The Role of Reactive Oxygen Species in Colorectal Cancer Initiation and Progression: Perspectives on Theranostic Approaches
Simple Summary
Abstract
1. Introduction
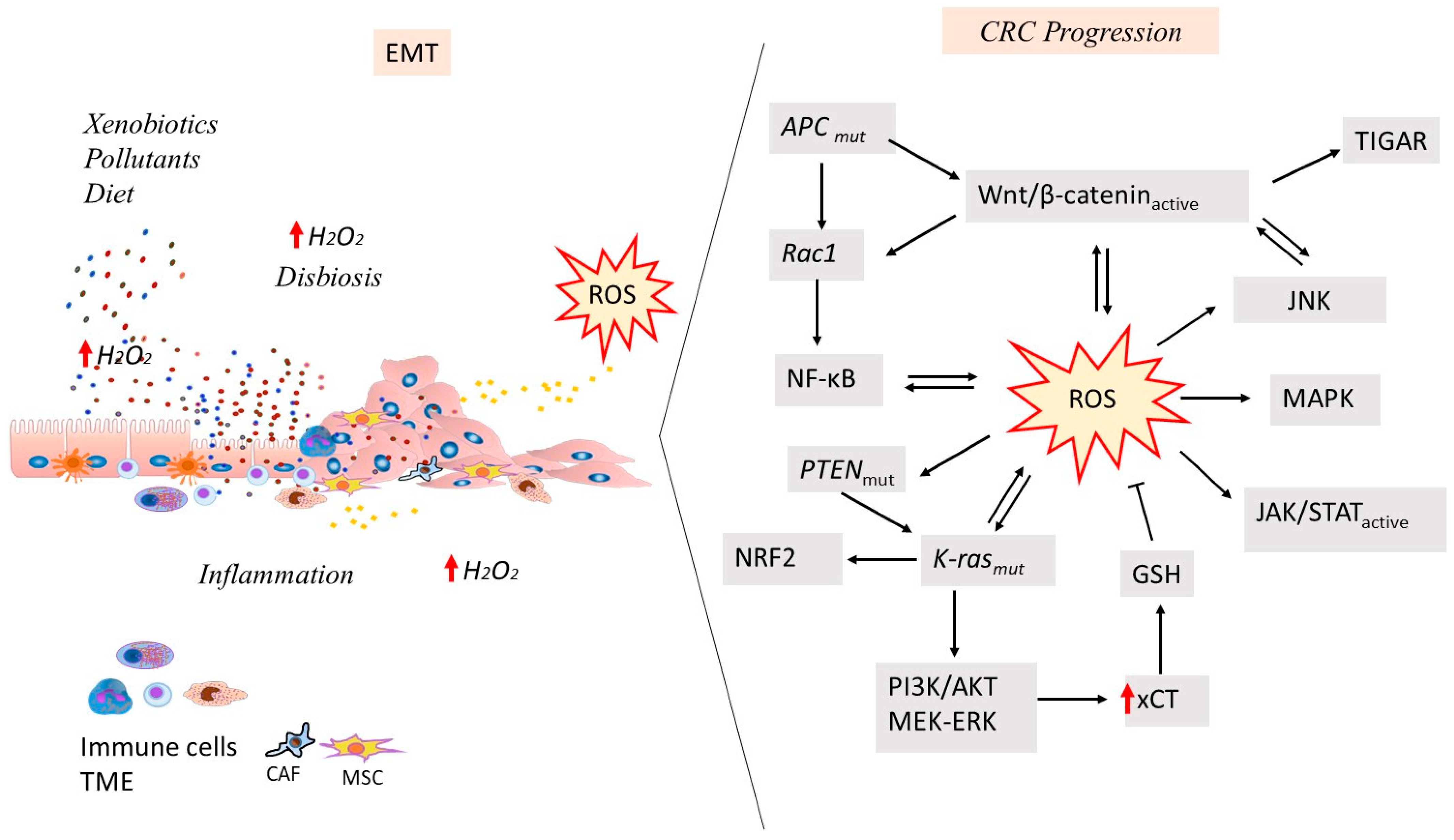
2. Reactive Oxygen Species Play a Role in Mediating CRC Initiation
3. Reactive Oxygen Species Play a Role in Mediating CRC Progression
| CRC Progression | |||
|---|---|---|---|
| Target Molecules or Pathways Activated by ROS | Effect | Cell Response | References |
| Wnt/β-catenin | induction of TIGAR and RAC1 | TIGAR regenerates the antioxidant GSH levels; RAC1 involved in ROS generation and nuclear localization of β-catenin | [96,98] |
| circMYH9 | overexpression of circMYH9; increased serine production and redox homeostasis | increased cell proliferation | [98] |
| KRAS mutations | activation of MAPK pathway; ROS production from mitochondria or COX2 resulting in additional mutations | drug resistance; activation of response to DNA damage, de-differentiation, genetic instability, hyperproliferation, activation of NADPH oxidase complex, inactivation of antioxidants | [87] |
| KRAS, p53 and SMAD4 mutations | changes in cell proliferation and nuclear accumulation of β-catenin | progression of adenoma to colon cancer | [94] |
| ERK and JNK in a concentration-dependent manner by MLK3 | equilibrium in the signal flow dependent from ROS concentration between the ERK and JNK pathways | increased cell proliferation | [100] |
| Loss of p53 | induction of protumorigenic inflammatory microenvironment NF-kB-dependent | increased gut permeability and EMT | [94] |
| JNK | regulation of transcriptional activities; interplay with Wnt signaling and other pathways | inflammation, apoptosis, cell proliferation, metastasis, and angiogenesis in TME | [35,102,103] |
| MAPK/JNK pathways | regulation of the Warburg effect | tumor progression | [102] |
| CXCL14 upregulation | regulation of EMT process and expression of cyclin A1/B1, CDK1/2 and E-cadherin, N-cadherin, vimentin | CRC progression | [104] |
| Increased exogenous cystine/cysteine uptake by SLC7A11, SLC3A2, SLC1A4, SLC1A5, SLC25A39 | process mediated by ATF4 | high intracellular cysteine levels; lower cytotoxic levels of ROS to support CRC progression | [109] |
| USP11 deubiquitinating enzyme overexpression | USP11 role in CRC as an oncogene | inhibition of mitochondrial apoptosis | [113] |
4. ROS Detection and Identification
5. Implications of ROS Production for CRC Treatment
5.1. Surgery
5.2. Prognosis
5.3. ROS as a Therapeutic Strategy in CRC
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
Abbreviations
| ACSL3 | Acyl-coenzyme A (CoA) synthetase long-chain family member 3; |
| AKT | Protein kinase B; |
| AMPK | 5′ AMP-activated protein kinase; |
| 6-AN | 6-anicotinamide; |
| Anlo | Anlotinib; |
| APC | Adenomatous polyposis coli; |
| ASK1 | Apoptosis signal-regulating kinase 1; |
| ATPP-DTPA | 5-(4-amino-phenyl)-10,15,20-triphenylporphyrin-diethylene-triaminopentaacetic acid; |
| ATF4 | Activating transcription factor 4; |
| BER | Base excision repair; |
| BMP | Bone morphogenetic protein; |
| BRAF | B-Raf proto-oncogene; |
| CAFs | Cancer-associated fibroblasts; |
| CAT | Catalase; |
| CDDP | Cisplatin; |
| CDK | Cyclin-dependent kinase; |
| CIN | Chromosomal instability; |
| CIOSS | CRC-integrated OS score; |
| CM-H2DCFDA | 5-(and 6)-chloromethyl-2′,7′-dichlorohydrofluorescein diacetate; |
| COX-2 | Cyclooxygenase-2; |
| CPT-11 | Irinotecan; |
| CRC | Colorectal cancer; |
| CTLA-4 | Cytotoxic T lymphocyte-associated protein 4; |
| CtBP1 | C-terminal binding protein-1; |
| CXCL2 | C-X-C motif chemokine ligand 2; |
| Cyt-c | Cytochrome-c; |
| DCF | 2′,7′-dichlorofluorescein; |
| DHA | Dihydroartemisinin; |
| DHE | Dihydroethidium; |
| d-ROMs | Reactive oxygen metabolite derivatives; |
| Dvl | Dishevelled protein 1; |
| DTNB | 5,5′-dithio-bis(2-nitrobenzoic acid); |
| EGFR | Epidermal growth factor receptor; |
| ELISA | Enzyme-linked immunosorbent assay; |
| EMT | Epithelial-to-mesenchymal transition; |
| ER | Endoplasmic reticulum; |
| ERK | Extracellular signal-regulated kinase; |
| FGFR | Fibroblast growth factor receptor; |
| FLIM | Fluorescence lifetime imaging microscopy; |
| 5-FU | 5-fluorouracil; |
| G6PD | Glucose-6-phosphatedehydrogenase; |
| GGT2 | Gamma-glutamyltransferase 2; |
| GOT1 | Glutamic-oxaloacetic transaminase 1; |
| GPXs | Glutathione peroxidases |
| GSH | Glutathione; |
| GSS | Glutathione synthase; |
| GSSG | Oxidized glutathione |
| GSTs | Glutathione S-transferases; |
| Gyp | Gypenosides; |
| H2DCF | 2′,7′-dichlorodihydrofluorescein; |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate; |
| H2O2 | Hydrogen peroxide; |
| HER | Human epidermal growth factor receptor; |
| HIF-1α | Hypoxia-inducible factor 1α; |
| HPLC | High-pressure liquid chromatography; |
| HY-PDT | Photodynamic therapy mediated by hypericin; |
| IBD | Inflammatory bowel diseases; |
| ICAM-1 | Intercellular adhesion molecule 1; |
| IFN | Interferon; |
| IFN-α | Interferon-alpha; |
| IKK | Inhibitor κB kinase; |
| ISC | Intestinal stem cell; |
| JAK | Janus kinase; |
| JNK | c-Jun N-terminal kinase; |
| Keap1 | Kelch-like ECH-associated protein 1; |
| KRAS | KRAS proto-oncogene, GTPase; |
| LncRNAs | Long noncoding RNAs; |
| L-OHP | Oxaliplatin; |
| mAbs | Monoclonal antibodies; |
| MAPK | Mitogen-activated protein kinase; |
| MAP3K | Mitogen-activated protein kinase kinase kinase; |
| MDA | Malondialdehyde; |
| MDR | Multidrug resistance; |
| MEK | Mitogen-activated protein kinase kinase-MAPKK; |
| MET | Metformin; |
| MLK3, MAP3K11 | Mixed lineage kinase 3; |
| MMR | Mismatch repair; |
| MPO | Myeloperoxidase; |
| MSI | Microsatellite instability; |
| MSI-H | Microsatellite instability-high; |
| mTOR | Mammalian target of rapamycin; |
| MUTYH | MutY homolog Escherichia coli, homolog of MYH, hMYH; |
| MVs | Membrane vesicles; |
| NaB | Sodium butyrate; |
| NAC | N-acety1 cysteine; |
| NADPH | Nicotinamide adenine dinucleotide phosphate; |
| NPs | Nanoparticles; |
| NER | Nucleotide excision repair; |
| NF-κB | Nuclear factor kappa B; |
| NIK | NF-κB inducing kinase; |
| NOTCH | Notch receptor; |
| NOX | NADPH oxidase; |
| NRAS | NRAS proto-oncogene, GTPase; |
| NRF2 | Nuclear factor-erythroid 2 p45-related factor 2; |
| NRX | Nucleoredoxin; |
| NSAIDs | Nonsteroidal anti-inflammatory drug; |
| O2•− | Superoxide anion; |
| OGG1 | 8-oxoguanine DNA glycosylase 1; |
| •OH | Hydroxyl radical; |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine; |
| 8-oxoG | 8-oxoguanine; |
| 8-oxodG | 8-oxo-7, 8-dihydro-2′-deoxyguanosine; |
| oxLDL | Oxidized low-density lipoprotein; |
| OS | Oxidative stress |
| P3C-Asp | Dextran–aspirin nanomedicine; |
| PD-1 | Programmed cell death 1; |
| PDGF-R | Platelet-derived growth factor receptors; |
| PDL1 | Programmed death ligand 1; |
| PDT | Photodynamic therapy; |
| PFL | Positive feedback loop; |
| P-gp | P-glycoprotein; |
| PI3K | Phosphatidyl inositol 3-OH kinase; |
| PKB | Protein kinase B; |
| PL | Piperlongumine; |
| PMN | Polymorphonuclear; |
| PPP | Pentose phosphate pathway; |
| PRX | Peroxiredoxin; |
| PTEN | Phosphatase and tensin homolog; |
| PTKs | Protein tyrosine kinases; |
| PTP | Protein tyrosine phosphatase; |
| PUFAs | Polyunsaturated fatty acids; |
| PL | Piperlongumine; |
| qPCR | Quantitative real-time polymerase chain reaction; |
| RAC1 | Rac family small GTPase 1; |
| ROS | Reactive oxygen species; |
| RTKs | Receptor tyrosine kinases; |
| SA | Salicylic acid; |
| SalB | Salvianolic acid B; |
| SCD1 | Stearoyl-CoA desaturase-1; |
| SLC7A11 | Solute carrier family 7 member 11; |
| SMAD4 | SMAD family member 4/mothers against decapentaplegic homolog 4; |
| SOD | Superoxide dismutase; |
| STAT | Signal transducer of activators of transcription; |
| TALDO1 | Transaldolase 1; |
| TAMs | Tumor-associated macrophages; |
| TANs | Tumor-associated neutrophils; |
| TBA | Modified 2-thiobarbituric acid; |
| TBARS | Thiobarbituric acid reactive; |
| TCA | Tricarboxylic acid cycle; |
| TCF-4 | T-cell factor-4; |
| TCRs | T-cell receptors; |
| TIGAR | TP53-induced glycolysis regulatory phosphatase; |
| TGF-β | Transforming growth factor-β; |
| TKI | Tyrosine kinase inhibitor; |
| TME | Tissue microenvironment; |
| TNFR | Tumor necrosis factor receptor; |
| TRAF3 | Tumor necrosis factor receptor-associated factor 3; |
| Trx/TrxR | Thioredoxin/thioredoxin reductase; |
| VCAM1 | Vascular cell adhesion molecule 1; |
| VEGF | Vascular endothelial growth factor; |
| VEGFR | Vascular endothelial growth factor receptor; |
| Wnt | Wingless/It; |
| Wnt-PCP | Wnt-planar cell polarity; |
| xCT | xc− transporter; |
| X-PDT | X-ray-induced photodynamic therapy. |
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Aviello, G.; Knaus, U.G. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol. 2018, 11, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Marjaneh, R.; Hassanian, S.M.; Mehramiz, M.; Rezayi, M.; Ferns, G.A.; Khazaei, M.; Avan, A. Reactive oxygen species in colorectal cancer: The therapeutic impact and its potential roles in tumor progression via perturbation of cellular and physiological dysregulated pathways. J. Cell Physiol. 2019, 234, 10072–10079. [Google Scholar] [CrossRef]
- Shu, P.; Liang, H.; Zhang, J.; Lin, Y.; Chen, W.; Zhang, D. Reactive oxygen species formation and its effect on CD4+ T cell-mediated inflammation. Front. Immunol. 2023, 14, 1199233. [Google Scholar] [CrossRef] [PubMed]
- Juanes, M.A. Cytoskeletal Control and Wnt Signaling-APC’s Dual Contributions in Stem Cell Division and Colorectal Cancer. Cancers 2020, 12, 3811. [Google Scholar] [CrossRef]
- Ahmad, R.; Singh, J.K.; Wunnava, A.; Al-Obeed, O.; Abdulla, M.; Srivastava, S.K. Emerging trends in colorectal cancer: Dysregulated signaling pathways (Review). Int. J. Mol. Med. 2021, 47, 14. [Google Scholar] [CrossRef]
- Coant, N.; Ben Mkaddem, S.; Pedruzzi, E.; Guichard, C.; Tréton, X.; Ducroc, R.; Freund, J.N.; Cazals-Hatem, D.; Bouhnik, Y.; Woerther, P.L.; et al. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol. Cell Biol. 2010, 30, 2636–2650. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.M.; Liou, M.J.; Zhang, L.F.; Nguyen, H.; Litvak, Y.; Schorr, E.M.; Jang, K.K.; Tiffany, C.R.; Butler, B.P.; Bäumler, A.J. Anaerobic Respiration of NOX1-Derived Hydrogen Peroxide Licenses Bacterial Growth at the Colonic Surface. Cell Host Microbe 2020, 28, 789–797.e5. [Google Scholar] [CrossRef]
- Nelson, V.K.; Nuli, M.V.; Mastanaiah, J.; Saleem, T.S.M.; Birudala, G.; Jamous, Y.F.; Alshargi, O.; Kotha, K.K.; Sudhan, H.H.; Mani, R.R.; et al. Reactive oxygen species mediated apoptotic death of colon cancer cells: Therapeutic potential of plant derived alkaloids. Front. Endocrinol. 2023, 14, 1201198. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.; Clevers, H. Cell fate specification and differentiation in the adult mammalian intestine. Nat. Rev. Mol. Cell Biol. 2021, 22, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Wharton, N.; Parris, A.; Mitchell, E.; Sobolewski, A.; Kam, C.; Bigwood, L.; El Hadi, A.; Münsterberg, A.; Lewis, M.; et al. Canonical Wnt signals combined with suppressed TGFβ/BMP pathways promote renewal of the native human colonic epithelium. Gut 2014, 63, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Aceto, G.M.; Catalano, T.; Curia, M.C. Molecular Aspects of Colorectal Adenomas: The Interplay among Microenvironment, Oxidative Stress, and Predisposition. Biomed. Res. Int. 2020, 2020, 1726309. [Google Scholar] [CrossRef]
- Morris, O.; Jasper, H. Reactive Oxygen Species in intestinal stem cell metabolism, fate and function. Free Radic. Biol. Med. 2021, 166, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, F.; Catalano, T.; Lattanzio, R.; Cotellese, R.; Aceto, G.M. Wingless/It/β-catenin signaling in liver metastasis from colorectal cancer: A focus on biological mechanisms and therapeutic opportunities. World J. Gastroenterol. 2023, 29, 2764–2783. [Google Scholar] [CrossRef] [PubMed]
- Yoboue, E.D.; Sitia, R.; Simmen, T. Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis. 2018, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS production by mitochondria: Function or dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, S.; Brookes, M.J.; Salgueiro, P.; Ridgway, R.A.; McGhee, E.; Anderson, K.; Sansom, O.J. Luminal iron levels govern intestinal tumorigenesis after apc loss in vivo. Cell Rep. 2012, 2, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Arcaro, A.; Pizzimenti, S.; Daga, M.; Cetrangolo, G.P.; Dianzani, C.; Lepore, A.; Graf, M.; Ames, P.R.J.; Barrera, G. DNA damage by lipid peroxidation products: Implications in cancer, inflammation and autoimmunity. AIMS Genet. 2017, 4, 103–137. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Basak, D.; Uddin, M.N.; Hancock, J. The Role of Oxidative Stress and Its Counteractive Utility in Colorectal Cancer (CRC). Cancers 2020, 12, 3336. [Google Scholar] [CrossRef]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen peroxide—Production, fate and role in redox signaling of tumor cells. Cell Commun. Signal 2015, 13, 39. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Zamyatnin, A.A., Jr.; Werner, J.; Bazhin, A.V. Reactive oxygen species and colorectal cancer. J. Cell Physiol. 2018, 233, 5119–5132. [Google Scholar] [CrossRef]
- Kohan, R.; Collin, A.; Guizzardi, S.; Tolosa de Talamoni, N.; Picotto, G. Reactive oxygen species in cancer: A paradox between pro- and anti-tumour activities. Cancer Chemother. Pharmacol. 2020, 86, 1–13. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, D.L.; Fantini, F.; Moscatello, C.; Ferrone, A.; Scaringi, S.; Valanzano, R.; Ficari, F.; Efthymakis, K.; Neri, M.; Aceto, G.M.; et al. The Interplay among Wnt/β-catenin Family Members in Colorectal Adenomas and Surrounding Tissues. Biomedicines 2024, 12, 1730. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, S.; Fang, L. Metabolic reprogramming in colorectal cancer: Regulatory networks and therapy. Cell Biosci. 2023, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, S.; Sebastián, C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin. Cell Dev. Biol. 2020, 98, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Catalano, T.; Curia, M.C.; Aceto, G.; Verginelli, F.; Cascinu, S.; Cama, A.; Mariani-Costantini, R.; Teti, D.; Battista, P. Mutations in the p53 and Ki-ras genes, microsatellite instability and site of tumor origin in colorectal cancer. Oncol. Rep. 2005, 14, 625–631. [Google Scholar] [CrossRef]
- Reischmann, N.; Andrieux, G.; Griffin, R.; Reinheckel, T.; Boerries, M.; Brummer, T. BRAFV600E drives dedifferentiation in small intestinal and colonic organoids and cooperates with mutant p53 and Apc loss in transformation. Oncogene 2020, 39, 6053–6070. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.D.G.; Vlahov, N.; Tsantoulis, P.; Ridgway, R.A.; Flanagan, D.J.; Gilroy, K.; Sphyris, N.; Vázquez, E.G.; Vincent, D.F.; Faller, W.J.; et al. Oncogenic BRAF, unrestrained by TGFβ-receptor signalling, drives right-sided colonic tumorigenesis. Nat. Commun. 2021, 12, 3464. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, D.J.; Amirkhah, R.; Vincent, D.F.; Gunduz, N.; Gentaz, P.; Cammareri, P.; McCooey, A.J.; McCorry, A.M.B.; Fisher, N.C.; Davis, H.L.; et al. Epithelial TGFβ engages growth-factor signalling to circumvent apoptosis and drive intestinal tumourigenesis with aggressive features. Nat. Commun. 2022, 13, 7551. [Google Scholar] [CrossRef]
- Catalano, T.; D’Amico, E.; Moscatello, C.; Di Marcantonio, M.C.; Ferrone, A.; Bologna, G.; Selvaggi, F.; Lanuti, P.; Cotellese, R.; Curia, M.C.; et al. Oxidative Distress Induces Wnt/β-Catenin Pathway Modulation in Colorectal Cancer Cells: Perspectives on APC Retained Functions. Cancers 2021, 13, 6045. [Google Scholar] [CrossRef] [PubMed]
- Aceto, G.M.; Pagotto, S.; Del Pizzo, F.D.; Saoca, C.; Selvaggi, F.; Visone, R.; Cotellese, R.; Aguennouz, M.; Lattanzio, R.; Catalano, T. Differential Regulation of Wingless-Wnt/c-Jun N-Terminal Kinase Crosstalk via Oxidative Eustress in Primary and Metastatic Colorectal Cancer Cells. Biomedicines 2024, 12, 1816. [Google Scholar] [CrossRef]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef]
- Sorolla, M.A.; Hidalgo, I.; Sorolla, A.; Montal, R.; Pallisé, O.; Salud, A.; Parisi, E. Microenvironmental Reactive Oxygen Species in Colorectal Cancer: Involved Processes and Therapeutic Opportunities. Cancers 2021, 13, 5037. [Google Scholar] [CrossRef]
- Kennel, K.B.; Greten, F.R. Immune cell—Produced ROS and their impact on tumor growth and metastasis. Redox Biol. 2021, 42, 101891. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Wang, Z.; Li, B.; Zhu, H. Modulation of redox homeostasis: A strategy to overcome cancer drug resistance. Front. Pharmacol. 2023, 14, 1156538. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Zuo, J.; Zhang, Z.; Li, M.; Yang, Y.; Zheng, B.; Wang, P.; Huang, C.; Zhou, S. The crosstalk between reactive oxygen species and noncoding RNAs: From cancer code to drug role. Mol. Cancer 2022, 21, 30. [Google Scholar] [CrossRef]
- González, A.; Fullaondo, A.; Odriozola, A. Microbiota-associated mechanisms in colorectal cancer. Adv. Genet. 2024, 112, 123–205. [Google Scholar] [CrossRef]
- Garvey, M. Intestinal Dysbiosis: Microbial Imbalance Impacts on Colorectal Cancer Initiation, Progression and Disease Mitigation. Biomedicines 2024, 12, 740. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, Y.; Otsuka, M.; Shibata, C.; Seimiya, T.; Yamamoto, K.; Ishibashi, R.; Kishikawa, T.; Tanaka, E.; Isagawa, T.; Takeda, N.; et al. Gut Bacteria-derived Membrane Vesicles Induce Colonic Dysplasia by Inducing DNA Damage in Colon Epithelial Cells. Cell Mol. Gastroenterol. Hepatol. 2024, 17, 745–767. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari Movahed, Z.; Rastegari-Pouyani, M.; Mohammadi, M.H.; Mansouri, K. Cancer cells change their glucose metabolism to overcome increased ROS: One step from cancer cell to cancer stem cell? Biomed. Pharmacother. 2019, 112, 108690. [Google Scholar] [CrossRef]
- Bekhet, O.H.; Eid, M.E. The interplay between reactive oxygen species and antioxidants in cancer progression and therapy: A narrative review. Transl. Cancer Res. 2021, 10, 4196–4206. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.; Ortega, Á.; Santeliz, R.; Garrido, B.; Chacín, M.; Galban, N.; Vera, I.; De Sanctis, J.B.; Bermúdez, V. Metabolic Reprogramming in Cancer Cells: Emerging Molecular Mechanisms and Novel Therapeutic Approaches. Pharmaceutics 2022, 14, 1303. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Bardelčíková, A.; Šoltys, J.; Mojžiš, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Banda, D.M.; Nuñez, N.N.; Burnside, M.A.; Bradshaw, K.M.; David, S.S. Repair of 8-oxoG:A mismatches by the MUTYH glycosylase: Mechanism, metals and medicine. Free Radic. Biol. Med. 2017, 107, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Moscatello, C.; Di Nicola, M.; Veschi, S.; Di Gregorio, P.; Cianchetti, E.; Stuppia, L.; Battista, P.; Cama, A.; Curia, M.C.; Aceto, G.M. Relationship between MUTYH, OGG1 and BRCA1 mutations and mRNA expression in breast and ovarian cancer predisposition. Mol. Clin. Oncol. 2021, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Moscatello, C.; Di Marcantonio, M.C.; Savino, L.; D’Amico, E.; Spacco, G.; Simeone, P.; Lanuti, P.; Muraro, R.; Mincione, G.; Cotellese, R.; et al. Emerging Role of Oxidative Stress on EGFR and OGG1-BER Cross-Regulation: Implications in Thyroid Physiopathology. Cells 2022, 11, 822. [Google Scholar] [CrossRef]
- Curia, M.C.; Catalano, T.; Aceto, G.M. MUTYH: Not just polyposis. World J. Clin. Oncol. 2020, 11, 428–449. [Google Scholar] [CrossRef] [PubMed]
- D’Augustin, O.; Huet, S.; Campalans, A.; Radicella, J.P. Lost in the Crowd: How Does Human 8-Oxoguanine DNA Glycosylase 1 (OGG1) Find 8-Oxoguanine in the Genome? Int. J. Mol. Sci. 2020, 21, 8360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhong, R.; Qu, X.; Xiang, Y.; Ji, M. Effect of 8-Hydroxyguanine DNA Glycosylase 1 on the Function of Immune Cells. Antioxidants 2023, 12, 1300. [Google Scholar] [CrossRef]
- Huang, L.; Li, W.; Lu, Y.; Ju, Q.; Ouyang, M. Iron metabolism in colorectal cancer. Front. Oncol. 2023, 13, 1098501. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.J.; Silverman, L.M.; Keku, T.; Lawrence, L.B.; Rohlfs, E.M.; Martin, C.F.; Galanko, J.; Sandler, R.S. Association between hemochromatosis (HFE) gene mutation carrier status and the risk of colon cancer. J. Natl. Cancer Inst. 2003, 95, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.J.; Gurrin, L.C.; Allen, K.J.; Constantine, C.C.; Delatycki, M.B.; McLaren, C.E.; Gertig, D.M.; Anderson, G.J.; Southey, M.C.; Olynyk, J.K.; et al. HFE C282Y homozygotes are at increased risk of breast and colorectal cancer. Hepatology 2010, 51, 1311–1318. [Google Scholar] [CrossRef]
- Asberg, A.; Thorstensen, K.; Irgens, W.Ø.; Romundstad, P.R.; Hveem, K. Cancer risk in HFE C282Y homozygotes: Results from the HUNT 2 study. Scand. J. Gastroenterol. 2013, 48, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.F.; Chang, X.; Hua, R.X.; Yan, G.N.; Meng, G.; Liao, X.Y.; Zhang, X.; Guo, Q.N. The risk of new-onset cancer associated with HFE C282Y and H63D mutations: Evidence from 87,028 participants. J. Cell Mol. Med. 2016, 20, 1219–1233. [Google Scholar] [CrossRef]
- Jarvik, G.P.; Wang, X.; Fontanillas, P.; Kim, E.; Chanprasert, S.; Gordon, A.S.; Bastarache, L.; Kowdley, K.V.; Harrison, T.; Rosenthal, E.A.; et al. Hemochromatosis risk genotype is not associated with colorectal cancer or age at its diagnosis. HGG Adv. 2020, 1, 100010. [Google Scholar] [CrossRef] [PubMed]
- Brookes, M.J.; Boult, J.; Roberts, K.; Cooper, B.T.; Hotchin, N.A.; Matthews, G.; Iqbal, T.; Tselepis, C. A role for iron in Wnt signalling. Oncogene 2008, 27, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Athena Aktipis, C. The Role of the Microbiome in Cancer Initiation and Progression: How Microbes and Cancer Cells Utilize Excess Energy and Promote One Another’s Growth. Curr. Nutr. Rep. 2019, 8, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Phipps, O.; Al-Hassi, H.O.; Quraishi, M.N.; Kumar, A.; Brookes, M.J. Influence of Iron on the Gut Microbiota in Colorectal Cancer. Nutrients 2020, 12, 2512. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.Z.; Ding, Y.D.; Xue, Q.M.; Cai, W.; Deng, H. Direct and indirect effects of pathogenic bacteria on the integrity of intestinal barrier. Therap Adv. Gastroenterol. 2023, 16, 17562848231176427. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.J.; Zhi-Min, Z.; Xu, X.N.; Shi, F.; Fu, X.L. Targeting critical pathways in ferroptosis and enhancing antitumor therapy of Platinum drugs for colorectal cancer. Sci. Prog. 2023, 106, 368504221147173. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yue, M.; Lu, Y.; Wang, Y.; Luo, S.; Liu, X.; Jiang, J. Advancing the frontiers of colorectal cancer treatment: Harnessing ferroptosis regulation. Apoptosis 2024, 29, 86–102. [Google Scholar] [CrossRef]
- Shen, J.; Sheng, X.; Chang, Z.; Wu, Q.; Wang, S.; Xuan, Z.; Li, D.; Wu, Y.; Shang, Y.; Kong, X.; et al. Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function. Cell Rep. 2014, 7, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sheng, X.; Chang, Z.; Wu, Q.; Xie, D.; Wang, F.; Hu, R. The heme-p53 interaction: Linking iron metabolism to p53 signaling and tumorigenesis. Mol. Cell Oncol. 2014, 3, e965642. [Google Scholar] [CrossRef][Green Version]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, X.; Huang, P. xCT: A Critical Molecule That Links Cancer Metabolism to Redox Signaling. Mol. Ther. 2020, 28, 2358–2366. [Google Scholar] [CrossRef] [PubMed]
- Magri, J.; Gasparetto, A.; Conti, L.; Calautti, E.; Cossu, C.; Ruiu, R.; Barutello, G.; Cavallo, F. Tumor-Associated Antigen xCT and Mutant-p53 as Molecular Targets for New Combinatorial Antitumor Strategies. Cells 2021, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Badana, A.K.; Murali Mohan, G.; Shailender, G.; Malla, R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, X.; Zhang, C.; Zhu, H.; Xu, Q.; Bu, Y.; Lei, Y. Redox Imbalance in the Development of Colorectal Cancer. J. Cancer 2017, 8, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Taléns-Visconti, R.; Rius-Pérez, S.; Finamor, I.; Sastre, J. Redox signaling in the gastrointestinal tract. Free Radic. Biol. Med. 2017, 104, 75–103. [Google Scholar] [CrossRef]
- Carethers, J.M.; Jung, B.H. Genetics and Genetic Biomarkers in Sporadic Colorectal Cancer. Gastroenterology 2015, 149, 1177–1190.e3. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, M. Molecular Network of Colorectal Cancer and Current Therapeutic Options. Front. Oncol. 2022, 12, 852927. [Google Scholar] [CrossRef]
- Dow, L.E.; O’Rourke, K.P.; Simon, J.; Tschaharganeh, D.F.; van Es, J.H.; Clevers, H.; Lowe, S.W. Apc Restoration Promotes Cellular Differentiation and Reestablishes Crypt Homeostasis in Colorectal Cancer. Cell 2015, 161, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shay, J.W. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109, djw332. [Google Scholar] [CrossRef] [PubMed]
- Myant, K.B.; Cammareri, P.; McGhee, E.J.; Ridgway, R.A.; Huels, D.J.; Cordero, J.B.; Schwitalla, S.; Kalna, G.; Ogg, E.L.; Athineos, D.; et al. ROS production and NF-κB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell 2013, 12, 761–773. [Google Scholar] [CrossRef]
- Ternet, C.; Kiel, C. Signaling pathways in intestinal homeostasis and colorectal cancer: KRAS at centre stage. Cell Commun. Signal 2021, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Rio-Vilariño, A.; Del Puerto-Nevado, L.; García-Foncillas, J.; Cebrián, A. Ras Family of Small GTPases in CRC: New Perspectives for Overcoming Drug Resistance. Cancers 2021, 13, 3757. [Google Scholar] [CrossRef]
- Müller, F.; Lim, J.K.M.; Bebber, C.M.; Seidel, E.; Tishina, S.; Dahlhaus, A.; Stroh, J.; Beck, J.; Yapici, F.I.; Nakayama, K.; et al. Elevated FSP1 protects KRAS-mutated cells from ferroptosis during tumor initiation. Cell Death Differ. 2023, 30, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.M.; Leprivier, G. The impact of oncogenic RAS on redox balance and implications for cancer development. Cell Death Dis. 2019, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.M.; Rolas, L.; El-Benna, J. The Dual Role of Reactive Oxygen Species-Generating Nicotinamide Adenine Dinucleotide Phosphate Oxidases in Gastrointestinal Inflammation and Therapeutic Perspectives. Antioxid. Redox Signal 2020, 33, 354–373. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Wang, Y.P.; Lei, Q.Y. Metabolic recoding of epigenetics in cancer. Cancer Commun. 2018, 38, 25. [Google Scholar] [CrossRef] [PubMed]
- Kirtonia, A.; Sethi, G.; Garg, M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell Mol. Life Sci. 2020, 77, 4459–4483. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J. Dysregulation of systemic immunity in colorectal cancer and its clinical applications as biomarkers and therapeutics. Crit. Rev. Oncol. Hematol. 2024, 204, 104543. [Google Scholar] [CrossRef]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Treviño, S.; Rosas-Murrieta, N.H.; Millán-Perez-Peña, L.; Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Pelosi, E.; Castelli, G. Colorectal cancer: Genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med. Sci. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Wilde, L.; Roche, M.; Domingo-Vidal, M.; Tanson, K.; Philp, N.; Curry, J.; Martinez-Outschoorn, U. Metabolic coupling and the Reverse Warburg Effect in cancer: Implications for novel biomarker and anticancer agent development. Semin. Oncol. 2017, 44, 198–203. [Google Scholar] [CrossRef]
- Wu, X.; Tu, X.; Joeng, K.S.; Hilton, M.J.; Williams, D.A.; Long, F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell 2008, 133, 340–353. [Google Scholar] [CrossRef]
- Cheung, E.C.; Lee, P.; Ceteci, F.; Nixon, C.; Blyth, K.; Sansom, O.J.; Vousden, K.H. Opposing effects of TIGAR- and RAC1-derived ROS on Wnt-driven proliferation in the mouse intestine. Genes Dev. 2016, 30, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Liu, Z.; Lin, C.; Meng, F.; Xu, L.; Zhang, X.; Zhang, C.; Zhang, P.; Gong, S.; et al. CircMYH9 drives colorectal cancer growth by regulating serine metabolism and redox homeostasis in a p53-dependent manner. Mol. Cancer 2021, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 2019, 38, 2223–2240. [Google Scholar] [CrossRef]
- Lee, H.S.; Hwang, C.Y.; Shin, S.Y.; Kwon, K.S.; Cho, K.H. MLK3 is part of a feedback mechanism that regulates different cellular responses to reactive oxygen species. Sci. Signal 2014, 7, ra52. [Google Scholar] [CrossRef] [PubMed]
- Sancho, R.; Nateri, A.S.; de Vinuesa, A.G.; Aguilera, C.; Nye, E.; Spencer-Dene, B.; Behrens, A. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J. 2009, 28, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Saadeddin, A.; Babaei-Jadidi, R.; Spencer-Dene, B.; Nateri, A.S. The links between transcription, beta-catenin/JNK signaling, and carcinogenesis. Mol. Cancer Res. 2009, 7, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wu, W.; Jacevic, V.; Franca, T.C.C.; Wang, X.; Kuca, K. Selective inhibitors for JNK signalling: A potential targeted therapy in cancer. J. Enzyme Inhib. Med. Chem. 2020, 35, 574–583. [Google Scholar] [CrossRef]
- Zeng, J.; Li, M.; Xu, J.Y.; Xiao, H.; Yang, X.; Fan, J.X.; Wu, K.; Chen, S. Aberrant ROS Mediate Cell Cycle and Motility in Colorectal Cancer Cells Through an Oncogenic CXCL14 Signaling Pathway. Front. Pharmacol. 2021, 12, 764015. [Google Scholar] [CrossRef]
- Sikder, M.O.F.; Sivaprakasam, S.; Brown, T.P.; Thangaraju, M.; Bhutia, Y.D.; Ganapathy, V. SLC6A14, a Na+/Cl--coupled amino acid transporter, functions as a tumor promoter in colon and is a target for Wnt signaling. Biochem. J. 2020, 477, 1409–1425. [Google Scholar] [CrossRef]
- Sniegowski, T.; Korac, K.; Bhutia, Y.D.; Ganapathy, V. SLC6A14 and SLC38A5 Drive the Glutaminolysis and Serine-Glycine-One-Carbon Pathways in Cancer. Pharmaceuticals 2021, 14, 216. [Google Scholar] [CrossRef]
- Gupta, N.; Miyauchi, S.; Martindale, R.G.; Herdman, A.V.; Podolsky, R.; Miyake, K.; Mager, S.; Prasad, P.D.; Ganapathy, M.E.; Ganapathy, V. Upregulation of the amino acid transporter ATB0,+ (SLC6A14) in colorectal cancer and metastasis in humans. Biochim. Biophys. Acta 2005, 1741, 215–223. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Sennoune, S.R.; Dharmalingam-Nandagopal, G.; Sivaprakasam, S.; Bhutia, Y.D.; Ganapathy, V. Impact of Oncogenic Changes in p53 and KRAS on Macropinocytosis and Ferroptosis in Colon Cancer Cells and Anticancer Efficacy of Niclosamide with Differential Effects on These Two Processes. Cells 2024, 13, 951. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yang, S.; Qiu, Q.; Cui, G.; Zhang, Y.; Yao, M.; Li, X.; Chen, C.; Gu, J.; Wang, T.; et al. Hypoxia-induced cysteine metabolism reprogramming is crucial for the tumorigenesis of colorectal cancer. Redox Biol. 2024, 75, 103286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ou, Z.; Tang, T.; Yang, T.; Li, Y.; Wu, H.; Li, L.; Liu, M.; Niu, L.; Zhu, J. Up-regulated SLC25A39 promotes cell growth and metastasis via regulating ROS production in colorectal cancer. J. Cancer 2024, 15, 5841–5854. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, H.; Mao, Y.; Jiang, S.; Yi, H.; Zhang, Z.; Wang, M.; Zong, Z. Myeloid-derived suppressor cells: Key immunosuppressive regulators and therapeutic targets in colorectal cancer (Review). Int. J. Oncol. 2024, 65, 85. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef]
- Lu, Y.; Wei, W.; Li, M.; Chen, D.; Li, W.; Hu, Q.; Dong, S.; Liu, L.; Zhao, Q. The USP11/Nrf2 positive feedback loop promotes colorectal cancer progression by inhibiting mitochondrial apoptosis. Cell Death Dis. 2024, 15, 873. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, T.; Qi, X. Balanced regulation of ROS production and inflammasome activation in preventing early development of colorectal cancer. Immunol. Rev. 2024, 329, e13417. [Google Scholar] [CrossRef] [PubMed]
- Veres, T.G.; Petrovics, L.; Sárvári, K.; Vereczkei, A.; Jancsó, G.; Farkas, K.B.; Takács, I. The effect of laparoscopic pre- and postconditioning on pneumoperitoneum induced injury of the peritoneum. Clin. Hemorheol. Microcirc. 2019, 73, 565–577. [Google Scholar] [CrossRef]
- Ng, M.L.; Ang, X.; Yap, K.Y.; Ng, J.J.; Goh, E.C.H.; Khoo, B.B.J.; Richards, A.M.; Drum, C.L. Novel Oxidative Stress Biomarkers with Risk Prognosis Values in Heart Failure. Biomedicines 2023, 11, 917. [Google Scholar] [CrossRef] [PubMed]
- Reitznerová, A.; Šuleková, M.; Nagy, J.; Marcinčák, S.; Semjon, B.; Čertík, M.; Klempová, T. Lipid Peroxidation Process in Meat and Meat Products: A Comparison Study of Malondialdehyde Determination between Modified 2-Thiobarbituric Acid Spectrophotometric Method and Reverse-Phase High-Performance Liquid Chromatography. Molecules 2017, 22, 1988. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Karimi Galougahi, K.; Liu, C.C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef]
- Hanning, N.; De Man, J.G.; De Winter, B.Y. Measuring Myeloperoxidase Activity as a Marker of Inflammation in Gut Tissue Samples of Mice and Rat. Bio Protoc. 2023, 13, e4758. [Google Scholar] [CrossRef]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef] [PubMed]
- Aqsa, A.; Prachi, K.; Nethravathy, V. Assessment of superoxide dismutase, catalase, and peroxidase activities in Aspergillus sp. and Cladosporium sp. J. Adv. Sci. Res. 2021, 12, 249–254. [Google Scholar]
- Chalhoub, V.; Pottecher, J.; Asehnoune, K.; Mazoit, J.X.; Duranteau, J.; Benhamou, D. Cytokine response and reactive oxygen species production after low- and intermediate-risk surgery. Acta Anaesthesiol. Scand 2011, 55, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Duanghathaipornsuk, S.; Farrell, E.J.; Alba-Rubio, A.C.; Zelenay, P.; Kim, D.S. Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications. Biosensors 2021, 11, 30. [Google Scholar] [CrossRef]
- Wojtala, A.; Bonora, M.; Malinska, D.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Methods to monitor ROS production by fluorescence microscopy and fluorometry. Methods Enzymol. 2014, 542, 243–262. [Google Scholar] [CrossRef]
- Krishnamurthy, H.K.; Pereira, M.; Rajavelu, I.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K.; Rajasekaran, J.J. Oxidative stress: Fundamentals and advances in quantification techniques. Front. Chem. 2024, 12, 1470458. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Harrison, D.G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal 2014, 20, 372–382. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, M.; Yang, X.; Zhang, S.; Han, J.; Wang, Z.; Chen, H.N. Reactive oxygen species in colorectal cancer adjuvant therapies. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166922. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef]
- Ward, J.F. Complexity of damage produced by ionizing radiation. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Wang, J.Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R., Jr.; Yang, D.H.; Chen, Z.S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updates 2018, 41, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Li, C.; Cheng, N.; Cui, X.; Xu, X.; Zhou, G. Redox Regulation in Cancer Stem Cells. Oxid. Med. Cell Longev. 2015, 2015, 750798. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Rizvi, S.F.; Parveen, S.; Pathak, N.; Nazir, A.; Mir, S.S. Crosstalk Between ROS and Autophagy in Tumorigenesis: Understanding the Multifaceted Paradox. Front. Oncol. 2022, 12, 852424. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Rashid, K.; AlAmodi, A.A.; Agha, M.V.; Akhtar, S.; Hakeem, I.; Raza, S.S.; Uddin, S. Reactive oxygen species (ROS) in cancer pathogenesis and therapy: An update on the role of ROS in anticancer action of benzophenanthridine alkaloids. Biomed. Pharmacother. 2021, 143, 112142. [Google Scholar] [CrossRef]
- El Sayed, S.M.; Mahmoud, A.A.; El Sawy, S.A.; Abdelaal, E.A.; Fouad, A.M.; Yousif, R.S.; Hashim, M.S.; Hemdan, S.B.; Kadry, Z.M.; Abdelmoaty, M.A.; et al. Warburg effect increases steady-state ROS condition in cancer cells through decreasing their antioxidant capacities (anticancer effects of 3-bromopyruvate through antagonizing Warburg effect). Med. Hypotheses 2013, 81, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, K.; Yhmed, A.M.A.; Nasef, M.M.; Georgopoulos, N.T. TRAF3/p38-JNK Signalling Crosstalk with Intracellular-TRAIL/Caspase-10-Induced Apoptosis Accelerates ROS-Driven Cancer Cell-Specific Death by CD40. Cells 2022, 11, 3274. [Google Scholar] [CrossRef]
- van Grevenstein, W.M.; Aalbers, A.G.; Ten Raa, S.; Sluiter, W.; Hofland, L.J.; Jeekel, H.; van Eijck, C.H. Surgery-derived reactive oxygen species produced by polymorphonuclear leukocytes promote tumor recurrence: Studies in an in vitro model. J. Surg. Res. 2007, 140, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Gül, N.; Bögels, M.; Grewal, S.; van der Meer, A.J.; Rojas, L.B.; Fluitsma, D.M.; van den Tol, M.P.; Hoeben, K.A.; van Marle, J.; de Vries, H.E.; et al. Surgery-induced reactive oxygen species enhance colon carcinoma cell binding by disrupting the liver endothelial cell lining. Gut 2011, 60, 1076–1086. [Google Scholar] [CrossRef]
- Sawai, K.; Goi, T.; Kimura, Y.; Koneri, K. Reduction of Blood Oxidative Stress Following Colorectal Cancer Resection. Cancers 2024, 16, 3550. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Deng, S.; Yan, L.; Gu, J.; Mao, F.; Xue, Y.; Zheng, C.; Yang, M.; Liu, H.; Liu, L.; et al. An Oxidative Stress Index-Based Score for Prognostic Prediction in Colorectal Cancer Patients Undergoing Surgery. Oxid. Med. Cell Longev. 2021, 2021, 6693707. [Google Scholar] [CrossRef]
- Lacy, A.M.; Delgado, S.; Castells, A.; Prins, H.A.; Arroyo, V.; Ibarzabal, A.; Pique, J.M. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann. Surg. 2008, 248, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shimotakahara, A.; Kuebler, J.F.; Vieten, G.; Kos, M.; Metzelder, M.L.; Ure, B.M. Carbon dioxide directly suppresses spontaneous migration, chemotaxis, and free radical production of human neutrophils. Surg. Endosc. 2008, 22, 1813–1817. [Google Scholar] [CrossRef]
- Potenza, L.; Calcabrini, C.; Bellis, R.D.; Mancini, U.; Polidori, E.; Zeppa, S.; Alloni, R.; Cucchiarini, L.; Dacha, M. Effect of surgical stress on nuclear and mitochondrial DNA from healthy sections of colon and rectum of patients with colorectal cancer. J. Biosci. 2011, 36, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shen, L.; Yang, Q.; Zhang, Q.; Cao, Y.; Liu, T. Oxidative stress index-based scoring for prediction of long-term prognosis in patients with colorectal cancer with liver metastases. Sci. Rep. 2025, 15, 5253. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.A.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; et al. Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values. Front. Mol. Biosci. 2019, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.A.; Kim, H.A.; Kang, B.Y.; Kim, K.H. Hemoglobin induces colon cancer cell proliferation by release of reactive oxygen species. World J. Gastroenterol. 2006, 12, 5644–5650. [Google Scholar] [CrossRef]
- Huang, L.; Su, Y.; Zhang, D.; Zeng, Z.; Hu, X.; Hong, S.; Lin, X. Recent theranostic applications of hydrogen peroxide-responsive nanomaterials for multiple diseases. RSC Adv. 2023, 13, 27333–27358. [Google Scholar] [CrossRef]
- Saxon, E.; Ali, T.; Peng, X. Hydrogen peroxide responsive theranostics for cancer-selective activation of DNA alkylators and real-time fluorescence monitoring in living cells. Eur. J. Med. Chem. 2024, 276, 116695. [Google Scholar] [CrossRef] [PubMed]
- Kasi, P.B.; Mallela, V.R.; Ambrozkiewicz, F.; Trailin, A.; Liška, V.; Hemminki, K. Theranostics Nanomedicine Applications for Colorectal Cancer and Metastasis: Recent Advances. Int. J. Mol. Sci. 2023, 24, 7922. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tian, Y.; Zhai, S.; Liu, Y.; Chu, S.; Xiong, Z. The progress of research on the application of redox nanomaterials in disease therapy. Front. Chem. 2023, 11, 1115440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ji, Y.; Hu, N.; Yu, Q.; Zhang, X.; Li, J.; Wu, F.; Xu, H.; Tang, Q.; Li, X. Ferroptosis-induced anticancer effect of resveratrol with a biomimetic nano-delivery system in colorectal cancer treatment. Asian J. Pharm. Sci. 2022, 17, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, C.; Ye, Y.; Wang, S.; Jiang, K. Therapeutic potentials of FexMoyS-PEG nanoparticles in colorectal cancer: A multimodal approach via ROS-ferroptosis-glycolysis regulation. J. Nanobiotechnol. 2024, 22, 253. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yao, H.; Si, X.; Huang, Z.; Wang, R.; Wan, R.; Tang, Z.; Wang, G.; Song, W. Orally available dextran-aspirin nanomedicine modulates gut inflammation and microbiota homeostasis for primary colorectal cancer therapy. J. Control Release 2024, 370, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Grassilli, E.; Cerrito, M.G. Emerging actionable targets to treat therapy-resistant colorectal cancers. Cancer Drug Resist 2022, 5, 36–63. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zheng, F.; Kang, K.; Xiao, L.; Bi, A.; Chen, Y.; Zhou, Q.; Feng, X.; Chen, Z.; Yin, H.; et al. Precise visualization and ROS-dependent photodynamic therapy of colorectal cancer with a novel mitochondrial viscosity photosensitive fluorescent probe. Biomater. Res. 2023, 27, 112. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lei, K.; Du, W.; Yang, L.; Shi, H.; Gao, Y.; Yin, P.; Liang, X.; Liu, J. Enhancement of oxaliplatin sensitivity in human colorectal cancer by hypericin mediated photodynamic therapy via ROS-related mechanism. Int. J. Biochem. Cell Biol. 2016, 71, 24–34. [Google Scholar] [CrossRef]
- Mei, Y.; Gu, L.; Chen, Y.; Zhang, P.; Cheng, Y.; Yuan, R.; Li, X.; Wang, X.; Guo, P.; He, D.; et al. A Novel Photosensitizer Based 450-nm Blue Laser-Mediated Photodynamic Therapy Induces Apoptosis in Colorectal Cancer—In Vitro and in Vivo Study. Front. Biosci. (Landmark Ed) 2024, 29, 199. [Google Scholar] [CrossRef]
- Mushtaq, A.; Iqbal, M.Z.; Tang, J.; Sun, W. The wonders of X-PDT: An advance route to cancer theranostics. J. Nanobiotechnol. 2024, 22, 655. [Google Scholar] [CrossRef]
- Shirmanova, M.V.; Gavrina, A.I.; Kovaleva, T.F.; Dudenkova, V.V.; Zelenova, E.E.; Shcheslavskiy, V.I.; Mozherov, A.M.; Snopova, L.B.; Lukyanov, K.A.; Zagaynova, E.V. Insight into redox regulation of apoptosis in cancer cells with multiparametric live-cell microscopy. Sci. Rep. 2022, 12, 4476, Erratum in Sci. Rep. 2022, 12, 9058. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, P.; Chen, Y.; Hu, J.; Deng, B.; Liu, C.; Yu, B.; Dong, W. Microbial metabolite sodium butyrate enhances the anti-tumor efficacy of 5-fluorouracil against colorectal cancer by modulating PINK1/Parkin signaling and intestinal flora. Sci. Rep. 2024, 14, 13063. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Liang, S.; Cheng, Z.; Zhang, X.; Luo, L.; Li, L.; Zhang, W.; Li, S.; Xu, Q.; Zhong, M.; et al. ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 41, 15. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wang, X.; Zhang, K.; Yuan, W.; Yang, Q.; Fan, J.; Wang, P.; Liu, Q. Gypenosides Synergistically Enhances the Anti-Tumor Effect of 5-Fluorouracil on Colorectal Cancer In Vitro and In Vivo: A Role for Oxidative Stress-Mediated DNA Damage and p53 Activation. PLoS ONE 2015, 10, e0137888. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, S.; Lu, X.; Shi, Z.; Liu, H.; Zhu, B. Metformin enhances the sensitivity of colorectal cancer cells to cisplatin through ROS-mediated PI3K/Akt signaling pathway. Gene 2020, 745, 144623. [Google Scholar] [CrossRef] [PubMed]
- Khader, E.I.; Ismail, W.W.; Mhaidat, N.M.; Alqudah, M.A. Effect of metformin on irinotecan-induced cell cycle arrest in colorectal cancer cell lines HCT116 and SW480. Int. J. Health Sci. 2021, 15, 34–41. [Google Scholar]
- Wang, X.; Zhang, H.; Yin, S.; Yang, Y.; Yang, H.; Yang, J.; Zhou, Z.; Li, S.; Ying, G.; Ba, Y. lncRNA-encoded pep-AP attenuates the pentose phosphate pathway and sensitizes colorectal cancer cells to Oxaliplatin. EMBO Rep. 2022, 23, e53140. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, S.; Liang, W.; Wang, W.; Wang, H.; Zhao, M.; Liu, X. Salvianolic acid B reverses multidrug resistance in HCT-8/VCR human colorectal cancer cells by increasing ROS levels. Mol. Med. Rep. 2017, 15, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.C.; Granja, S.; Almeida, A.F.; Baltazar, F.; Gonçalves, M.S.T.; Preto, A.; Sousa, M.J. Targeting Lysosomes in Colorectal Cancer: Exploring the Anticancer Activity of a New Benzo[a]phenoxazine Derivative. Int. J. Mol. Sci. 2022, 24, 614. [Google Scholar] [CrossRef]
- Matschke, J.; Riffkin, H.; Klein, D.; Handrick, R.; Lüdemann, L.; Metzen, E.; Shlomi, T.; Stuschke, M.; Jendrossek, V. Targeted Inhibition of Glutamine-Dependent Glutathione Metabolism Overcomes Death Resistance Induced by Chronic Cycling Hypoxia. Antioxid. Redox Signal 2016, 25, 89–107. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Zhang, R.; Wang, F.; Wang, T.; Jiao, Y. The Role of Erastin in Ferroptosis and Its Prospects in Cancer Therapy. Onco Targets Ther. 2020, 13, 5429–5441. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, H.; Corbet, C.; de Mey, S.; Law, K.; Gevaert, T.; Feron, O.; De Ridder, M. Piperlongumine increases sensitivity of colorectal cancer cells to radiation: Involvement of ROS production via dual inhibition of glutathione and thioredoxin systems. Cancer Lett. 2019, 450, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Bader, S.; Wilmers, J.; Pelzer, M.; Jendrossek, V.; Rudner, J. Activation of anti-oxidant Keap1/Nrf2 pathway modulates efficacy of dihydroartemisinin-based monotherapy and combinatory therapy with ionizing radiation. Free Radic. Biol. Med. 2021, 168, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.Q.; Luo, Q.; Wang, L.; Song, G.B.; Sheng, J.P.; Xu, B. Signaling pathways involved in colorectal cancer: Pathogenesis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Sawyers, C. Targeted cancer therapy. Nature 2004, 432, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Rowland, A.; Dias, M.M.; Wiese, M.D.; Kichenadasse, G.; McKinnon, R.A.; Karapetis, C.S.; Sorich, M.J. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br. J. Cancer 2015, 112, 1888–1894. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, T.Y.; Jeon, S.E.; Kim, J.H.; Lee, C.J.; Yun, H.J.; Jung, J.Y.; Park, S.Y.; Nam, J.S. The Small-Molecule Wnt Inhibitor ICG-001 Efficiently Inhibits Colorectal Cancer Stemness and Metastasis by Suppressing MEIS1 Expression. Int. J. Mol. Sci. 2021, 22, 13413. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Zhao, Y.; Yuan, Y.C.; Goel, A. Metformin and ICG-001 Act Synergistically to Abrogate Cancer Stem Cells-Mediated Chemoresistance in Colorectal Cancer by Promoting Apoptosis and Autophagy. Cancers 2022, 14, 1281. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Ros, J.; Baraibar, I.; Saoudi, N.; Rodriguez, M.; Salvà, F.; Tabernero, J.; Élez, E. Immunotherapy for Colorectal Cancer with High Microsatellite Instability: The Ongoing Search for Biomarkers. Cancers 2023, 15, 4245. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Zhang, S.; Tan, D.; Yu, X.; Lin, J.; Wang, M. Anlotinib Benefits the αPDL1 Immunotherapy by Activating ROS/JNK/AP-1 Pathway to Upregulate PDL1 Expression in Colorectal Cancer. Oxid. Med. Cell Longev. 2022, 2022, 8965903. [Google Scholar] [CrossRef]
- Carter, L.; Fouser, L.A.; Jussif, J.; Fitz, L.; Deng, B.; Wood, C.R.; Collins, M.; Honjo, T.; Freeman, G.J.; Carreno, B.M. PD-1: PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur. J. Immunol. 2002, 32, 634–643. [Google Scholar] [CrossRef] [PubMed]

| CRC Initiation | |||
|---|---|---|---|
| Oxidative Molecules | Genotoxic Effects of ROS | Cell Response | References |
| 8-oxodG | oxidative DNA damage | activation of OGG1 and MUTYH | [55] |
| Target molecules or pathways activated by ROS | Effect | Cell response | References |
| Wnt/β-catenin | deficit of β-catenin degradation | activation of carcinogenesis target genes | [76] |
| KRAS mutation | activation of Raf-MEK-ERK and PI3K-AKT pathways | superoxide production by upregulation of NOX1 | [85] |
| resistance to apoptosis | [86] | ||
| changes in intracellular metabolism; activation of pro-oxidant pathways resulting in additional mutations | [87] | ||
| EGFR | activation of PI3K pathway | proliferation, survival, migration, invasion, angiogenesis, et al. | [76] |
| PTEN cys124 | activation of PI3K/AKT pathway Wnt/β-catenin pathway deregulation | cell cycle progression/proliferation; changes in intracellular metabolism; cell survival | [22] |
| JAK/STAT | overexpression of cyclin D1; STAT3 dimerization | inhibition of cell apoptosis; nuclear translocation of STAT3 | [22] |
| MAPK | inhibition of MEK1/2; oxidization of p38 cysteine residue | decreased phosphorylation of p38, ERK1/2 and JNK; suppressed activity of p38 | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalano, T.; Selvaggi, F.; Cotellese, R.; Aceto, G.M. The Role of Reactive Oxygen Species in Colorectal Cancer Initiation and Progression: Perspectives on Theranostic Approaches. Cancers 2025, 17, 752. https://doi.org/10.3390/cancers17050752
Catalano T, Selvaggi F, Cotellese R, Aceto GM. The Role of Reactive Oxygen Species in Colorectal Cancer Initiation and Progression: Perspectives on Theranostic Approaches. Cancers. 2025; 17(5):752. https://doi.org/10.3390/cancers17050752
Chicago/Turabian StyleCatalano, Teresa, Federico Selvaggi, Roberto Cotellese, and Gitana Maria Aceto. 2025. "The Role of Reactive Oxygen Species in Colorectal Cancer Initiation and Progression: Perspectives on Theranostic Approaches" Cancers 17, no. 5: 752. https://doi.org/10.3390/cancers17050752
APA StyleCatalano, T., Selvaggi, F., Cotellese, R., & Aceto, G. M. (2025). The Role of Reactive Oxygen Species in Colorectal Cancer Initiation and Progression: Perspectives on Theranostic Approaches. Cancers, 17(5), 752. https://doi.org/10.3390/cancers17050752







