, F.; Weckesser, M.; et al. Positron emission tomography-guided therapy of aggressive non-Hodgkin lymphomas (PETAL): A multicenter, randomized phase III trial. J. Clin. Oncol. 2018, 36, 2024–2034. [Google Scholar] [CrossRef] [PubMed]
- Boo, S.H.; O, J.H.; Kwon, S.J.; Yoo, I.R.; Kim, S.H.; Park, G.S.; Choi, B.O.; Jung, S.E.; Cho, S.G. Predictive value of interim and end-oftherapy 18F-FDG PET/CT in patients with follicular lymphoma. Nucl. Med. Mol. Imaging 2019, 53, 263–269. [Google Scholar] [CrossRef]
- Merryman, R.W.; Michaud, L.; Redd, R.; Mondello, P.; Park, H.; Spilberg, G.; Robertson, M.; Taranto, E.; Ahmed, G.; Chase, M.; et al. Interim Positron Emission Tomography During Frontline Chemoimmunotherapy for Follicular Lymphoma. Hemasphere 2023, 7, e826. [Google Scholar] [CrossRef] [PubMed]
- Bishu, S.; Quigley, J.M.; Bishu, S.R.; Olsasky, S.M.; Stem, R.A.; Shostrom, V.K.; Holdeman, K.P.; Paknikar, S.; Armitage, J.O.; Hankins, J.H.; et al. Predictive value and diagnostic accuracy of F-18-fluoro-deoxy-glucose positron emission tomography treated grade 1 and 2 follicular lymphoma. Leuk. Lymphoma 2007, 48, 1548–1555. [Google Scholar] [CrossRef]
- Lu, Z.; Lin, M.; Downe, P.; Chong, S.; Ling, S. The prognostic value of mid- and post-treatment [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) in indolent follicular lymphoma. Ann. Nucl. Med. 2014, 28, 805–811. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Kurch, L.; Hüttmann, A.; Georgi, T.W.; Rekowski, J.; Sabri, O.; Schmitz, C.; Kluge, R.; Dührsen, U.; Hasenclever, D. Interim PET in Diffuse Large B-Cell Lymphoma. J. Nucl. Med. 2021, 62, 1068–1074. [Google Scholar] [CrossRef]
- Luminari, S.; Manni, M.; Galimberti, S.; Versari, A.; Tucci, A.; Boccomini, C.; Farina, L.; Olivieri, J.; Marcheselli, L.; Guerra, L.; et al. Response-adapted postinduction strategy in patients with advanced-stage follicular lymphoma: The FOLL12 study. J. Clin. Oncol. 2022, 40, 729–739. [Google Scholar] [CrossRef]
- Jiménez-Ubieto, A.; Poza, M.; Martin-Muñoz, A.; Ruiz-Heredia, Y.; Dorado, S.; Figaredo, G.; Rosa-Rosa, J.M.; Rodriguez, A.; Barcena, C.; Navamuel, L.P.; et al. Real-life disease monitoring in follicular lymphoma patients using liquid biopsy ultra-deep sequencing and PET/CT. Leukemia 2023, 37, 659–669. [Google Scholar] [CrossRef]
- Dreyling, M.; Fowler, N.H.; Dickinson, M.; Martinez-Lopez, J.; Kolstad, A.; Butler, J.; Ghosh, M.; Popplewell, L.; Chavez, J.C.; Bachy, E.; et al. Durable response after tisagenlecleucel in adults with relapsed/refractory follicular lymphoma: ELARA trial update. Blood 2024, 143, 1713–1725. [Google Scholar] [CrossRef]
- Sehn, L.H.; Bartlett, N.L.; Matasar, M.J.; Schuster, S.J.; Assouline, S.E.; Giri, P.; Kuruvilla, J.; Shadman, M.; Cheah, C.Y.; Dietrich, S.; et al. Long-term 3-year follow-up of mosunetuzumab in relapsed or refractory follicular lymphoma after ≥2 prior therapies. Blood 2025, 145, 708–719. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Barrington, S.F.; Mir, F.; El-Galaly, T.C.; Knapp, A.; Nielsen, T.G.; Sahin, D.; Wenger, M.; Kostakoglu, L.; Trotman, J.; Meignan, M. Follicular Lymphoma Treated with First-Line Immunochemotherapy: A Review of PET/CT in Patients Who Did Not Achieve a Complete Metabolic Response in the GALLIUM Study. J. Nucl. Med. 2022, 63, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Armitage, J.O. Positron Emission Tomographic Scans in Lymphoma: Convention and Controversy. Mayo Clin. Proc. 2012, 87, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, A.R.; Barrington, S.; Kalakonda, N.; Khan, U.T.; Jackson, R.; Carruthers, S.; Oates, M.; Lin, K.; Ardeshna, K.; Eyre, T.; et al. NCRI Petrea Trial: A Phase 3 Evaluation of Pet-Guided, Response-Adapted Therapy in Patients with Previously Untreated, Advanced-Stage, High-Tumour-Burden Follicular Lymphoma. Hematol. Oncol. 2019, 37, 67–68. [Google Scholar] [CrossRef]


| Parameter | Total n = 117 |
|---|---|
| Median age, years (range) | 62 (27–87) |
| Sex, male, % | 60 |
| Grade, % | |
| 1–2 | 88 |
| 3A | 29 |
| Ann Arbor stage, % | |
| 2 | 9 |
| 3 | 25 |
| 4 | 83 |
| B symptoms, % | 23 |
| Bulky disease, % | 38 |
| Extranodal involvement, % * | 31 |
| Bone marrow involvement, % | 57 |
| FLIPI, n = 113, % | |
| Low risk | 9 |
| Intermediate risk | 38 |
| High risk | 66 |
| FLIPI2, n = 113, % | |
| Low risk | 35 |
| Intermediate risk | 27 |
| High risk | 51 |
| PRIMA-PI, n = 111, % | |
| Low risk | 35 |
| Intermediate risk | 42 |
| High risk | 34 |
| Hemoglobin (median g/dL, range) | 13.9 (8.8–17.7) |
| Increased LDH, % | 37 |
| Increased β2-microglobulin, % | 57 |
| Treatment regimen, % | |
| R-CHOP | 77 |
| Rituximab and bendamustine | 21 |
| Other treatments | 2 |
| Rituximab maintenance, n = 105, % | 87 |
| Univariate p Value | Univariate HR (95% CI) | Multivariate p Value | Multivariate HR (95% CI) | |
|---|---|---|---|---|
| Age ≥ 60 | 0.585 | 1.22 (0.60–2.45) | * | |
| Female (vs. male) | 0.414 | 0.78 (0.37–1.50) | * | |
| Grade FL 3A (vs. 1–2) | 0.101 | 0.41 (0.15–1.19) | * | |
| Advanced Ann Arbor stage | 0.310 | 2.81 (0.38–20.68) | * | |
| B symptoms | 0.929 | 1.04 (0.47–2.31) | * | |
| >4 nodal areas | 0.033 | 8.74 (1.19–64.03) | 0.072 | 6.58 (0.84–51.3) |
| BM involvement | 0.133 | 1.76 (0.84–3.76) | * | |
| Bulky disease | 0.778 | 1.11 (0.53–2.31) | * | |
| Extranodal involvement 1 | 0.943 | 1.03 (0.46–2.31) | * | |
| Elevated β2 microglobulin | 0.002 | 3.61 (1.61–8.09) | 0.226 | 1.81 (0.69–4.71) |
| Hemoglobin < 12 | 0.109 | 1.88 (0.89–4.08) | * | |
| Elevated LDH | <0.001 | 4.17 (1.99–8.78) | 0.521 | 1.38 (0.51–3.73) |
| RB (vs. RCHOP) | 0.655 | 1.20 (0.54–4.66) | * | |
| Rituximab maintenance | 0.040 | 0.38 (0.15–0.96) | 0.055 | 0.37 (0.13–1.02) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poza, M.; Martin-Muñoz, A.; López-Pereira, P.; Figaredo, G.; Zamanillo, I.; Íñiguez, R.; Oliveira, A.C.; Baumann, T.; Rodríguez-Izquierdo, A.; Grande, C.; et al. Exploratory Study to Evaluate the Impact of Interim PET/CT Assessment in First-Line Follicular Lymphoma. Cancers 2025, 17, 1065. https://doi.org/10.3390/cancers17071065
Poza M, Martin-Muñoz A, López-Pereira P, Figaredo G, Zamanillo I, Íñiguez R, Oliveira AC, Baumann T, Rodríguez-Izquierdo A, Grande C, et al. Exploratory Study to Evaluate the Impact of Interim PET/CT Assessment in First-Line Follicular Lymphoma. Cancers. 2025; 17(7):1065. https://doi.org/10.3390/cancers17071065
Chicago/Turabian StylePoza, María, Alejandro Martin-Muñoz, Patricia López-Pereira, Gloria Figaredo, Irene Zamanillo, Rodrigo Íñiguez, Ana Carla Oliveira, Tycho Baumann, Antonia Rodríguez-Izquierdo, Carlos Grande, and et al. 2025. "Exploratory Study to Evaluate the Impact of Interim PET/CT Assessment in First-Line Follicular Lymphoma" Cancers 17, no. 7: 1065. https://doi.org/10.3390/cancers17071065
APA StylePoza, M., Martin-Muñoz, A., López-Pereira, P., Figaredo, G., Zamanillo, I., Íñiguez, R., Oliveira, A. C., Baumann, T., Rodríguez-Izquierdo, A., Grande, C., Sarandeses, P., Revilla, E., Cortés, M., Ayala, R., Calbacho, M., Martínez, J., Barrio, S., & Jiménez-Ubieto, A. (2025). Exploratory Study to Evaluate the Impact of Interim PET/CT Assessment in First-Line Follicular Lymphoma. Cancers, 17(7), 1065. https://doi.org/10.3390/cancers17071065






