Extended Lymph Node Dissection May Not Provide a Therapeutic Benefit in Patients with Intermediate-to High-Risk Prostate Cancer Treated with Robotic-Assisted Radical Prostatectomy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Surgical Approach
2.3. Endpoints
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Perioperative Clinical Characteristics and Pathological Outcomes (Propensity-Score-Matched Cohort)
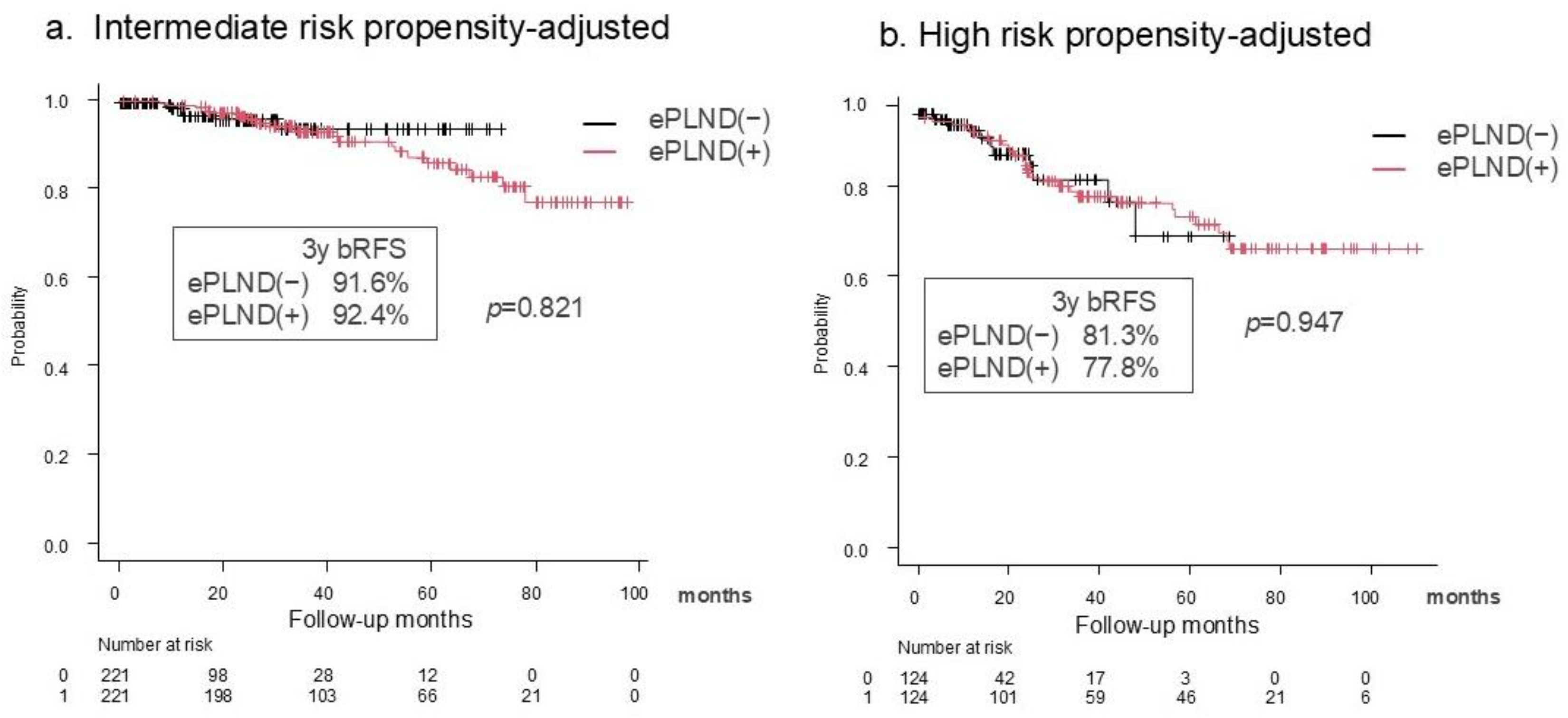
3.3. Oncological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
- The following abbreviations are used in this manuscript:
| AUA | American Urological Association |
| bRFS | biochemical recurrence-free survival |
| BCR | biochemical recurrence |
| CIs | confidence intervals |
| CSM | cancer-specific mortality |
| EAU | European Association of Urology |
| ePLND | extended pelvic lymph node dissection |
| GS | Gleason score |
| HR | high risk |
| HRs | hazard ratios |
| IR | intermediate risk |
| IRB | Institutional Review Board |
| MFS | metastasis-free survival |
| NCCN | National Comprehensive Cancer Network |
| PLND | pelvic lymph node dissection |
| PSA | prostate-specific antigen |
| RARP | robotic-assisted radical prostatectomy |
| RP | radical prostatectomy |
References
- Comford, P.; Tilki, D.; van den Bergh, R.C.N.; Briers, E.; Eberli, D.; De Meerleer, G.; De Santis, M.; Gillessen, S.; Henry, A.M.; van Leenders, G.J.L.H.; et al. EAU Guidelines. Available online: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines-on-Prostate-Cancer-2024_2024-04-09-132035_ypmy_2024-04-16-122605_lqpk.pdf (accessed on 26 December 2024).
- NCCN Guidelines: Prostate Cancer version 1.2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 26 December 2024).
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part II: Principles of Active Surveillance, Principles of Surgery, and Follow-Up. J. Urol. 2022, 208, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Preisser, F.; van den Bergh, R.C.N.; Gandaglia, G.; Ost, P.; Surcel, C.I.; Sooriakumaran, P.; Montorsi, F.; Graefen, M.; van der Poel, H.; de la Taille, A.; et al. Effect of Extended Pelvic Lymph Node Dissection on Oncologic Outcomes in Patients with D’Amico Intermediate and High Risk Prostate Cancer Treated with Radical Prostatectomy: A Multi-Institutional Study. J. Urol. 2020, 203, 338–343. [Google Scholar] [CrossRef]
- Choo, M.S.; Kim, M.; Ku, J.H.; Kwak, C.; Kim, H.H.; Jeong, C.W. Extended versus Standard Pelvic Lymph Node Dissection in Radical Prostatectomy on Oncological and Functional Outcomes: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2017, 24, 2047–2054. [Google Scholar] [CrossRef]
- Withrow, D.R.; DeGroot, J.M.; Siemens, D.R.; Groome, P.A. Therapeutic Value of Lymph Node Dissection at Radical Prostatectomy: A Population-Based Case-Cohort Study. BJU Int. 2011, 108, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Briganti, A.; Larcher, A.; Abdollah, F.; Capitanio, U.; Gallina, A.; Suardi, N.; Bianchi, M.; Sun, M.; Freschi, M.; Salonia, A.; et al. Updated Nomogram Predicting Lymph Node Invasion in Patients with Prostate Cancer Undergoing Extended Pelvic Lymph Node Dissection: The Essential Importance of Percentage of Positive Cores. Eur. Urol. 2012, 61, 480–487. [Google Scholar] [CrossRef]
- Abdollah, F.; Gandaglia, G.; Suardi, N.; Capitanio, U.; Salonia, A.; Nini, A.; Moschini, M.; Sun, M.; Karakiewicz, P.I.; Shariat, S.F.; et al. More Extensive Pelvic Lymph Node Dissection Improves Survival in Patients with Node-Positive Prostate Cancer. Eur. Urol. 2015, 67, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Preisser, F.; Bandini, M.; Marchioni, M.; Nazzani, S.; Tian, Z.; Pompe, R.S.; Fossati, N.; Briganti, A.; Saad, F.; Shariat, S.F.; et al. Extent of Lymph Node Dissection Improves Survival in Prostate Cancer Patients Treated with Radical Prostatectomy Without Lymph Node Invasion. Prostate 2018, 78, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Joslyn, S.A.; Konety, B.R. Impact of Extent of Lymphadenectomy on Survival After Radical Prostatectomy for Prostate Cancer. Urology 2006, 68, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Moschini, M.; Fossati, N.; Abdollah, F.; Gandaglia, G.; Cucchiara, V.; Dell’Oglio, P.; Luzzago, S.; Shariat, S.F.; Dehò, F.; Salonia, A.; et al. Determinants of Long-Term Survival of Patients with Locally Advanced Prostate Cancer: The Role of Extensive Pelvic Lymph Node Dissection. Prostate Cancer Prostatic Dis. 2016, 19, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Touijer, K.A.; Sjoberg, D.D.; Benfante, N.; Laudone, V.P.; Ehdaie, B.; Eastham, J.A.; Scardino, P.T.; Vickers, A. Limited Versus Extended Pelvic Lymph Node Dissection for Prostate Cancer: A Randomized Clinical Trial. Eur. Urol. Oncol. 2021, 4, 532–539. [Google Scholar] [CrossRef]
- Lestingi, J.F.P.; Guglielmetti, G.B.; Trinh, Q.D.; Coelho, R.F.; Pontes, J.; Bastos, D.A.; Cordeiro, M.D.; Sarkis, A.S.; Faraj, S.F.; Mitre, A.I.; et al. Extended Versus Limited Pelvic Lymph Node Dissection During Radical Prostatectomy for Intermediate- and High-risk Prostate Cancer: Early Oncological Outcomes from a Randomized Phase 3 Trial. Eur. Urol. 2021, 79, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Maas, M.; Nassiri, N.; Ortega, D.; Gill, K.; Dell’Oglio, P.; Thalmann, G.N.; Heidenreich, A.; Eastham, J.A.; Evans, C.P.; et al. Impact of Pelvic Lymph Node Dissection and Its Extent on Perioperative Morbidity in Patients Undergoing Radical Prostatectomy for Prostate Cancer: A Comprehensive Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2021, 4, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.; Zhao, J.; Zhu, S.; Sun, G.; Liu, J.; Zhang, H.; Zhang, X.; Shen, P.; Shi, M.; et al. Pelvic Lymph Node Dissection and Its Extent on Survival Benefit in Prostate Cancer Patients with a Risk of Lymph Node Invasion >5%: A Propensity Score Matching Analysis From SEER Database. Sci. Rep. 2019, 9, 17985. [Google Scholar] [CrossRef]
- Fujimoto, N.; Shiota, M.; Tomisaki, I.; Minato, A.; Yahara, K. Reconsideration on Clinical Benefit of Pelvic Lymph Node Dissection during Radical Prostatectomy for Clinically Localized Prostate Cancer. Urol. Int. 2019, 103, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Ploussard, G.; Briganti, A.; de la Taille, A.; Haese, A.; Heidenreich, A.; Menon, M.; Sulser, T.; Tewari, A.K.; Eastham, J.A. Pelvic Lymph Node Dissection During Robot-Assisted Radical Prostatectomy: Efficacy, Limitations, And Complications-A Systematic Review of the Literature. Eur. Urol. 2014, 65, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Mattei, A.; Fuechsel, F.G.; Bhatta Dhar, N.; Warncke, S.H.; Thalmann, G.N.; Krause, T.; Studer, U.E. The Template of the Primary Lymphatic Landing Sites of the Prostate Should Be Revisited: Results of a Multimodality Mapping Study. Eur. Urol. 2008, 53, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort Of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the Freely Available Easy-To-Use Software ’EZR’ For Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Touijer, K.A.; Vertosick, E.A.; Sjoberg, D.D.; Liso, N.; Nalavenkata, S.; Melao, B.; Laudone, V.P.; Ehdaie, B.; Carver, B.; Eastham, J.A.; et al. Pelvic Lymph Node Dissection in Prostate Cancer: Update from a Randomized Clinical Trial of Limited Versus Extended Dissection. Eur. Urol. 2025, 87, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Mandel, P.; Kriegmair, M.C.; Veleva, V.; Salomon, G.; Graefen, M.; Huland, H.; Tilki, D. The Role of Pelvic Lymph Node Dissection During Radical Prostatectomy in Patients With Gleason 6 Intermediate-risk Prostate Cancer. Urology 2016, 93, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Namiki, S.; Kawase, M.; Ebara, S.; Tatenuma, T.; Sasaki, T.; Ikehata, Y.; Nakayama, A.; Toide, M.; Yoneda, T.; Sakaguchi, K.; et al. Pelvic Lymphadenectomy May Not Improve Biochemical Recurrence-Free Survival in Patients with Prostate Cancer Treated with Robot-Assisted Radical Prostatectomy in Japan (The MSUG94 Group). Cancers 2022, 14, 5803. [Google Scholar] [CrossRef] [PubMed]
- Yuh, B.; Artibani, W.; Heidenreich, A.; Kimm, S.; Menon, M.; Novara, G.; Tewari, A.; Touijer, K.; Wilson, T.; Zorn, K.C.; et al. The Role of Robot-Assisted Radical Prostatectomy and Pelvic Lymph Node Dissection in the Management of High-Risk Prostate Cancer: A Systematic Review. Eur. Urol. 2014, 65, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Colicchia, M.; Sharma, V.; Abdollah, F.; Briganti, A.; Jeffrey Karnes, R. Therapeutic Value of Standard Versus Extended Pelvic Lymph Node Dissection During Radical Prostatectomy for High-Risk Prostate Cancer. Curr. Urol. Rep. 2017, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, L.; Pacini, M.; Calvo, R.S.; Morgantini, L.; Cannoletta, D.; Di Maida, F.; Valastro, F.; Mari, A.; Bignante, G.; Lasorsa, F.; et al. Extraperitoneal Single Port vs Transperitoneal Multiport Robot assisted radical prostatectomy in frail patients: A propensity score matched comparative analysis. Eur. J. Surg. Oncol. 2024, 50, 108741. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.J.; Kattan, M.W.; Eastham, J.A.; Dotan, Z.A.; Bianco, F.J.; Lilja, H.; Scardino, P.T. Defining Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy: A Proposal For a Standardized Definition. J. Clin. Oncol. 2006, 24, 3973–3978. [Google Scholar] [CrossRef] [PubMed]
- Williams, S. Surrogate Endpoints in Early Prostate Cancer Research. Transl. Androl. Urol. 2018, 7, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Regan, M.M.; Buyse, M.; Halabi, S.; Kantoff, P.W.; Sartor, O.; Soule, H.; Clarke, N.W.; Collette, L.; Dignam, J.J.; et al. Metastasis-Free Survival Is a Strong Surrogate of Overall Survival in Localized Prostate Cancer. J. Clin. Oncol. 2017, 35, 3097–3104. [Google Scholar] [CrossRef] [PubMed]
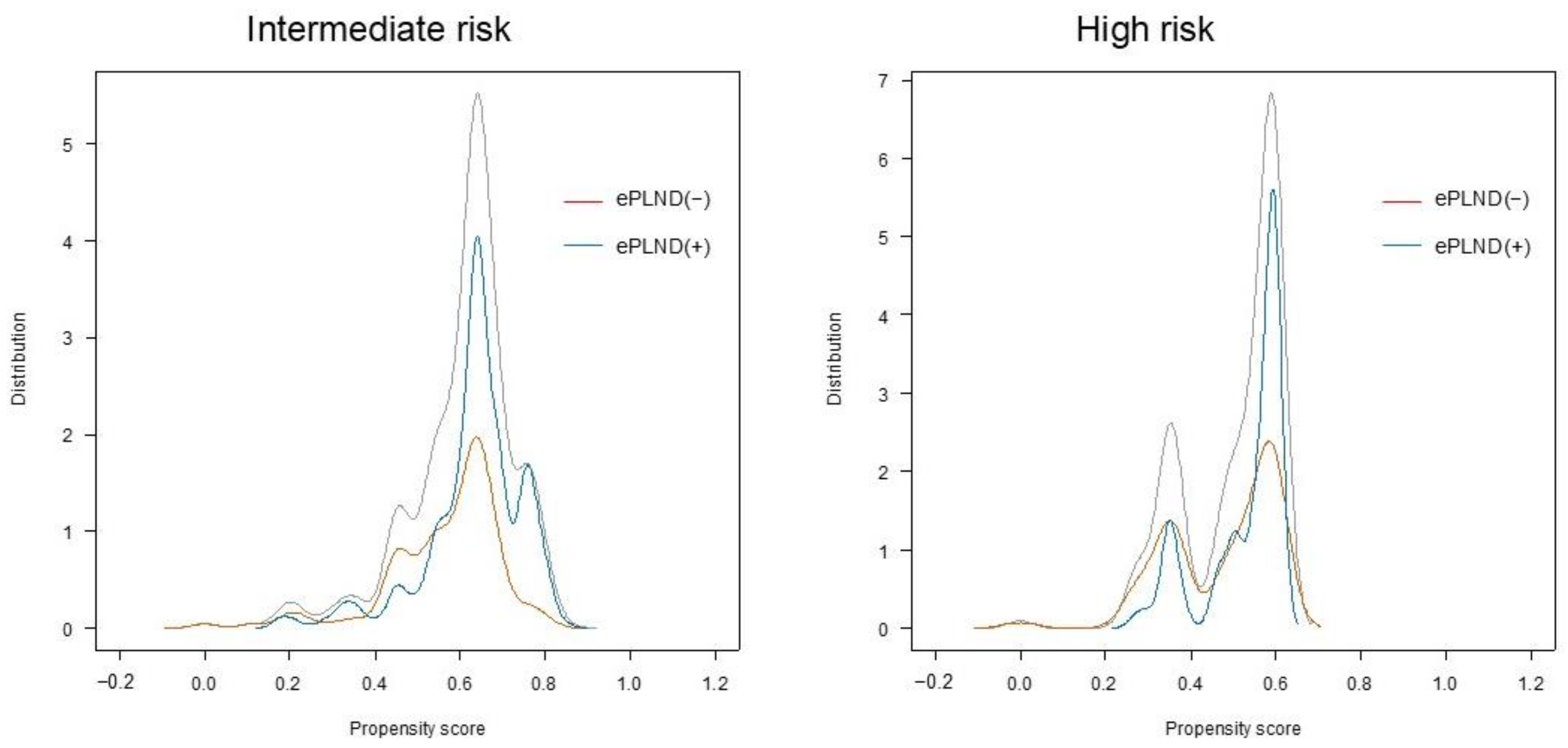
| Variable (Original Cohort) | ePLND (−) (n = 315) | ePLND (n = 317) | p Value | ePLND (–) (n = 146) | ePLND (n = 224) | p Value |
|---|---|---|---|---|---|---|
| Age, median (IQR) yr | 71 (67–83) | 67 (62–79) | <0.001 | 71 (66–76) | 68 (62–73) | <0.001 |
| Preoperative PSA (ng/mL) | ||||||
| <10 | 232 (73.7%) | 254 (80.1%) | 0.0592 | 91 (70.1%) | 165 (77.4%) | 0.0101 |
| 10≤□<20 | 83 (26.3%) | 63 (19.9%) | 37 (26.0%) | 49 (20.1%) | ||
| 20≤ | 18 (3.9%) | 10 (1.8%) | ||||
| Prostate biopsy ISUP Gleason Grading | ||||||
| 1 | 38 (12.1%) | 3 (0.9%) | <0.001 | 6 (4.1%) | 3 (1.3%) | <0.001 |
| 2 | 156 (49.5%) | 220 (69.4%) | 12 (8.2%) | 18 (8.0%) | ||
| 3 | 121 (29.7%) | 94 (29.7%) | 15 (10.3%) | 10 (4.5%) | ||
| 4 | 0 (0%) | 0 (0%) | 94 (64.4%) | 125 (55.8%) | ||
| 5 | 0 (0%) | 0 (0%) | 19 (13.0%) | 68 (30.4%) | ||
| cT stage T1a-c | 88 (28.0%) | 42 (13.2%) | <0.001 | 27 (18.5%) | 24 (10.7%) | 0.234 |
| T2a | 143 (45.4%) | 195 (61.5%) | 70 (47.9%) | 120 (53.6%) | ||
| T2b | 24 (7.6%) | 13 (4.1%) | 11 (7.5%) | 15 (6.7%) | ||
| T2c | 60 (19.0%) | 67 (21.1%) | 23 (15.8%) | 45 (20.1%) | ||
| T3a | 0 (0%) | 0 (0%) | 15 (10.3%) | 20 (8.9%) | ||
| T3b | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Variable (Propensity-score-matched cohort) | ePLND (–) (n = 221) | ePLND (n = 221) | p value | ePLND (–) (n = 124) | ePLND (n = 124) | p value |
| Age, median(IQR) yr | 71 (66–74) | 67 (62–71) | <0.001 | 71 (65–76) | 67 (62–73) | <0.001 |
| Preoperative PSA (ng/mL) | ||||||
| <10 | 178 (80.5%) | 180 (81.4%) | 0.904 | 81 (65.3%) | 87 (70.2%) | 0.733 |
| 10≤□<20 | 43 (19.5%) | 41 (18.6%) | 34 (27.4%) | 30 (24.2%) | ||
| 20≤ | 9 (7.3%) | 7 (5.6%) | ||||
| Prostate biopsy ISUP Gleason Grading | ||||||
| 1 | 4 (1.8%) | 3 (1.4%) | 1 | 3 (2.4%) | 2 (1.6%) | 0.930 |
| 2 | 138 (62.4%) | 138 (62.4%) | 11 (8.9%) | 14 (11.3%) | ||
| 3 | 79 (35.7%) | 80 (36.2%) | 9 (7.3%) | 7 (5.6%) | ||
| 4 | 0 (0%) | 0 (0%) | 82 (66.1%) | 83 (66.9%) | ||
| 5 | 0 (0%) | 0 (0%) | 19 (15.3%) | 18 (14.5%) | ||
| cT stage T1a–c | 39 (17.6%) | 38 (17.2%) | 0.753 | 16 (12.9%) | 18 (14.5%) | 0.995 |
| T2a | 133 (60.2%) | 125 (56.6%) | 65 (52.4%) | 61 (49.2%) | ||
| T2b | 9 (4.1%) | 9 (4.1%) | 9 (7.3%) | 9 (7.3%) | ||
| T2c | 40 (18.1%) | 49 (22.2%) | 20 (16.1%) | 21 (16.9%) | ||
| T3a | 0 (0%) | 0(0%) | 14 (11.3%) | 15 (12.1%) | ||
| T3b | 0 (0%) | 0(0%) | 0 (0%) | 0 (0%) |
| Variable | ePLND (–) (n = 221) | ePLND (n = 221) | p Value | ePLND (–) (n = 124) | ePLND (n = 124) | p Value |
|---|---|---|---|---|---|---|
| Operation time median(IQR) min | 175 (150–207) | 325 (299–355) | <0.001 | 180 (153–217) | 327 (293–354) | <0.001 |
| Console time median(IQR) min | 126 (99–149) | 267 (240–292) | <0.001 | 125 (101–161) | 271 (249–302) | <0.001 |
| Bleeding median(IQR) mL | 0 (0–100) | 150 (100–250) | <0.001 | 0 (0–100) | 150 (100–250) | <0.001 |
| Prostatectomy specimen ISUP Gleason Grading | ||||||
| 0 | 0 (0%) | 1 (0.5%) | <0.001 | 0 (0%) | 0 (0%) | <0.001 |
| 1 | 11 (5.0%) | 13 (5.9%) | 4 (3.2%) | 1 (0.8%) | ||
| 2 | 68 (30.8%) | 163 (73.8%) | 15 (12.1%) | 51 (41.1%) | ||
| 3 | 75 (33.9%) | 38 (17.2%) | 28 (22.6%) | 41 (33.1%) | ||
| 4 | 21 (9.5%) | 3 (1.4%) | 34 (27.4%) | 22 (17.7%) | ||
| 5 | 46 (20.8%) | 3 (1.4%) | 43 (34.7%) | 9 (7.3%) | ||
| pT stage | ||||||
| T0 | 0 (0%) | 1 (0.3%) | 0.930 | 0 (0%) | 0 (0%) | 0.254 |
| T2 | 184 (80.6%) | 185 (84.2%) | 87 (70.2%) | 92 (74.2%) | ||
| T3a | 29 (15.2%) | 29 (12.9%) | 30 (24.2%) | 21 (16.9%) | ||
| T3b | 8 (3.8%) | 6 (2.5%) | 7 (5.6%) | 11 (8.9%) | ||
| pN stage | ||||||
| N0 | 0 (0%) | 213 (96.4%) | 0 (0%) | 115 (92.7%) | - | |
| N1 | 0 (0%) | 8 (3.6%) | 0 (0%) | 9 (7.3%) | ||
| Nx | 221 (100%) | 0 (0%) | 124 (100%) | 0 (0%) | ||
| Surgical margin | ||||||
| positive | 34 (15.4%) | 20(9.0%) | 0.00246 | 24 (19.4%) | 23 (18.5%) | 0.325 |
| unknown | 2 (0.9%) | 13(5.9%) | 0 (0%) | 3 (2.4%) |
| Complications | ePLND (–) (n = 461) | ePLND (n = 541) | p Value |
|---|---|---|---|
| All Grade | 37 (8.0%) | 159 (29.4%) | |
| 1 | 12 (2.6%) | 89 (16.5%) | <0.0001 |
| 2 | 15 (3.3%) | 37 (6.8%) | |
| 3 | 10 (2.2%) | 33 (6.1%) | |
| <3 | 27 | 126 | |
| 3≤ | 10 | 33 | |
| Complications related with PLND | 0 (0%) | 69 (12.8%) | |
| extremity edema | 0 (0%) | 53 (9.8%) | <0.001 |
| Pelvic hematoma | 0 (0%) | 9 (1.7%) | 0.00467 |
| Neuropathy | 0 (0%) | 7 (1.3%) | 0.0174 |
| Intermediate Risk | High Risk | |||||
|---|---|---|---|---|---|---|
| Variable | Univariate p Value | Multivariable | Univariate p Value | Multivariable | ||
| HR 95% CI | p Value | HR 95% CI | p Value | |||
| Age (years) | 0.32 | 0.85 | ||||
| PSA (ng/mL) | ||||||
| <10 | ref | ref | ||||
| 10–20 | 0.018 | 0.38 (0.18–0.81) | 0.013 | <0.001 | 1.97 (1.00–3.86) | 0.050 |
| 20< | n.a. | n.a. | n.a. | 0.014 | 2.87 (1.07–7.70) | 0.037 |
| ePLND yes | 0.62 | 1.31 (0.55–3.07) | 0.54 | 0.95 | 1.06 (0.54–2.07) | 0.87 |
| Prostatectomy specimen ISUP Gleason Grading | ||||||
| 0 | ref | ref | ||||
| 1 | 1.0 | 0.88 | ||||
| 2 | 0.99 | 0.14 | ||||
| 3 | 0.99 | 0.90 | ||||
| 4 | 0.99 | n.a | ||||
| 5 | 0.99 | n.a | ||||
| pT stage | ||||||
| T0 | ref | |||||
| T2 | 0.99 | ref | ref | Ref | ||
| T3a | 0.99 | <0.001 | 2.22 (1.08–4.56) | 0.029 | ||
| T3b | 0.99 | 0.0011 | 1.98 (0.77–5.08) | 0.155 | ||
| Surgical margin | ||||||
| negative | ref | ref | ref | ref | ref | ref |
| positive | 0.002 | 3.68 (1.67–8.10) | 0.001 | <0.001 | 2.41 (1.24–4.70) | 0.010 |
| unknown | 0.072 | 3.68 (0.82–16.5) | 0.088 | 0.99 | n.a | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, N.; Shimbo, M.; Okawa, D.; Sakamoto, M.; Sugihara, N.; Sawada, T.; Haga, S.; Arai, H.; Nishida, K.; Arai, O.; et al. Extended Lymph Node Dissection May Not Provide a Therapeutic Benefit in Patients with Intermediate-to High-Risk Prostate Cancer Treated with Robotic-Assisted Radical Prostatectomy. Cancers 2025, 17, 655. https://doi.org/10.3390/cancers17040655
Miura N, Shimbo M, Okawa D, Sakamoto M, Sugihara N, Sawada T, Haga S, Arai H, Nishida K, Arai O, et al. Extended Lymph Node Dissection May Not Provide a Therapeutic Benefit in Patients with Intermediate-to High-Risk Prostate Cancer Treated with Robotic-Assisted Radical Prostatectomy. Cancers. 2025; 17(4):655. https://doi.org/10.3390/cancers17040655
Chicago/Turabian StyleMiura, Noriyoshi, Masaki Shimbo, Dai Okawa, Miki Sakamoto, Naoya Sugihara, Takatora Sawada, Shunsuke Haga, Haruna Arai, Keigo Nishida, Osuke Arai, and et al. 2025. "Extended Lymph Node Dissection May Not Provide a Therapeutic Benefit in Patients with Intermediate-to High-Risk Prostate Cancer Treated with Robotic-Assisted Radical Prostatectomy" Cancers 17, no. 4: 655. https://doi.org/10.3390/cancers17040655
APA StyleMiura, N., Shimbo, M., Okawa, D., Sakamoto, M., Sugihara, N., Sawada, T., Haga, S., Arai, H., Nishida, K., Arai, O., Onishi, T., Watanabe, R., Nishimura, K., Fukumoto, T., Miyauchi, Y., Kikugawa, T., Nishino, T., Endo, F., Hattori, K., & Saika, T. (2025). Extended Lymph Node Dissection May Not Provide a Therapeutic Benefit in Patients with Intermediate-to High-Risk Prostate Cancer Treated with Robotic-Assisted Radical Prostatectomy. Cancers, 17(4), 655. https://doi.org/10.3390/cancers17040655





