Radiological and Immunohistochemical Characteristics of PitNETs in 79 Patients Undergoing Neurosurgery
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Materials and Methods
2.2.1. Magnetic Resonance Imaging of the Tumor
2.2.2. Immunohistochemical Assessment of the Tumor
3. Statistics
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef]
- McDowell, B.D.; Wallace, R.B.; Carnahan, R.M.; Chrischilles, E.A.; Lynch, C.F.; Schlechte, J.A. Demographic differences in incidence for Pituitary adenoma. Pituitary 2011, 14, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Raverot, G.; Burman, P.; McCormack, A.; Heaney, A.; Petersenn, S.; Popovic, V.; Trouillas, J.; Dekkers, O.M.; European Society of Endocrinology. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive Pituitary tumors and carcinomas. Eur. J. Endocrinol. 2018, 178, G1–G24. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, S.M.C.; McCormack, A.I. Aggressive Pituitary tumors and Pituitary carcinomas. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Lopes, M.B.S. The 2017 World Health Organization classification of tumors of the Pituitary gland: A summary. Acta Neuropathol. 2017, 134, 521–535. [Google Scholar] [CrossRef]
- Ezzat, S.; Asa, S.L.; Couldwell, W.T.; Barr, C.E.; Dodge, W.E.; Vance, M.L.; McCutcheon, I.E. The prevalence of Pituitary adenomas: A systematic review. Cancer 2004, 101, 613–619. [Google Scholar] [CrossRef]
- Knosp, E.; Steiner, E.; Kitz, K.; Matula, C. Pituitary adenomas with invasion of the cavernous sinus space: A magnetic resonance imaging classification compared with surgical findings. Neurosurgery 1993, 33, 610–617; discussion 617–618. [Google Scholar] [CrossRef]
- Fang, Y.; Pei, Z.; Chen, H.; Wang, R.; Feng, M.; Wei, L.; Li, J.; Zhang, H.; Wang, S. Diagnostic value of Knosp grade and modified Knosp grade for cavernous sinus invasion in Pituitary adenomas: A systematic review and meta-analysis. Pituitary 2021, 24, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Vezina, J.L. Transsphenoidal neurosurgery of intracranial neoplasm. Adv. Neurol. 1976, 15, 261–273. [Google Scholar] [PubMed]
- Zhu, X.; Rosenfeld, M.G. Transcriptional control of precursor proliferation in the early phases of Pituitary development. Curr. Opin. Genet. Dev. 2004, 14, 567–574. [Google Scholar] [CrossRef]
- Osamura, R.Y.; Grossman, A.; Korbonits, M.; Kovacs, K.; Lopes, M.B.S.; Matsuno, A.; Trouillas, J. Pituitary adenoma. In Tumors of the Pituitary Gland. WHO Classification of Endocrine Tumors, 4th ed.; IARC: Lyon, France, 2017; pp. 14–18. [Google Scholar]
- Trouillas, J.; Jaffrain-Rea, M.L.; Vasiljevic, A.; Raverot, G.; Roncaroli, F.; Villa, C. How to classify the Pituitary neuroendocrine tumors (PitNET)S in 2020. Cancers 2020, 12, 514. [Google Scholar] [CrossRef]
- Asa, S.L. Challenges in the Diagnosis of Pituitary Neuroendocrine Tumors. Endocr. Pathol. 2021, 32, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.Y.; Chen, J.; Wang, J.W.; Liu, Y.C.; Shu, K.; Lei, T. Overview of the 2022 WHO Classification of Pituitary Adenomas/Pituitary Neuroendocrine Tumors: Clinical Practices, Controversies, and Perspectives. Curr. Med. Sci. 2022, 42, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Obari, A.; Sano, T.; Ohyama, K.; Kudo, E.; Qian, Z.R.; Yoneda, A.; Rayhan, N.; Mustafizur Rahman, M.; Yamada, S. Clinicopathological features of growth hormone-producing Pituitary adenomas: Difference among various types defined by cytokeratin distribution pattern including a transitional form. Endocr. Pathol. 2008, 19, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, H.; Inoshita, N.; Mete, O.; Asa, S.L.; Hayashi, K.; Takeshita, A.; Fukuhara, N.; Yamaguchi-Okada, M.; Takeuchi, Y.; Yamada, S. The Complementary Role of Transcription Factors in the Accurate Diagnosis of Clinically Nonfunctioning Pituitary Adenomas. Endocr. Pathol. 2015, 26, 349–355. [Google Scholar] [CrossRef]
- Nishioka, H.; Inoshita, N.; Sano, T.; Fukuhara, N.; Yamada, S. Correlation Between Histological Subtypes and MRI Findings in Clinically Nonfunc-tioning Pituitary Adenomas. Endocr. Pathol. 2012, 23, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, B.W.; Gaffey, T.A.; Lloyd, R.V.; Sebo, T.J.; Kovacs, K.T.; Horvath, E.; Yapicier, O.; Young, W.F., Jr.; Meyer, F.B.; Kuroki, T.; et al. Pathobiology of pituitary adenomas and carcinomas. Neurosurgery 2006, 59, 341–353; discussion 341–353. [Google Scholar] [CrossRef]
- Yamada, S.; Osamura, R.Y.; Righi, A.; Trouillas, J. Gonadotroph adenoma. In Tumors of the Pituitary Gland. WHO Classification of Endocrine Tumors; IARC: Lyon, France, 2017; pp. 34–36. [Google Scholar]
- Rak, B.; Maksymowicz, M.; Grzywa, T.M.; Sajjad, E.; Pękul, M.; Włodarski, P.; Zieliński, G. Pituitary tumours—A large retrospective single-centre study of over 2300 cases. Experience of a tertiary reference centre. Endokrynol. Pol. 2020, 71, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Mete, O.; Grossman, A.; Trouillas, J.; Asa, S.L.; Ezzat, S.; Fadare, O.; Ferone, D.; Gattuso, P.; Horvath, E.; Kovacs, K.; et al. Corticotroph adenoma. In Tumors of the Pituitary Gland. WHO Classification of Endocrine Tumors, 4th ed.; IARC: Lyon, France, 2017; pp. 30–33. [Google Scholar]
- Ben-Shlomo, A.; Cooper, O. Silent corticotroph adenomas. Pituitary 2018, 21, 183–193. [Google Scholar] [CrossRef]
- Cooper, O. Silent corticotroph adenomas. Pituitary 2015, 18, 225–231. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Pirchio, R.; Pivonello, C.; Garifalos, F.; Colao, A.; Pivonello, R. Approach to the Patient With Prolactinoma. J. Clin. Endocrinol. Metab. 2023, 108, 2400–2423. [Google Scholar] [CrossRef] [PubMed]
- Shimon, I. Giant Prolactinomas. Neuroendocrinology 2019, 109, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Shimon, I.; Sosa, E.; Mendoza, V.; Greenman, Y.; Tirosh, A.; Espinosa, E.; Popovic, V.; Glezer, A.; Bronstein, M.D.; Mercado, M. Giant prolactinomas larger than 60 mm in size: A cohort of massive and aggressive prolactin-secreting pituitary adenomas. Pituitary 2016, 19, 429–436. [Google Scholar] [CrossRef]
- Maiter, D. Management of Dopamine Agonist-Resistant Prolactinoma. Neuroendocrinology 2019, 109, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Pinzone, J.J.; Katznelson, L.; Danila, D.C.; Pauler, D.K.; Miller, C.S.; Klibanski, A. Primary medical therapy of micro- and macroprolactinomas in men. J. Clin. Endocrinol. Metab. 2000, 85, 3053–3057. [Google Scholar] [CrossRef] [PubMed]
- Chanson, P.; Maiter, D. The epidemiology, diagnosis and treatment of Prolactinomas: The old and the new. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101290. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Arcano, K.; Berrocal, V.R.; Bernal, C.; Villabona, C.; Díez, J.J. Giant Prolactinoma in Men: Clinical Features and Therapeutic Outcomes. Horm. Metab. Res. 2018, 50, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.L.; Qian, Z.R.; Yamada, S.; Rahman, M.M.; Inosita, N.; Kageji, T.; Endo, H.; Kudo, E.; Sano, T. Clinicopathological characterization of TSH-producing adenomas: Special reference to TSH-immunoreactive but clinically non-functioning adenomas. Endocr. Pathol. 2009, 20, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgos, G.; Kovacs, K.; Lloyd, R.V.; Trouillas, J.; Osamura, R.Y.; Lopes, M.B.S.; Grossman, A.; Matsuno, M.; Korbonits, M.; Kovacs, K. Plurihormonal and double adenomas. In Tumors of the Pituitary Gland. WHO Classification of Endocrine Tumors, 4th ed.; IARC: Lyon, France, 2017; pp. 39–40. [Google Scholar]
- Nishioka, H.; Kontogeorgos, G.; Lloyd, R.V.; Lopes, B.S.; Mete, O.; Nose, V. Null cell adenoma. In Tumors of the Pituitary Gland. WHO Classification of Endocrine Tumors, 4th ed.; IARC: Lyon, France, 2017; pp. 37–38. [Google Scholar]
- Picó, A. Agressive Pituitary tumors: A diagnostic and therapeutic challenge for multidisciplinary Pituitary units. Endocrinol. Diabetes Nutr. Engl. Ed. 2020, 67, 75–77, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Dekkers, O.M.; Karavitaki, N.; Pereira, A.M. The epidemiology of aggressive Pituitary tumors (and its challenges). Rev. Endocr. Metab. Disord. 2020, 21, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Raverot, G.; Ilie, M.D.; Lasolle, H.; Amodru, V.; Trouillas, J.; Castinetti, F.; Brue, T. Aggressive Pituitary tumors and Pituitary carcinomas. Nat. Rev. Endocrinol. 2021, 17, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, Z.; Tian, T.; Wu, X.; He, D.; Zhu, Y.; Liu, D.; Wang, H. Prevalence and clinical characteristics of Crooke’s cell adenomas in 101 patients with T-PIT-positive Pituitary adenomas: Case series and literature review. Front. Endocrinol. 2022, 13, 947085. [Google Scholar] [CrossRef] [PubMed]
- Giraldi, E.A.; Neill, S.G.; Mendoza, P.; Saindane, A.; Oyesiku, N.M.; Ioachimescu, A.G. Functioning Crooke Cell Adenomas: Case Series and Literature Review. World Neurosurg. 2022, 158, e754–e765. [Google Scholar] [CrossRef] [PubMed]
- Findlay, M.C.; Drexler, R.; Azab, M.; Karbe, A.; Rotermund, R.; Ricklefs, F.L.; Flitsch, J.; Smith, T.R.; Kilgallon, J.L.; Honegger, J.; et al. Crooke Cell Adenoma Confers Poorer Endocrinological Outcomes Compared with Corticotroph Adenoma: Results of a Multicenter, International Analysis. World Neurosurg. 2023, 180, e376–e391. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Wang, Q.; Wang, W.; Cheng, L.Q.; Wang, Y.E.; Huang, L.L.; Li, Y.J.; Wu, H.B.; Zhang, A.L. Pituitary Crooke cell neuroendocrine tumor of adrenocorticotropic hormone differentiation-specific transcription factor lineage: A clinicopathological analysis of six cases. Zhonghua Bing Li Xue Za Zhi 2024, 53, 722–727. (In Chinese) [Google Scholar] [CrossRef]
- Scheithauer, B.W.; Jaap, A.J.; Horvath, E.; Kovacs, K.; Lloyd, R.V.; Meyer, F.B.; Laws, E.R., Jr.; Young, W.F., Jr. Clinically silent corticotroph tumors of the pituitary gland. Neurosurgery 2000, 47, 723–729; discussion 729–730. [Google Scholar] [CrossRef]
- Webb, K.M.; Laurent, J.J.; Okonkwo, D.O.; Lopes, M.B.; Vance, M.L.; Laws, E.R., Jr. Clinical characteristics of silent corticotrophic adenomas and creation of an internet-accessible database to facilitate their multi-institutional study. Neurosurgery 2003, 53, 1076–1084; discussion 1084–1085. [Google Scholar] [CrossRef] [PubMed]
- Chacko, G.; Chacko, A.G.; Lombardero, M.; Mani, S.; Seshadri, M.S.; Kovacs, K.; Scheithauer, B.W. Clinicopathologic correlates of giant pituitary adenomas. J. Clin. Neurosci. 2009, 16, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Arasho, B.D.; Schaller, B.; Sandu, N.; Zenebe, G. Gender-related differences in pituitary adenomas. Exp. Clin. Endocrinol. Diabetes 2009, 117, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, C.; Scarano, E.; de Alteriis, G.; Barrea, L.; Riccio, E.; Arianna, R.; Savastano, S.; Colao, A. Is there any gender difference in epidemiology, clinical presentation and co-morbidities of non-functioning pituitary adenomas? A prospective survey of a National Referral Center and review of the literature. J. Endocrinol. Investig. 2021, 44, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Schaller, B. Gender-related differences in growth hormone-releasing pituitary adenomas. A clinicopathological study. Pituitary 2002, 5, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Theiler, S.; Hegetschweiler, S.; Staartjes, V.E.; Spinello, A.; Brandi, G.; Regli, L.; Serra, C. Influence of gender and sexual hormones on outcomes after pituitary surgery: A systematic review and meta-analysis. Acta Neurochir. 2023, 165, 2445–2460. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.A.; Zhou, Q.; Siegelin, M.D.; Angelastro, J.M. Targeting Transcription Factors ATF5, CEBPB and CEBPD with Cell-Penetrating Peptides to Treat Brain and Other Cancers. Cells 2023, 12, 581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giannopoulou, A.I.; Kanakoglou, D.S.; Piperi, C. Transcription Factors with Targeting Potential in Gliomas. Int. J. Mol. Sci. 2022, 23, 3720. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Lv, C.; Zhang, N.; Xing, K.; Wang, Z.; Lv, R.; Yu, M.; Xu, C.; Wang, Y. Single-cell RNA sequencing identifies critical transcription factors of tumor cell invasion induced by hypoxia microenvironment in glioblastoma. Theranostics 2023, 13, 3744–3760. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wan, X.; Xu, Y.; Chen, J.; Shu, K.; Lei, T. Prognostic Factors for Recurrence in Pituitary Adenomas: Recent Progress and Future Directions. Diagnostics 2022, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, P.B.; Greene, C.; McCombe, K.D.; Sidi, F.A.; McQuaid, S.; Cooke, S.; Hunter, S.J.; Herron, B.; Korbonits, M.; Craig, S.G.; et al. Assessment of Ki-67 and mitoses in pituitary neuroendocrine tumours-Consistency counts. Brain Pathol. 2025, 35, e13285. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Saeger, K.; Saeger, W. Quantifizierung von Ki-67 in PitNET (“pituitary neuroendocrine tumors”)/Adenomen [Quantification of Ki-67 in PitNET (pituitary neuroendocrine tumors)/adenomas]. Pathologie 2024, 45, 339–343. (In German) [Google Scholar] [CrossRef]
- Tóth, M. Agresszív hypophysisadenoma és hypophysiscarcinoma [Aggressive pituitary adenoma and pituitary carcinoma]. Orvosi Hetil. 2023, 164, 1167–1175. (In Hungarian) [Google Scholar] [CrossRef]
| Overall (N = 79) | |
|---|---|
| Age | |
| Mean (SD) | 57.2 (13.9) |
| Median [Q1–Q3] | 60.0 [47.5–68.5] |
| Min–Max | 23.0–82.0 |
| Gender | |
| F | 32 (40.5%) |
| M | 47 (59.5%) |
| Tumor size AP (mm) | |
| Mean (SD) | 21.2 (8.40) |
| Median [Q1–Q3] | 20.0 [16.0–25.8] |
| Min–Max | 4.50–50.0 |
| Missing | 1 (1.3%) |
| Tumor size ML (mm) | |
| Mean (SD) | 25.5 (8.06) |
| Median [Q1–Q3] | 25.0 [20.0–30.0] |
| Min–Max | 5.50–45.0 |
| Missing | 1 (1.3%) |
| Tumor size CC (mm) | |
| Mean (SD) | 24.3 (10.7) |
| Median [Q1–Q3] | 22.0 [17.0–30.8] |
| Min–Max | 4.50–56.0 |
| Missing | 1 (1.3%) |
| Volume of tumor (cm3) | |
| Mean (SD) | 8.51 (8.66) |
| Median [Q1–Q3] | 5.20 [3.15–10.1] |
| Min–Max | 0.200–50.0 |
| Missing | 4 (5.1%) |
| Transcriptions factors: | |
| Pit-1 | 21 (26.6%) |
| SF 1 | 55 (69.6%) |
| TPit | 14 (17.7%) |
| Type of PitNET | |
| Gonadotroph | 44 (55.69%) |
| Gonadotroph/lactotroph | 2 (2.53%) |
| Corticotroph | 10 (12.65%) |
| Lactotroph | 4 (5.06%) |
| Multiple synchronous | 4 (5.06%) |
| Somatotroph | 1 (1.26%) |
| Thyrotroph | 1 (1.26%) |
| Null cell adenoma | 3 (3.79%) |
| Mature Pit-1 lineage tumor | 3 (3.8%) |
| Immature Pit-1 lineage tumor | 7 (8.86%) |
| Hormonal activity of PitNET | |
| Non-active | 62 (78.48%) |
| Active | 17 (21.52%) |
| Hardy scale | |
| Non-invasive (grades 1, 2) | 15 (19.0%) |
| Invasive (grades 3 and above) | 62 (78.5%) |
| Missing | 2 (2.5%) |
| Knosp scale | |
| Non-invasive (grades 1, 2) | 37 (46.8%) |
| Invasive (grades 3, 4) | 40 (50.6%) |
| Missing | 2 (2.5%) |
| Non-Invasive (N = 37) | Invasive (N = 40) | p-Value | |
|---|---|---|---|
| Age | 0.87 | ||
| Mean (SD) | 57.4 (14.1) | 56.9 (13.9) | |
| Median [Q1–Q3] | 59.0 [48.0−69.0] | 61.0 [46.5–5.3] | |
| Min–Max | 31.0–0.0 | 23.0–0.0 | |
| Gender | 0.52 | ||
| F | 14 (37.8%) | 18 (45.0%) | |
| M | 23 (62.2%) | 22 (55.0%) | |
| Max size (mm) | <0.0001 * | ||
| Mean (SD) | 22.9 (7.32) | 32.1 (9.79) | |
| Median [Q1–Q3] | 22.5 [19.0–0.0] | 30.5 [23.8–8.5] | |
| Min–Max | 5.50–0.0 | 18.0–0.0 | |
| Tumor volume (cm3) | <0.0001 * | ||
| Mean (SD) | 4.90 (4.57) | 11.8 (10.1) | |
| Median [Q1–Q3] | 3.30 [1.83–3.88] | 9.00 [4.60–0.2] | |
| Min–Max | 0.200–0.0 | 1.70–0.0 | |
| Missing | 1 (2.7%) | 1 (2.5%) | |
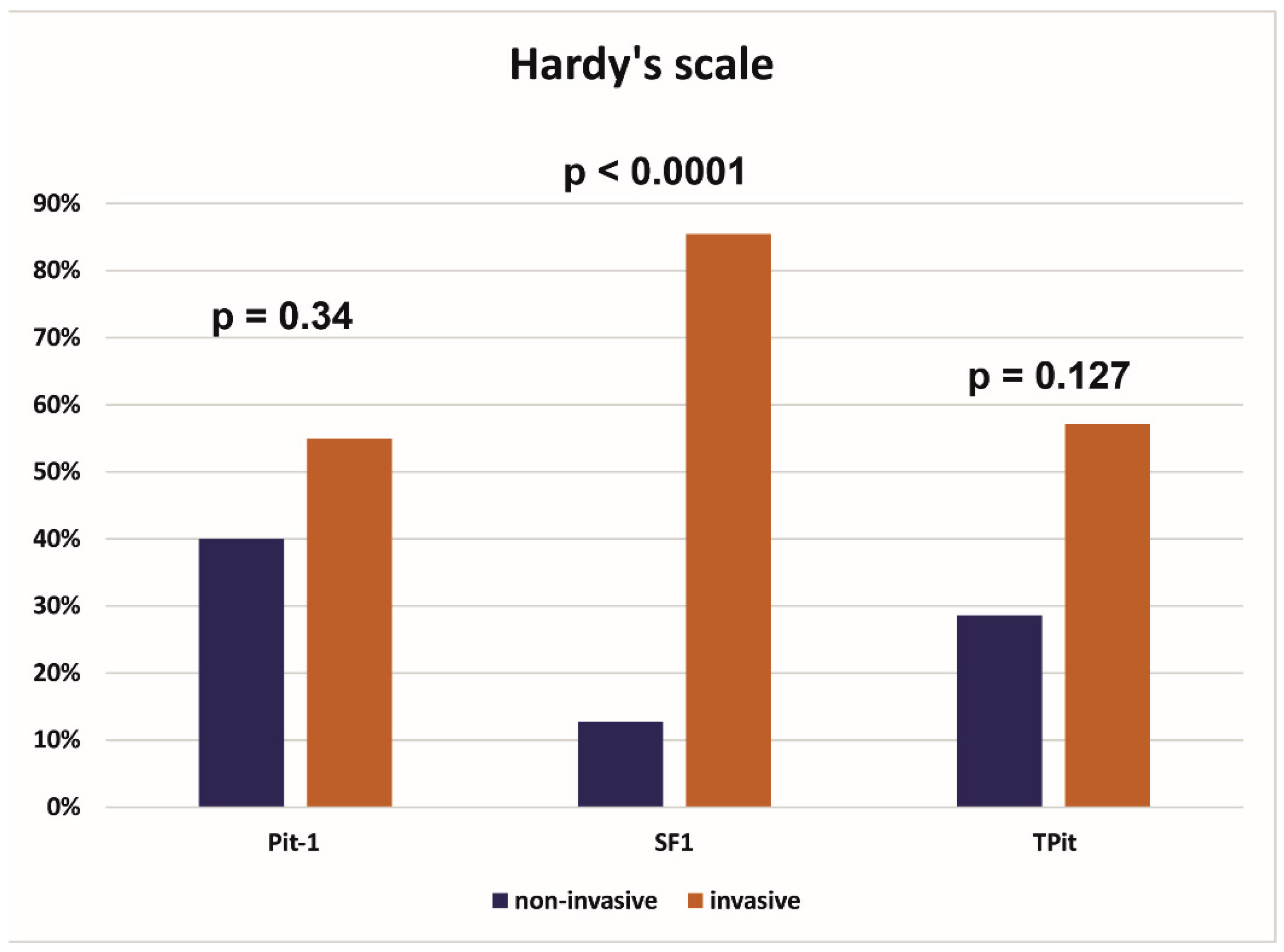
| Pit-1 | 0.65 | ||
| Negative | 27 (73.0%) | 31 (77.5%) | |
| Positive | 10 (27.0%) | 9 (22.5%) | |
| SF 1 | 0.64 | ||
| Negative | 12 (32.4%) | 11 (27.5%) | |
| Positive | 25 (67.6%) | 29 (72.5%) | |
| TPit | 0.44 | ||
| Negative | 30 (81.1%) | 35 (87.5%) | |
| Positive | 7 (18.9%) | 5 (12.5%) | |
| Type of PitNET | 0.37 | ||
| Gonadotroph | 20 (54.1%) | 24 (60.0%) | |
| Gonadotroph/lactotroph | 0 (0%) | 2 (5.0%) | |
| Corticotroph | 4 (10.8%) | 5 (12.5%) | |
| Lactotroph | 3 (8.1%) | 1 (2.5%) | |
| Multiple synchronous | 1 (2.7%) | 3 (7.5%) | |
| Somatotroph | 1 (2.7%) | 0 (0%) | |
| Thyrotroph | 0 (0%) | 1 (2.5%) | |
| Null cell adenoma | 2 (5.4%) | 1 (2.5%) | |
| Mature Pit-1 lineage tumor | 3 (8.1%) | 0 (0%) | |
| Immature Pit-1 lineage tumor | 3 (8.1%) | 3 (7.5%) | |
| Hormonal activity of PitNET | 0.65 | ||
| Non-active | 28 (75.7%) | 32 (80.0%) | |
| Active | 9 (24.3%) | 8 (20.0%) | |
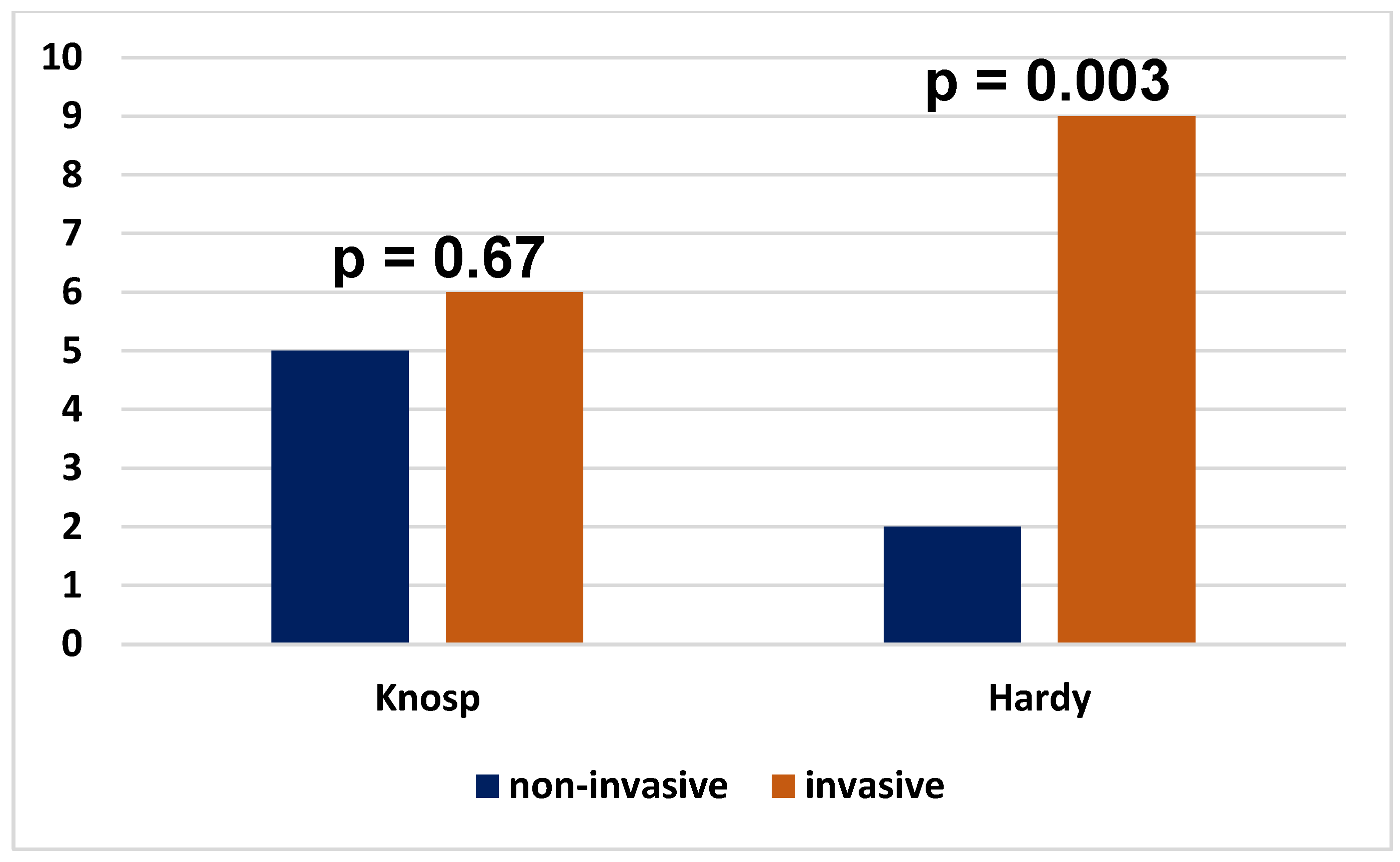
| Invasiveness on the Hardy scale | <0.0001 * | ||
| Non-invasive | 14 (37.8%) | 1 (2.5%) | |
| Invasive | 23 (62.2%) | 39 (97.5%) |
| Non-Invasive (N = 15) | Invasive (N = 62) | p-Value | |
|---|---|---|---|
| Age | 0.64 | ||
| Mean (SD) | 55.4 (14.7) | 57.6 (13.8) | |
| Median [Q1–Q3] | 61.0 [42.0–65.5] | 59.5 [49.0–68.8] | |
| Min–Max | 31.0–75.0 | 23.0–82.0 | |
| Gender | 0.89 | ||
| F | 6 (40.0%) | 26 (41.9%) | |
| M | 9 (60.0%) | 36 (58.1%) | |
| Max size (mm) | <0.0001 * | ||
| Mean (SD) | 18.7 (6.18) | 29.9 (9.28) | |
| Median [Q1–Q3] | 20.0 [16.5–23.0] | 28.3 [23.0–34.0] | |
| Min–Max | 5.50–29.0 | 16.0–56.0 | |
| Volume of tumor (cm3) | <0.0001 * | ||
| Mean (SD) | 2.22 (1.98) | 9.96 (8.96) | |
| Median [Q1–Q3] | 1.65 [1.18–2.93] | 8.20 [4.00–12.0] | |
| Min-Max | 0.200–8.00 | 1.30–50.0 | |
| Missing | 1 (6.7%) | 1 (1.6%) | |
| Pit-1 | 0.008 * | ||
| Negative | 7 (46.7%) | 51 (82.3%) | |
| Positive | 8 (53.3%) | 11 (17.7%) | |
| SF 1 | 0.055 | ||
| Negative | 8 (53.3%) | 15 (24.2%) | |
| Positive | 7 (46.7%) | 47 (75.8%) | |
| TPit | 0.23 | ||
| Negative | 11 (73.3%) | 54 (87.1%) | |
| Positive | 4 (26.7%) | 8 (12.9%) | |
| Type of PitNET | 0.011 * | ||
| Gonadotroph | 5 (33.3%) | 39 (62.9%) | |
| Gonadotroph/lactotroph | 0 (0%) | 2 (3.2%) | |
| Corticotroph | 2 (13.3%) | 7 (11.3%) | |
| Lactotroph | 3 (20.0%) | 1 (1.6%) | |
| Multiple synchronous | 0 (0%) | 4 (6.5%) | |
| Somatotroph | 1 (6.7%) | 0 (0%) | |
| Thyrotroph | 0 (0%) | 1 (1.6%) | |
| Null cell adenoma | 0 (0%) | 3 (4.8%) | |
| Mature Pit-1 lineage tumor | 2 (13.3%) | 1 (1.6%) | |
| Immature Pit-1 lineage tumor | 2 (13.3%) | 4 (6.5%) | |
| Hormonal activity of PitNETs | 0.084 | ||
| Non—active | 9 (60.0%) | 51 (82.3%) | |
| Active | 6 (40.0%) | 11 (17.7%) | |
| Invasiveness on the Knosp scale | <0.0001 * | ||
| Non-invasive | 14 (93.3%) | 23 (37.1%) | |
| Invasive | 1 (6.7%) | 39 (62.9%) |
| Age | Sex | AP (mm) | ML (mm) | CC (mm) | KS | HS | V cm3 | Type PitNET | PRL | ACTH | GH | TSH | LH | FSH | Pit-1 | SF 1 | TPit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 62 | F | 33 | 27 | 41 | 1 | 4D | 18 | Gonadotroph | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ++ | 0 |
| 64 | M | 40 | 37 | 45 | 4 | 4E | 33 | Multiple Synchronous | 0 | 0 | 0 | 0 | + | 0 | 0 | + | 0 |
| 65 | M | 29 | 36 | 46 | 4 | 4D | 21 | Gonadotroph | 0 | 0 | 0 | 0 | 0 | 0 | 0 | +++ | 0 |
| 73 | F | 41 | 41 | 43 | 4 | 4E | 25 | Gonadotroph | 0 | 0 | 0 | 0 | 0 | 0 | 0 | +++ | 0 |
| 62 | M | 33 | 44 | 35 | 4 | 4A | Immature Pit-1 | 0 | 0 | 0 | 0 | 0 | 0 | + | −/+ | 0 | |
| 43 | M | 31 | 29 | 51 | 4 | 4E | 21 | Immature pit-1 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 |
| 57 | F | 30 | 45 | 31 | 3B | 4E | 21 | Corticotroph | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | + |
| 63 | F | 26 | 34 | 43 | 4 | 4E | 19 | Corticotroph | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + |
| 44 | M | 37 | 44 | 56 | 4 | 4E | 50 | Gonadotroph | 0 | 0 | 0 | 0 | 0 | + | 0 | + | 0 |
| 70 | F | 50 | 38 | 43 | 4 | 4E | 33 | Gonadotroph | 0 | 0 | 0 | 0 | 0 | + | 0 | + | 0 |
| 57 | M | 32 | 40 | 40 | 4 | 4D | 13 | Gonadotroph | 0 | 0 | 0 | 0 | 0 | + | 0 | + | 0 |
| Hormonally Inactive (N = 62) | Hormonally Active (N = 17) | p-Value | |
|---|---|---|---|
| Age | 0.089 | ||
| Mean (SD) | 58.7 (13.0) | 51.8 (15.9) | |
| Median [Q1–Q3] | 62.0 [50.0–69.0] | 52.0 [39.0–64.0] | |
| Min–Max | 27.0–82.0 | 23.0–77.0 | |
| Gender | 0.24 | ||
| F | 23 (37.1%) | 9 (52.9%) | |
| M | 39 (62.9%) | 8 (47.1%) | |
| Max size (mm) | 0.37 | ||
| Mean (SD) | 28.3 (9.75) | 25.8 (9.80) | |
| Median [Q1–Q3] | 26.0 [22.0–33.0] | 23.0 [20.0–29.0] | |
| Min–Max | 5.50–56.0 | 8.00–45.0 | |
| Missing | 1 (1.6%) | 0 (0%) | |
| Tumor volume (cm3) | 0.13 | ||
| Mean (SD) | 8.94 (8.72) | 7.05 (8.53) | |
| Median [Q1–Q3] | 5.60 [3.23–10.8] | 4.00 [1.40–8.60] | |
| Min–Max | 0.800–50.0 | 0.200–33.0 | |
| Missing | 4 (6.5%) | 0 (0%) | |
| Type of PitNETs | <0.0001 * | ||
| Gonadotroph | 44 (71.0%) | 0 (0%) | |
| Gonadotroph/lactotroph | 0 (0%) | 2 (11.8%) | |
| Corticotroph | 5 (8.1%) | 5 (29.4%) | |
| Lactotroph | 1 (1.6%) | 3 (17.6%) | |
| Null cell adenoma | 3 (4.8%) | 0 (0%) | |
| Multiple synchronous | 3 (4.8%) | 1 (5.9%) | |
| Thyrotroph | 0 (0%) | 1 (5.9%) | |
| Somatotroph | 0 (0%) | 1 (5.9%) | |
| Mature Pit-1-lineage tumor | 0 (0%) | 3 (17.6%) | |
| Immature Pit-1 lineage tumor | 6 (9.7%) | 1 (5.9%) | |
| Invasiveness on the Hardy scale | 0.084 | ||
| Non-invasive | 9 (14.5%) | 6 (35.3%) | |
| Invasive | 51 (82.3%) | 11 (64.7%) | |
| Missing | 2 (3.2%) | 0 (0%) | |
| Invasiveness on the Knosp scale | 0.65 | ||
| Non-invasive | 28 (45.2%) | 9 (52.9%) | |
| Invasive | 32 (51.6%) | 8 (47.1%) | |
| Missing | 2 (3.2%) | 0 (0%) |
| Type of Pit-NET | PRL | ACTH | GH | TSH | LH | FSH | Pit-1 | SF 1 | TPit |
|---|---|---|---|---|---|---|---|---|---|
| Gonadotroph | 0 | 0 | 0 | 0 | 0 | 0 | −/+ | ++ | 0 |
| Immature Pit-1 lineage tumor | 0 | 0 | 0 | 0 | 0 | 0 | + | −/+ | 0 |
| Gonadotroph | 0 | 0 | 0 | 0 | 0 | 1 | −/+ | + | 0 |
| Immature Pit-1 lineage tumor | 0 | 0 | 0 | 0 | 0 | 0 | + | + | 0 |
| Immature Pit-1 lineage tumor | 0 | 0 | 0 | 0 | 0 | 0 | + | −/+ | −/+ |
| Gonadotroph | 0 | 0 | 0 | 0 | 0 | 0 | −/+ | + | 0 |
| Gonadotroph/lactotroph | 1 | 0 | 0 | 0 | 0 | 1 | + | + | 0 |
| Gonadotroph/lactotroph | 1 | 0 | 0 | 0 | 1 | 1 | + | + | 0 |
| Immature Pit-1 lineage tumor | 0 | 0 | 0 | 0 | 0 | 0 | + | −/+ | + |
| Mature Pit-1 lineage tumor | 1 | 1 | 1 | 0 | 1 | 1 | + | + | + |
| Immature Pit-1 lineage tumor | 0 | 0 | 0 | 0 | 0 | 0 | −/+ | + | 0 |
| Age | Sex | AP (mm) | ML (mm) | CC (mm) | Knosp Scale | Hardy Scale | V cm3 | Subtype | ACTH |
|---|---|---|---|---|---|---|---|---|---|
| 41 | F | 14 | 17 | 11 | 2 | 2A | 1.9 | SGCT | 0 |
| 31 | M | 20 | 25 | 20 | 4 | 3B | 3.7 | Crooke | 1 |
| 72 | F | 23 | 22 | 20 | 4 | 3E | 4.6 | SGCT | 0 |
| 71 | M | No data | No data | No data | No data | No data | No data | Silent | 0 |
| 38 | F | 4.5 | 5.5 | 4.5 | 1 | 1A | 0.8 | SGCT | 1 |
| 77 | M | 34 | 21 | 18 | 1 | 3C | 6.2 | Crooke | 1 |
| 57 | F | 30 | 45 | 31 | 3B | 4E | 21 | No data | 1 |
| 63 | F | 26 | 34 | 43 | 4 | 4E | 19 | Silent | 0 |
| 49 | F | 27 | 26 | 27 | 2 | 3C | 8.6 | Crooke | 1 |
| 68 | F | 20 | 27 | 21 | 3A | 3 | 6 | SGCT | 1 |
| Age | Sex | AP (mm) | ML (mm) | CC (mm) | Knosp Scale | Hardy Scale | V cm3 | Type of PitNET | Sub Type | PRL | ACTH | GH | TSH | LH | FSH | Pit-1 | SF 1 | TPit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 35 | M | 20 | 22 | 29 | 1 | 2C | 8 | Lactotroph | 1 | 0 | 0 | 0 | 0 | 0 | + | 0 | ||
| 23 | M | 28 | 35 | 29 | 4 | 4D | 12 | Lactotroph | 1 | 0 | 1 | 0 | 0 | 0 | + | 0 | 0 | |
| 31 | M | 20 | 25 | 20 | 4 | 3B | 3.7 | Corticotroph | CA | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | +++ |
| 77 | M | 34 | 21 | 18 | 1 | 2C | 6.2 | Corticotroph | CA | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | + |
| 49 | F | 27 | 26 | 27 | 2 | 3C | 8.6 | Corticotroph | CA | 0 | 1 | 0 | 0 | 0 | 0 | 0 | + | |
| 63 | F | 26 | 34 | 43 | 4 | 4E | 19 | Corticotroph | Silent | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + |
| 71 | M | No date | No date | No date | No date | No date | No date | Corticotroph | Silent | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | +++ |
| 75 | M | 6 | 17 | 6 | 1 | 2A | 0.5 | Plurihormonal | 1 | 1 | 1 | 0 | 1 | 1 | + | + | + | |
| 66 | F | 8 | 8 | 6 | 0 | no date | 0.2 | Plurihormonal | 1 | 0 | 1 | 1 | 0 | 0 | + | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzentowska, A.; Czepko, R.; Adamek, D.; Filipowicz, M.; Broniatowska, E.; Gołkowski, F. Radiological and Immunohistochemical Characteristics of PitNETs in 79 Patients Undergoing Neurosurgery. Cancers 2025, 17, 666. https://doi.org/10.3390/cancers17040666
Krzentowska A, Czepko R, Adamek D, Filipowicz M, Broniatowska E, Gołkowski F. Radiological and Immunohistochemical Characteristics of PitNETs in 79 Patients Undergoing Neurosurgery. Cancers. 2025; 17(4):666. https://doi.org/10.3390/cancers17040666
Chicago/Turabian StyleKrzentowska, Anna, Ryszard Czepko, Dariusz Adamek, Michał Filipowicz, Elżbieta Broniatowska, and Filip Gołkowski. 2025. "Radiological and Immunohistochemical Characteristics of PitNETs in 79 Patients Undergoing Neurosurgery" Cancers 17, no. 4: 666. https://doi.org/10.3390/cancers17040666
APA StyleKrzentowska, A., Czepko, R., Adamek, D., Filipowicz, M., Broniatowska, E., & Gołkowski, F. (2025). Radiological and Immunohistochemical Characteristics of PitNETs in 79 Patients Undergoing Neurosurgery. Cancers, 17(4), 666. https://doi.org/10.3390/cancers17040666







