Prognostic Frailty-Based Determinants of Long-Term Mortality in Older Patients with Newly Diagnosed Multiple Myeloma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Study Designs
2.2. Laboratory and Cancer-Specific Variables
2.3. Demographic and Clinical Assessments
2.4. Statistical Analysis
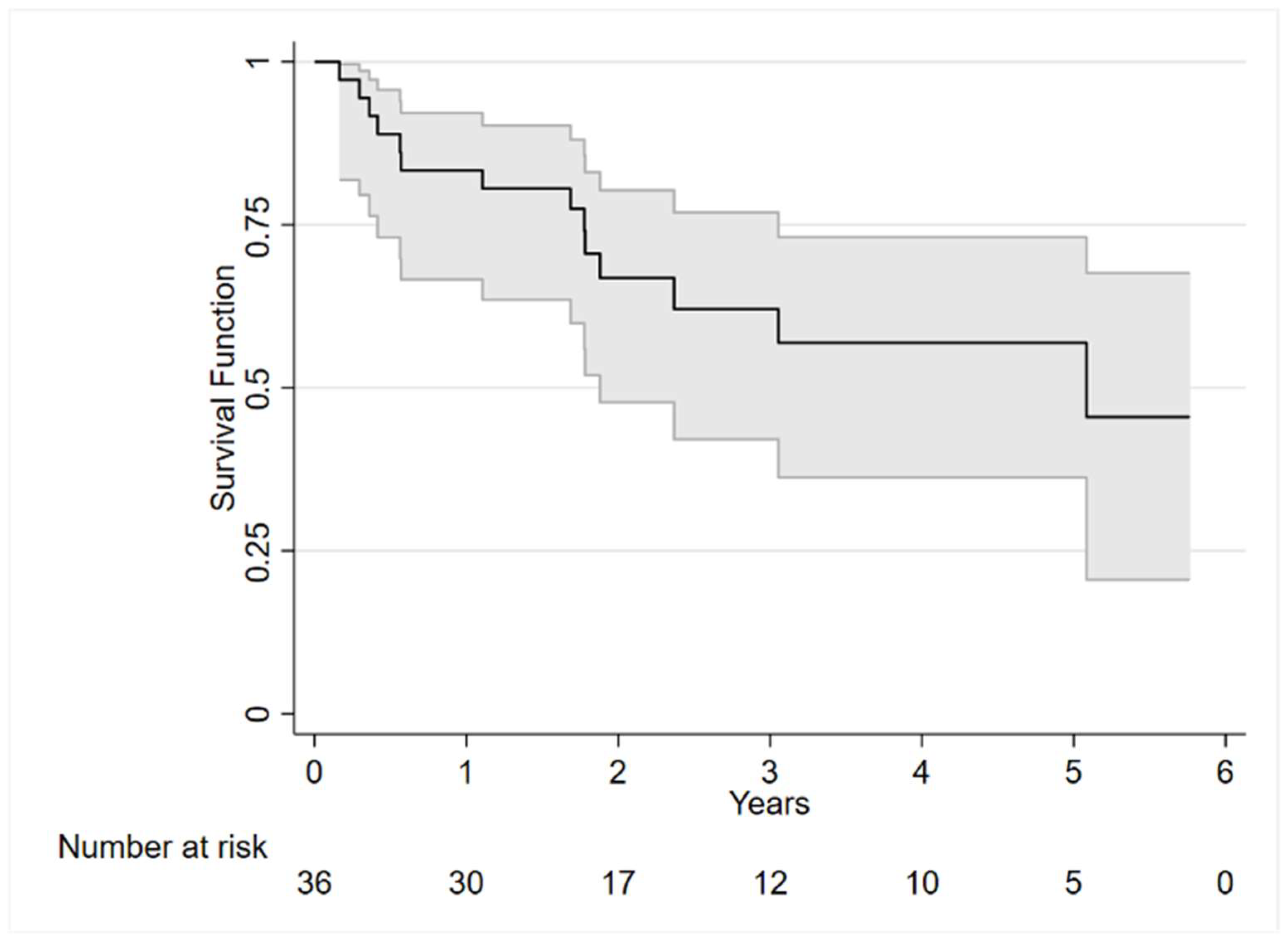
3. Results
3.1. Cohort Characteristics
3.2. CGA and Frailty Assessment
3.3. Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajkumar, S.V. Multiple Myeloma: 2022 Update on Diagnosis, Risk Stratification, and Management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Allen, C.; Barac, A.; Basaleem, H.; Bensenor, I.; Curado, M.P.; Foreman, K.; Gupta, R.; Harvey, J.; Hosgood, H.D.; et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018, 4, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.S.; Barker, K.A.; Anderson, W.F. Future Distribution of Multiple Myeloma in the United States by Sex, Age, and Race/Ethnicity. Blood 2015, 125, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Bringhen, S.; Ludwig, H.; Dimopoulos, M.A.; Bladé, J.; Mateos, M.V.; Rosiñol, L.; Boccadoro, M.; Cavo, M.; Lokhorst, H.; et al. Personalized Therapy in Multiple Myeloma According to Patient Age and Vulnerability: A Report of the European Myeloma Network (EMN). Blood 2011, 118, 4519–4529. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved Survival in Multiple Myeloma and the Impact of Novel Therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Oriol, A.; Martínez-López, J.; Gutiérrez, N.; Teruel, A.-I.; De Paz, R.; García-Laraña, J.; Bengoechea, E.; Martín, A.; Mediavilla, J.D.; et al. Bortezomib, Melphalan, and Prednisone versus Bortezomib, Thalidomide, and Prednisone as Induction Therapy Followed by Maintenance Treatment with Bortezomib and Thalidomide versus Bortezomib and Prednisone in Elderly Patients with Untreated Multiple Myeloma: A Randomised Trial. Lancet Oncol. 2010, 11, 934–941. [Google Scholar] [CrossRef]
- Pozzi, S.; Marcheselli, L.; Bari, A.; Liardo, E.V.; Marcheselli, R.; Luminari, S.; Quaresima, M.; Cirilli, C.; Ferri, P.; Federico, M.; et al. Survival of Multiple Myeloma Patients in the Era of Novel Therapies Confirms the Improvement in Patients Younger than 75 Years: A Population-Based Analysis. Br. J. Haematol. 2013, 163, 40–46. [Google Scholar] [CrossRef]
- Pawlyn, C.; Cairns, D.; Kaiser, M.; Striha, A.; Jones, J.; Shah, V.; Jenner, M.; Drayson, M.; Owen, R.; Gregory, W.; et al. The Relative Importance of Factors Predicting Outcome for Myeloma Patients at Different Ages: Results from 3894 Patients in the Myeloma XI Trial. Leukemia 2020, 34, 604–612. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Mateos, M.-V.; Larocca, A.; Facon, T.; Kumar, S.K.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric Assessment Predicts Survival and Toxicities in Elderly Myeloma Patients: An International Myeloma Working Group Report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef]
- Larocca, A.; Dold, S.M.; Zweegman, S.; Terpos, E.; Wäsch, R.; D’Agostino, M.; Scheubeck, S.; Goldschmidt, H.; Gay, F.; Cavo, M.; et al. Patient-Centered Practice in Elderly Myeloma Patients: An Overview and Consensus from the European Myeloma Network (EMN). Leukemia 2018, 32, 1697–1712. [Google Scholar] [CrossRef]
- Soto-Perez-de-Celis, E.; Li, D.; Yuan, Y.; Lau, Y.M.; Hurria, A. Functional versus Chronological Age: Geriatric Assessments to Guide Decision Making in Older Patients with Cancer. Lancet Oncol. 2018, 19, e305–e316. [Google Scholar] [CrossRef] [PubMed]
- De Alfieri, W.; Costanzo, S.; Borgogni, T. Biological Resilience of Older Adults versus Frailty. Med. Hypotheses 2011, 76, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of Deficits as a Proxy Measure of Aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef]
- Engelhardt, M.; Domm, A.-S.; Dold, S.M.; Ihorst, G.; Reinhardt, H.; Zober, A.; Hieke, S.; Baayen, C.; Müller, S.J.; Einsele, H.; et al. A Concise Revised Myeloma Comorbidity Index as a Valid Prognostic Instrument in a Large Cohort of 801 Multiple Myeloma Patients. Haematologica 2017, 102, 910–921. [Google Scholar] [CrossRef]
- Scheepers, E.R.M.; Vondeling, A.M.; Thielen, N.; Van Der Griend, R.; Stauder, R.; Hamaker, M.E. Geriatric Assessment in Older Patients with a Hematologic Malignancy: A Systematic Review. Haematologica 2020, 105, 1484–1493. [Google Scholar] [CrossRef]
- Larocca, A.; Palumbo, A. How I Treat Fragile Myeloma Patients. Blood 2015, 126, 2179–2185. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Kint, N.; Delforge, M. Concise Review—Treatment of Multiple Myeloma in the Very Elderly: How Do Novel Agents Fit In? J. Geriatr. Oncol. 2016, 7, 383–389. [Google Scholar] [CrossRef]
- Zweegman, S.; Engelhardt, M.; Larocca, A. EHA SWG on ‘Aging and Hematology’ Elderly Patients with Multiple Myeloma: Towards a Frailty Approach? Curr. Opin. Oncol. 2017, 29, 315–321. [Google Scholar] [CrossRef]
- Buckstein, R.; Wells, R.A.; Zhu, N.; Leitch, H.A.; Nevill, T.J.; Yee, K.W.L.; Leber, B.; Sabloff, M.; St Hilaire, E.; Kumar, R.; et al. Patient-Related Factors Independently Impact Overall Survival in Patients with Myelodysplastic Syndromes: An MDS-CAN Prospective Study. Br. J. Haematol. 2016, 174, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Mian, H.S.; Wildes, T.M.; Fiala, M.A. Development of a Medicare Health Outcomes Survey Deficit-Accumulation Frailty Index and Its Application to Older Patients With Newly Diagnosed Multiple Myeloma. JCO Clin. Cancer Inform. 2018, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.G.; Luo, S.; Wildes, T.M.; Sanfilippo, K.M. Frailty in Older Adults With Multiple Myeloma: A Study of US Veterans. JCO Clin. Cancer Inform. 2020, 4, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Abete, P.; Basile, C.; Bulli, G.; Curcio, F.; Liguori, I.; Della-Morte, D.; Gargiulo, G.; Langellotto, A.; Testa, G.; Galizia, G.; et al. The Italian Version of the “Frailty Index” Based on Deficits in Health: A Validation Study. Aging Clin. Exp. Res. 2017, 29, 913–926. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Salmon, S.E. A Clinical Staging System for Multiple Myeloma Correlation of Measured Myeloma Cell Mass with Presenting Clinical Features, Response to Treatment, and Survival. Cancer 1975, 36, 842–854. [Google Scholar] [CrossRef]
- Greipp, P.R.; San Miguel, J.; Durie, B.G.M.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International Staging System for Multiple Myeloma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Rostoft, S.; O’Donovan, A.; Soubeyran, P.; Alibhai, S.M.H.; Hamaker, M.E. Geriatric Assessment and Management in Cancer. J. Clin. Oncol. 2021, 39, 2058–2067. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Rozzini, R.; Bianchetti, A.; Trabucchi, M. Principal Lifetime Occupation and MMSE Score in Elderly Persons. J. Gerontol. 1993, 48, S310–S314. [Google Scholar] [CrossRef]
- Shulman, K.I.; Pushkar Gold, D.; Cohen, C.A.; Zucchero, C.A. Clock-drawing and Dementia in the Community: A Longitudinal Study. Int. J. Geriatr. Psychiatry 1993, 8, 487–496. [Google Scholar] [CrossRef]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Assessing the Nutritional Status of the Elderly: The Mini Nutritional Assessment as Part of the Geriatric Evaluation. Nutr. Rev. 1996, 54, S59–S65. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel index. MD State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative Illness Rating Scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Sheikh, J.I. 9/Geriatric Depression Scale (GDS): Recent Evidence and Development of a Shorter Version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Tinetti, M.E. Performance-Oriented Assessment of Mobility Problems in Elderly Patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef]
- Downie, W.W.; Leatham, P.A.; Rhind, V.M.; Wright, V.; Branco, J.A.; Anderson, J.A. Studies with Pain Rating Scales. Ann. Rheum. Dis. 1978, 37, 378–381. [Google Scholar] [CrossRef]
- García González, J.V.; Díaz Palacios, E.; Salamea García, A.; Cabrera González, D.; Menéndez Caicoya, A.; Fernández Sánchez, A.; Acebal García, V. An evaluation of the feasibility and validity of a scale of social assessment of the elderly. Aten. Primaria 1999, 23, 434–440. [Google Scholar]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A Symptom Score to Predict Persons with Sarcopenia at Risk for Poor Functional Outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Bohannon, R.W. Muscle Strength: Clinical and Prognostic Value of Hand-Grip Dynamometry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 465–470. [Google Scholar] [CrossRef]
- Tjia, J.; Velten, S.J.; Parsons, C.; Valluri, S.; Briesacher, B.A. Studies to Reduce Unnecessary Medication Use in Frail Older Adults: A Systematic Review. Drugs Aging 2013, 30, 285–307. [Google Scholar] [CrossRef] [PubMed]
- EuroQol Group. EuroQol—A New Facility for the Measurement of Health-Related Quality of Life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Stege, C.A.M.; Nasserinejad, K.; Klein, S.K.; Timmers, G.-J.; Hoogendoorn, M.; Ypma, P.F.; Nijhof, I.S.; Velders, G.A.; Strobbe, L.; Durdu-Rayman, N.; et al. Improving the Identification of Frail Elderly Newly Diagnosed Multiple Myeloma Patients. Leukemia 2021, 35, 2715–2719. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics Corner: A Guide to Appropriate Use of Correlation Coefficient in Medical Research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- D’Agostino, M.; Larocca, A.; Offidani, M.; Liberati, A.M.; Gaidano, G.; Petrucci, M.T.; Derudas, D.; Capra, A.; Zambello, R.; Cascavilla, N.; et al. Octogenarian Newly Diagnosed Multiple Myeloma Patients without Geriatric Impairments: The Role of Age >80 in the IMWG Frailty Score. Blood Cancer J. 2021, 11, 73. [Google Scholar] [CrossRef]
- Murillo, A.; Cronin, A.M.; Laubach, J.P.; Hshieh, T.T.; Tanasijevic, A.M.; Richardson, P.G.; Driver, J.A.; Abel, G.A. Performance of the International Myeloma Working Group Myeloma Frailty Score among Patients 75 and Older. J. Geriatr. Oncol. 2019, 10, 486–489. [Google Scholar] [CrossRef]
- Tyczyńska, A.; Krzempek, M.K.; Cortez, A.J.; Jurczyszyn, A.; Godlewska, K.; Ciepłuch, H.; Subocz, E.; Hałka, J.; Kulikowska de Nałęcz, A.; Wiśniewska, A.; et al. The Real-World Evidence on the Fragility and Its Impact on the Choice of Treatment Regimen in Newly Diagnosed Patients with Multiple Myeloma over 75 Years of Age. Cancers 2023, 15, 3469. [Google Scholar] [CrossRef]
- Bird, S.; Cairns, D.; Menzies, T.; Boyd, K.; Davies, F.; Cook, G.; Drayson, M.; Gregory, W.; Jenner, M.; Jones, J.; et al. Sex Differences in Multiple Myeloma Biology but Not Clinical Outcomes: Results from 3894 Patients in the Myeloma XI Trial. Clin. Lymphoma Myeloma Leuk. 2021, 21, 667–675. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Katz, S. Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J. Am. Geriatr. Soc. 1983, 31, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef] [PubMed]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Shumway Cook, A.; Brauer, S.; Woollacott, M. Predicting the Probability for Falls in CommunityDwelling Older Adults Using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896903. [Google Scholar]
| Overall, N = 36 (100%) | Dead, N = 14 (39%) | Alive, N = 22 (61%) | p-Value | |
|---|---|---|---|---|
| Female sex | 12 (33.33%) | 3 (21.43%) | 9 (40.91%) | 0.227 |
| Age | 76 (6.22) | 78.21 (6.76) | 74.59 (5.56) | 0.089 |
| IMWG-FI, median (IQR) | 1 [0–2] | 1.5 [1–3] | 1 [0–2] | 0.367 |
| G8 (≤14), median (IQR) | N = 35 13 [12–15] | N = 13 12 [11–14] | N = 22 13.25 [12–16] | 0.130 |
| MMSE, median (IQR) | 28 [26.6–29] | 27.85 [26.4–28.7] | 28 [27–29] | 0.442 |
| Clock drawing test, median (IQR) | N = 35 2 [1–2] | N = 14 2 [1–3] | N = 21 1 [1–2] | 0.137 |
| MNA | 23.93 (3.68) | 22.54 (3.37) | 24.82 (3.66) | 0.069 |
| IADL, median (IQR) | 7 [7–8] | 8 [7–8] | 8 [8–8] | 0.017 |
| Barthel, median (IQR) | 100 [95–100] | 97.5 [90–100] | 100 [95–100] | 0.381 |
| CIRS comorbidity index | 4.29 (1.92) | 4.74 (2.11) | 4 (1.77) | 0.263 |
| GDS, median (IQR) | N = 35 3 [1–4] | N = 14 3.5 [1–6] | N = 21 3 [2–3] | 0.388 |
| Tinetti test, median (IQR) | N = 34 26 [22–28] | N = 13 23 [21–27] | N = 21 26 [2–28] | 0.276 |
| NRS, median (IQR) | 4 [0–5] | 5 [3–6] | 2 [0–5] | 0.057 |
| Gjion | 8.75 (2.66) | 8.57 (2.38) | 8.86 (2.87) | 0.753 |
| TUG, median (IQR) | N = 34 9.5 [7.2–12] | N = 13 10 [7.2–12] | N = 21 8.5 [8–11] | 0.644 |
| HG | 27.92 (9.16) | 29.76 (8.32) | 26.75 (9.67) | 0.345 |
| SARC-F | N = 20 2.85 (2.39) | N = 8 3.13 (2.42) | N = 12 2.67 (2.46) | 0.686 |
| Cut-off CGA (≥3) | 3.19 (2.15) | 3.71 (2.16) | 2.86 (2.12) | 0.253 |
| Polypharmacy | 6.22 (3.45) | 6.93 (3.79) | 5.77 (3.22) | 0.334 |
| Rockwood’s FI, median (IQR) | 0.18 [0.11–0.23] | 0.21 [0.18–0.27] | 0.16 [0.1–0.2] | 0.066 |
| EuroQoL | N = 35 0.70 (0.17) | N = 13 0.63 (0.20) | N = 22 0.74 (0.14) | 0.072 |
| RT, yes | N = 32 10 (31.25%) | N = 14 4 (28.57%) | N = 18 6 (33.33%) | 0.773 |
| Stage D-S | N = 35 | N = 14 | N = 21 | 0.048 |
| IA | 5 (14.29%) | 0 (0.00%) | 5 (23.81%) | |
| IIA | 11 (31.43%) | 6 (42.86%) | 5 (23.81%) | |
| IIIA | 12 (34.29%) | 7 (50.00%) | 5 (23.81%) | |
| IIIB | 7 (20.00%) | 1 (7.14%) | 6 (28.57%) | |
| ISS stage | 0.221 | |||
| I | 8 (22.22%) | 1 (7.14%) | 7 (31.82%) | |
| II | 13 (36.11%) | 6 (42.86%) | 7 (31.82%) | |
| III | 15 (41.67%) | 7 (50.00%) | 8 (36.36%) |
| Univariate | Backward Stepwise Multivariate # | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Sex (female vs. male) | 0.55 (0.15–1.96) | 0.354 | 0.15 (0.03–0.75) | 0.021 |
| Age | 1.10 (1.01–1.19) | 0.022 * | - | - |
| IMWG-FI | 1.67 (1.09–2.57) | 0.019 | - | - |
| G8 (≤14) (N = 35) | 0.76 (0.60–0.95) | 0.016 | - | - |
| MMSE | 0.90 (0.70–1.14) | 0.368 | - | - |
| Clock drawing test (N = 35) | 1.43 (0.90–2.27) | 0.127 | - | - |
| MNA | 0.81 (0.69–0.95) | 0.008 * | - | - |
| IADL | 0.73 (0.56–0.96) | 0.024 * | - | - |
| Barthel index | 0.96 (0.91–1.01) | 0.158 | - | - |
| CIRS comorbidity index | 1.21 (0.94–1.54) | 0.137 | - | - |
| GDS (N = 35) | 1.27 (1.04–1.55) | 0.021 | - | - |
| Tinetti (N = 34) | 0.92 (0.81–1.04) | 0.195 | - | - |
| NRS | 1.25 (1.02–1.54) | 0.034 | 1.40 (1.09–1.78) | 0.008 |
| Gjion | 1.05 (0.86–1.29) | 0.626 | - | - |
| TUG (N = 34) | 1.07 (0.94–1.22) | 0.311 | - | - |
| HG | 1.01 (0.96–1.06) | 0.781 | - | - |
| SARC F (N = 20) | 1.10 (0.80–1.51) | 0.556 | - | - |
| Cut-off CGA (≥3) | 1.36 (1.03–1.79) | 0.027 * | - | - |
| Polypharmacy | 1.12 (0.96–1.31) | 0.139 | - | - |
| Rockwood’s FI (0.1 increase) | 1.57 (1.07–2.31) | 0.021 | 2.23 (1.29–3.87) | 0.004 |
| EuroQoL (0.1 increase) (N = 35) | 0.75 (0.55–1.02) | 0.066 | - | - |
| RT (yes vs. no) (N = 32) | 0.68 (0.21–2.22) | 0.525 | - | - |
| Stage D-S (IA or IIA vs. IIIA or IIIB) (N = 35) | 0.59 (0.20–1.76) | 0.348 | - | - |
| ISS stage (I or II vs. III) | 0.36 (0.12–1.10) | 0.072 | 6.09 (1.36–27.21) | 0.018 |
| AIC | BIC | C-Index | |
|---|---|---|---|
| Model A | 83.45 | 89.78 | 0.7745 |
| Model B | 88.35 | 94.68 | 0.7493 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzyka, M.; Ottaviani, S.; Caffa, I.; Bonfiglio, T.; Parisi, E.; Guijarro, A.; Tagliafico, L.; Lemoli, R.M.; Ponzano, M.; Marelli, C.; et al. Prognostic Frailty-Based Determinants of Long-Term Mortality in Older Patients with Newly Diagnosed Multiple Myeloma. Cancers 2025, 17, 789. https://doi.org/10.3390/cancers17050789
Muzyka M, Ottaviani S, Caffa I, Bonfiglio T, Parisi E, Guijarro A, Tagliafico L, Lemoli RM, Ponzano M, Marelli C, et al. Prognostic Frailty-Based Determinants of Long-Term Mortality in Older Patients with Newly Diagnosed Multiple Myeloma. Cancers. 2025; 17(5):789. https://doi.org/10.3390/cancers17050789
Chicago/Turabian StyleMuzyka, Mariya, Silvia Ottaviani, Irene Caffa, Tommaso Bonfiglio, Erica Parisi, Ana Guijarro, Luca Tagliafico, Roberto Massimo Lemoli, Marta Ponzano, Cristina Marelli, and et al. 2025. "Prognostic Frailty-Based Determinants of Long-Term Mortality in Older Patients with Newly Diagnosed Multiple Myeloma" Cancers 17, no. 5: 789. https://doi.org/10.3390/cancers17050789
APA StyleMuzyka, M., Ottaviani, S., Caffa, I., Bonfiglio, T., Parisi, E., Guijarro, A., Tagliafico, L., Lemoli, R. M., Ponzano, M., Marelli, C., Signori, A., Nencioni, A., Cea, M., & Monacelli, F. (2025). Prognostic Frailty-Based Determinants of Long-Term Mortality in Older Patients with Newly Diagnosed Multiple Myeloma. Cancers, 17(5), 789. https://doi.org/10.3390/cancers17050789









