Dermoscopy of External Ear Melanocytic Lesions: Performance of Selected Dermoscopic Screening Algorithms and Proposal of a New Predictive Model for Malignancy (AuriCheck Dermoscopic Algorithm)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dermoscopy Protocol
2.2. Statistical Analysis and Model Development
3. Results
3.1. Clinical Assessment
3.2. Dermoscopic Analysis
3.3. Performance of the Predictive Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EEMLs | External ear melanocytic lesions |
| SSL | Special site location |
| EEMs | External ear melanomas |
| SAMPUS | Superficial Atypical Melanocytic Proliferations of Unknown Significance |
| MELTUMP | Melanocytic Tumours of Uncertain Malignant Potential |
| IAMPUS | Intraepidermal Atypical Melanocytic Proliferation of Uncertain Significance |
| N/A | Not applicable |
| SD | Standard deviation |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
| LMM | Lentigo maligna melanoma |
| SSM | Superficial spreading melanoma |
| PLP | Perifollicular linear projections |
References
- Mandel, V.D.; Ardigo, M. Non-Invasive Diagnostic Techniques in Dermatology. J. Clin. Med. 2023, 12, 1081. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Pampena, R.; Moscarella, E.; Chester, J.; Starace, M.; Cinotti, E.; Piraccini, B.M.; Argenziano, G.; Peris, K.; Pellacani, G. Dermoscopy of melanoma according to different body sites: Head and neck, trunk, limbs, nail, mucosal and acral. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1718–1730. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Phan, A.; Pralong, P.; Poulalhon, N.; Debarbieux, S.; Dalle, S. Special locations dermoscopy: Facial, acral, and nail. Dermatol. Clin. 2013, 31, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Lallas, A.; Lallas, K.; Tschandl, P.; Kittler, H.; Apalla, Z.; Longo, C.; Argenziano, G. The dermoscopic inverse approach significantly improves the accuracy of human readers for lentigo maligna diagnosis. J. Am. Acad. Dermatol. 2021, 84, 381–389. [Google Scholar] [CrossRef]
- Micantonio, T.; Neri, L.; Longo, C.; Grassi, S.; Di Stefani, A.; Antonini, A.; Coco, V.; Fargnoli, M.C.; Argenziano, G.; Peris, K. A new dermoscopic algorithm for the differential diagnosis of facial lentigo maligna and pigmented actinic keratosis. Eur. J. Dermatol. 2018, 28, 162–168. [Google Scholar] [CrossRef]
- Jellinek, N. Nail matrix biopsy of longitudinal melanonychia: Diagnostic algorithm including the matrix shave biopsy. J. Am. Acad. Dermatol. 2007, 56, 803–810. [Google Scholar] [CrossRef]
- Lallas, A.; Korecka, K.; Apalla, Z.; Sgouros, D.; Liopyris, K.; Argenziano, G.; Thomas, L. Seven Plus One Steps to Assess Pigmented Nail Bands (Melanonychia Striata Longitudinalis). Dermatol. Pract. Concept. 2023, 13, e2023204. [Google Scholar] [CrossRef]
- Rishpon, A.; Sprecher, E.; Dusza, S.W.; Kleinman, E.; Haupt, S.; Rabinovitz, H.; Scope, A. Morphological features of benign pigmented ear lesions: A cross-sectional study. Int. J. Dermatol. 2022, 61, 208–215. [Google Scholar] [CrossRef]
- Scampa, M.; Megevand, V.; Viscardi, J.A.; Giordano, S.; Kalbermatten, D.F.; Oranges, C.M. Melanoma of the Scalp and Neck: A Population-Based Analysis of Survival and Treatment Patterns. Cancers 2022, 14, 6052. [Google Scholar] [CrossRef]
- Toia, F.; Garbo, G.; Tripoli, M.; Rinaldi, G.; Moschella, F.; Cordova, A. A systematic review on external ear melanoma. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 883–894. [Google Scholar] [CrossRef]
- Tammaro, A.; Adebanjo, G.A.R.; Chello, C.; Parisella, F.R.; Cantisani, C.; Farnetani, F.; Pellacani, G. Malignant lesions of the ear. Arch. Dermatol. Res. 2022, 314, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Kaminska-Winciorek, G.; Slowinska, M.; Krotowski, J.; Nasierowska-Guttmejer, A.; Musial, J.; Cybulska-Stopa, B. Dermoscopy of external ear melanoma (EEM). Arch. Dermatol. Res. 2023, 315, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Peralta, R.; Cabo, H.; Sabban, E.C.; Salerni, G.; Gonzalez, V.M. Dermoscopic Features of External Ear Melanoma: A Case Series. Dermatol. Pract. Concept. 2023, 13, e2023033. [Google Scholar] [CrossRef]
- Fiorio, L.M.; Diniz, L.M.; Spelta, K.; Badaro, B.A. Ear melanoma: A four-case series. Bras. Dermatol. 2021, 96, 64–67. [Google Scholar] [CrossRef]
- Kittler, H.; Marghoob, A.A.; Argenziano, G.; Carrera, C.; Curiel-Lewandrowski, C.; Hofmann-Wellenhof, R.; Malvehy, J.; Menzies, S.; Puig, S.; Rabinovitz, H.; et al. Standardization of terminology in dermoscopy/dermatoscopy: Results of the third consensus conference of the International Society of Dermoscopy. J. Am. Acad. Dermatol. 2016, 74, 1093–1106. [Google Scholar] [CrossRef]
- Deinlein, T.; Blum, A.; Schulter, G.; Haenssle, H.A.; Braun, R.; Giuffrida, R.; Hofmann-Wellenhof, R. Clinical and Dermoscopic Features of Melanocytic Lesions on the Face Versus the External Ear. Dermatol. Pract. Concept. 2021, 11, e2021124. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Jaimes, N.; Dusza, S.W.; Liopyris, K.; Marchetti, M.A.; Cordova, M.; Oliviero, M.; Villaseca, M.A.; Pulitzer, M.; Busam, K.J.; et al. Perifollicular linear projections: A dermatoscopic criterion for the diagnosis of lentigo maligna on the face. J. Am. Acad. Dermatol. 2024, 90, 52–57. [Google Scholar] [CrossRef]
- Argenziano, G.; Catricala, C.; Ardigo, M.; Buccini, P.; De Simone, P.; Eibenschutz, L.; Ferrari, A.; Mariani, G.; Silipo, V.; Sperduti, I.; et al. Seven-point checklist of dermoscopy revisited. Br. J. Dermatol. 2011, 164, 785–790. [Google Scholar] [CrossRef]
- Henning, J.S.; Dusza, S.W.; Wang, S.Q.; Marghoob, A.A.; Rabinovitz, H.S.; Polsky, D.; Kopf, A.W. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J. Am. Acad. Dermatol. 2007, 56, 45–52. [Google Scholar] [CrossRef]
- Menzies, S.W.; Ingvar, C.; Crotty, K.A.; McCarthy, W.H. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch. Dermatol. 1996, 132, 1178–1182. [Google Scholar] [CrossRef]
- Rosendahl, C.; Cameron, A.; McColl, I.; Wilkinson, D. Dermatoscopy in routine practice—‘Chaos and clues’. Aust. Fam. Physician 2012, 41, 482–487. [Google Scholar] [PubMed]
- Soyer, H.P.; Argenziano, G.; Zalaudek, I.; Corona, R.; Sera, F.; Talamini, R.; Barbato, F.; Baroni, A.; Cicale, L.; Di Stefani, A.; et al. Three-point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology 2004, 208, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Tschandl, P.; Gambardella, A.; Boespflug, A.; Deinlein, T.; de Giorgi, V.; Kittler, H.; Lallas, A.; Malvehy, J.; Moscarella, E.; Puig, S.; et al. Seven Non-melanoma Features to Rule Out Facial Melanoma. Acta Derm. Venereol. 2017, 97, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Tschandl, P.; Rosendahl, C.; Kittler, H. The HAM10000 dataset, a large collection of multi-source dermatoscopic images of common pigmented skin lesions. Sci. Data 2018, 5, 180161. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.D.; Chin, O.Y.; Baredes, S.; Eloy, J.A.; Ying, Y.M. A Population Based Analysis of Melanoma of the External Ear. Otol. Neurotol. 2018, 39, e137–e142. [Google Scholar] [CrossRef]
- Deep, N.L.; Glasgow, A.E.; Habermann, E.B.; Kasperbauer, J.L.; Carlson, M.L. Melanoma of the external ear: A population-based study. Am. J. Otolaryngol. 2017, 38, 309–315. [Google Scholar] [CrossRef]
- Green, A.C.; Kimlin, M.; Siskind, V.; Whiteman, D.C. Hypothesis: Hair cover can protect against invasive melanoma on the head and neck (Australia). Cancer Causes Control 2006, 17, 1263–1266. [Google Scholar] [CrossRef]
- Sawyer, J.D.; Wilson, M.L.; Neumeister, M.W. A Systematic Review of Surgical Management of Melanoma of the External Ear. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1755. [Google Scholar] [CrossRef]
- Pajak, J.; Lelonek, E.; Chlebicka, I.; Szepietowski, J.C. Nodular melanoma of the external ear: A rare tumour successfully treated with simple excision. Postep. Dermatol. Alergol. 2022, 39, 635–636. [Google Scholar] [CrossRef]
- Ankad, B.S.; Behera, B.; Lallas, A.; Akay, B.N.; Bhat, Y.J.; Chauhan, P.; Enechukwu, N.A.; Geller, S.; Jha, A.K.; Kaliyadan, F.; et al. International Dermoscopy Society (IDS) Criteria for Skin Tumors: Validation for Skin of Color Through a Delphi Expert Consensus by the “Imaging in Skin of Color” IDS Task Force. Dermatol. Pract. Concept. 2023, 13, e2023067. [Google Scholar] [CrossRef]
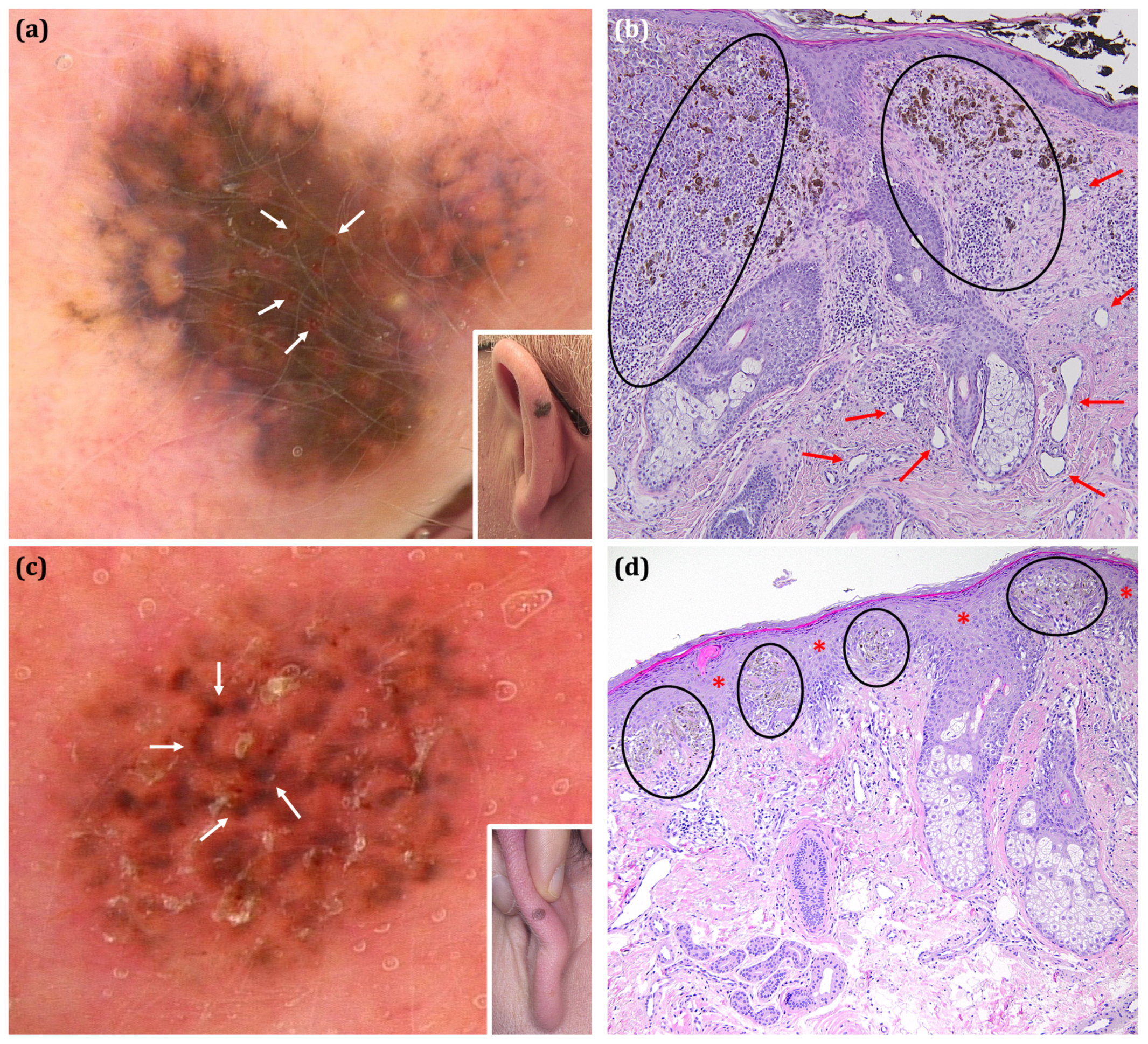

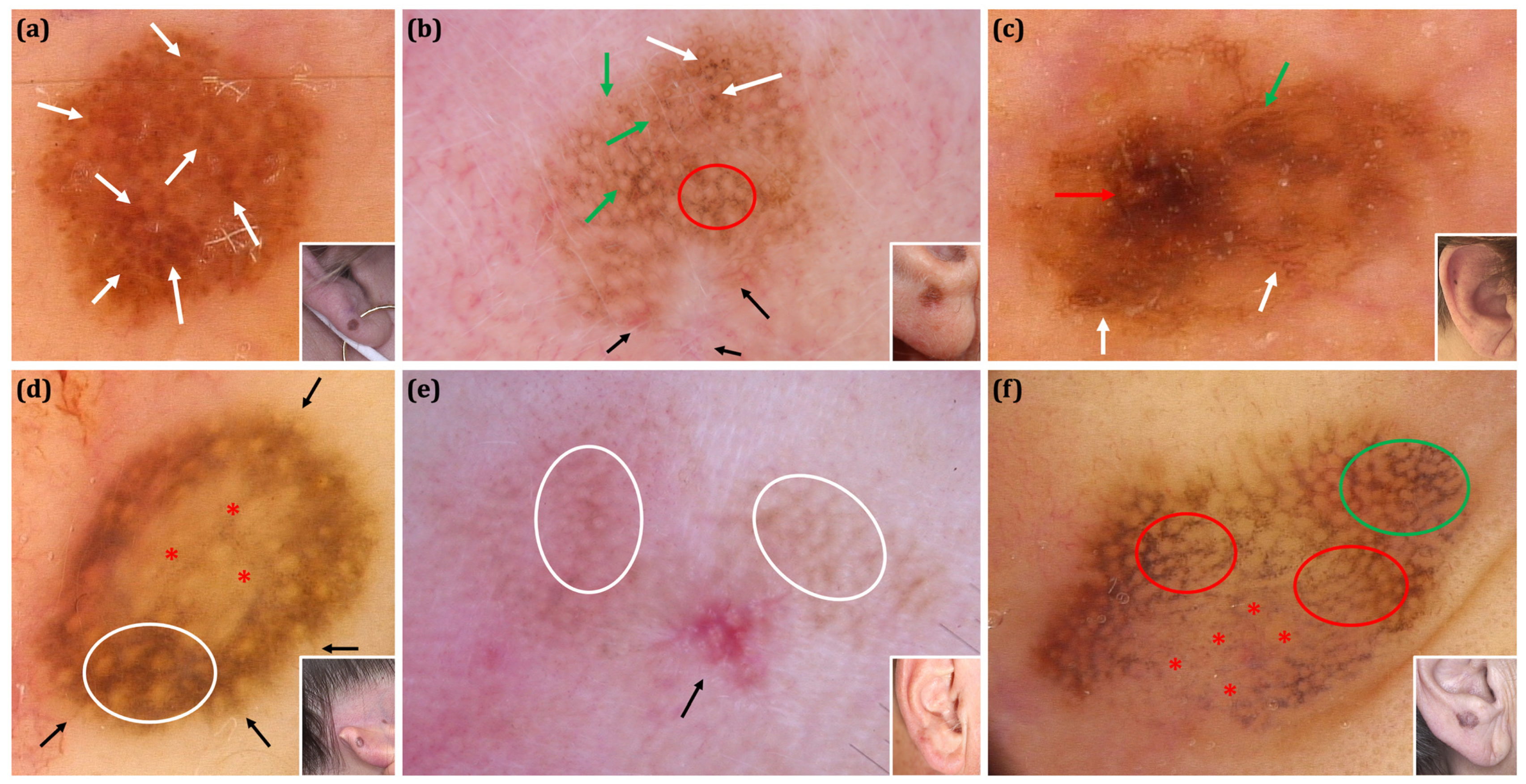
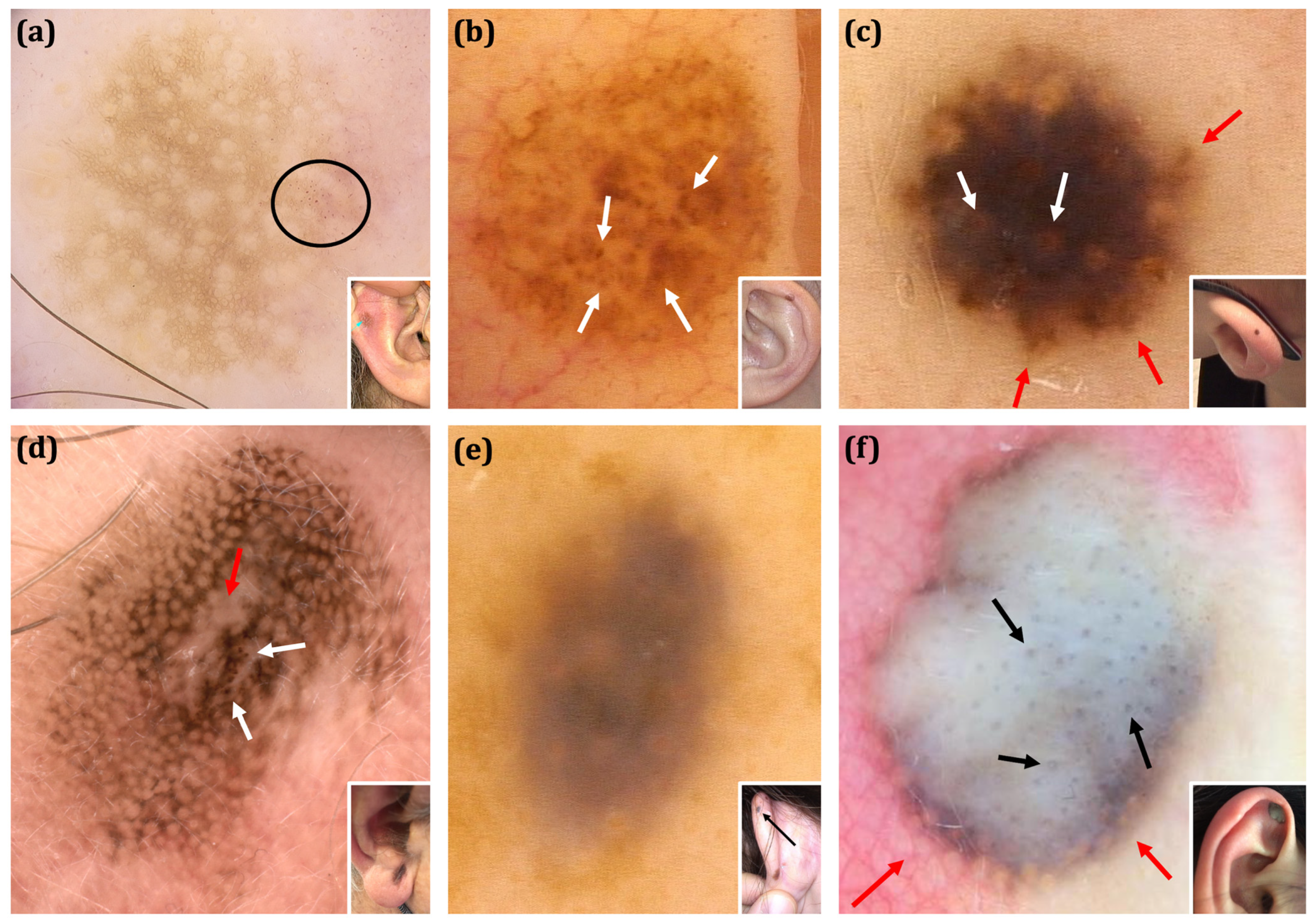
| Malignant | Benign | Total Malignant vs. Total Benign p Value | Total Malignant vs. Excised Benign Lesions 6 p Value | Total Melanoma vs. Nevi Excised p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanoma 1 (n = 48) | Other Entities 2 (n = 8) | Total Malignant (n = 56) | Nevi Excised 3 (n = 37) | Benign Melanocytic Nevi— Followed-Up 4 (n = 40) | Spitz Nevus (n = 6) | Other Entities 5 (n = 6) | Total Benign (n = 89) | ||||
| Female, No (%) | 18/48; 37.5% | 4/8; 50% | 22/56; 39.3% | 19/37; 51.4% | 21/40; 52.5% | 0/6; 0% | 5/6; 83.3% | 45/89; 50.6% | 0.2315 | 0.3324 | 0.2704 |
| Age at diagnosis, mean years ± SD (range) | 61.7 ± 18.3 (7–88) | 37.5 ± 28.6 (4–80) | 58.3 ± 21.6 (4–88) | 40.2 ± 17.1 (11–72) | 37.9 ± 13.1 (19–74) | 21.8 ± 20.4 (7–54) | 37.2 ± 20.8 (17–74) | 37.7 ± 16.2 (7–74) | <0.0001 *** | <0.0001 *** | <0.0001 *** |
| Followed-up lesions, No (%) | 4/48; 8.3% | 2/8; 25% | 6/56; 10.7% | 6/37; 16.2% | 40/40; 100% | 1/6; 16.7% | 2/6; 33.3% | 49/89; 55.1% | <0.0001 *** | 0.2806 | 0.3196 |
| Follow-up period, mean months ± SD (range) | 33 ± 35.1 (8–83) | 29 ± 32.5 (6–52) | 31.7 ± 30.9 (6–83) | 18.3 ± 13.3 (4–35) | 45 ± 31.9 (24–157) | 8 ± NA (8–8) | 32.5 ± 38.9 (5–60) | 40.4 ± 31.3 (4–157) | 0.7232 | 0.7232 | 1 |
| Anatomical location, No (%) | |||||||||||
| Antihelix | 0/48; 0% | 0/8; 0% | 0/56; 0% | 1/37; 2.7% | 0/40; 0% | 1/6; 16.7% | 1/6; 16.7% | 3/89; 3.4% | 0.2839 | 0.0983 | 0.4353 |
| Antitragus | 3/48; 6.2% | 0/8; 0% | 3/56; 5.4% | 2/37; 5.4% | 5/40; 12.5% | 0/6; 0% | 0/6; 0% | 7/89; 7.9% | 0.7411 | 1 | 1 |
| Concha | 0/48; 0% | 0/8; 0% | 0/56; 0% | 1/37; 2.7% | 1/40; 2.5% | 0/6; 0% | 1/6; 16.7% | 3/89; 3.4% | 0.2839 | 0.2154 | 0.4353 |
| Crus of antihelix | 0/48; 0% | 0/8; 0% | 0/56; 0% | 2/37; 5.4% | 1/40; 2.5% | 0/6; 0% | 0/6; 0% | 3/89; 3.4% | 0.2839 | 0.2154 | 0.1866 |
| Crus of helix | 1/48; 2.1% | 0/8; 0% | 1/56; 1.8% | 0/37; 0% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 0/89; 0% | 0.3862 | 1 | 1 |
| Helix | 26/48; 54.2% | 5/8; 62.5% | 31/56; 55.4% | 19/37; 51.4% | 23/40; 57.5% | 5/6; 83.3% | 3/6; 50% | 50/89; 56.2% | 1 | 1 | 0.8294 |
| Lobule | 11/48; 22.9% | 2/8; 25% | 13/56; 23.2% | 4/37; 10.8% | 4/40; 10% | 0/6; 0% | 0/6; 0% | 8/89; 9% | 0.0276 * | 0.0609 | 0.1657 |
| Posterior part of auricle | 0/48; 0% | 1/8; 12.5% | 1/56; 1.8% | 3/37; 8.1% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 3/89; 3.4% | 1 | 0.337 | 0.0787 |
| Scaphoid fossa | 5/48; 10.4% | 0/8; 0% | 5/56; 8.9% | 5/37; 13.5% | 6/40; 15% | 0/6; 0% | 1/6; 16.7% | 12/89; 13.5% | 0.5969 | 0.7515 | 0.741 |
| Tragus | 2/48; 4.2% | 0/8; 0% | 2/56; 3.6% | 0/37; 0% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 0/89; 0% | 0.1475 | 0.4974 | 0.5025 |
| Left side, No (%) | 26/48; 54.2% | 3/8; 37.5% | 29/56; 51.8% | 18/37; 48.6% | 21/40; 52.5% | 4/6; 66.7% | 2/6; 33.3% | 45/89; 50.6% | 1 | 0.8459 | 0.6654 |
| Lesion diameter, mean millimetres ± SD (range) | 11.2 ± 8 (3.2–50) | 6.4 ± 2.6 (3.4–11.1) | 10.5 ± 7.6 (3.2–50) | 5.3 ± 3 (1.6–17) | 4.2 ± 1.6 (1.7–7.6) | 4 ± 1.2 (2.1–5.2) | 5.4 ± 3.2 (1.4–9) | 4.7 ± 2.4 (1.4–17) | <0.0001 *** | <0.0001 *** | <0.0001 *** |
| Dermoscopic Features | Malignant | Benign | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanoma (n = 48) | Other Entities (n = 8) | Total Malignant (n = 56) | Nevi Excised (n = 37) | Benign Melanocytic Nevi—Followed-Up (n = 40) | Spitz Nevus (n = 6) | Other Entities (n = 6) | Total Benign (n = 89) | Total Malignant vs. Total Benign p Value | Total Malignant vs. Excised Benign Lesions p Value | Total Melanoma vs. Nevi Excised p Value | ||
| Presence of colour | Light brown | 35/48; 72.9% | 6/8; 75% | 41/56; 73.2% | 30/37; 81.1% | 37/40; 92.5% | 3/6; 50% | 2/6; 33.3% | 72/89; 80.9% | 0.3078 | 1 | 0.4459 |
| Dark brown | 42/48; 87.5% | 7/8; 87.5% | 49/56; 87.5% | 34/37; 91.9% | 23/40; 57.5% | 6/6; 100% | 3/6; 50% | 66/89; 74.2% | 0.0604 | 1 | 0.7253 | |
| Black | 17/48; 35.4% | 0/8; 0% | 17/56; 30.4% | 2/37; 5.4% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 2/89; 2.2% | <0.0001 *** | 0.0006 *** | 0.0012 ** | |
| White | 31/48; 64.6% | 4/8; 50% | 35/56; 62.5% | 6/37; 16.2% | 0/40; 0% | 1/6; 16.7% | 1/6; 16.7% | 8/89; 9% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Grey | 29/48; 60.4% | 5/8; 62.5% | 34/56; 60.7% | 14/37; 37.8% | 3/40; 7.5% | 5/6; 83.3% | 2/6; 33.3% | 24/89; 27% | 0.0001 *** | 0.08 | 0.05 | |
| Red | 35/48; 72.9% | 7/8; 87.5% | 42/56; 75% | 8/37; 21.6% | 1/40; 2.5% | 2/6; 33.3% | 0/6; 0% | 11/89; 12.4% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Blue | 9/48; 18.8% | 1/8; 12.5% | 10/56; 17.9% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 3/6; 50% | 4/89; 4.5% | 0.0177 * | 0.1638 | 0.0379 * | |
| Total number of colours, mean number ± SD (range) | 4.1 ± 1.3 (1–6) | 3.8 ± 1.3 (2–5) | 4.1 ± 1.3 (1–6) | 2.6 ± 1.1 (1–5) | 1.6 ± 0.6 (1–3) | 2.8 ± 0.4 (2–3) | 1.8 ± 1 (1–3) | 2.1 ± 1 (1–5) | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Features characteristic for facial melanoma | Annular granular pattern | 11/48; 22.9% | 1/8; 12.5% | 12/56; 21.4% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 1/89; 1.1% | <0.0001 *** | 0.0025 ** | 0.0102 * |
| Asymmetric pigmented follicular openings | 30/48; 62.5% | 4/8; 50% | 34/56; 60.7% | 15/37; 40.5% | 2/40; 5% | 1/6; 16.7% | 0/6; 0% | 18/89; 20.2% | <0.0001 *** | 0.0059 ** | 0.0516 | |
| Circle within a circle | 8/48; 16.7% | 0/8; 0% | 8/56; 14.3% | 0/37; 0% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 0/89; 0% | 0.0004 *** | 0.0067 ** | 0.0086 ** | |
| Dark blotches and obliterated hair follicles | 18/48; 37.5% | 1/8; 12.5% | 19/56; 33.9% | 5/37; 13.5% | 1/40; 2.5% | 3/6; 50% | 0/6; 0% | 9/89; 10.1% | 0.0009 *** | 0.0462 * | 0.0152 * | |
| Hyperpigmented follicular openings | 32/48; 66.7% | 4/8; 50% | 36/56; 64.3% | 16/37; 43.2% | 2/40; 5% | 2/6; 33.3% | 0/6; 0% | 20/89; 22.5% | <0.0001 *** | 0.0062 ** | 0.0466 * | |
| Increased density of the vascular network | 34/48; 70.8% | 3/8; 37.5% | 37/56; 66.1% | 11/37; 29.7% | 3/40; 7.5% | 0/6; 0% | 0/6; 0% | 14/89; 15.7% | <0.0001 *** | <0.0001 *** | 0.0002 *** | |
| Polygons/zig zag | 24/48; 50% | 3/8; 37.5% | 27/56; 48.2% | 10/37; 27% | 0/40; 0% | 1/6; 16.7% | 0/6; 0% | 11/89; 12.4% | <0.0001 *** | 0.0081 ** | 0.0445 * | |
| Red rhomboidal structures | 12/48; 25% | 0/8; 0% | 12/56; 21.4% | 0/37; 0% | 0/40; 0% | 1/6; 16.7% | 0/6; 0% | 1/89; 1.1% | <0.0001 *** | 0.0025 ** | 0.0009 *** | |
| Rhomboids | 23/48; 47.9% | 3/8; 37.5% | 26/56; 46.4% | 7/37; 18.9% | 2/40; 5% | 1/6; 16.7% | 0/6; 0% | 10/89; 11.2% | <0.0001 *** | 0.0015 ** | 0.0064 ** | |
| Total number of features characteristic for facial melanoma, mean number ± SD (range) | 4.3 ± 2.6 (0–8) | 2.5 ± 2.1 (0–5) | 4.1 ± 2.6 (0–8) | 2.1 ± 2 (0–6) | 0.7 ± 0.9 (0–4) | 1.8 ± 2.1 (0–6) | 0.5 ± 0.5 (0–1) | 1.3 ± 1.6 (0–6) | <0.0001 *** | 0.0001 *** | <0.0001 *** | |
| Features characteristic for non-facial melanoma | Irregular globules | 22/48; 45.8% | 4/8; 50% | 26/56; 46.4% | 15/37; 40.5% | 3/40; 7.5% | 2/6; 33.3% | 0/6; 0% | 20/89; 22.5% | 0.0034 ** | 0.2396 | 0.6643 |
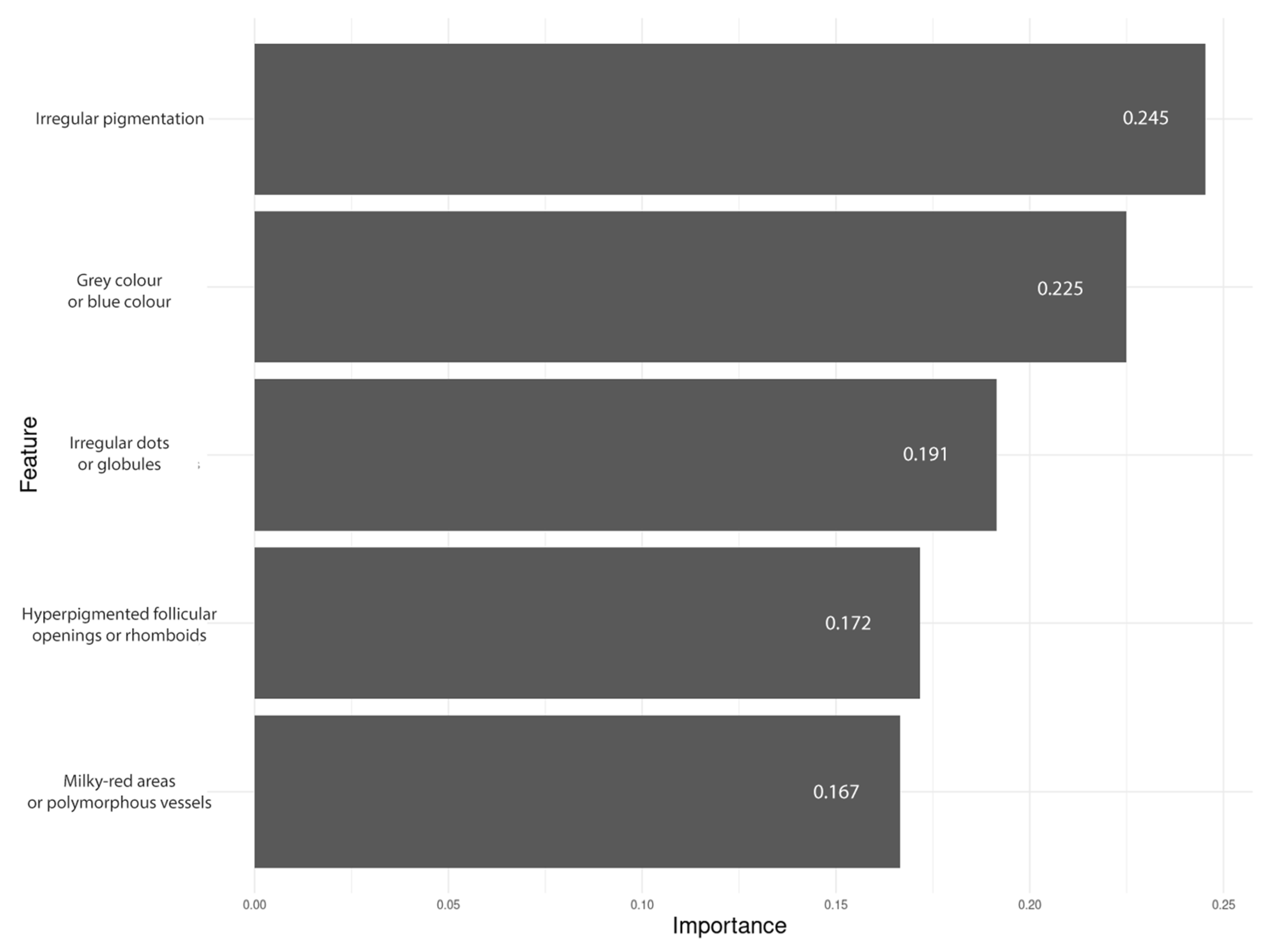
| Irregular pigmentation | 44/48; 91.7% | 6/8; 75% | 50/56; 89.3% | 18/37; 48.6% | 2/40; 5% | 1/6; 16.7% | 0/6; 0% | 21/89; 23.6% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Milky-red areas | 32/48; 66.7% | 7/8; 87.5% | 39/56; 69.6% | 4/37; 10.8% | 0/40; 0% | 1/6; 16.7% | 0/6; 0% | 5/89; 5.6% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Multiple small hyperpigmented areas/patchy pigmented islands | 11/48; 22.9% | 3/8; 37.5% | 14/56; 25% | 9/37; 24.3% | 4/40; 10% | 1/6; 16.7% | 0/6; 0% | 14/89; 15.7% | 0.1974 | 0.6455 | 1 | |
| Negative network | 6/48; 12.5% | 0/8; 0% | 6/56; 10.7% | 0/37; 0% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 0/89; 0% | 0.0028 ** | 0.0289 * | 0.0334 * | |
| Regression erythema/vessels/scar-like depigmentation | 12/48; 25% | 2/8; 25% | 14/56; 25% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 1/89; 1.1% | <0.0001 *** | 0.0006 *** | 0.0052 ** | |
| Regression granularity/peppering | 5/48; 10.4% | 1/8; 12.5% | 6/56; 10.7% | 0/37; 0% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 0/89; 0% | 0.0028 ** | 0.0289 * | 0.0655 | |
| Regular globules | 2/48; 4.2% | 0/8; 0% | 2/56; 3.6% | 5/37; 13.5% | 7/40; 17.5% | 0/6; 0% | 0/6; 0% | 12/89; 13.5% | 0.0801 | 0.2473 | 0.2312 | |
| Tan peripheral structureless area | 2/48; 4.2% | 0/8; 0% | 2/56; 3.6% | 1/37; 2.7% | 3/40; 7.5% | 0/6; 0% | 0/6; 0% | 4/89; 4.5% | 1 | 1 | 1 | |
| Atypical network | 3/48; 6.2% | 0/8; 0% | 3/56; 5.4% | 2/37; 5.4% | 1/40; 2.5% | 0/6; 0% | 0/6; 0% | 3/89; 3.4% | 0.6765 | 1 | 1 | |
| Blue-white veil | 7/48; 14.6% | 0/8; 0% | 7/56; 12.5% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 1/89; 1.1% | 0.0055 ** | 0.0647 | 0.13 | |
| Irregular streaks | 2/48; 4.2% | 1/8; 12.5% | 3/56; 5.4% | 0/37; 0% | 0/40; 0% | 1/6; 16.7% | 0/6; 0% | 1/89; 1.1% | 0.2986 | 0.6211 | 0.5025 | |
| Irregular blotches [Black, brown and ⁄ or grey structureless areas asymmetrically distributed within the lesion] | 11/48; 22.9% | 2/8; 25% | 13/56; 23.2% | 8/37; 21.6% | 1/40; 2.5% | 0/6; 0% | 0/6; 0% | 9/89; 10.1% | 0.0551 | 0.4663 | 1 | |
| Total number of features characteristic for non-facial melanoma, mean number ± SD (range) | 3.3 ± 1.5 (0–7) | 3.2 ± 1.4 (1–5) | 3.3 ± 1.5 (0–7) | 1.7 ± 1.4 (0–4) | 0.5 ± 1 (0–5) | 1 ± 0.9 (0–2) | 0 ± 0 (0–0) | 1 ± 1.3 (0–5) | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Vascular structures | Presence of vessels | 33/48; 68.8% | 7/8; 87.5% | 40/56; 71.4% | 12/37; 32.4% | 4/40; 10% | 1/6; 16.7% | 0/6; 0% | 17/89; 19.1% | <0.0001 *** | <0.0001 *** | 0.0011 ** |
| Milky-red areas/globules | 32/48; 66.7% | 7/8; 87.5% | 39/56; 69.6% | 4/37; 10.8% | 0/40; 0% | 1/6; 16.7% | 0/6; 0% | 5/89; 5.6% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Coiled (glomerular) vessels | 2/48; 4.2% | 0/8; 0% | 2/56; 3.6% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 1/89; 1.1% | 0.5593 | 1 | 1 | |
| Looped (hairpin) vessels | 0/48; 0% | 0/8; 0% | 0/56; 0% | 0/37; 0% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 0/89; 0% | - | - | - | |
| Linear irregular (serpentine) vessels | 23/48; 47.9% | 2/8; 25% | 25/56; 44.6% | 7/37; 18.9% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 7/89; 7.9% | <0.0001 *** | 0.0012 ** | 0.0064 ** | |
| Helical (corkscrew) vessels | 0/48; 0% | 0/8; 0% | 0/56; 0% | 0/37; 0% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 0/89; 0% | - | - | - | |
| Curved (comma) vessels | 0/48; 0% | 0/8; 0% | 0/56; 0% | 2/37; 5.4% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 2/89; 2.2% | 0.5226 | 0.2154 | 0.1866 | |
| Dotted vessels | 7/48; 14.6% | 2/8; 25% | 9/56; 16.1% | 5/37; 13.5% | 4/40; 10% | 0/6; 0% | 0/6; 0% | 9/89; 10.1% | 0.3105 | 0.4068 | 1 | |
| Monomorphous vessels (exactly 1 type of vessel) | 9/48; 18.8% | 4/8; 50% | 13/56; 23.2% | 8/37; 21.6% | 4/40; 10% | 1/6; 16.7% | 0/6; 0% | 13/89; 14.6% | 0.2659 | 0.6339 | 0.7891 | |
| Polymporphous vessels (>1 type of vessel) | 24/48; 50% | 3/8; 37.5% | 27/56; 48.2% | 4/37; 10.8% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 4/89; 4.5% | <0.0001 *** | <0.0001 *** | 0.0002 *** | |
| SELECTED DERMOSCOPIC ALGORITHMS | ||||||||||||
| 7-non melanoma features to rule out melanoma | Scales (pigmented or non-pigmented) | 4/48; 8.3% | 1/8; 12.5% | 5/56; 8.9% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 1/89; 1.1% | 0.032 * | 0.2115 | 0.3816 |
| White follicles (white circles, follicular white clods and/or 4 dots clods (rosettes)) | 11/48; 22.9% | 0/8; 0% | 11/56; 19.6% | 0/37; 0% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 0/89; 0% | <0.0001 *** | 0.0007 *** | 0.0019 ** | |
| Erythema/reticular vessels | 32/48; 66.7% | 5/8; 62.5% | 37/56; 66.1% | 5/37; 13.5% | 0/40; 0% | 2/6; 33.3% | 0/6; 0% | 7/89; 7.9% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Reticular lines/parallel lines (fingerprints) | 3/48; 6.2% | 0/8; 0% | 3/56; 5.4% | 1/37; 2.7% | 5/40; 12.5% | 0/6; 0% | 0/6; 0% | 6/89; 6.7% | 1 | 0.6211 | 0.629 | |
| Brown structureless | 27/48; 56.2% | 5/8; 62.5% | 32/56; 57.1% | 27/37; 73% | 33/40; 82.5% | 5/6; 83.3% | 4/6; 66.7% | 69/89; 77.5% | 0.0153 * | 0.1024 | 0.1723 | |
| Sharp demarcation | 2/48; 4.2% | 1/8; 12.5% | 3/56; 5.4% | 7/37; 18.9% | 2/40; 5% | 2/6; 33.3% | 0/6; 0% | 11/89; 12.4% | 0.2487 | 0.062 | 0.0371 * | |
| Seborrheic keratosis features | 0/48; 0% | 0/8; 0% | 0/56; 0% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 1/89; 1.1% | 1 | 0.4667 | 0.4353 | |
| Positive test | 6/48; 12.5% | 0/8; 0% | 6/56; 10.7% | 6/37; 16.2% | 6/40; 15% | 0/6; 0% | 2/6; 33.3% | 14/89; 15.7% | 0.465 | 0.5664 | 0.7561 | |
| 7-point checklist | Atypical network | 3/48; 6.2% | 0/8; 0% | 3/56; 5.4% | 2/37; 5.4% | 1/40; 2.5% | 0/6; 0% | 0/6; 0% | 3/89; 3.4% | 0.6765 | 1 | 1 |
| Blue-white veil | 7/48; 14.6% | 0/8; 0% | 7/56; 12.5% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 1/89; 1.1% | 0.0055 ** | 0.0647 | 0.13 | |
| Atypical vascular pattern [Linear-irregular vessels, dotted vessels and ⁄ or milky-red areas not clearly seen within regression structures] | 33/48; 68.8% | 7/8; 87.5% | 40/56; 71.4% | 10/37; 27% | 4/40; 10% | 1/6; 16.7% | 0/6; 0% | 15/89; 16.9% | <0.0001 *** | <0.0001 *** | 0.0002 *** | |
| Irregular dots ⁄globules | 31/48; 64.6% | 4/8; 50% | 35/56; 62.5% | 18/37; 48.6% | 6/40; 15% | 2/6; 33.3% | 0/6; 0% | 26/89; 29.2% | 0.0001 *** | 0.0322 * | 0.1849 | |
| Irregular streaks | 2/48; 4.2% | 1/8; 12.5% | 3/56; 5.4% | 0/37; 0% | 0/40; 0% | 1/6; 16.7% | 0/6; 0% | 1/89; 1.1% | 0.2986 | 0.6211 | 0.5025 | |
| Irregular blotches [Black, brown and ⁄ or grey structureless areas asymmetrically distributed within the lesion] | 11/48; 22.9% | 2/8; 25% | 13/56; 23.2% | 8/37; 21.6% | 1/40; 2.5% | 0/6; 0% | 0/6; 0% | 9/89; 10.1% | 0.0551 | 0.4663 | 1 | |
| Regression structures | 13/48; 27.1% | 2/8; 25% | 15/56; 26.8% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 1/89; 1.1% | <0.0001 *** | 0.0003 *** | 0.0026 ** | |
| Positive test | 44/48; 91.7% | 8/8; 100% | 52/56; 92.9% | 23/37; 62.2% | 10/40; 25% | 4/6; 66.7% | 0/6; 0% | 37/89; 41.6% | <0.0001 *** | <0.0001 *** | 0.0013 ** | |
| Chaos and clues | Asymmetry of pattern | 42/48; 87.5% | 7/8; 87.5% | 49/56; 87.5% | 17/37; 45.9% | 5/40; 12.5% | 1/6; 16.7% | 0/6; 0% | 23/89; 25.8% | <0.0001 *** | <0.0001 *** | <0.0001 *** |
| Asymmetry of colour | 43/48; 89.6% | 5/8; 62.5% | 48/56; 85.7% | 18/37; 48.6% | 5/40; 12.5% | 1/6; 16.7% | 0/6; 0% | 24/89; 27% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Grey or blue structures | 33/48; 68.8% | 5/8; 62.5% | 38/56; 67.9% | 14/37; 37.8% | 3/40; 7.5% | 5/6; 83.3% | 3/6; 50% | 25/89; 28.1% | <0.0001 *** | 0.0292 * | 0.0079 ** | |
| Thick lines reticular or branched | 3/48; 6.2% | 0/8; 0% | 3/56; 5.4% | 1/37; 2.7% | 5/40; 12.5% | 0/6; 0% | 0/6; 0% | 6/89; 6.7% | 1 | 0.6211 | 0.629 | |
| White lines | 15/48; 31.2% | 2/8; 25% | 17/56; 30.4% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 1/89; 1.1% | <0.0001 *** | <0.0001 *** | 0.0006 *** | |
| Eccentric structureless areas | 12/48; 25% | 1/8; 12.5% | 13/56; 23.2% | 3/37; 8.1% | 2/40; 5% | 0/6; 0% | 0/6; 0% | 5/89; 5.6% | 0.0034 ** | 0.0271 * | 0.0496 * | |
| Peripheral black dots or clods | 3/48; 6.2% | 0/8; 0% | 3/56; 5.4% | 0/37; 0% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 0/89; 0% | 0.0557 | 0.2462 | 0.2538 | |
| Radial lines or pseudopods, segmental | 3/48; 6.2% | 1/8; 12.5% | 4/56; 7.1% | 2/37; 5.4% | 0/40; 0% | 5/6; 83.3% | 0/6; 0% | 7/89; 7.9% | 1 | 0.3399 | 1 | |
| Polymorphous vessels | 24/48; 50% | 3/8; 37.5% | 27/56; 48.2% | 4/37; 10.8% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 4/89; 4.5% | <0.0001 *** | <0.0001 *** | 0.0002 *** | |
| Polygons/zig zag | 24/48; 50% | 3/8; 37.5% | 27/56; 48.2% | 10/37; 27% | 0/40; 0% | 1/6; 16.7% | 0/6; 0% | 11/89; 12.4% | <0.0001 *** | 0.0081 ** | 0.0445 * | |
| Positive test | 44/48; 91.7% | 6/8; 75% | 50/56; 89.3% | 17/37; 45.9% | 5/40; 12.5% | 0/6; 0% | 0/6; 0% | 22/89; 24.7% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| CASH algorithm | Light brown | 35/48; 72.9% | 6/8; 75% | 41/56; 73.2% | 30/37; 81.1% | 37/40; 92.5% | 3/6; 50% | 2/6; 33.3% | 72/89; 80.9% | 0.3078 | 1 | 0.4459 |
| Dark brown | 42/48; 87.5% | 7/8; 87.5% | 49/56; 87.5% | 34/37; 91.9% | 23/40; 57.5% | 6/6; 100% | 3/6; 50% | 66/89; 74.2% | 0.0604 | 1 | 0.7253 | |
| Black | 17/48; 35.4% | 0/8; 0% | 17/56; 30.4% | 2/37; 5.4% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 2/89; 2.2% | <0.0001 *** | 0.0006 *** | 0.0012 ** | |
| Red | 35/48; 72.9% | 7/8; 87.5% | 42/56; 75% | 8/37; 21.6% | 1/40; 2.5% | 2/6; 33.3% | 0/6; 0% | 11/89; 12.4% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| White | 31/48; 64.6% | 4/8; 50% | 35/56; 62.5% | 6/37; 16.2% | 0/40; 0% | 1/6; 16.7% | 1/6; 16.7% | 8/89; 9% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Blue | 9/48; 18.8% | 1/8; 12.5% | 10/56; 17.9% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 3/6; 50% | 4/89; 4.5% | 0.0177 * | 0.1638 | 0.0379 * | |
| Architectural disorder [0-none/mild; 1-moderate; 2-marked] | 0: 3/48; 6.2% 1: 15/48; 31.2% 2: 30/48; 62.5% | 0: 2/8; 25% 1: 3/8; 37.5% 2: 3/8; 37.5% | 0: 5/56; 8.9% 1: 18/56; 32.1% 2: 33/56; 58.9% | 0: 16/37; 43.2% 1: 16/37; 43.2% 2: 5/37; 13.5% | 0: 35/40; 87.5% 1: 5/40; 12.5% 2: 0/40; 0% | 0: 4/6; 66.7% 1: 2/6; 33.3% 2: 0/6; 0% | 0: 6/6; 100% 1: 0/6; 0% 2: 0/6; 0% | 0: 61/89; 68.5% 1: 23/89; 25.8% 2: 5/89; 5.6% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Symmetry, shape and dermoscopic structures [0-biaxial symmetry; 1-monoaxial symmetry; 2-biaxial asymmetry] | 0: 3/48; 6.2% 1: 17/48; 35.4% 2: 28/48; 58.3% | 0: 2/8; 25% 1: 3/8; 37.5% 2: 3/8; 37.5% | 0: 5/56; 8.9% 1: 20/56; 35.7% 2: 31/56; 55.4% | 0: 16/37; 43.2% 1: 17/37; 45.9% 2: 4/37; 10.8% | 0: 33/40; 82.5% 1: 7/40; 17.5% 2: 0/40; 0% | 0: 4/6; 66.7% 1: 2/6; 33.3% 2: 0/6; 0% | 0: 6/6; 100% 1: 0/6; 0% 2: 0/6; 0% | 0: 59/89; 66.3% 1: 26/89; 29.2% 2: 4/89; 4.5% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Network | 9/48; 18.8% | 0/8; 0% | 9/56; 16.1% | 5/37; 13.5% | 10/40; 25% | 0/6; 0% | 1/6; 16.7% | 16/89; 18% | 0.8248 | 0.7808 | 0.5696 | |
| Dots/globules | 34/48; 70.8% | 4/8; 50% | 38/56; 67.9% | 24/37; 64.9% | 17/40; 42.5% | 4/6; 66.7% | 0/6; 0% | 45/89; 50.6% | 0.0575 | 0.313 | 0.6408 | |
| Streaks/pseudopods | 3/48; 6.2% | 1/8; 12.5% | 4/56; 7.1% | 2/37; 5.4% | 0/40; 0% | 5/6; 83.3% | 0/6; 0% | 7/89; 7.9% | 1 | 0.3399 | 1 | |
| Blue-white veil | 7/48; 14.6% | 0/8; 0% | 7/56; 12.5% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 1/89; 1.1% | 0.0055 ** | 0.0647 | 0.13 | |
| Regression structures [grey areas with or without peppering; scarring] | 14/48; 29.2% | 2/8; 25% | 16/56; 28.6% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 1/89; 1.1% | <0.0001 *** | 0.0001 *** | 0.0013 ** | |
| Blotches [structureless regions of any colour occupying [10% of the area of the lesion] | 12/48; 25% | 2/8; 25% | 14/56; 25% | 9/37; 24.3% | 1/40; 2.5% | 0/6; 0% | 0/6; 0% | 10/89; 11.2% | 0.039 * | 0.4825 | 1 | |
| Polymorphous blood vessels [including dotted and irregular linear] | 24/48; 50% | 3/8; 37.5% | 27/56; 48.2% | 4/37; 10.8% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 4/89; 4.5% | <0.0001 *** | <0.0001 *** | 0.0002 *** | |
| Positive test | 34/48; 70.8% | 4/8; 50% | 38/56; 67.9% | 6/37; 16.2% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 6/89; 6.7% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Menzies algorithm | Point and axial symmetry of pigmentation | 5/48; 10.4% | 3/8; 37.5% | 8/56; 14.3% | 19/37; 51.4% | 33/40; 82.5% | 5/6; 83.3% | 6/6; 100% | 63/89; 70.8% | <0.0001 *** | <0.0001 *** | <0.0001 *** |
| Presence of only a single colour | 1/48; 2.1% | 0/8; 0% | 1/56; 1.8% | 5/37; 13.5% | 18/40; 45% | 0/6; 0% | 3/6; 50% | 26/89; 29.2% | <0.0001 *** | 0.0116 * | 0.0812 | |
| Blue-white veil | 7/48; 14.6% | 0/8; 0% | 7/56; 12.5% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 1/89; 1.1% | 0.0055 ** | 0.0647 | 0.13 | |
| Multiple brown dots | 21/48; 43.8% | 2/8; 25% | 23/56; 41.1% | 12/37; 32.4% | 12/40; 30% | 1/6; 16.7% | 0/6; 0% | 25/89; 28.1% | 0.1467 | 0.1501 | 0.3705 | |
| Pseudopods | 1/48; 2.1% | 1/8; 12.5% | 2/56; 3.6% | 2/37; 5.4% | 0/40; 0% | 5/6; 83.3% | 0/6; 0% | 7/89; 7.9% | 0.4827 | 0.0788 | 0.5774 | |
| Radial streaming [asymmetric] | 2/48; 4.2% | 1/8; 12.5% | 3/56; 5.4% | 0/37; 0% | 0/40; 0% | 1/6; 16.7% | 0/6; 0% | 1/89; 1.1% | 0.2986 | 0.6211 | 0.5025 | |
| Scar-like depigmentation | 11/48; 22.9% | 0/8; 0% | 11/56; 19.6% | 1/37; 2.7% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 1/89; 1.1% | 0.0001 *** | 0.0049 ** | 0.0102 * | |
| Peripheral black dots/globules | 3/48; 6.2% | 0/8; 0% | 3/56; 5.4% | 0/37; 0% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 0/89; 0% | 0.0557 | 0.2462 | 0.2538 | |
| Multiple (5–6) colours | 22/48; 45.8% | 3/8; 37.5% | 25/56; 44.6% | 2/37; 5.4% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 2/89; 2.2% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Multiple blue/grey dots | 11/48; 22.9% | 1/8; 12.5% | 12/56; 21.4% | 0/37; 0% | 0/40; 0% | 0/6; 0% | 0/6; 0% | 0/89; 0% | <0.0001 *** | 0.0003 *** | 0.0019 ** | |
| Broadened network | 3/48; 6.2% | 0/8; 0% | 3/56; 5.4% | 2/37; 5.4% | 1/40; 2.5% | 0/6; 0% | 1/6; 16.7% | 4/89; 4.5% | 1 | 1 | 1 | |
| Positive test | 34/48; 70.8% | 4/8; 50% | 38/56; 67.9% | 11/37; 29.7% | 4/40; 10% | 1/6; 16.7% | 0/6; 0% | 16/89; 18% | <0.0001 *** | <0.0001 *** | 0.0002 *** | |
| 3-point checklist | Asymmetry [Asymmetrical distribution of colours and dermoscopic structures] | 43/48; 89.6% | 5/8; 62.5% | 48/56; 85.7% | 16/37; 43.2% | 7/40; 17.5% | 1/6; 16.7% | 0/6; 0% | 24/89; 27% | <0.0001 *** | <0.0001 *** | <0.0001 *** |
| Atypical network [Pigmented network with irregular holes and thick lines] | 3/48; 6.2% | 0/8; 0% | 3/56; 5.4% | 2/37; 5.4% | 1/40; 2.5% | 0/6; 0% | 1/6; 16.7% | 4/89; 4.5% | 1 | 1 | 1 | |
| Blue-white structures [Any type of blue and/or white colour] | 32/48; 66.7% | 5/8; 62.5% | 37/56; 66.1% | 6/37; 16.2% | 0/40; 0% | 1/6; 16.7% | 3/6; 50% | 10/89; 11.2% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| Positive test | 33/48; 68.8% | 3/8; 37.5% | 36/56; 64.3% | 5/37; 13.5% | 1/40; 2.5% | 1/6; 16.7% | 0/6; 0% | 7/89; 7.9% | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| OTHERS | ||||||||||||
| Facial vs. extra-facial vs. equivocal phenotype | Facial 27/48; 56.2% Extra-facial 13/48; 27.1% Equivocal 8/48; 16.7% | Facial 2/8; 25% Extra-facial 5/8; 62.5% Equivocal 1/8; 12.5% | Facial 29/56; 51.8% Extra-facial 18/56; 32.1% Equivocal 9/56; 16.1% | Facial 17/37; 45.9% Extra-facial 13/37; 35.1% Equivocal 7/37; 18.9% | Facial 20/40; 50% Extra-facial 9/40; 22.5% Equivocal 11/40; 27.5% | Facial 3/6; 50% Extra-facial 2/6; 33.3% Equivocal 1/6; 16.7% | Facial 3/6; 50% Extra-facial 0/6; 0% Equivocal 3/6; 50% | Facial 43/89; 48.3% Extra-facial 24/89; 27% Equivocal 22/89; 24.7% | 0.4703 | 0.8824 | 0.6151 | |
| Annular globular pattern | 3/48; 6.2% | 1/8; 12.5% | 4/56; 7.1% | 5/37; 13.5% | 2/40; 5% | 0/6; 0% | 0/6; 0% | 7/89; 7.9% | 1 | 0.7307 | 0.2867 | |
| Perifollicular linear projections/ pigmented circles with thick line(s) originating from them | 1/48; 2.1% | 0/8; 0% | 1/56; 1.8% | 0/37; 0% | 4/40; 10% | 0/6; 0% | 0/6; 0% | 4/89; 4.5% | 0.649 | 1 | 1 | |
| Red circles | 5/48; 10.4% | 1/8; 12.5% | 6/56; 10.7% | 0/37; 0% | 0/40; 0% | 1/6; 16.7% | 0/6; 0% | 1/89; 1.1% | 0.0135 * | 0.1183 | 0.0655 | |
| Pseudonetwork | 15/48; 31.2% | 1/8; 12.5% | 16/56; 28.6% | 11/37; 29.7% | 17/40; 42.5% | 2/6; 33.3% | 3/6; 50% | 33/89; 37.1% | 0.3677 | 0.6761 | 1 | |
| Combined Performance on Training and Validation Sets | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity, % | Specificity, % | Compared to Our Model, p | Balanced Accuracy | PPV | NPV | Sensitivity, p | Specificity, p | Balanced Accuracy, p | |
| 3-point checklist | 64.3% | 92.1% | 0.0046 ** | 78.2% | 83.7% | 80.4% | 0.008 ** | 0.61 | 0.1407 |
| 7-non melanoma features to rule out melanoma | 10.7% | 84.6% | <0.0001 *** | 47.5% | 30% | 60% | <0.0001 *** | 0.5103 | <0.0001 *** |
| 7-point checklist | 92.9% | 58.4% | <0.0001 *** | 75.6% | 58.4% | 92.9% | 0.5254 | <0.0001 *** | 0.0007 *** |
| ‘Chaos and Clues’ | 89.3% | 75.3% | <0.0001 *** | 82.3% | 69.4% | 91.8% | 1 | 0.0318 * | 0.1048 |
| CASH algorithm | 67.9% | 93.3% | 0.0148 * | 74.9% | 86.4% | 82.2% | 0.0233 * | 0.4318 | 0.3119 |
| Menzies method | 67.9% | 82% | 0.499 | 74.9% | 70.4% | 80.2% | 0.0233 * | 0.2886 | 0.0136 * |
| Proposed algorithm | 87.5% | 88.8% | N/A | 88.1% | 83.1% | 91.9% | N/A | N/A | N/A |
| Performance on training set | |||||||||
| Sensitivity, % | Specificity, % | Compared to our model, p | Balanced accuracy | PPV | NPV | Sensitivity, p | Specificity, p | Balanced accuracy, p | |
| 3-point checklist | 63.2% | 95.2% | 0.0218 * | 79.2% | 88.9% | 81.1% | 0.0682 | 0.489 | 0.4222 |
| 7-non melanoma features to rule out melanoma | 10.5% | 87.3% | 0.0002 ** | 48.9% | 33.3% | 61.8% | <0.0001 *** | 0.7768 | <0.0001 *** |
| 7-point checklist | 97.4% | 57.1% | <0.0001 *** | 77.3% | 57.8% | 97.3% | 0.1126 | <0.0001 *** | 0.0081 ** |
| ‘Chaos and Clues’ | 89.5% | 77.8% | 0.0339 * | 83.6% | 70.8% | 92.5% | 0.7344 | 0.0879 | 0.3225 |
| CASH algorithm | 79% | 95.2% | 0.3588 | 87.1% | 90.9% | 88.2% | 0.7673 | 0.489 | 1 |
| Menzies method | 73.7% | 84.1% | 1 | 78.9% | 73.7% | 84.1% | 0.3986 | 0.4222 | 0.1774 |
| Proposed algorithm | 84.2% | 90.5% | N/A | 87.3% | 84.2% | 90.5% | N/A | N/A | N/A |
| Performance on validation set | |||||||||
| Sensitivity, % | Specificity, % | Compared to our model, p | Balanced accuracy | PPV | NPV | Sensitivity, p | Specificity, p | Balanced accuracy, p | |
| 3-point checklist | 66.7% | 84.6% | 0.1824 | 75.6% | 75% | 78.6% | 0.0921 | 1 | 0.2568 |
| 7-non melanoma features to rule out melanoma | 11.1% | 76.9% | 0.0164 * | 44% | 25% | 55.6% | <0.0001 *** | 0.7249 | 0.0002 *** |
| 7-point checklist | 83.3% | 61.5% | 0.2888 | 72.4% | 60% | 84.2% | 0.5959 | 0.118 | 0.0643 |
| ‘Chaos and Clues’ | 88.9% | 69.2% | 0.3711 | 79.1% | 66.7% | 90% | 1 | 0.3234 | 0.2568 |
| CASH algorithm | 44.4% | 88.5% | 0.0162 * | 66.5% | 72.7% | 69.7% | 0.0038 ** | 1 | 0.0643 |
| Menzies method | 55.6% | 76.9% | 0.2278 | 79.1% | 62.5% | 71.4% | 0.0209 * | 0.7249 | 0.0382 * |
| Proposed algorithm | 94.4% | 84.6% | N/A | 89.5% | 81% | 95.7% | N/A | N/A | N/A |
| Performance on test set | |||||||||
| Sensitivity, % | Specificity, % | Compared to our model, p | Balanced accuracy | PPV | NPV | Sensitivity, p | Specificity, p | Balanced accuracy, p | |
| 3-point checklist | 50% | 86.7% | 0.2207 | 68.3% | 71.4% | 72.2% | 0.1432 | 1 | 0.2888 |
| 7-non melanoma features to rule out melanoma | 20% | 86.7% | 0.0961 | 53.3% | 50% | 61.9% | 0.007 ** | 1 | 0.0531 |
| 7-point checklist | 90% | 6.7% | 0.0033 ** | 48.3% | 39.1% | 50% | 1 | <0.0001 *** | 0.0012 ** |
| ‘Chaos and Clues’ | 100% | 60% | 0.0736 | 80.0% | 62.5% | 100% | 1 | 0.2155 | 0.4616 |
| CASH algorithm | 60% | 73.3% | 1 | 66.7% | 61.5% | 83.3% | 0.3017 | 0.6481 | 0.1721 |
| Menzies method | 80% | 66.7% | 0.6831 | 73.3% | 60% | 73.3% | 1 | 0.388 | 0.2888 |
| Proposed algorithm | 90% | 86.7% | N/A | 88.3% | 81.8% | 92.9% | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żółkiewicz, J.; Thomas, L.; Kamińska-Winciorek, G.; Pastuszak, K.; Kunc, M.; Maińska, U.; Sobjanek, M.; Sławińska, M. Dermoscopy of External Ear Melanocytic Lesions: Performance of Selected Dermoscopic Screening Algorithms and Proposal of a New Predictive Model for Malignancy (AuriCheck Dermoscopic Algorithm). Cancers 2025, 17, 679. https://doi.org/10.3390/cancers17040679
Żółkiewicz J, Thomas L, Kamińska-Winciorek G, Pastuszak K, Kunc M, Maińska U, Sobjanek M, Sławińska M. Dermoscopy of External Ear Melanocytic Lesions: Performance of Selected Dermoscopic Screening Algorithms and Proposal of a New Predictive Model for Malignancy (AuriCheck Dermoscopic Algorithm). Cancers. 2025; 17(4):679. https://doi.org/10.3390/cancers17040679
Chicago/Turabian StyleŻółkiewicz, Jakub, Luc Thomas, Grażyna Kamińska-Winciorek, Krzysztof Pastuszak, Michał Kunc, Urszula Maińska, Michał Sobjanek, and Martyna Sławińska. 2025. "Dermoscopy of External Ear Melanocytic Lesions: Performance of Selected Dermoscopic Screening Algorithms and Proposal of a New Predictive Model for Malignancy (AuriCheck Dermoscopic Algorithm)" Cancers 17, no. 4: 679. https://doi.org/10.3390/cancers17040679
APA StyleŻółkiewicz, J., Thomas, L., Kamińska-Winciorek, G., Pastuszak, K., Kunc, M., Maińska, U., Sobjanek, M., & Sławińska, M. (2025). Dermoscopy of External Ear Melanocytic Lesions: Performance of Selected Dermoscopic Screening Algorithms and Proposal of a New Predictive Model for Malignancy (AuriCheck Dermoscopic Algorithm). Cancers, 17(4), 679. https://doi.org/10.3390/cancers17040679







