1. Introduction
Despite evolving oncological neoadjuvant and adjuvant treatments, surgery still remains the mainstay curative option for patients with rectal cancer, and total mesorectal excision (TME) is the gold standard technique in rectal cancer surgery, as it provides improved control of local recurrence and overall survival [
1]. Although it has been traditionally approached through open laparotomy, minimally invasive approaches are increasingly utilized in patients with rectal cancer, and randomized clinical trials including the COLOR II and the COREAN trials have demonstrated laparoscopic rectal cancer surgery to have similar or better short-term surgical outcomes and similar long-term oncological outcomes compared with open rectal cancer surgery [
2,
3,
4]. However, laparoscopic rectal cancer surgery might be very challenging due to the confines of the narrow bony pelvis and the restricted flexibility of the rigid laparoscopic arms, hence raising concerns regarding tumor clearance and controlling distal and circumferential resection margins. The recent ACOSOG Z6051 and ALaCaRT trials failed to demonstrate the non-inferiority of laparoscopy compared with open surgery concerning pathological outcomes, questioning the true oncological safety of laparoscopic surgery for rectal cancer [
5,
6]. Those limitations of the laparoscopic platform could be potentially overcome by the robotic approach with its articulating instruments, three-dimensional depth of field view, stable camera platform, and possibly more precise tissue dissection [
7]. Robotic-assisted TME was first described in 2006 [
8], and since then, different retrospective studies compared between the robotic and laparoscopic surgical approach, and the majority of them showed no significant differences in postoperative morbidity and oncological outcomes [
9,
10,
11]. The ROLARR randomized clinical trial comparing robotic-assisted surgery with laparoscopic surgery for rectal cancer failed to show clear superiority of the robotic approach in terms of conversion rates [
12], and the COLARAR randomized trial concluded that robotic-assisted surgery did not significantly improve the TME quality compared with conventional laparoscopic surgery [
13]. Despite the growing utilization of robotic surgery in rectal cancer treatment, the question of whether it offers substantial clinical benefits over the more traditional laparoscopic or open approaches remains unanswered. A recent meta-analysis comparing the robotic and laparoscopic approach for rectal cancers found no significant difference in overall survival and postoperative complications between the groups [
14]. Most of the previous literature comparing rectal cancer surgery approach outcomes was limited to pairwise comparison only, and just a few studies have compared the open, laparoscopic, and robotic approaches together. Therefore, the aim of this study was to present a contemporary large cohort long-term comprehensive analysis of robotic rectal cancer surgery compared with laparoscopic and open approach outcomes.
2. Materials and Methods
Study design/population: This was a single-center retrospective study. After the approval of the institutional review board, we searched our electronic medical records to extract all patients who underwent radical rectal resection between the years 2010 and 2020 using all appropriate surgery codes including anterior resection of rectum, proctectomy with coloanal anastomosis, and abdomino-perineal resection (APR). From this database, we chose only patients with a diagnosis of primary non-metastatic rectal adenocarcinoma, designated clinically as stage I-III according to the American Joint Committee on Cancer Guidelines 7th edition [
15]. We excluded non-elective, emergency cases, and palliative procedures. The final cohort was divided into three groups according to the primary surgical approach: robotic (Da Vinci Si or Xi Surgical System Intuitive Surgical, Sunnyvale, CA, USA), laparoscopic, or open approach. The decision regarding the surgical approach was taken according to the surgeon’s discretion. Intraoperative conversion to a different surgical approach was analyzed as the original intention to treat.
Study variables and outcome measures: Patients’ characteristics and demographics included age, gender, body mass index (BMI), smoking status, personal surgical history, comorbidities, and American Society of Anesthesiologists score (ASA). Preoperative disease characteristics included presenting symptoms, disease work-up and imaging, tumor location in the rectum (lower rectum ≤ 5 cm, mid rectum 5–10 cm, upper rectum > 10 cm), pretreatment clinical staging, neoadjuvant oncological therapy, and preoperative laboratory values. Operative characteristics included the extent of resection performed, such as abdomino-perineal resection (APR), low anterior resection of rectum (with TME), and anterior resection of rectum (with tumor-specific TME), type of stoma constructed (which was performed at the discretion of the surgeon), additional procedure undertaken during surgery, type of anastomosis, skin incision location, conversion to open procedure, and intraoperative complications. Postoperative surgical outcomes included length of hospital stay (LOS), 30-day readmission rates and in hospital or 30-day postoperative complications, and their severity grading according to the Clavien–Dindo classification system [
16]. A Clavien–Dindo grade > 2 was defined as a major complication. Histopathological surgical results included tumor size, differentiation, pathological stage, number of harvested lymph nodes, and distance of tumor from distal margins. Long-term oncological outcomes included details about adjuvant radiation therapy or adjuvant chemotherapy, the presence of local or distant disease recurrence, and mortality data. Recurrence-free survival (RFS) was defined as the period from the date of surgery to the date of the first recurrence. If tumor recurrence was not recorded, RFS was defined as the time between the date of surgery and the date of the last follow-up. Overall survival (OS) was calculated from the date of surgery to either the date of death or the date of the last follow-up visit.
Statistical analysis: For numerical variables, we reported the median and range for each group and tested their difference using the Wilcoxon rank test. For categorical variables, we used the chi-squared test of independence using bootstrap to obtain a more accurate
p-value when there were categories with low frequencies.
p value < 0.05 was considered statistically significant. Univariate and multivariate analyses for risk factors for major complications were performed using the logistic regression model. The multivariate analysis was adjusted for the covariables which had a
p < 0.3 in the univariate analysis (age, gender, tumor distance from anal verge, surgical approach, neoadjuvant radiation, and stoma creation). We used the Kaplan–Meier procedure for the estimated survival curves and their 95% confidence intervals. Univariate and multivariate analyses for disease recurrence were based on the Cox proportional hazard regression. The multivariate analysis for disease recurrence was adjusted for the covariables which had a
p < 0.3 in the univariate analysis (age, surgical approach, tumor distance from anal verge, and positive margins).
4. Discussion
There is still a continuous unsolved debate regarding the optimal surgical approach to treat rectal cancer [
3,
4,
5,
6]. Therefore, this present study aimed to retrospectively review the short- and long-term outcomes of robotic-assisted approach compared with the laparoscopic-assisted approach and the open approach.
Interestingly, in our study, the robotic group included more patients with distal and clinically locally advanced tumors compared with the other two groups. This was probably the reason for the higher rates of neoadjuvant radiation treatment observed among the robotic group, and also for the higher proportion of abdomino-perineal resections among the robotic group. Nevertheless, those differences did not translate into worse oncological outcomes as could possibly be expected. Similarly, in a study analyzing the short-term outcomes of 2114 consecutive patients in a single center in Korea, the robotic group also had the most distal tumors among the three groups, and the laparoscopic group included less patients with advanced and lower rectal tumors. The authors concluded that laparoscopic-assisted rectal cancer surgery tends to be preferred for upper rectal cancers and less advanced tumors, irrespective of the surgeon’s competence, and that the robotic procedure was probably chosen to overcome surgical complexity in patients with locally advanced and lower rectal cancers [
17].
Despite operating on more distal challenging tumors, our robotic group had significantly lower conversion rates to an open procedure compared with the laparoscopic approach. This is in concordance with multiple prior studies that also described significant lower conversion rates among the robotic approach [
17,
18], while some studies have reported similar conversion rates between the laparoscopic and robotic approach [
19,
20]. This has clinical significance, as patients converted from the minimally invasive approach to open surgery were found to be at a greater risk for perioperative morbidity and to have worse oncological outcomes [
21]. We speculate whether those lower conversion rates among the robotic group are partially related to better patients’ selection, and maybe surgeons using the robotic platform early on in their learning curve have a higher threshold for conversion compared with traditional laparoscopy. In addition, the higher proportion of neoadjuvant radiation among the robotic group, which is known to cause fibrosis and tissue edema that can make surgery technically more difficult [
22], has not translated into higher rates of intraoperative complications, nor in intraoperative bleeding, both of which were significantly less prevalent among the robotic approach. Previous meta-analyses and systematic reviews have also observed lower intraoperative blood loss during robotic-assisted rectal cancer surgery compared with laparoscopic and open surgery [
20,
23]. This could be theoretically explained by the technical advantages of the robotic platform, including three-dimensional high-definition visualization, providing a detailed, stable, and magnified view of the surgical field, instrumental dexterity, and precise and stable dissection, all of which could potentially lead to decreased surgical blood loss, yet we acknowledge the need for cautious interpretation of these results. Still, higher blood loss during surgery has been associated with poor prognosis in colorectal cancer [
24]; therefore, these findings suggest that robotic surgery may indirectly improve the prognosis of patients undergoing rectal surgery for cancer.
Consistent with previous studies, we also found a significant shorter hospital stay in the robotic group compared with the other two groups [
20,
25], and early postoperative complications, specifically ileus and wound infection, occurred more frequently in the open group than in the robotic and laparoscopic groups [
17,
26,
27]. Moreover, major complications (Clavien–Dindo grade > 2) were more prevalent among the open group as well, and this is clinically meaningful as major complications after proctectomy for cancer are associated with earlier disease recurrence, ultimately leading to decreased survival [
28]. Our multivariate analysis has found loop ileostomy creation, open surgery, and male gender to be associated with major complications. The latter two factors may be related to limited visibility and a confined narrow workspace, eventually leading to a more technically difficult surgery, possibly dissecting along non-anatomical planes, increasing blood loss, and altogether resulting in an increased postoperative morbidity. In a similar manner, Kim et al. reported that anastomotic complications, including leakage, abscess, fistula, and stricture, were significantly associated with male patients in multivariate analysis (OR, 1.85; 95 % CI, 1.049–3.263;
p = 0.034), and so performed postoperative ileus (OR, 1.775; 95 % CI, 1.092–2.887;
p = 0.021) [
17]. This strengthens the notion that rectal cancer surgery in male patients is different than in females and could be very challenging and associated with an increased potential for major morbidity. Surgeons and oncologists should consider these issues upon deciding on preoperative treatment, while considering and planning surgery in male patients differently from females. For example, it should be considered to lower the “threshold” for non-operative management in male patients that seem to have a complete clinical response following neoadjuvant therapy.
Our overall postoperative complication rates were relatively high compared with previous studies [
23,
26]. We believe that this may be related to the definition and classification applied to postoperative morbidity documentation. We followed a very broad interpretation and included in our postoperative complication recordings any deviation from the normal postoperative course (such as electrolyte disturbances).
In line with the previous literature [
17,
26], the three groups did not differ in the number of retrieved lymph nodes, nor in terms of positive distal/radial margins, both of which serve as benchmarks for surgical oncology quality in rectal cancer surgery. On the other hand, a systematic review by Khajeh et al. found that the robotic approach had significantly higher rates of negative radial margins and a higher number of harvested lymph nodes than the open approach did, and also higher rates of negative radial margins than the laparoscopic approach [
23]. The authors stated that this might be related to better visualization and improved access to the pelvis, utilizing the robotic platform. However, the superior histopathological outcomes of the robotic approach have not translated into differences in OS or DFS in this meta-analysis. Our robotic group did show significantly longer distal margins compared with the two other groups, which might also be related to the higher proportions of abdomino-perineal resections performed among this group. Unfortunately, our pathological reports lack reference regarding the completeness of mesorectal excision, which also serves as a surrogate marker for quality rectal cancer surgery.
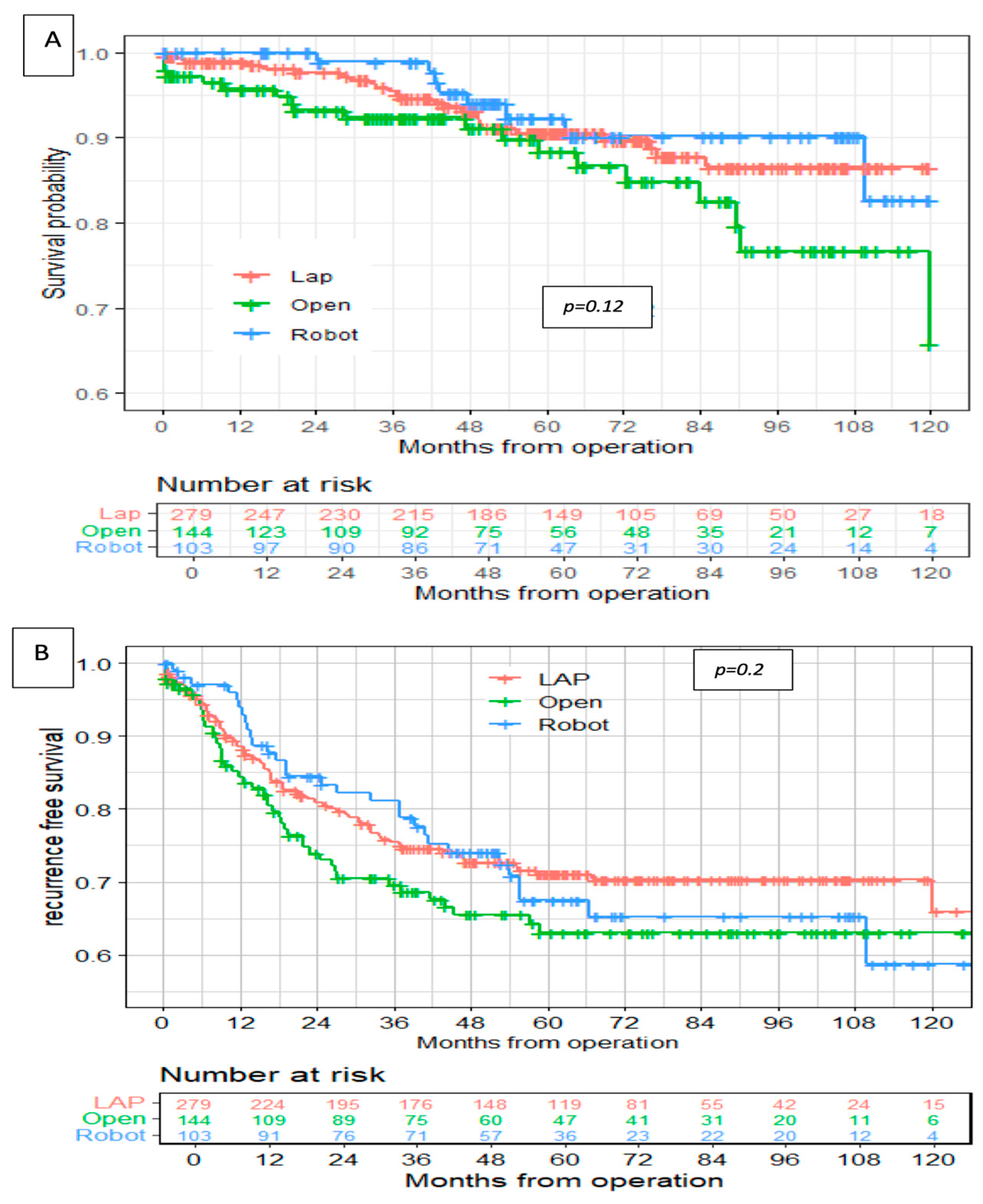
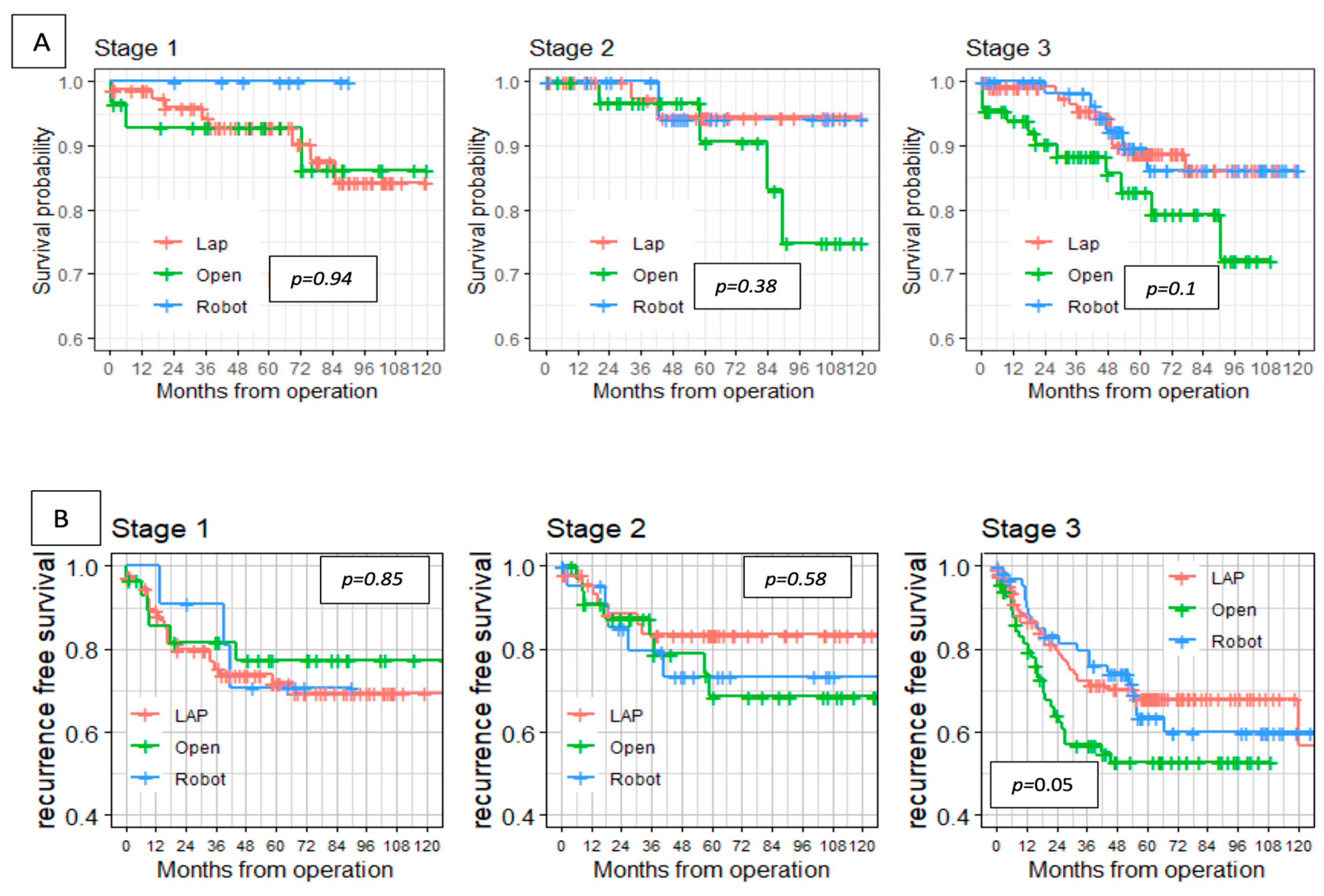
In this study, the robotic group showed similar long-term oncological outcomes compared with the laparoscopic and open approaches, and there were no significant differences between the groups in overall survival and disease-free survival rates. Those results are similar to previously published studies [
23,
26,
29]. Our multivariate analysis has found shorter tumor distances from the anal verge and involved distal/radial surgical margins to be significantly associated with disease relapse. Similarly, Kim et al. found the local recurrence rates to be closely related to postoperative hemorrhage (OR, 14.02; 95% CI, 2.592–75.84;
p = 0.002), DRM+ (OR, 13.4; 95% CI, 2.319– 77.439;
p = 0.004), anastomotic complications (OR, 5.514; 95% CI, 1.416–21.475;
p = 0.014), and tumor location (OR, 0.364; 95% CI, 0.134–0.989;
p = 0.047) in multivariate analysis, but their study had a limited follow-up period [
17]. The minimal acceptable DRM is controversial, but clearly, a threatened DRM is associated with local recurrence, and approximately three times more local recurrence was reported in patients with DRM <2 cm than in those with DRM >2 cm in one study [
30]. This again emphasizes the importance of precise surgical techniques and controlling the distal resection margins in relation to disease-free survival and long-term oncological outcomes. Although we have not found differences in the margin positivity rate, the robotic group in our study presented significantly larger DRMs compared with the other groups, and this can imply that the robotic platform might be better in clear and safe distal resection margins compared with the open and laparoscopic approaches. However, Mirza et al. found that robotic and open TME were associated with higher margin positivity rates (8.2% versus 6.6% versus 1.9%, respectively,
p = 0.17) compared with laparoscopic TME, and they thought it was related to the higher percentage of low rectal cancers in the robotic and open cohorts [
29].
Our study has some limitations. First of all, the non-randomized, retrospective nature of our study is subject to a selection bias due to the surgeon’s preference for each procedure. In addition, this was a single-institution study, which limits the ability to generalize our results. The impact of the learning curve on surgical outcomes, specifically in minimally invasive approaches, such as robotic and laparoscopic, is substantial and should be considered. Our analysis included heterogenous populations of the respective groups regarding tumor location in the rectum, tumor stage, and preoperative radiation therapy, all of which could have an effect on surgical and oncological outcomes. However, this was confronted by subgroup analysis. Despite comprehensive data collection, our report lacks information on the completeness of mesorectal excision, estimated blood loss, and length of surgery, all of which are important markers in comparing the different surgical approaches.