Low Intratumoral CD200 Protein Expression in Primary Merkel Cell Carcinoma Is a Strong Predictor for Disease Relapse
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Analysis of Merkel Cell Polyomavirus (MCPyV) in FFPE Tissue
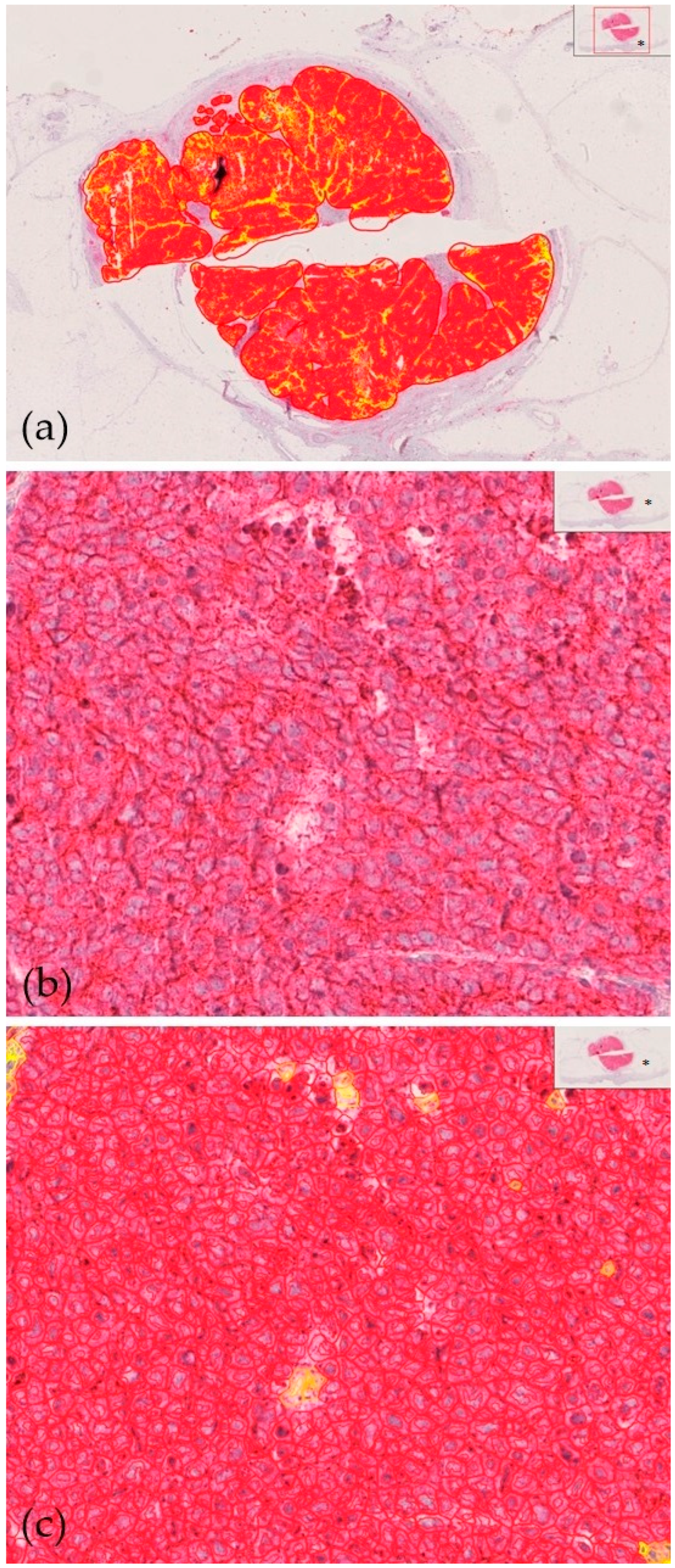
2.3. Immunohistochemistry of MCC Tumor Samples
2.4. Microscopic Evaluation
2.5. Statistics
3. Results
3.1. Patients’ Characteristics
3.2. Expression of CD200/CD200R
3.3. Patients’ Treatment and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, J.C.; Beer, A.J.; DeTemple, V.K.; Eigentler, T.; Flaig, M.; Gambichler, T.; Grabbe, S.; Höller, U.; Klumpp, B.; Lang, S.; et al. S2k Guideline-Merkel cell carcinoma (MCC, neuroendocrine carcinoma of the skin)-Update 2022. J. Der Dtsch. Dermatol. Ges. 2023, 21, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Stang, A.; DeCaprio, J.A.; Cerroni, L.; Lebbé, C.; Veness, M.; Nghiem, P. Merkel cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17077. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Stang, A.; Schrama, D.; Ugurel, S. Merkel Cell Carcinoma: Integrating Epidemiology, Immunology, and Therapeutic Updates. Am. J. Clin. Dermatol. 2024, 25, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Stang, A.; Möller, L.; Wellmann, I.; Claaßen, K.; Kajüter, H.; Ugurel, S.; Becker, J.C. Incidence and Relative Survival of Patients with Merkel Cell Carcinoma in North Rhine-Westphalia, Germany, 2008–2021. Cancers 2024, 16, 2158. [Google Scholar] [CrossRef]
- Nghiem, P.; Kaufman, H.L.; Bharmal, M.; Mahnke, L.; Phatak, H.; Becker, J.C. Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Future Oncol. 2017, 13, 1263–1279. [Google Scholar] [CrossRef]
- Becker, J.C.; Lorenz, E.; Ugurel, S.; Eigentler, T.K.; Kiecker, F.; Pföhler, C.; Kellner, I.; Meier, F.; Kähler, K.; Mohr, P.; et al. Evaluation of real-world treatment outcomes in patients with distant metastatic Merkel cell carcinoma following second-line chemotherapy in Europe. Oncotarget 2017, 8, 79731–79741. [Google Scholar] [CrossRef]
- Angeles, C.V.; Sabel, M.S. Immunotherapy for Merkel cell carcinoma. J. Surg. Oncol. 2021, 123, 775–781. [Google Scholar] [CrossRef]
- Kacew, A.J.; Dharaneeswaran, H.; Starrett, G.J.; Thakuria, M.; LeBoeuf, N.R.; Silk, A.W.; DeCaprio, J.A.; Hanna, G.J. Predictors of immunotherapy benefit in Merkel cell carcinoma. Oncotarget 2020, 11, 4401–4410. [Google Scholar] [CrossRef]
- Abu Rached, N.; Becker, J.C.; Lonsdorf, A.S.; Keller, A.; Zeglis, I.A.; Gambichler, T. Introducing MCC-PS: A novel prognostic score for Merkel cell carcinoma. Front. Oncol. 2024, 14, 1427740. [Google Scholar] [CrossRef]
- Gambichler, T.; Abu Rached, N.; Susok, L.; Becker, J.C. Serum neuron-specific enolase independently predicts outcomes of patients with Merkel cell carcinoma. Br. J. Dermatol. 2022, 187, 806–808. [Google Scholar] [CrossRef]
- Guénolé, M.; Bénigni, P.; Bourbonne, V.; Lucia, F.; Legoupil, D.; Pradier, O.; Misery, L.; Uguen, A.; Schick, U. The prognostic significance of PD-L1 expression on tumor and immune cells in Merkel cell carcinoma. J. Cancer Res. Clin. Oncol. 2021, 147, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Moreaux, J.; Veyrune, J.L.; Reme, T.; De Vos, J.; Klein, B. CD200: A putative therapeutic target in cancer. Biochem. Biophys. Res. Commun. 2008, 366, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Hu, A.; Zhu, J.; Yu, J.; Talebian, F.; Bai, X.F. CD200-CD200R Pathway in the Regulation of Tumor Immune Microenvironment and Immunotherapy. Adv. Exp. Med. Biol. 2020, 1223, 155–165. [Google Scholar] [PubMed]
- Lee, M.H.; Kim, Y.J.; Yun, K.A.; Won, C.H.; Lee, M.W.; Choi, J.H.; Chang, S.E.; Lee, W.J. Prognostic significance of CD200 protein expression and its correlation with COX-2 in cutaneous melanoma. J. Am. Acad. Dermatol. 2020, 82, 1526–1528. [Google Scholar] [CrossRef]
- Talebian, F.; Liu, J.Q.; Liu, Z.; Khattabi, M.; He, Y.; Ganju, R.; Bai, X.F. Melanoma cell expression of CD200 inhibits tumor formation and lung metastasis via inhibition of myeloid cell functions. PLoS ONE 2012, 7, e31442. [Google Scholar] [CrossRef]
- Talebian, F.; Yu, J.; Lynch, K.; Liu, J.Q.; Carson, W.E.; Bai, X.F. CD200 Blockade Modulates Tumor Immune Microenvironment but Fails to Show Efficacy in Inhibiting Tumor Growth in a Murine Model of Melanoma. Front. Cell Dev. Biol. 2021, 9, 739816. [Google Scholar] [CrossRef]
- Petermann, K.B.; Rozenberg, G.I.; Zedek, D.; Groben, P.; McKinnon, K.; Buehler, C.; Kim, W.Y.; Shields, J.M.; Penland, S.; Bear, J.E.; et al. CD200 is induced by ERK and is a potential therapeutic target in melanoma. J. Clin. Investig. 2007, 117, 3922–3929. [Google Scholar] [CrossRef]
- Tonks, A.; Hills, R.; White, P.; Rosie, B.; Mills, K.I.; Burnett, A.K.; Darley, R.L. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia 2007, 21, 566–568. [Google Scholar] [CrossRef]
- Rygiel, T.P.; Meyaard, L. CD200R signaling in tumor tolerance and inflammation: A delicate balance. Curr. Opin. Immunol. 2011, 23, 697–702. [Google Scholar]
- Erin, N.; Podnos, A.; Tanriover, G.; Duymuş, Ö.; Cote, E.; Khatri, I.; Gorczynski, R.M. Bidirectional effect of CD200 on breast cancer development and metastasis, with ultimate outcome determined by tumor aggressiveness and a cancer-induced inflammatory response. Oncogene 2015, 34, 3860–3870. [Google Scholar] [CrossRef]
- Su, Y.; Yamazaki, S.; Morisue, R.; Suzuki, J.; Yoshikawa, T.; Nakatsura, T.; Tsuboi, M.; Ochiai, A.; Ishii, G. Tumor-Infiltrating T Cells Concurrently Overexpress CD200R with Immune Checkpoints PD-1, CTLA-4, and TIM-3 in Non-Small-Cell Lung Cancer. Pathobiology 2021, 88, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Abu Rached, N.; Nick, M.; Susok, L.; Becker, J.C.; Gambichler, T. Intra-tumoral CD200/200R protein expression in advanced melanoma patients who underwent treatment with immune checkpoint inhibitors: Preliminary data. J. Dtsch. Dermatol. Ges. 2023, 21, 120–121. [Google Scholar]
- Zheng, Y.; Tang, L.; Chen, J. CD200/CD200R axis in cancer immunotherapy: New perspectives on an emerging target. Front. Immunol. 2020, 11, 1788. [Google Scholar]
- Gaiser, M.R.; Weis, C.A.; Gaiser, T.; Jiang, H.; Buder-Bakhaya, K.; Herpel, E.; Warth, A.; Xiao, Y.; Miao, L.; Brownell, I. Merkel cell carcinoma expresses the immunoregulatory ligand CD200 and induces immunosuppressive macrophages and regulatory T cells. Oncoimmunology 2018, 7, e1426517. [Google Scholar] [CrossRef]
- Love, J.E.; Thompson, K.; Kilgore, M.R.; Westerhoff, M.; Murphy, C.E.; Papanicolau-Sengos, A.; McCormick, K.A.; Shankaran, V.; Vandeven, N.; Miller, F.; et al. CD200 Expression in Neuroendocrine Neoplasms. Am. J. Clin. Pathol. 2017, 148, 236–242. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer. AJCC Cancer Staging Handbook. Merkel Cell Carcinoma; Springer: New York, NY, USA, 2017. [Google Scholar]
- Wieland, U.; Mauch, C.; Kreuter, A.; Krieg, T.; Pfister, H. Merkel cell polyomavirus DNA in persons without merkel cell carcinoma. Emerg. Infect. Dis. 2009, 15, 1496–1498. [Google Scholar] [CrossRef]
- Gambichler, T.; Dreißigacker, M.; Kasakovski, D.; Skrygan, M.; Wieland, U.; Silling, S.; Gravemeyer, J.; Melior, A.; Cherouny, A.; Stücker, M.; et al. Patched 1 expression in Merkel cell carcinoma. J. Dermatol. 2021, 48, 64–74. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Gambichler, T.; Gnielka, M.; Rüddel, I.; Stockfleth, E.; Stücker, M.; Schmitz, L. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol. Immunother. 2017, 66, 1199–1204. [Google Scholar] [CrossRef]
- Khan, I.Z.; Del Guzzo, C.A.; Shao, A.; Cho, J.; Du, R.; Cohen, A.O.; Owens, D.M. The CD200-CD200R Axis Promotes Squamous Cell Carcinoma Metastasis via Regulation of Cathepsin, K. Cancer Res. 2021, 81, 5021–5032. [Google Scholar] [CrossRef]
- Liu, J.Q.; Talebian, F.; Wu, L.; Liu, Z.; Li, M.S.; Wu, L.; Zhu, J.; Markowitz, J.; Carson WE 3rd Basu, S.; Bai, X.F. A Critical Role for CD200R Signaling in Limiting the Growth and Metastasis of CD200+ Melanoma. J. Immunol. 2016, 197, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Nip, C.; Wang, L.; Liu, C. CD200/CD200R: Bidirectional Role in Cancer Progression and Immunotherapy. Biomedicines 2023, 11, 3326. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.L.; Lu, C.T.; Karmakar, R.; Nampalley, K.; Mukundan, A.; Hsiao, Y.-P.; Hsieh, S.-C.; Wang, H.-C. Assessing the efficacy of the spectrum-aided vision enhancer (SAVE) to detect acral lentiginous melanoma, melanoma in situ, nodular melanoma, and superficial spreading melanoma. Diagnostics 2024, 14, 1672. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Hsiao, Y.-P.; Mukundan, A.; Tsao, Y.-M.; Chang, W.Y.; Wang, H.-C. Classification of skin cancer using novel hyperspectral imaging engineering via YOLOv5. J. Clin. Med. 2023, 12, 1134. [Google Scholar] [CrossRef]


| Parameters | Data |
|---|---|
| Age at diagnosis * | |
| Years | 76.2 (50–94) |
| Gender | |
| Male | 34 (50%) |
| female | 34 (50%) |
| Primary MCC localization | |
| Head/neck | 34 (50%) |
| Upper extremities | 23 (33.8%) |
| Lower extremities | 11 (16.2%) |
| MCPyV | |
| Not tested | 50 (73.5%) |
| Positive | 17 (25%) |
| Negative | 1 (1.5%) |
| Tumor stage at diagnosis (AJCC 2017) | |
| I | 19 (27.9%) |
| II | 24 (35.3%) |
| III | 21 (30.9%) |
| IV | 4 (5.9%) |
| Immunosuppression | |
| No | 54 (79.4%) |
| Yes | 14 (20.6%) |
| Treatments | |
| None ** | 35 (51.5%) |
| Immune checkpoint inhibitors | 11 (16.2%) |
| Chemotherapy | 7 (10.3%) |
| Others *** | 15 (22%) |
| Outcomes | |
| MCC relapse | |
| No | 46 (67.6%) |
| Yes | 22 (32.4%) |
| Median time to relapse (months) * | 12 (3–113) |
| MCC death | |
| No | 46 (67.6%) |
| Yes | 22 (32.4%) |
| Median time to death (months) * | 18 (3–133) |
| Parameters | Primary MCC | MCC Metastasis | p-Value |
|---|---|---|---|
| CD200 | |||
| Median (range) H-score | 171.5 (3–284) | 197 (20–271) | p = 0.68 |
| CD200R | |||
| Median (range) H-score | 167 (53–243) | 171 (91–259) | p = 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambichler, T.; Girke, S.; Abu Rached, N.; Susok, L.; Becker, J.C.; Schulze, H.-J.; Hirsch, T.; Kückelhaus, M.; Wellenbrock, S. Low Intratumoral CD200 Protein Expression in Primary Merkel Cell Carcinoma Is a Strong Predictor for Disease Relapse. Cancers 2025, 17, 822. https://doi.org/10.3390/cancers17050822
Gambichler T, Girke S, Abu Rached N, Susok L, Becker JC, Schulze H-J, Hirsch T, Kückelhaus M, Wellenbrock S. Low Intratumoral CD200 Protein Expression in Primary Merkel Cell Carcinoma Is a Strong Predictor for Disease Relapse. Cancers. 2025; 17(5):822. https://doi.org/10.3390/cancers17050822
Chicago/Turabian StyleGambichler, Thilo, Sophia Girke, Nessr Abu Rached, Laura Susok, Jürgen C. Becker, Hans-Joachim Schulze, Tobias Hirsch, Maximilian Kückelhaus, and Sascha Wellenbrock. 2025. "Low Intratumoral CD200 Protein Expression in Primary Merkel Cell Carcinoma Is a Strong Predictor for Disease Relapse" Cancers 17, no. 5: 822. https://doi.org/10.3390/cancers17050822
APA StyleGambichler, T., Girke, S., Abu Rached, N., Susok, L., Becker, J. C., Schulze, H.-J., Hirsch, T., Kückelhaus, M., & Wellenbrock, S. (2025). Low Intratumoral CD200 Protein Expression in Primary Merkel Cell Carcinoma Is a Strong Predictor for Disease Relapse. Cancers, 17(5), 822. https://doi.org/10.3390/cancers17050822