Performance Comparison of Diffusion Kurtosis Imaging (DKI), Neurite Orientation Dispersion and Density Imaging (NODDI), and Diffusion Microstructure Imaging (DMI) in Predicting Adult-Type Glioma Subtype—A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. MR Imaging
2.4. Image Analysis
2.5. Postoperative Tumor Subtyping
- (i)
- glioblastomas, IDH wildtype
- (ii)
- astrocytomas, IDH mutant
- (iii)
- oligodendrogliomas, IDH mutant
2.6. Statistical Analysis
3. Results
3.1. Patients
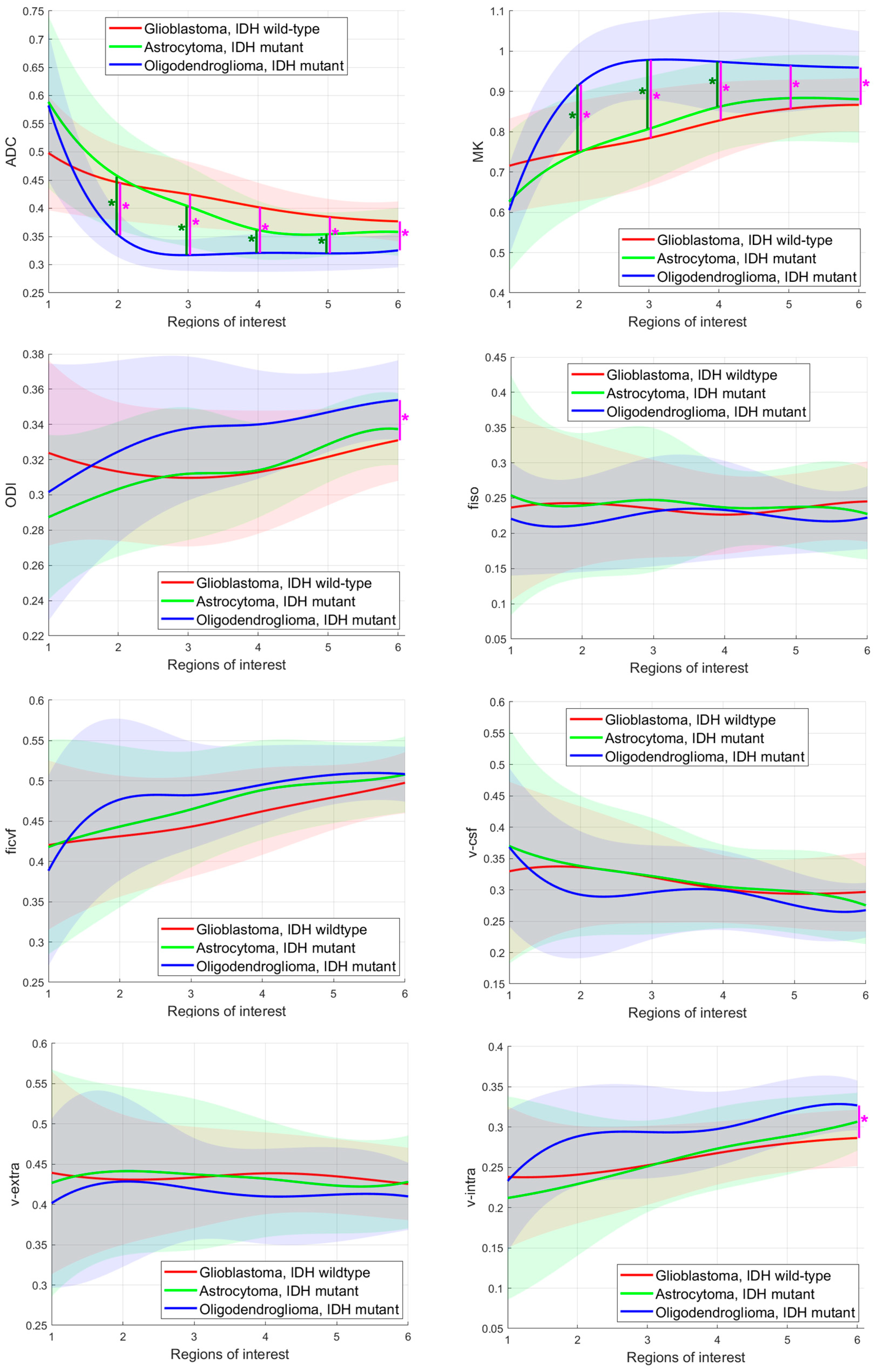
3.2. Comparison of DKI, NODDI, and DMI Parameters in Each ROI
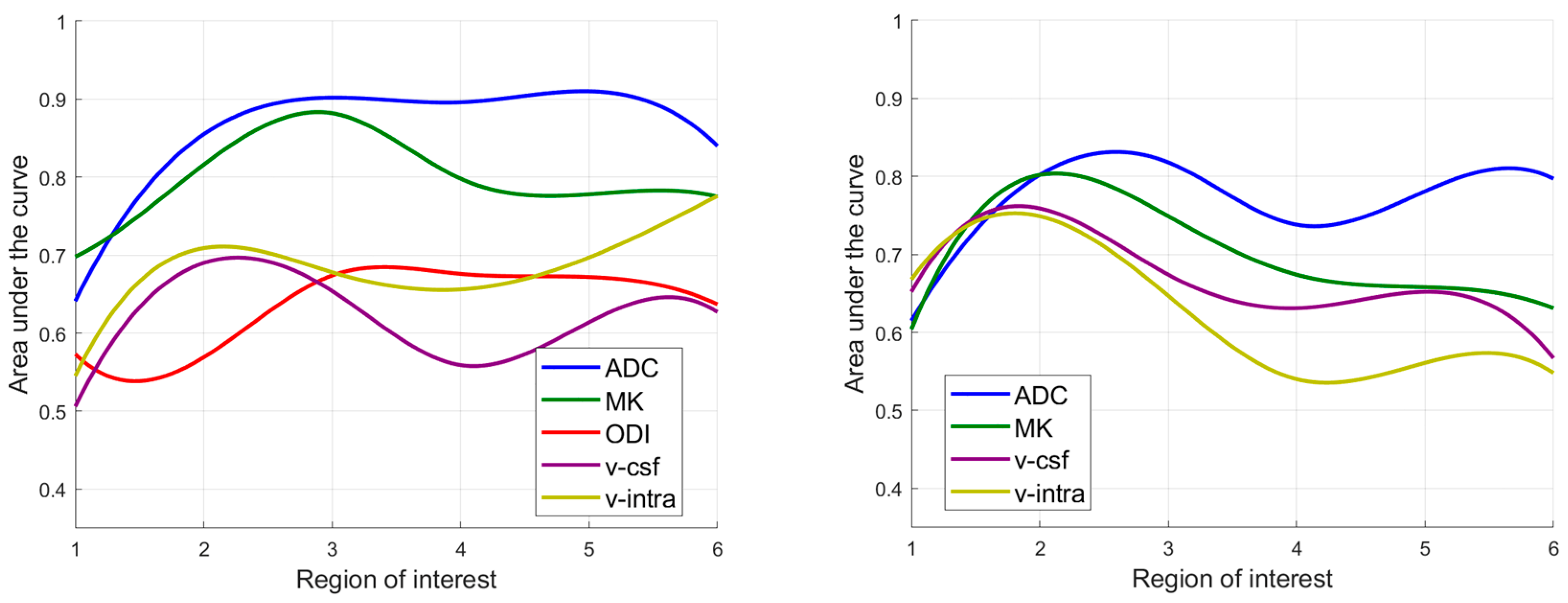
3.3. Diagnostic Performance in Differating IDH Mutant from IDH Wildtype Gliomas
3.4. Diagnostic Performance in Differating Astocytomas, IDH Mutant from Oligodendrogliomas, IDH Mutant
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Alkanhal, H.; Das, K.; Poptani, H. Diffusion- and Perfusion-Weighted Magnetic Resonance Imaging Methods in Nonenhancing Gliomas. World Neurosurg. 2020, 141, 123–130. [Google Scholar] [CrossRef]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non–Contrast-Enhanced Tumor with Survival Within Molecular Subgroups of Patients with Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Vivas-Buitrago, T.; Domingo, R.A.; Tripathi, S.; De Biase, G.; Brown, D.; Akinduro, O.O.; Ramos-Fresnedo, A.; Sabsevitz, D.S.; Bendok, B.R.; Sherman, W.; et al. Influence of supramarginal resection on survival outcomes after gross-total resection of IDH-wild-type glioblastoma. J. Neurosurg. 2022, 136, 1–8. [Google Scholar] [CrossRef]
- Hempel, J.M.; Bisdas, S.; Schittenhelm, J.; Brendle, C.; Bender, B.; Wassmann, H.; Skardelly, M.; Tabatabai, G.; Vega, S.C.; Ernemann, U.; et al. In vivo molecular profiling of human glioma using diffusion kurtosis imaging. J. Neurooncol. 2017, 131, 93–101. [Google Scholar] [CrossRef]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Sledzinska-Bebyn, P.; Furtak, J.; Bebyn, M.; Serafin, Z. Beyond conventional imaging: Advancements in MRI for glioma malignancy prediction and molecular profiling. Magn. Reson. Imaging 2024, 112, 63–81. [Google Scholar] [CrossRef]
- Verburg, N.; de Witt Hamer, P.C. State-of-the-art imaging for glioma surgery. Neurosurg. Rev. 2021, 44, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Jiang, R.F.; Zhang, J.; Su, C.L.; Chen, X.W.; Zhang, J.X.; Jiang, J.J.; Zhu, W.Z. Application of Neurite Orientation Dispersion and Density Imaging in Assessing Glioma Grades and Cellular Proliferation. World Neurosurg. 2019, 131, e247–e254. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, G.; Dixon, L.; Sanverdi, E.; Machado, P.M.; Kwong, J.S.W.; Panovska-Griffiths, J.; Rojas-Garcia, A.; Yoneoka, D.; Veraart, J.; Van Cauter, S.; et al. The diagnostic role of diffusional kurtosis imaging in glioma grading and differentiation of gliomas from other intra-axial brain tumours: A systematic review with critical appraisal and meta-analysis. Neuroradiology 2020, 62, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.H.; Helpern, J.A. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010, 23, 698–710. [Google Scholar] [CrossRef]
- Cauter, S.V.; Veraart, J.; Sijbers, J.; Peeters, R.R.; Himmelreich, U.; Keyzer, F.D.; Gool, S.W.V.; Calenbergh, F.V.; Vleeschouwer, S.D.; Hecke, W.V.; et al. Gliomas: Diffusion Kurtosis MR Imaging in Grading. Radiology 2012, 263, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, H.; Zhao, R.F.; Wang, X.C.; Qin, J.B.; Wu, X.F. Comparison of the values of MRI diffusion kurtosis imaging and diffusion tensor imaging in cerebral astrocytoma grading and their association with aquaporin-4. Neurol. India 2016, 64, 265–272. [Google Scholar] [CrossRef]
- Rau, A.; Schroeter, N.; Blazhenets, G.; Dressing, A.; Walter, L.I.; Kellner, E.; Bormann, T.; Mast, H.; Wagner, D.; Urbach, H.; et al. Widespread white matter oedema in subacute COVID-19 patients with neurological symptoms. Brain 2022, 145, 3203–3213. [Google Scholar] [CrossRef]
- Wurtemberger, U.; Diebold, M.; Rau, A.; Akgun, V.; Becker, L.; Beck, J.; Reinacher, P.C.; Taschner, C.A.; Reisert, M.; Fehrenbacher, L.; et al. Advanced diffusion imaging reveals microstructural characteristics of primary CNS lymphoma, allowing differentiation from glioblastoma. Neurooncol. Adv. 2024, 6, vdae093. [Google Scholar] [CrossRef]
- Wurtemberger, U.; Erny, D.; Rau, A.; Hosp, J.A.; Akgun, V.; Reisert, M.; Kiselev, V.G.; Beck, J.; Jankovic, S.; Reinacher, P.C.; et al. Mesoscopic Assessment of Microstructure in Glioblastomas and Metastases by Merging Advanced Diffusion Imaging with Immunohistopathology. AJNR Am. J. Neuroradiol. 2023, 44, 1262–1269. [Google Scholar] [CrossRef]
- Wurtemberger, U.; Rau, A.; Reisert, M.; Kellner, E.; Diebold, M.; Erny, D.; Reinacher, P.C.; Hosp, J.A.; Hohenhaus, M.; Urbach, H.; et al. Differentiation of Perilesional Edema in Glioblastomas and Brain Metastases: Comparison of Diffusion Tensor Imaging, Neurite Orientation Dispersion and Density Imaging and Diffusion Microstructure Imaging. Cancers 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, J.B.; Wang, J.Y.; Wang, Y.L.; Liu, D.W.; Li, X.B.; Song, Y.K.; Tian, Y.S.; Yan, X.; Li, Z.H.; et al. Quantitative analysis of neurite orientation dispersion and density imaging in grading gliomas and detecting IDH-1 gene mutation status. Neuroimage Clin. 2018, 19, 174–181. [Google Scholar] [CrossRef]
- Wurtemberger, U.; Diebold, M.; Erny, D.; Hosp, J.A.; Schnell, O.; Reinacher, P.C.; Rau, A.; Kellner, E.; Reisert, M.; Urbach, H.; et al. Diffusion Microstructure Imaging to Analyze Perilesional T2 Signal Changes in Brain Metastases and Glioblastomas. Cancers 2022, 14, 1155. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Hori, M.; Aoki, S. NODDI in clinical research. J. Neurosci. Methods 2020, 346, 108908. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kim, J.W.; Ahn, S.J.; Suh, S.H. Evaluation of brain tumors using NODDI technique: A promising tool. J. Neuroradiol. 2020, 47, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012, 61, 1000–1016. [Google Scholar] [CrossRef]
- Chung, A.W.; Seunarine, K.K.; Clark, C.A. NODDI reproducibility and variability with magnetic field strength: A comparison between 1.5 T and 3 T. Hum. Brain Mapp. 2016, 37, 4550–4565. [Google Scholar] [CrossRef] [PubMed]
- Reisert, M.; Kellner, E.; Dhital, B.; Hennig, J.; Kiselev, V.G. Disentangling micro from mesostructure by diffusion MRI: A Bayesian approach. Neuroimage 2017, 147, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shu, X.; He, P.; Cai, Y.; Geng, Y.; Hu, X.; Sun, Y.; Xiao, H.; Zheng, W.; Song, Y.; et al. Ultra-high b-value DWI accurately identifies isocitrate dehydrogenase genotypes and tumor subtypes of adult-type diffuse gliomas. Eur. Radiol. 2024, 34, 6751–6762. [Google Scholar] [CrossRef]
- Weller, M.; Knobbe-Thomsen, C.B.; Le Rhun, E.; Reifenberger, G. Die WHO-Klassifikation der Tumoren des zentralen Nervensystems 2021. Onkologe 2022, 28, 155–163. [Google Scholar] [CrossRef]
- Veraart, J.; Novikov, D.S.; Christiaens, D.; Ades-Aron, B.; Sijbers, J.; Fieremans, E. Denoising of diffusion MRI using random matrix theory. Neuroimage 2016, 142, 394–406. [Google Scholar] [CrossRef]
- Kellner, E.; Dhital, B.; Kiselev, V.G.; Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 2016, 76, 1574–1581. [Google Scholar] [CrossRef]
- Diaz-Pinto, A.; Alle, S.; Nath, V.; Tang, Y.; Ihsani, A.; Asad, M.; Perez-Garcia, F.; Mehta, P.; Li, W.; Flores, M.; et al. MONAI Label: A framework for AI-assisted interactive labeling of 3D medical images. Med. Image Anal. 2024, 95, 103207. [Google Scholar] [CrossRef]
- Menze, B.H.; Jakab, A.; Bauer, S.; Kalpathy-Cramer, J.; Farahani, K.; Kirby, J.; Burren, Y.; Porz, N.; Slotboom, J.; Wiest, R.; et al. The Multimodal Brain Tumor Image Segmentation Benchmark (BRATS). IEEE Trans. Med. Imaging 2015, 34, 1993–2024. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Su, C.Q.; Tang, W.T.; Lin, J.; Lu, S.S.; Hong, X.N. Combined texture analysis of dynamic contrast-enhanced MRI with histogram analysis of diffusion kurtosis imaging for predicting IDH mutational status in gliomas. Acta Radiol. 2023, 64, 2552–2560. [Google Scholar] [CrossRef] [PubMed]
- Kickingereder, P.; Sahm, F.; Radbruch, A.; Wick, W.; Heiland, S.; Deimling, A.; Bendszus, M.; Wiestler, B. IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci. Rep. 2015, 5, 16238. [Google Scholar] [CrossRef] [PubMed]
- Hempel, J.M.; Schittenhelm, J.; Brendle, C.; Bender, B.; Bier, G.; Skardelly, M.; Tabatabai, G.; Castaneda Vega, S.; Ernemann, U.; Klose, U. Histogram analysis of diffusion kurtosis imaging estimates for in vivo assessment of 2016 WHO glioma grades: A cross-sectional observational study. Eur. J. Radiol. 2017, 95, 202–211. [Google Scholar] [CrossRef]
- Chen, L.; Liu, M.; Bao, J.; Xia, Y.; Zhang, J.; Zhang, L.; Huang, X.; Wang, J. The correlation between apparent diffusion coefficient and tumor cellularity in patients: A meta-analysis. PLoS ONE 2013, 8, e79008. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagels, F.W.; De Witt Hamer, P.; Sanz-Arigita, E.; Idema, S.; Kuijer, J.P.; Pouwels, P.J.; Barkhof, F.; Vandertop, W.P. Differentiation of edema and glioma infiltration: Proposal of a DTI-based probability map. J. Neurooncol. 2014, 120, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, A.; Yadav, V.; Parvaze, S.P.; Singh, A.; Saini, J.; Patir, R.; Vaishya, S.; Ahlawat, S.; Gupta, R.K. Comparative evaluation of intracranial oligodendroglioma and astrocytoma of similar grades using conventional and T1-weighted DCE-MRI. Neuroradiology 2021, 63, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- van der Meulen, M.; Mason, W.P. First-line chemotherapeutic treatment for oligodendroglioma, WHO grade 3-PCV or temozolomide? Neurooncol. Pract. 2022, 9, 163–164. [Google Scholar] [CrossRef]
- Yang, X.; Lin, Y.; Xing, Z.; She, D.; Su, Y.; Cao, D. Predicting 1p/19q codeletion status using diffusion-, susceptibility-, perfusion-weighted, and conventional MRI in IDH-mutant lower-grade gliomas. Acta Radiol. 2021, 62, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Figini, M.; Riva, M.; Graham, M.; Castelli, G.M.; Fernandes, B.; Grimaldi, M.; Baselli, G.; Pessina, F.; Bello, L.; Zhang, H.; et al. Prediction of Isocitrate Dehydrogenase Genotype in Brain Gliomas with MRI: Single-Shell versus Multishell Diffusion Models. Radiology 2018, 289, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Maximov, I.I.; Tonoyan, A.S.; Pronin, I.N. Differentiation of glioma malignancy grade using diffusion MRI. Phys. Med. 2017, 40, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Richter, V.; Nagele, T.; Erb, G.; Klose, U.; Ernemann, U.; Hauser, T.K. Improved diagnostic confidence and tumor type prediction in adult-type diffuse glioma by multimodal imaging including DCE perfusion and diffusion kurtosis mapping—A standardized multicenter study. Eur. J. Radiol. 2024, 171, 111293. [Google Scholar] [CrossRef] [PubMed]
- Zerweck, L.; Hauser, T.K.; Klose, U.; Han, T.; Nagele, T.; Shen, M.; Gohla, G.; Estler, A.; Xie, C.; Hu, H.; et al. Glioma Type Prediction with Dynamic Contrast-Enhanced MR Imaging and Diffusion Kurtosis Imaging-A Standardized Multicenter Study. Cancers 2024, 16, 2644. [Google Scholar] [CrossRef] [PubMed]
- Zerweck, L.; Klose, U.; Würtemberger, U.; Richter, V.; Nägele, T.; Gohla, G.; Grundmann-Hauser, K.; Estler, A.; Ruff, C.; Erb, G.; et al. Preoperative Adult-Type Diffuse Glioma Subtype Prediction with Dynamic Contrast-Enhanced MR Imaging and Diffusion Weighted Imaging in Tumor Cores and Peritumoral Tissue—A Standardized Multicenter Study. Diagnostics 2025, 15, 532. [Google Scholar] [CrossRef]
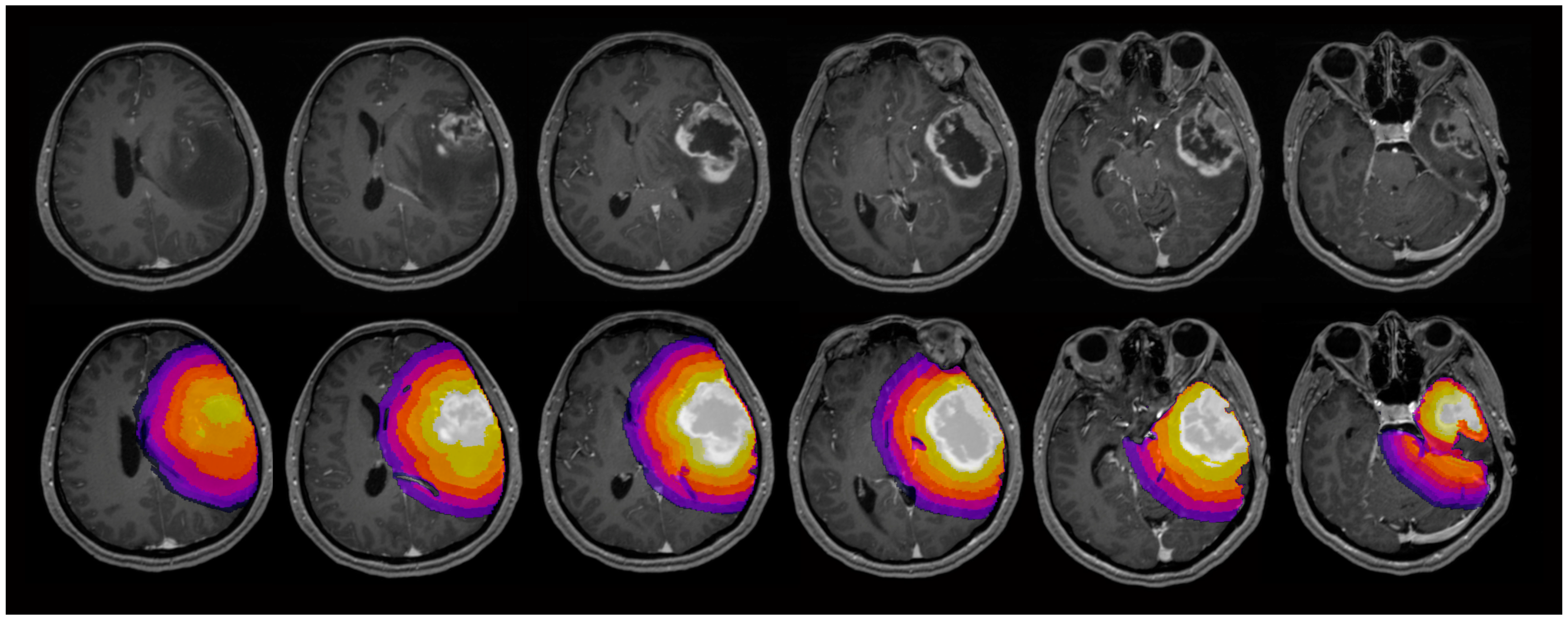

| Patients enrolled in the study | 108 |
| Patients included in the data analysis | 59 |
| Patients excluded due to histopathological/molecular diagnosis | 24 |
| Patients excluded due to insufficient MRI quality | 25 |
| Mean age of the included patients ± SD | 45.3 ± 15.7 |
| Female:male ratio | 1:1.6 |
| Glioblastoma, IDH wildtype (WHO grade 4) | 31 (47.4%) |
| Astrocytoma, IDH mutant (WHO grade 2) | 12 (18.5%) |
| Astrocytoma, IDH mutant (WHO grade 3) | 1 (1.5%) |
| Astrocytoma, IDH mutant (WHO grade 4) | 4 (6.2%) |
| Oligodendroglioma, IDH mutant (WHO grade 2) | 3 (4.6%) |
| Oligodendroglioma, IDH mutant (WHO grade 3) | 8 (12.3%) |
| ADC | MK | ODI | fiso | ficvf | v-csf | v-Extra | v-Intra | ||
|---|---|---|---|---|---|---|---|---|---|
| ROI1 | AUC (95% Confidence interval) | 0.641 (0.452–0.830) | 0.698 (0.522–0.874) | 0.573 (0.397–0.749) | 0.547 (0.377–0.717) | 0.525 (0.348–0.703) | 0.506 (0.319–0.693) | 0.549 (0.379–0.719) | 0.545 (0.367–0.723) |
| Cutoff value 1 | 0.536 | 0.561 | 0.638 | 0.164 | 0.624 | 0.283 | 0.416 | 0.619 | |
| Sensitivity | 0.933 | 0.867 | 0.667 | 0.767 | 0.767 | 0.700 | 0.733 | 0.767 | |
| Specificity | 0.529 | 0.588 | 0.588 | 0.412 | 0.412 | 0.412 | 0.412 | 0.353 | |
| ROI2 | AUC (95% Confidence interval) | 0.855 (0.738–0.972) | 0.816 (0.672–0.959) | 0.569 (0.395–0.742) | 0.622 (0.458–0.785) | 0.643 (0.477–0.810) | 0.690 (0.531–0.849) | 0.459 (0.284–0.633) | 0.709 (0.555–0.863) |
| Cutoff value 1 | 0.534 | 0.454 | 0.650 | 0.204 | 0.663 | 0.232 | 0.330 | 0.645 | |
| Sensitivity | 0.867 | 0.933 | 0.533 | 0.600 | 0.567 | 0.900 | 0.800 | 0.667 | |
| Specificity | 0.824 | 0.706 | 0.765 | 0.647 | 0.765 | 0.412 | 0.059 | 0.606 | |
| ROI3 | AUC (95% Confidence interval) | 0.902 (0.812–0.992) | 0.882 (0.680–0.963) | 0.674 (0.516–0.831) | 0.558 (0.384–0.731) | 0.655 (0.494–0.816) | 0.654 (0.487–0.821) | 0.529 (0.351–0.708) | 0.678 (0.516–0.841) |
| Cutoff value 1 | 0.723 | 0.485 | 0.664 | 0.191 | 0.601 | 0.304 | 0.439 | 0.652 | |
| Sensitivity | 0.767 | 0.993 | 0.633 | 0.800 | 0.700 | 0.633 | 0.633 | 0.600 | |
| Specificity | 0.941 | 0.665 | 0.765 | 0.412 | 0.588 | 0.765 | 0.647 | 0.606 | |
| ROI4 | AUC (95% Confidence interval) | 0.894 (0.797–0.991) | 0.798 (0.638–0.958) | 0.676 (0.520–0.833) | 0.504 (0.328–0.680) | 0.642 (0.481–0.803) | 0.559 (0.387–0.731) | 0.564 (0.387–0.741) | 0.656 (0.489–0.823) |
| Cutoff value 1 | 0.737 | 0.627 | 0.690 | 0.226 | 0.668 | 0.262 | 0.429 | 0.626 | |
| Sensitivity | 0.833 | 0.800 | 0.533 | 0.367 | 0.533 | 0.767 | 0.633 | 0.633 | |
| Specificity | 0.882 | 0.824 | 0.824 | 0.471 | 0.824 | 0.412 | 0.647 | 0.647 | |
| ROI5 | AUC (95% Confidence interval) | 0.910 (0.824–0.995) | 0.778 (0.609–0.948) | 0.672 (0.513–0.830) | 0.594 (0.428–0.760) | 0.651 (0.483–0.819) | 0.614 (0.449–0.778) | 0.567 (0.385–0.749) | 0.697 (0.529–0.865) |
| Cutoff value 1 | 0.666 | 0.543 | 0.680 | 0.224 | 0.574 | 0.315 | 0.419 | 0.613 | |
| Sensitivity | 0.833 | 0.867 | 0.567 | 0.600 | 0.833 | 0.433 | 0.633 | 0.800 | |
| Specificity | 0.882 | 0.665 | 0.765 | 0.529 | 0.471 | 0.824 | 0.588 | 0.647 | |
| ROI6 | AUC (95% Confidence interval) | 0.840 (0.718–0.962) | 0.775 (0.598–0.951) | 0.637 (0.466–0.808) | 0.629 (0.470–0.789) | 0.572 (0.396–0.4747) | 0.627 (0.468–0.786) | 0.522 (0.349–0.694) | 0.776 (0.640–0.913) |
| Cutoff value 1 | 0.711 | 0.581 | 0.597 | 0.245 | 0.637 | 0.317 | 0.397 | 0.598 | |
| Sensitivity | 0.700 | 0.833 | 0.767 | 0.533 | 0.533 | 0.422 | 0.733 | 0.833 | |
| Specificity | 0.882 | 0.824 | 0.529 | 0.765 | 0.647 | 1.000 | 0.412 | 0.606 | |
| ADC | MK | ODI | fiso | ficvf | v-csf | v-Extra | v-Intra | ||
|---|---|---|---|---|---|---|---|---|---|
| ROI1 | AUC (95% Confidence interval) | 0.615 (0.398–0.832) | 0.604 (0.381–0.827) | 0.647 (0.431–0.863) | 0.631 (0.413–0.849) | 0.529 (0.294–0.764) | 0.652 (0.443–0.862) | 0.572 (0.339–0.805) | 0.668 (0.443–0.894) |
| Cutoff value 1 | 0.558 | 0.549 | 0.301 | 0.732 | 0.594 | 0.694 | 0.586 | 0.472 | |
| Sensitivity | 0.824 | 0.824 | 0.529 | 0.353 | 0.941 | 0.529 | 0.824 | 1.000 | |
| Specificity | 0.455 | 0.455 | 0.727 | 0.909 | 0.273 | 0.818 | 0.364 | 0.455 | |
| ROI2 | AUC (95% Confidence interval) | 0.802 (0.621–0.984) | 0.802 (0.619–0.986) | 0.625 (0.398–0.853) | 0.636 (0.421–0.852) | 0.652 (0.421–0.884) | 0.759 (0.576–0.942) | 0.540 (0.308–0.772) | 0.749 (0.548–0.950) |
| Cutoff value 1 | 0.514 | 0.808 | 0.300 | 0.558 | 0.584 | 0.468 | 0.570 | 0.439 | |
| Sensitivity | 0.882 | 0.824 | 0.824 | 0.824 | 0.765 | 0.941 | 0.882 | 0.941 | |
| Specificity | 0.455 | 0.818 | 0.455 | 0.455 | 0.555 | 0.455 | 0.273 | 0.555 | |
| ROI3 | AUC (95% Confidence interval) | 0.818 (0.649–0.988) | 0.749 (0.547–0.950) | 0.658 (0.440–0.876) | 0.599 (0.374–0.824) | 0.599 (0.364–0.834) | 0.674 (0.475–0.890) | 0.460 (0.229–0.691) | 0.647 (0.423–0.871) |
| Cutoff value 1 | 0.606 | 0.909 | 0.290 | 0.587 | 0.590 | 0.621 | 0.611 | 0.467 | |
| Sensitivity | 0.824 | 0.765 | 0.882 | 0.824 | 0.706 | 0.765 | 0.412 | 0.941 | |
| Specificity | 0.727 | 0.636 | 0.455 | 0.364 | 0.636 | 0.627 | 0.364 | 0.455 | |
| ROI4 | AUC (95% Confidence interval) | 0.738 (0.544–0.932) | 0.674 (0.454–0.893) | 0.674 (0.472–0.876) | 0.604 (0.382–0.826) | 0.460 (0.231–0.707) | 0.631 (0.402–0.860) | 0.516 (0.287–0.745) | 0.540 (0.305–0.775) |
| Cutoff value1 | 0.535 | 0.907 | 0.339 | 0.584 | 0.617 | 0.514 | 0.636 | 0.579 | |
| Sensitivity | 0.824 | 0.765 | 0.529 | 0.765 | 0.059 | 0.941 | 0.294 | 0.765 | |
| Specificity | 0.636 | 0.627 | 0.436 | 0.545 | 0.636 | 0.455 | 0.909 | 0.455 | |
| ROI5 | AUC (95% Confidence interval) | 0.781 (0.600–0.961) | 0.658 (0.442–0.874) | 0.620 (0.411–0.830) | 0.647 (0.428–0.866) | 0.481 (0.240–0.723) | 0.652 (0.438–0.867) | 0.551 (0.327–0.774) | 0.561 (0.331–0.792) |
| Cutoff value 1 | 0.489 | 0.995 | 0.338 | 0.512 | 0.585 | 0.631 | 0.547 | 0.488 | |
| Sensitivity | 0.882 | 0.588 | 0.588 | 0.941 | 1.000 | 0.588 | 0.765 | 1–000 | |
| Specificity | 0.636 | 0.818 | 0.627 | 0.364 | 0.273 | 0.536 | 0.273 | 0.273 | |
| ROI6 | AUC (95% Confidence interval) | 0.797 (0.620–0.974) | 0.631 (0.408–0.854) | 0.508 (0.290–0.726) | 0.556 (0.317–0.795) | 0.503 (0.265–0.740) | 0.567 (0.325–0.809) | 0.561 (0.322–0.801) | 0.548 (0.317–0.779) |
| Cutoff value 1 | 0.523 | 0.935 | 0.340 | 0.518 | 0.499 | 0.531 | 0.585 | 0.521 | |
| Sensitivity | 0.882 | 0.765 | 0.529 | 1.000 | 1.000 | 1.000 | 0.882 | 1.000 | |
| Specificity | 0.727 | 0.545 | 0.182 | 0.273 | 0.273 | 0.364 | 0.364 | 0.182 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerweck, L.; Würtemberger, U.; Klose, U.; Reisert, M.; Richter, V.; Nägele, T.; Staber, D.; Han, T.; Shen, M.; Xie, C.; et al. Performance Comparison of Diffusion Kurtosis Imaging (DKI), Neurite Orientation Dispersion and Density Imaging (NODDI), and Diffusion Microstructure Imaging (DMI) in Predicting Adult-Type Glioma Subtype—A Pilot Study. Cancers 2025, 17, 876. https://doi.org/10.3390/cancers17050876
Zerweck L, Würtemberger U, Klose U, Reisert M, Richter V, Nägele T, Staber D, Han T, Shen M, Xie C, et al. Performance Comparison of Diffusion Kurtosis Imaging (DKI), Neurite Orientation Dispersion and Density Imaging (NODDI), and Diffusion Microstructure Imaging (DMI) in Predicting Adult-Type Glioma Subtype—A Pilot Study. Cancers. 2025; 17(5):876. https://doi.org/10.3390/cancers17050876
Chicago/Turabian StyleZerweck, Leonie, Urs Würtemberger, Uwe Klose, Marco Reisert, Vivien Richter, Thomas Nägele, Deborah Staber, Tong Han, Mi Shen, Chuanmiao Xie, and et al. 2025. "Performance Comparison of Diffusion Kurtosis Imaging (DKI), Neurite Orientation Dispersion and Density Imaging (NODDI), and Diffusion Microstructure Imaging (DMI) in Predicting Adult-Type Glioma Subtype—A Pilot Study" Cancers 17, no. 5: 876. https://doi.org/10.3390/cancers17050876
APA StyleZerweck, L., Würtemberger, U., Klose, U., Reisert, M., Richter, V., Nägele, T., Staber, D., Han, T., Shen, M., Xie, C., Hu, H., Yang, S., Cao, Z., Erb, G., Ernemann, U., & Hauser, T.-K. (2025). Performance Comparison of Diffusion Kurtosis Imaging (DKI), Neurite Orientation Dispersion and Density Imaging (NODDI), and Diffusion Microstructure Imaging (DMI) in Predicting Adult-Type Glioma Subtype—A Pilot Study. Cancers, 17(5), 876. https://doi.org/10.3390/cancers17050876





