Fluorescence-Guided Resection of GL261 Red-FLuc and TRP-mCherry-FLuc Mouse Glioblastoma Tumors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. In Vitro Fluorescence Assay
2.3. Animals
2.4. Orthotopic Mouse Glioblastoma Models
2.5. Bioluminescence Imaging
2.6. T2-Weighted Magnetic Resonance Imaging
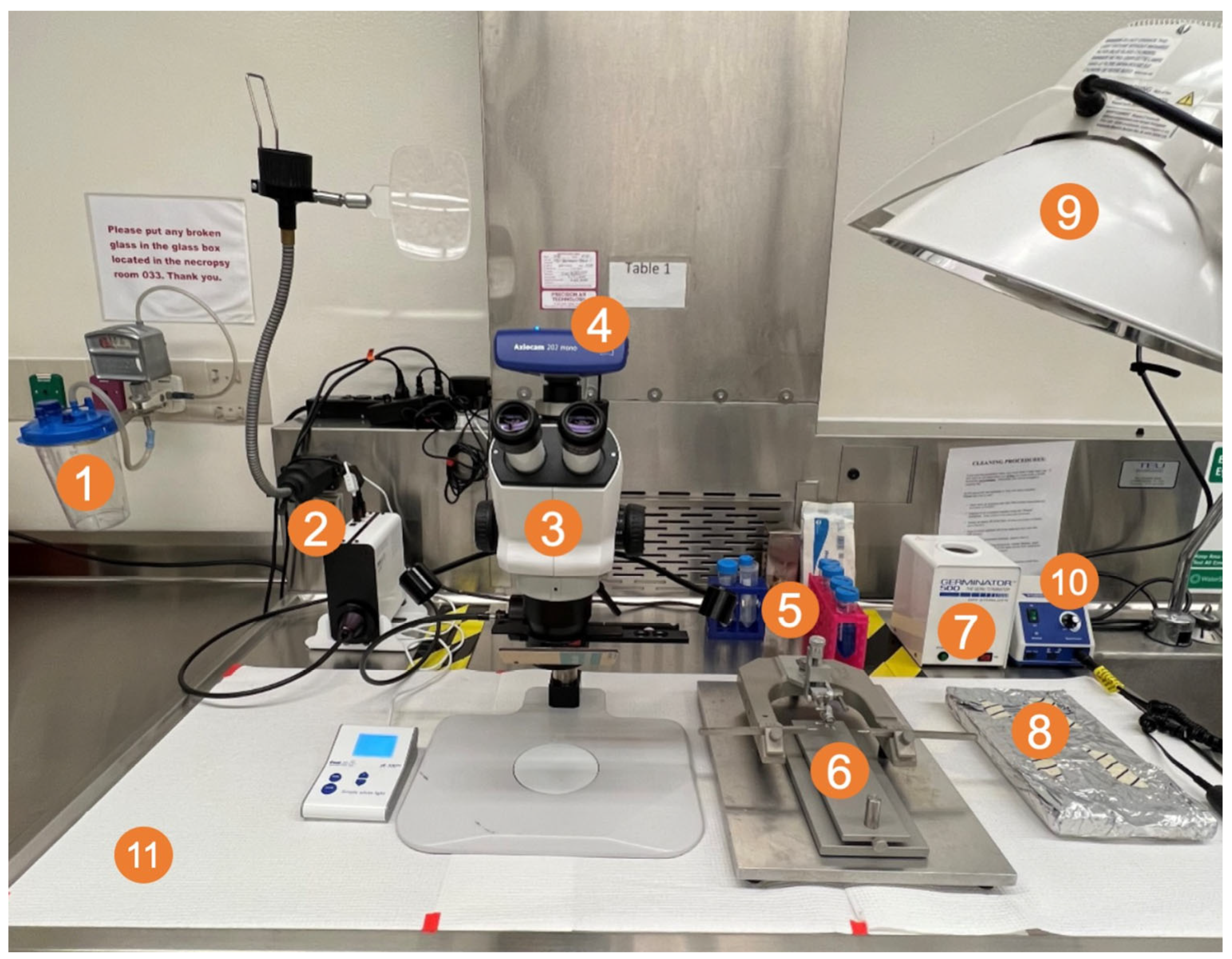
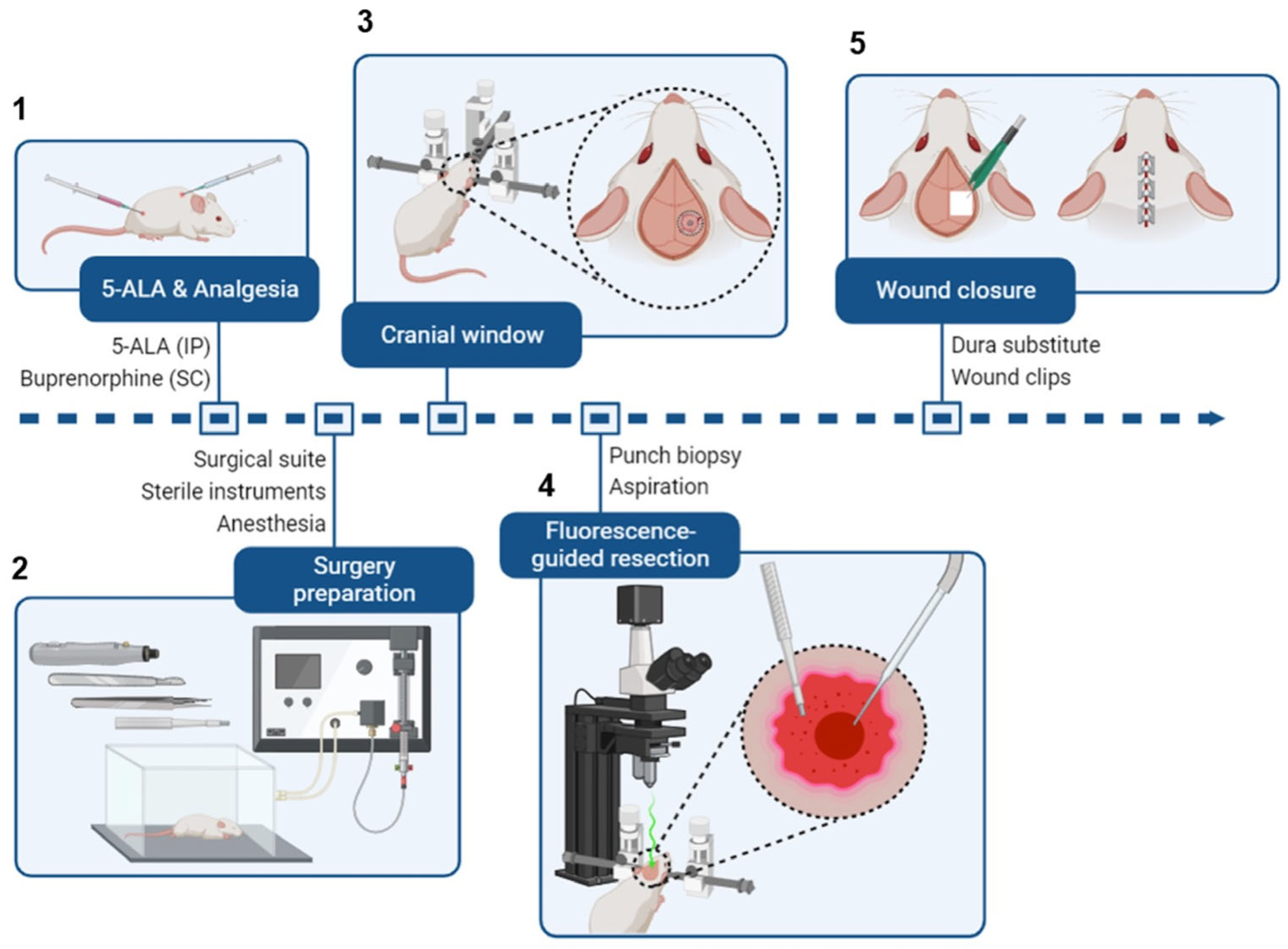
2.7. Fluorescence-Guided Tumor Resection
2.8. Data Analysis and Statistics
3. Results
3.1. In Vitro 5-ALA-Induced Fluorescence of GL261 Red-FLuc and TRP-mCF Cells
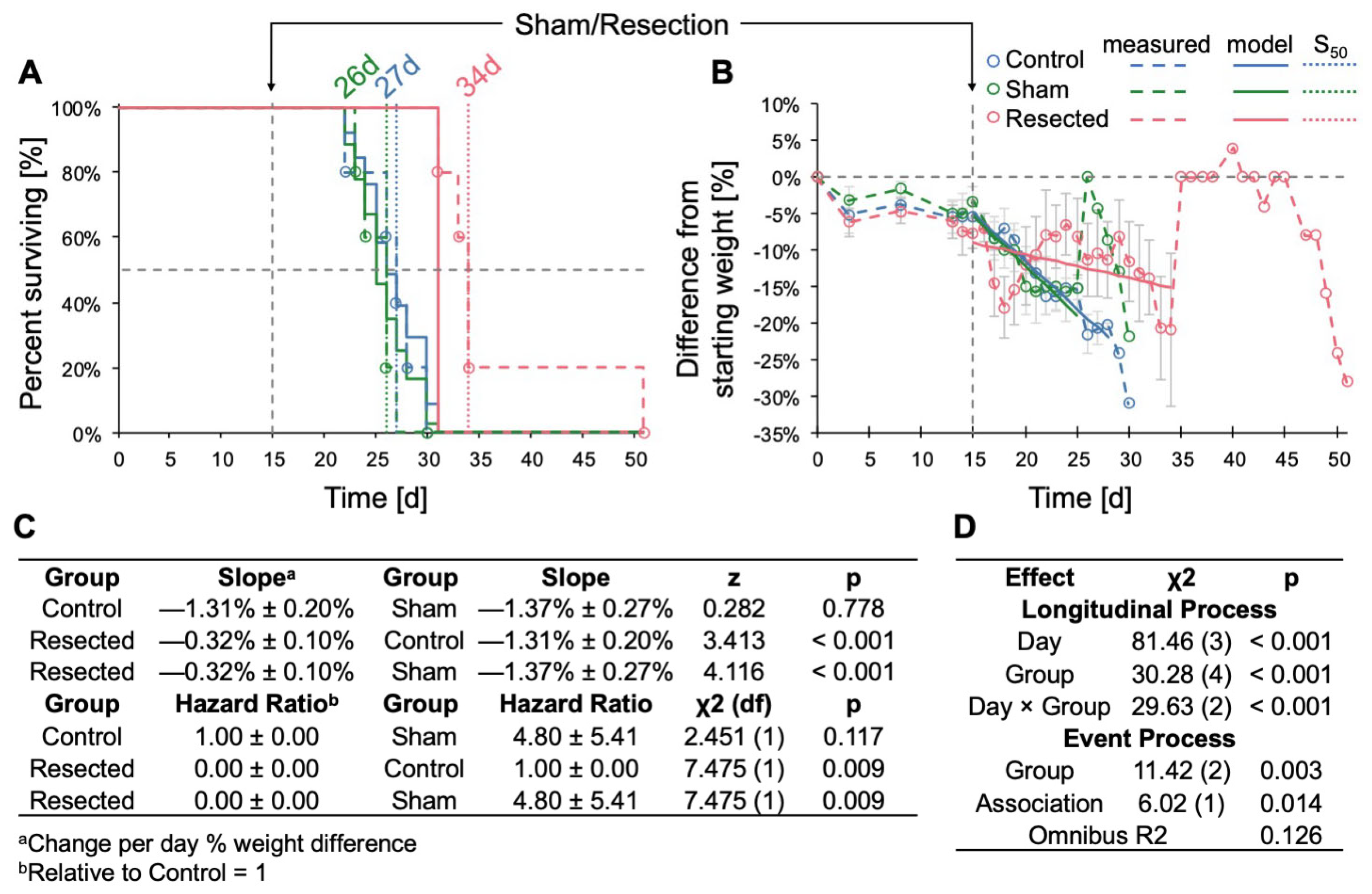
3.2. Resection of TRP-mCF Tumors Significantly Extended Mouse Survival and Slowed Body Weight Loss
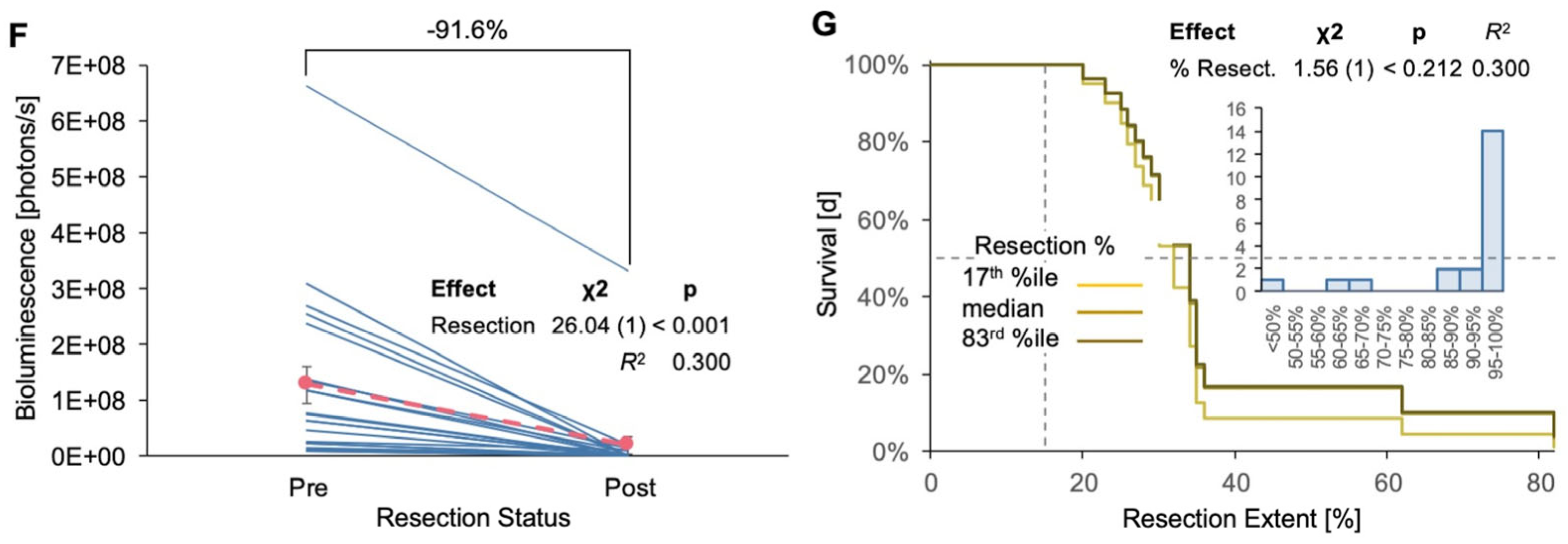
3.3. Resection of GL261 Red-FLuc Tumors Extended Survival, Reduced Body Weight Loss, and Slowed Tumor Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro. Oncol. 2023, 25, iv1–iv99. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Vanderbeek, A.M.; Rahman, R.; Fell, G.; Ventz, S.; Chen, T.; Redd, R.; Parmigiani, G.; Cloughesy, T.F.; Wen, P.Y.; Trippa, L.; et al. The clinical trials landscape for glioblastoma: Is it adequate to develop new treatments? Neuro. Oncol. 2018, 20, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Oster, C.; Schmidt, T.; Agkatsev, S.; Lazaridis, L.; Kleinschnitz, C.; Sure, U.; Scheffler, B.; Kebir, S.; Glas, M. Are we providing best-available care to newly diagnosed glioblastoma patients? Systematic review of phase III trials in newly diagnosed glioblastoma 2005–2022. Neuro. Oncol. Adv. 2023, 5, vdad105. [Google Scholar] [CrossRef]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Amara, D.; Berger, M.S.; Raleigh, D.R.; Aghi, M.K.; Butowski, N.A. Mouse models of glioblastoma for the evaluation of novel therapeutic strategies. Neurooncol. Adv. 2021, 3, vdab100. [Google Scholar] [CrossRef]
- Akter, F.; Simon, B.; Leonie de Boer, N.; Redjal, N.; Wakimoto, H.; Shah, K. Pre-clinical tumor models of primary brain tumors: Challenges and opportunities. BBA Rev. Cancer 2021, 1875, 188458. [Google Scholar] [CrossRef]
- Gunjur, A.; Balasubramanian, A.; Hafeez, U.; Menon, S.; Cher, L.; Parakh, S.; Gan, H.K. Poor correlation between preclinical and patient efficacy data for tumor targeted monotherapies in glioblastoma: The results of a systematic review. J. Neurooncol. 2022, 159, 539–549. [Google Scholar] [CrossRef]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.J.; Than, K.; Olivi, A.; Weingart, J.D.; Brem, H.; Quiñones-Hinojosa, A. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J. Neurosurg. 2009, 110, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Polley, M.-Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef]
- Stummer, W.; Kamp, M.A. The importance of surgical resection in malignant glioma. Curr. Opin. Neurol. 2009, 22, 645–649. [Google Scholar] [CrossRef]
- Alieva, M.; Margarido, A.S.; Wieles, T.; Abels, E.R.; Colak, B.; Boquetale, C.; Jan Noordmans, H.; Snijders, T.J.; Broekman, M.L.; van Rheenen, J. Preventing inflammation inhibits biopsy-mediated changes in tumor cell behavior. Sci. Rep. 2017, 7, 7529. [Google Scholar] [CrossRef]
- Okolie, O.; Bago, J.R.; Schmid, R.S.; Irvin, D.M.; Bash, R.E.; Miller, C.R.; Hingtgen, S.D. Reactive astrocytes potentiate tumor aggressiveness in a murine glioma resection and recurrence model. Neuro. Oncol. 2016, 18, 1622–1633. [Google Scholar] [CrossRef]
- Knudsen, A.M.; Halle, B.; Cedile, O.; Burton, M.; Baun, C.; Thisgaard, H.; Anand, A.; Hubert, C.; Thomassen, M.; Michaelsen, S.R.; et al. Surgical resection of glioblastomas induces pleiotrophin-mediated self-renewal of glioblastoma stem cells in recurrent tumors. Neuro. Oncol. 2022, 24, 1074–1087. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
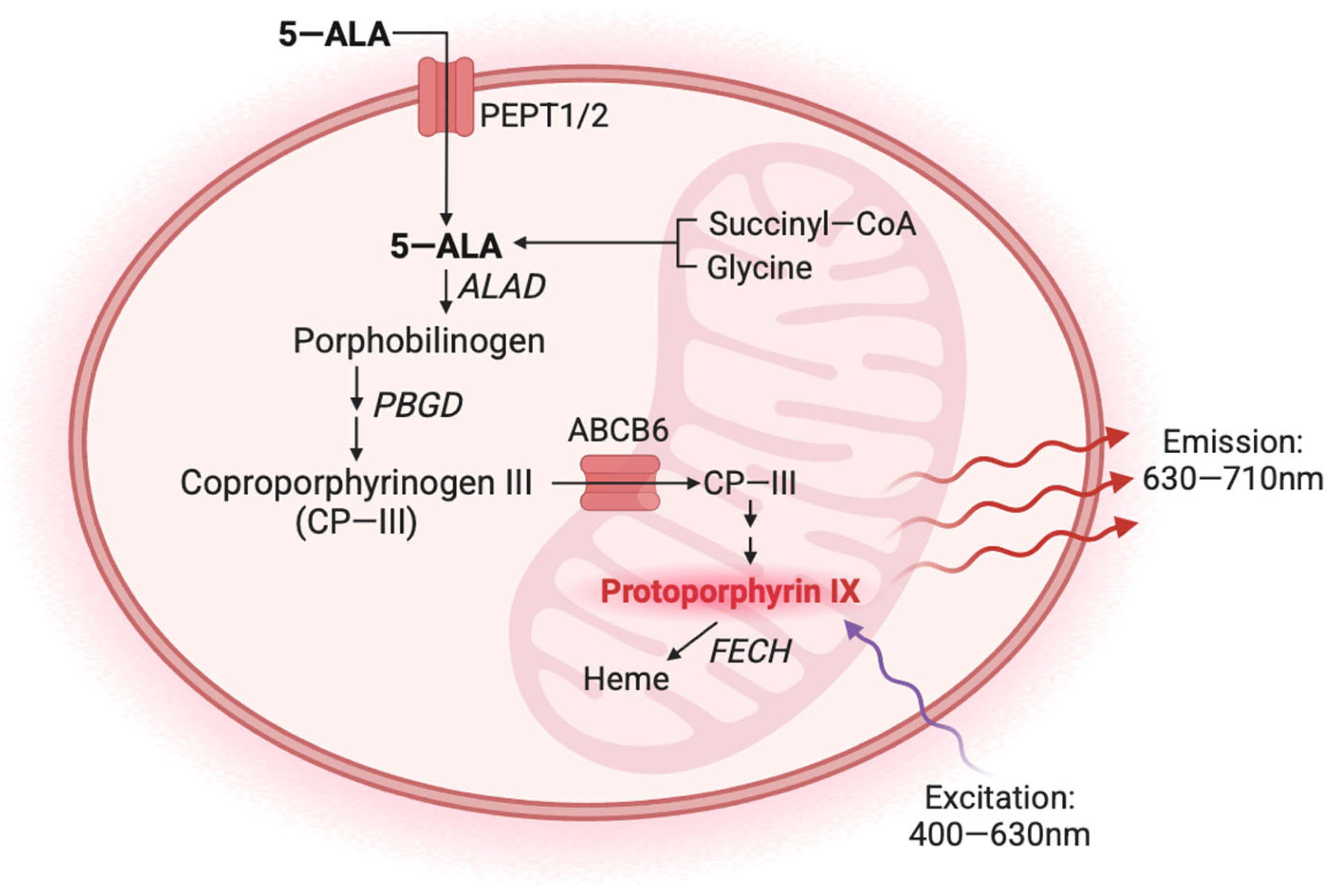
- Jonker, J.W.; Buitelaar, M.; Wagenaar, E.; van der Valk, M.A.; Scheffer, G.L.; Scheper, R.J.; Plösch, T.; Kuipers, F.; Elferink, R.P.J.O.; Rosing, H.; et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. USA 2002, 99, 15649–15654. [Google Scholar] [CrossRef]
- Zhao, S.G.; Chen, X.F.; Wang, L.G.; Yang, G.; Han, D.Y.; Teng, L.; Yang, M.C.; Wang, D.Y.; Shi, C.; Liu, Y.H.; et al. Increased expression of ABCB6 enhances protoporphyrin IX accumulation and photodynamic effect in human glioma. Ann. Surg. Oncol. 2013, 20, 4379–4388. [Google Scholar] [CrossRef] [PubMed]
- Traylor, J.I.; Pernik, M.N.; Sternisha, A.C.; McBrayer, S.K.; Abdullah, K.G. Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas. Cancers 2021, 13, 580. [Google Scholar] [CrossRef]
- Mazurek, M.; Szczepanek, D.; Orzyłowska, A.; Rola, R. Analysis of Factors Affecting 5-ALA Fluorescence Intensity in Visualizing Glial Tumor Cells—Literature Review. Int. J. Mol. Sci. 2022, 23, 926. [Google Scholar] [CrossRef] [PubMed]
- Kauer, T.M.; Figueiredo, J.L.; Hingtgen, S.; Shah, K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nat. Neurosci. 2011, 15, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Hingtgen, S.; Figueiredo, J.L.; Farrar, C.; Duebgen, M.; Martinez-Quintanilla, J.; Bhere, D.; Shah, K. Real-time multi-modality imaging of glioblastoma tumor resection and recurrence. J. Neurooncol. 2013, 111, 153–161. [Google Scholar] [CrossRef]
- Momiyama, M.; Hiroshima, Y.; Suetsugu, A.; Tome, Y.; Mii, S.; Yano, S.; Bouvet, M.; Chishima, T.; Endo, I.; Hoffman, R.M. Enhanced resection of orthotopic red-fluorescent-protein-expressing human glioma by fluorescence-guided surgery in nude mice. Anticancer. Res. 2013, 33, 107–111. [Google Scholar]
- Sheets, K.T.; Bago, J.R.; Paulk, I.L.; Hingtgen, S.D. Image-Guided Resection of Glioblastoma and Intracranial Implantation of Therapeutic Stem Cell-seeded Scaffolds. J. Vis. Exp. 2018, 137, 57452. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Georges, J.; Eschbacher, J.M.; Cavalcanti, D.D.; Elhadi, A.M.; Abdelwahab, M.G.; Scheck, A.C.; Nakaji, P.; Spetzler, R.F.; Preul, M.C. Potential application of a handheld confocal endomicroscope imaging system using a variety of fluorophores in experimental gliomas and normal brain. Neurosurg. Focus 2014, 36, E16. [Google Scholar] [CrossRef]
- Belykh, E.; Miller, E.J.; Hu, D.; Martirosyan, N.L.; Woolf, E.C.; Scheck, A.C.; Byvaltsev, V.A.; Nakaji, P.; Nelson, L.Y.; Seibel, E.J.; et al. Scanning Fiber Endoscope Improves Detection of 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence at the Boundary of Infiltrative Glioma. World Neurosurg. 2018, 113, e51–e69. [Google Scholar] [CrossRef]
- El Meskini, R.; Iacovelli, A.J.; Kulaga, A.; Gumprecht, M.; Martin, P.L.; Baran, M.; Householder, D.B.; Van Dyke, T.; Weaver Ohler, Z. A preclinical orthotopic model for glioblastoma recapitulates key features of human tumors and demonstrates sensitivity to a combination of MEK and PI3K pathway inhibitors. Dis. Model. Mech. 2015, 8, 45–56. [Google Scholar] [CrossRef]
- Rodgers, L.T.; Schulz Pauly, J.A.; Maloney, B.J.; Hartz, A.M.S.; Bauer, B. Optimization, Characterization, and Comparison of Two Luciferase-Expressing Mouse Glioblastoma Models. Cancers 2024, 16, 1997. [Google Scholar] [CrossRef]
- Enriquez Perez, J.; Kopecky, J.; Visse, E.; Darabi, A.; Siesjo, P. Convection-enhanced delivery of temozolomide and whole cell tumor immunizations in GL261 and KR158 experimental mouse gliomas. BMC Cancer 2020, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Garbow, J.R.; Johanns, T.M.; Ge, X.; Engelbach, J.A.; Yuan, L.; Dahiya, S.; Tsien, C.I.; Gao, F.; Rich, K.M.; Ackerman, J.J.H. Irradiation-Modulated Murine Brain Microenvironment Enhances GL261-Tumor Growth and Inhibits Anti-PD-L1 Immunotherapy. Front. Oncol. 2021, 11, 693146. [Google Scholar] [CrossRef] [PubMed]
- Renner, D.N.; Malo, C.S.; Jin, F.; Parney, I.F.; Pavelko, K.D.; Johnson, A.J. Improved Treatment Efficacy of Antiangiogenic Therapy when Combined with Picornavirus Vaccination in the GL261 Glioma Model. Neurotherapeutics 2016, 13, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, V.E.; Lynes, J.P.; Walbridge, S.; Wang, X.; Edwards, N.A.; Nwankwo, A.K.; Sur, H.P.; Dominah, G.A.; Obungu, A.; Adamstein, N.; et al. GL261 luciferase-expressing cells elicit an anti-tumor immune response: An evaluation of murine glioma models. Sci. Rep. 2020, 10, 11003. [Google Scholar] [CrossRef]
- Carlson, B.L.; Pokorny, J.L.; Schroeder, M.A.; Sarkaria, J.N. Establishment, maintenance and in vitro and in vivo applications of primary human glioblastoma multiforme (GBM) xenograft models for translational biology studies and drug discovery. In Current Protocols in Pharmacology, 2011/07/12 ed.; James Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 1–14. [Google Scholar]
- Schulz, J.A.; Rodgers, L.T.; Kryscio, R.J.; Hartz, A.M.S.; Bauer, B. Characterization and comparison of human glioblastoma models. BMC Cancer 2022, 22, 844. [Google Scholar] [CrossRef]
- El Meskini, R.; Atkinson, D.; Weaver Ohler, Z. Translational Orthotopic Models of Glioblastoma Multiforme. J. Vis. Exp. 2023, 192, e64482. [Google Scholar] [CrossRef]
- Toth, L.A. Defining the Moribound Condition as an Experimental Endpoint for Animal Research. ILAR J. 2000, 41, 72–79. [Google Scholar] [CrossRef]
- Wallace, J. Humane endpoints and cancer research. ILAR J. 2000, 41, 87–93. [Google Scholar] [CrossRef]
- Rizopoulos, D. Joint Models for Longitudinal and Time-to-Event Data: With Applications in R; CRC Press: Boca Raton, FL, USA, 2012; 261p. [Google Scholar]
- Nakagawa, S.; Johnson, P.C.; Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef]
- Nagelkerke, N.J.D. A Note on a General Definition of the Coefficient of Determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Society. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar] [CrossRef]
- Rizopoulos, D. JM: An R Package for the Joint Modelling of Longitudinal and Time-to-Event Data. J. Stat. Softw. 2010, 35, 1–33. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Therneau, T. A Package for Survival Analysis in R. Available online: https://CRAN.R-project.org/package=survival (accessed on 23 April 2024).
- Bianco, J.; Bastiancich, C.; Joudiou, N.; Gallez, B.; des Rieux, A.; Danhier, F. Novel model of orthotopic U-87 MG glioblastoma resection in athymic nude mice. J. Neurosci. Methods 2017, 284, 96–102. [Google Scholar] [CrossRef]
- de Gooijer, M.C.; Guillen Navarro, M.; Bernards, R.; Wurdinger, T.; van Tellingen, O. An Experimenter’s Guide to Glioblastoma Invasion Pathways. Trends Mol. Med. 2018, 24, 763–780. [Google Scholar] [CrossRef]
- Lilja-Cyron, A.; Andresen, M.; Kelsen, J.; Andreasen, T.; Petersen, L.; Fugleholm, K.; Juhler, M. Intracranial pressure before and after cranioplasty: Insights into intracranial physiology. J. Neurosurg. 2020, 133, 1548–1558. [Google Scholar] [CrossRef]
- Lilja-Cyron, A.; Andresen, M.; Kelsen, J.; Andreasen, T.; Fugleholm, K.; Juhler, M. Long-Term Effect of Decompressive Craniectomy on Intracranial Pressure and Possible Implications for Intracranial Fluid Movements. Neurosurgery 2020, 86, 231–240. [Google Scholar] [CrossRef]
- Riva, M.; Bevers, S.; Wouters, R.; Thirion, G.; Vandenbrande, K.; Vankerckhoven, A.; Berckmans, Y.; Verbeeck, J.; Keersmaecker, K.D.; Coosemans, A. Towards more accurate preclinical glioblastoma modelling: Reverse translation of clinical standard of care in a glioblastoma mouse model. bioRxiv 2021, 2021, 17.448792. [Google Scholar] [CrossRef]
- Gleolan. Available online: https://gleolan.com/ (accessed on 23 April 2024).
- Johnson, B.E.; Mazor, T.; Hong, C.; Barnes, M.; Aihara, K.; McLean, C.Y.; Fouse, S.D.; Yamamoto, S.; Ueda, H.; Tatsuno, K.; et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 2014, 343, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Burns, T.C. Radiation-Induced Alterations in the Recurrent Glioblastoma Microenvironment: Therapeutic Implications. Front. Oncol. 2018, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Ochocka, N.; Segit, P.; Wojnicki, K.; Cyranowski, S.; Swatler, J.; Jacek, K.; Grajkowska, W.; Kaminska, B. Specialized functions and sexual dimorphism explain the functional diversity of the myeloid populations during glioma progression. Cell Rep. 2023, 42, 111971. [Google Scholar] [CrossRef]
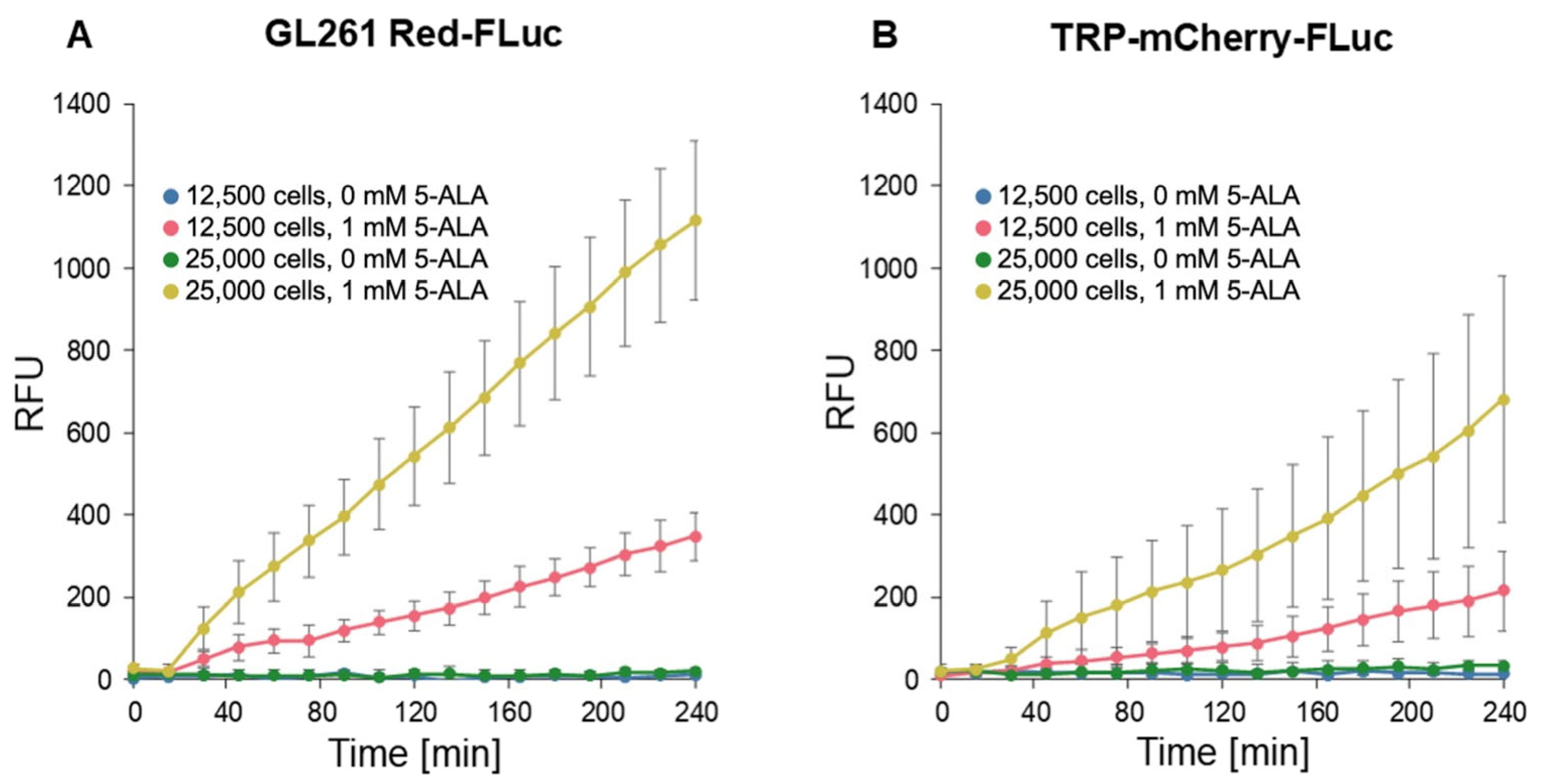

| Effect | χ2 (df) | p | R2 |
|---|---|---|---|
| 5-ALA | 326 (1) | <0.001 | 0.512 |
| Time | 1337 (1) | <0.001 | 0.268 |
| Cells | 104 (1) | <0.001 | 0.241 |
| Line | 28 (1) | <0.001 | 0.085 |
| 5-ALA × Time | 1285 (1) | <0.001 | 0.131 |
| 5-ALA × Cells | 97 (1) | <0.001 | 0.117 |
| Time × Cells | 392 (1) | <0.001 | 0.060 |
| 5-ALA × Line | 35 (1) | <0.001 | 0.046 |
| Cells × Line | 9 (1) | 0.002 | 0.020 |
| Time × Line | 88 (1) | <0.001 | 0.018 |
| 5-ALA × Time × Cells | 361 (1) | <0.001 | 0.029 |
| 5-ALA × Cells × Line | 10 (1) | 0.002 | 0.011 |
| 5-ALA × Time × Line | 93 (1) | <0.001 | 0.009 |
| Time × Cells × Line | 28 (1) | <0.001 | 0.005 |
| 5-ALA × Time × Cells × Line | 33 (1) | <0.001 | 0.002 |
| Overall | 2282 (15) | <0.001 | 0.761 |
| Cells | 5-ALA | RFU/min | Cells | 5-ALA | RFU/min | t (df) | p |
|---|---|---|---|---|---|---|---|
| GL261 Red-FLuc | |||||||
| 12,500 | 0 | 0.001 ± 0.019 | 25,000 | 0 | 0.005 ± 0.019 | 0.164 (1448) | 0.869 |
| 12,500 | 0 | 0.001 ± 0.019 | 12,500 | 1 | 0.276 ± 0.019 | 10.360 (1448) | <0.001 |
| 25,000 | 0 | 0.005 ± 0.019 | 25,000 | 1 | 0.935 ± 0.019 | 35.064 (1448) | <0.001 |
| 12,500 | 1 | 0.276 ± 0.019 | 25,000 | 1 | 0.935 ± 0.019 | 24.868 (1448) | <0.001 |
| TRP-mCF | |||||||
| 12,500 | 0 | −0.002 ± 0.042 | 25,000 | 0 | 0.015 ± 0.042 | 35.064 (1448) | 0.869 |
| 12,500 | 0 | −0.002 ± 0.042 | 12,500 | 1 | 0.169 ± 0.042 | 24.868 (1448) | 0.005 |
| 25,000 | 0 | 0.015 ± 0.042 | 25,000 | 1 | 0.537 ± 0.042 | 9.087 (36,192) | <0.001 |
| 12,500 | 1 | 0.169 ± 0.042 | 25,000 | 1 | 0.537 ± 0.042 | 2.605 (36,192) | <0.001 |
| Effect | χ2 (df) | p |
|---|---|---|
| Longitudinal Process | ||
| Day | 111.85 (2) | <0.001 |
| Group | 134.43 (2) | <0.001 |
| Day × Group | 111.43 (1) | <0.001 |
| Event Process | ||
| Group | 5.48 (1) | <0.019 |
| Association | 35.78 (1) | <0.001 |
| Omnibus R2 | 0.105 | |
| Effect | χ2 (df) | p |
|---|---|---|
| Longitudinal Process | ||
| Day | 154.98 (2) | <0.001 |
| Group | 165.29 (2) | <0.001 |
| Day × Group | 111.69 (1) | <0.001 |
| Event Process | ||
| Group | 15.04 (1) | <0.001 |
| Association | 0.09 (1) | 0.769 |
| Omnibus R2 | 0.049 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodgers, L.T.; Maloney, B.J.; Hartz, A.M.S.; Bauer, B. Fluorescence-Guided Resection of GL261 Red-FLuc and TRP-mCherry-FLuc Mouse Glioblastoma Tumors. Cancers 2025, 17, 734. https://doi.org/10.3390/cancers17050734
Rodgers LT, Maloney BJ, Hartz AMS, Bauer B. Fluorescence-Guided Resection of GL261 Red-FLuc and TRP-mCherry-FLuc Mouse Glioblastoma Tumors. Cancers. 2025; 17(5):734. https://doi.org/10.3390/cancers17050734
Chicago/Turabian StyleRodgers, Louis T., Bryan J. Maloney, Anika M. S. Hartz, and Björn Bauer. 2025. "Fluorescence-Guided Resection of GL261 Red-FLuc and TRP-mCherry-FLuc Mouse Glioblastoma Tumors" Cancers 17, no. 5: 734. https://doi.org/10.3390/cancers17050734
APA StyleRodgers, L. T., Maloney, B. J., Hartz, A. M. S., & Bauer, B. (2025). Fluorescence-Guided Resection of GL261 Red-FLuc and TRP-mCherry-FLuc Mouse Glioblastoma Tumors. Cancers, 17(5), 734. https://doi.org/10.3390/cancers17050734






