Diagnostic and Therapeutic Implications of the SUMOylation Pathway in Acute Myeloid Leukemia
Simple Summary
Abstract
1. Introduction
2. SUMOylation Cascade and Its Tumorigenic Potential
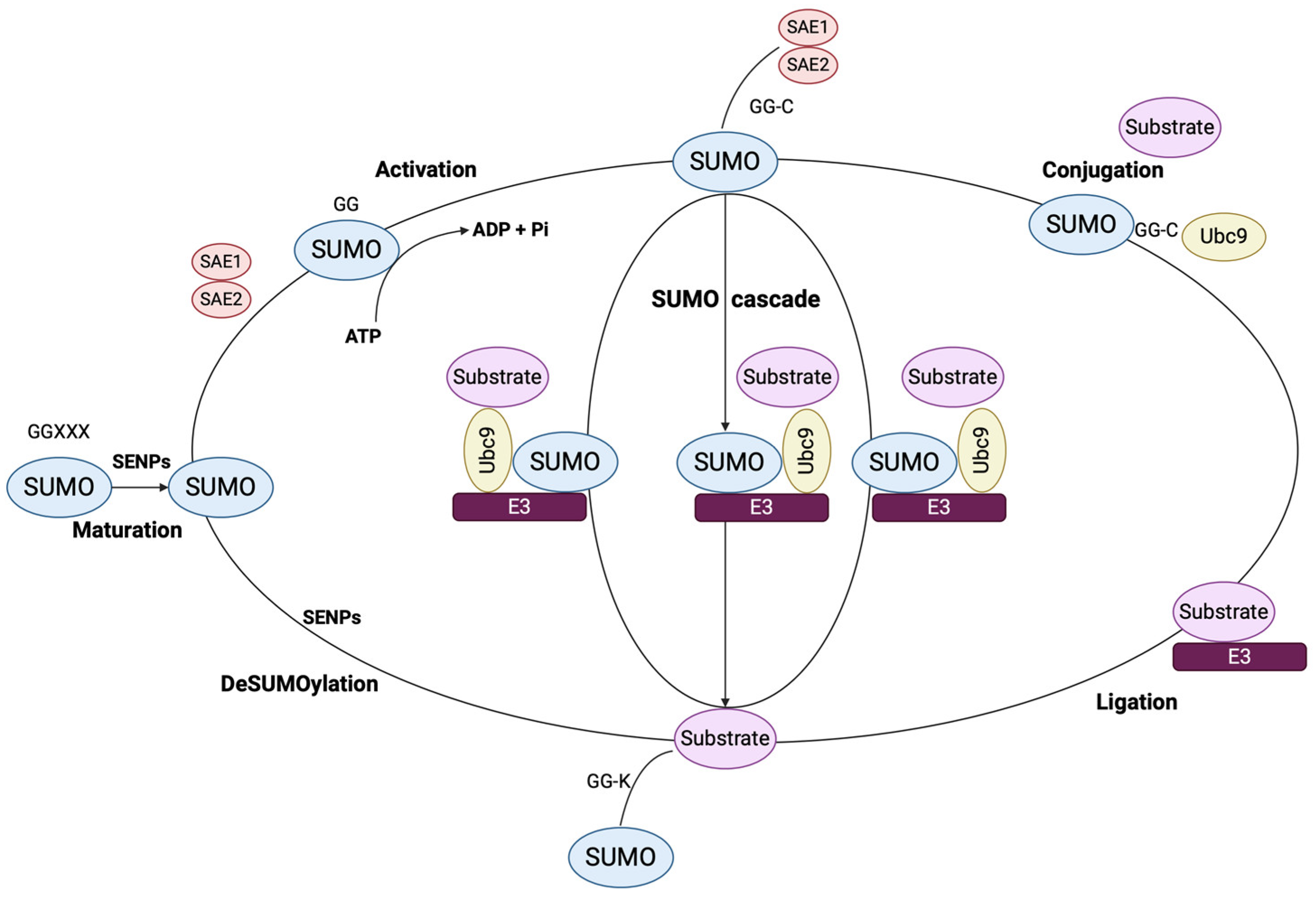
2.1. Isoforms, Structure and Signaling Mechanisms
2.2. Implications in Carcinogenesis
2.3. SUMOylation as a Part of Epigenetic Modification Patterns of AML
3. Role of the SUMO Pathway in AML: Novel Insights in Leukemogenesis and Response to Treatment
3.1. SUMO in Acute Promyelocytic Leukemia (APL)
3.2. SUMO in Non-APL Acute Leukemia
4. Targeting SUMOylation in AML: Ongoing Clinical Trials and Pre-Clinical Studies
5. Discussion and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gibney, E.R.; Nolan, C.M. Epigenetics and Gene Expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Tuscher, J.J.; Day, J.J. Multigenerational Epigenetic Inheritance: One Step Forward, Two Generations Back. Neurobiol. Dis. 2019, 132, 104591. [Google Scholar] [CrossRef]
- Yuan, M.; Yang, B.; Rothschild, G.; Mann, J.J.; Sanford, L.D.; Tang, X.; Huang, C.; Wang, C.; Zhang, W. Epigenetic Regulation in Major Depression and Other Stress-Related Disorders: Molecular Mechanisms, Clinical Relevance and Therapeutic Potential. Signal Transduct. Target. Ther. 2023, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Fatima, S.I.; Schmitz, M.; Zerr, I. Current Technologies Unraveling the Significance of Post-Translational Modifications (PTMs) as Crucial Players in Neurodegeneration. Biomolecules 2024, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.J. Protein Prenylation: A Mediator of Protein-Protein Interactions. Science 1993, 259, 1865–1866. [Google Scholar] [CrossRef] [PubMed]
- Del Monte, F.; Agnetti, G. Protein Post-translational Modifications and Misfolding: New Concepts in Heart Failure. PROTEOMICS–Clinical Appl. 2014, 8, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Audagnotto, M.; Dal Peraro, M. Protein Post-Translational Modifications: In Silico Prediction Tools and Molecular Modeling. Comput. Struct. Biotechnol. J. 2017, 15, 307–319. [Google Scholar] [CrossRef]
- Huang, C.-H.; Yang, T.-T.; Lin, K.-I. Mechanisms and Functions of SUMOylation in Health and Disease: A Review Focusing on Immune Cells. J. Biomed. Sci. 2024, 31, 16. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-N.; Li, M.-Y.; Qi, G.-Q.; Wei, L.-N.; Zhang, D.-K. SUMOylation at the Crossroads of Gut Health: Insights into Physiology and Pathology. Cell Commun. Signal. 2024, 22, 404. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, S.P.; Chatzikalil, E.; Vakadaris, G.; Reppas, L.; Arvanitakis, K.; Koufakis, T.; Siakavellas, S.I.; Manolakopoulos, S.; Germanidis, G.; Theocharis, S. Exploring the Role of GITR/GITRL Signaling: From Liver Disease to Hepatocellular Carcinoma. Cancers 2024, 16, 2609. [Google Scholar] [CrossRef]
- Arvanitakis, K.; Papadakos, S.P.; Vakadaris, G.; Chatzikalil, E.; Stergiou, I.E.; Kalopitas, G.; Theocharis, S.; Germanidis, G. Shedding Light on the Role of LAG-3 in Hepatocellular Carcinoma: Unraveling Immunomodulatory Pathways. Hepatoma Res. 2024, 10, 20. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Chatzikalil, E.; Arvanitakis, K.; Vakadaris, G.; Stergiou, I.E.; Koutsompina, M.-L.; Argyrou, A.; Lekakis, V.; Konstantinidis, I.; Germanidis, G.; et al. Understanding the Role of Connexins in Hepatocellular Carcinoma: Molecular and Prognostic Implications. Cancers 2024, 16, 1533. [Google Scholar] [CrossRef]
- Sahin, U.; de Thé, H.; Lallemand-Breitenbach, V. Sumoylation in Physiology, Pathology and Therapy. Cells 2022, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, Y.; Chan, Y.-T.; Zhang, C.; Wang, N.; Feng, Y. The Role of Protein SUMOylation in Human Hepatocellular Carcinoma: A Potential Target of New Drug Discovery and Development. Cancers 2021, 13, 5700. [Google Scholar] [CrossRef] [PubMed]
- Wemyss, C.; Jones, E.; Stentz, R.; Carding, S.R. Acute Myeloid Leukaemia and Acute Lymphoblastic Leukaemia Classification and Metabolic Characteristics for Informing and Advancing Treatment. Cancers 2024, 16, 4136. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Boscaro, E.; Urbino, I.; Catania, F.M.; Arrigo, G.; Secreto, C.; Olivi, M.; D’Ardia, S.; Frairia, C.; Giai, V.; Freilone, R.; et al. Modern Risk Stratification of Acute Myeloid Leukemia in 2023: Integrating Established and Emerging Prognostic Factors. Cancers 2023, 15, 3512. [Google Scholar] [CrossRef] [PubMed]
- Bono, R.; Sapienza, G.; Tringali, S.; Rotolo, C.; Patti, C.; Mulè, A.; Calafiore, V.; Santoro, A.; Castagna, L. Allogeneic Stem Cell Transplantation in Refractory Acute Myeloid Leukaemia. Cells 2024, 13, 755. [Google Scholar] [CrossRef]
- Canichella, M.; de Fabritiis, P. Cell-Based Treatment in Acute Myeloid Leukemia Relapsed after Allogeneic Stem Cell Transplantation. Biomedicines 2024, 12, 1721. [Google Scholar] [CrossRef]
- Delaporta, P.; Chatzikalil, E.; Ladis, V.; Moraki, M.; Kattamis, A. Evolving Changes in the Characteristics of Death in Transfusion Dependent Thalassemia in Greece. Blood 2023, 142, 1103. [Google Scholar] [CrossRef]
- Chatzikalil, E.; Roka, K.; Diamantopoulos, P.T.; Rigatou, E.; Avgerinou, G.; Kattamis, A.; Solomou, E.E. Venetoclax Combination Treatment of Acute Myeloid Leukemia in Adolescents and Young Adult Patients. J. Clin. Med. 2024, 13, 2046. [Google Scholar] [CrossRef]
- Totiger, T.M.; Ghoshal, A.; Zabroski, J.; Sondhi, A.; Bucha, S.; Jahn, J.; Feng, Y.; Taylor, J. Targeted Therapy Development in Acute Myeloid Leukemia. Biomedicines 2023, 11, 641. [Google Scholar] [CrossRef]
- Baylin, S.B.; Fearon, E.R.; Vogelstein, B.; de Bustros, A.; Sharkis, S.J.; Burke, P.J.; Staal, S.P.; Nelkin, B.D. Hypermethylation of the 5’region of the Calcitonin Gene Is a Property of Human Lymphoid and Acute Myeloid Malignancies. Blood 1987, 70, 412–417. [Google Scholar] [CrossRef]
- Pfeifer, G.P.; Steigerwald, S.; Boehm, T.L.J.; Drahovsky, D. DNA Methylation Levels in Acute Human Leukemia. Cancer Lett. 1988, 39, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Celen, A.B.; Sahin, U. Sumoylation on Its 25th Anniversary: Mechanisms, Pathology, and Emerging Concepts. FEBS J. 2020, 287, 3110–3140. [Google Scholar] [CrossRef]
- Guo, D.; Li, M.; Zhang, Y.; Yang, P.; Eckenrode, S.; Hopkins, D.; Zheng, W.; Purohit, S.; Podolsky, R.H.; Muir, A. A Functional Variant of SUMO4, a New IκBα Modifier, Is Associated with Type 1 Diabetes. Nat. Genet. 2004, 36, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Bohren, K.M.; Nadkarni, V.; Song, J.H.; Gabbay, K.H.; Owerbach, D. A M55V Polymorphism in a Novel SUMO Gene (SUMO-4) Differentially Activates Heat Shock Transcription Factors and Is Associated with Susceptibility to Type I Diabetes Mellitus. J. Biol. Chem. 2004, 279, 27233–27238. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wansleeben, C.; Zhao, S.; Miao, P.; Paschen, W.; Yang, W. SUMO 2 Is Essential While SUMO 3 Is Dispensable for Mouse Embryonic Development. EMBO Rep. 2014, 15, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Gareau, J.R.; Lima, C.D. The SUMO Pathway: Emerging Mechanisms That Shape Specificity, Conjugation and Recognition. Nat. Rev. Mol. cell Biol. 2010, 11, 861–871. [Google Scholar] [CrossRef]
- Tatham, M.H.; Jaffray, E.; Vaughan, O.A.; Desterro, J.M.P.; Botting, C.H.; Naismith, J.H.; Hay, R.T. Polymeric Chains of SUMO-2 and SUMO-3 Are Conjugated to Protein Substrates by SAE1/SAE2 and Ubc9. J. Biol. Chem. 2001, 276, 35368–35374. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, I.A.; D’souza, R.C.J.; Yang, B.; Verlaan-de Vries, M.; Mann, M.; Vertegaal, A.C.O. Uncovering Global SUMOylation Signaling Networks in a Site-Specific Manner. Nat. Struct. Mol. Biol. 2014, 21, 927–936. [Google Scholar] [CrossRef]
- Sundvall, M. Role of Ubiquitin and SUMO in Intracellular Trafficking. Curr. Issues Mol. Biol. 2020, 35, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Vijay-Kumar, S.; Bugg, C.E.; Cook, W.J. Structure of Ubiquitin Refined at 1.8 Åresolution. J. Mol. Biol. 1987, 194, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Bayer, P.; Arndt, A.; Metzger, S.; Mahajan, R.; Melchior, F.; Jaenicke, R.; Becker, J. Structure Determination of the Small Ubiquitin-Related Modifier SUMO-1. J. Mol. Biol. 1998, 280, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Pichler, A.; Fatouros, C.; Lee, H.; Eisenhardt, N. SUMO Conjugation–a Mechanistic View. Biomol. Concepts 2017, 8, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Hutten, S.; Chachami, G.; Winter, U.; Melchior, F.; Lamond, A.I. A Role for the CB-Associated SUMO Isopeptidase USPL1 in RNAPII-Mediated SnRNA Transcription. J. Cell Sci. 2014, 127, 1065. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Flotho, A.; Melchior, F. The RanBP2/RanGAP1∗ SUMO1/Ubc9 Complex Is a Multisubunit SUMO E3 Ligase. Mol. Cell 2012, 46, 287–298. [Google Scholar] [CrossRef]
- Eisenhardt, N.; Chaugule, V.K.; Koidl, S.; Droescher, M.; Dogan, E.; Rettich, J.; Sutinen, P.; Imanishi, S.Y.; Hofmann, K.; Palvimo, J.J. A New Vertebrate SUMO Enzyme Family Reveals Insights into SUMO-Chain Assembly. Nat. Struct. Mol. Biol. 2015, 22, 959–967. [Google Scholar] [CrossRef]
- Cappadocia, L.; Pichler, A.; Lima, C.D. Structural Basis for Catalytic Activation by the Human ZNF451 SUMO E3 Ligase. Nat. Struct. Mol. Biol. 2015, 22, 968–975. [Google Scholar] [CrossRef]
- Boulanger, M.; Paolillo, R.; Piechaczyk, M.; Bossis, G. The SUMO Pathway in Hematomalignancies and Their Response to Therapies. Int. J. Mol. Sci. 2019, 20, 3895. [Google Scholar] [CrossRef] [PubMed]
- Stankovic-Valentin, N.; Melchior, F. Control of SUMO and Ubiquitin by ROS: Signaling and Disease Implications. Mol. Aspects Med. 2018, 63, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.; Schofield, P.; Barton, G.J.; Hay, R.T. Proteotoxic Stress Reprograms the Chromatin Landscape of SUMO Modification. Sci. Signal. 2015, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Gervais, C.; Dano, L.; Perrusson, N.; Helias, C.; Jeandidier, E.; Galoisy, A.C.; Ittel, A.; Herbrecht, R.; Bilger, K.; Mauvieux, L. A Translocation t (2; 8)(Q12; P11) Fuses FGFR1 to a Novel Partner Gene, RANBP2/NUP358, in a Myeloproliferative/Myelodysplastic Neoplasm. Leukemia 2013, 27, 1186–1188. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kang, S.Y.; Takeuchi, K.; Ko, Y.H. Identification of RANBP2–ALK Fusion in ALK Positive Diffuse Large B-cell Lymphoma. Hematol. Oncol. 2014, 32, 221–224. [Google Scholar] [CrossRef]
- Lim, J.-H.; Jang, S.; Park, C.-J.; Cho, Y.-U.; Lee, J.-H.; Lee, K.-H.; Lee, J.-O.; Shin, J.-Y.; Kim, J.-I.; Huh, J. RANBP2-ALK Fusion Combined with Monosomy 7 in Acute Myelomonocytic Leukemia. Cancer Genet. 2014, 207, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Bettermann, K.; Benesch, M.; Weis, S.; Haybaeck, J. SUMOylation in Carcinogenesis. Cancer Lett. 2012, 316, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ma, Y.; Li, Z.; Zhang, K.; Zheng, M.; Zhang, S. The Function of SUMOylation and Its Role in the Development of Cancer Cells under Stress Conditions: A Systematic Review. Stem Cells Int. 2020, 2020, 8835714. [Google Scholar] [CrossRef]
- Lee, J.; Chu, I.; Heo, J.; Calvisi, D.F.; Sun, Z.; Roskams, T.; Durnez, A.; Demetris, A.J.; Thorgeirsson, S.S. Classification and Prediction of Survival in Hepatocellular Carcinoma by Gene Expression Profiling. Hepatology 2004, 40, 667–676. [Google Scholar] [CrossRef]
- Chen, X.-L.; Wang, S.-F.; Liang, X.-T.; Liang, H.-X.; Wang, T.-T.; Wu, S.-Q.; Qiu, Z.-J.; Zhan, R.; Xu, Z.-S. SENP2 Exerts an Anti-Tumor Effect on Chronic Lymphocytic Leukemia Cells through the Inhibition of the Notch and NF-ΚB Signaling Pathways. Int. J. Oncol. 2018, 54, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Oskooei, V.K.; Ghafouri-Fard, S. Protein Inhibitor of Activated STAT Genes Are Differentially Expressed in Breast Tumor Tissues. Per. Med. 2019, 16, 277–285. [Google Scholar] [CrossRef]
- Tuccilli, C.; Baldini, E.; Sorrenti, S.; Di Gioia, C.; Bosco, D.; Ascoli, V.; Mian, C.; Barollo, S.; Rendina, R.; Coccaro, C. PAPILLARY THYROID CANCER IS CHARACTERIZED BY ALTERED EXPRESSION OF GENES INVOLVED IN THE SUMOYLATION PROCESS. J. Biol. Regul. Homeost. Agents 2015, 29, 655–662. [Google Scholar]
- Qian, J.; Luo, Y.; Gu, X.; Wang, X. Inhibition of SENP6-Induced Radiosensitization of Human Hepatocellular Carcinoma Cells by Blocking Radiation-Induced NF-ΚB Activation. Cancer Biother. Radiopharm. 2013, 28, 196–200. [Google Scholar] [CrossRef]
- Stefanska, B.; Cheishvili, D.; Suderman, M.; Arakelian, A.; Huang, J.; Hallett, M.; Han, Z.-G.; Al-Mahtab, M.; Akbar, S.M.F.; Khan, W.A. Genome-Wide Study of Hypomethylated and Induced Genes in Patients with Liver Cancer Unravels Novel Anticancer Targets. Clin. Cancer Res. 2014, 20, 3118–3132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Tan, X.; Zhao, A.; Zhu, L.; Yin, B.; Yuan, J.; Qiang, B.; Peng, X. MicroRNA-214-Mediated UBC9 Expression in Glioma. BMB Rep. 2012, 45, 641. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tang, Y.; Guo, W.; Du, Y.; Wang, Y.; Li, P.; Zang, W.; Yin, X.; Wang, H.; Chu, H. Up-Regulation of MicroRNA-138 Induce Radiosensitization in Lung Cancer Cells. Tumor Biol. 2014, 35, 6557–6565. [Google Scholar] [CrossRef]
- Zheng, C.; Li, J.; Wang, Q.; Liu, W.; Zhou, J.; Liu, R.; Zeng, Q.; Peng, X.; Huang, C.; Cao, P. MicroRNA-195 Functions as a Tumor Suppressor by Inhibiting CBX4 in Hepatocellular Carcinoma Retraction in/10.3892/or. 2021.8145. Oncol. Rep. 2015, 33, 1115–1122. [Google Scholar] [CrossRef]
- Wang, C.; Tao, W.; Ni, S.; Chen, Q.; Zhao, Z.; Ma, L.; Fu, Y.; Jiao, Z. Tumor-suppressive Micro RNA-145 Induces Growth Arrest by Targeting SENP 1 in Human Prostate Cancer Cells. Cancer Sci. 2015, 106, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, W.; Zhang, R.; Liu, P.; Ye, Y.; Yu, W.; Guo, X.; Yu, J. Cancer Exosome-Derived MiR-9 and MiR-181a Promote the Development of Early-Stage MDSCs via Interfering with SOCS3 and PIAS3 Respectively in Breast Cancer. Oncogene 2020, 39, 4681–4694. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The Clinical Role of the TME in Solid Cancer. Br. J. Cancer 2019, 120, 45–53. [Google Scholar] [CrossRef]
- Mastrogeorgiou, M.; Chatzikalil, E.; Theocharis, S.; Papoudou-Bai, A.; Péoc’h, M.; Mobarki, M.; Karpathiou, G. The Immune Microenvironment of Cancer of the Uterine Cervix. Histol. Histopathol. 2024, 39, 1245–1271. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Xie, Z.; Zhang, N.; Zhang, Y.; Xiao, D.; Liu, S.; Zhuang, W.; Li, L.; Tao, Y. Signaling Pathways in Cancer Metabolism: Mechanisms and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 196. [Google Scholar] [CrossRef]
- Hannoun, Z.; Maarifi, G.; Chelbi-Alix, M.K. The Implication of SUMO in Intrinsic and Innate Immunity. Cytokine Growth Factor Rev. 2016, 29, 3–16. [Google Scholar] [CrossRef]
- Desterro, J.M.P.; Rodriguez, M.S.; Hay, R.T. SUMO-1 Modification of IκBα Inhibits NF-ΚB Activation. Mol. Cell 1998, 2, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Sarkar, A.; Bonni, S. The SUMO System and TGFβ Signaling Interplay in Regulation of Epithelial-Mesenchymal Transition: Implications for Cancer Progression. Cancers 2018, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, H.; Zhao, W.; Fu, S.; Li, Y.; Ni, W.; Xin, Y.; Li, W.; Yang, C.; Bai, Y. SUMO1 Modification of Methyltransferase-like 3 Promotes Tumor Progression via Regulating Snail MRNA Homeostasis in Hepatocellular Carcinoma. Theranostics 2020, 10, 5671. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, X.; Yang, W.; Qi, Y.; Yu, H.; Wang, X.; Gong, Y.; Chen, Q.; Zhong, B.; Dai, L. The SUMOylation of TAB2 Mediated by TRIM60 Inhibits MAPK/NF-ΚB Activation and the Innate Immune Response. Cell. Mol. Immunol. 2021, 18, 1981–1994. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, C.; Lin, W.; Lin, C.; Han, D.; Xie, Q.; Lai, J.; Yang, C. Importation of Chloroplast Proteins under Heat Stress Is Facilitated by Their SUMO Conjugations. New Phytol. 2022, 235, 173–187. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Q.; Yang, W.; Mackensen, G.B.; Paschen, W. Moderate Hypothermia Induces Marked Increase in Levels and Nuclear Accumulation of SUMO 2/3-conjugated Proteins in Neurons. J. Neurochem. 2012, 123, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Keiten-Schmitz, J.; Wagner, K.; Piller, T.; Kaulich, M.; Alberti, S.; Müller, S. The Nuclear SUMO-Targeted Ubiquitin Quality Control Network Regulates the Dynamics of Cytoplasmic Stress Granules. Mol. Cell 2020, 79, 54–67. [Google Scholar] [CrossRef]
- Ferdaoussi, M.; Dai, X.; Jensen, M.V.; Wang, R.; Peterson, B.S.; Huang, C.; Ilkayeva, O.; Smith, N.; Miller, N.; Hajmrle, C. Isocitrate-to-SENP1 Signaling Amplifies Insulin Secretion and Rescues Dysfunctional β Cells. J. Clin. Investig. 2015, 125, 3847–3860. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhou, H.J.; Zhang, H.; Qin, L.; Hwa, J.; Yun, Z.; Ji, W.; Min, W. SENP1-Mediated NEMO DeSUMOylation in Adipocytes Limits Inflammatory Responses and Type-1 Diabetes Progression. Nat. Commun. 2015, 6, 8917. [Google Scholar] [CrossRef]
- Corces, M.R.; Buenrostro, J.D.; Wu, B.; Greenside, P.G.; Chan, S.M.; Koenig, J.L.; Snyder, M.P.; Pritchard, J.K.; Kundaje, A.; Greenleaf, W.J.; et al. Lineage-Specific and Single-Cell Chromatin Accessibility Charts Human Hematopoiesis and Leukemia Evolution. Nat. Genet. 2016, 48, 1193–1203. [Google Scholar] [CrossRef]
- Eriksson, A.; Lennartsson, A.; Lehmann, S. Epigenetic Aberrations in Acute Myeloid Leukemia: Early Key Events during Leukemogenesis. Exp. Hematol. 2015, 43, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.L.; Hassan, C.; Khunte, M.; Soldatenko, A.; Jong, Y.; Afshinnekoo, E.; Mason, C.E. Epigenetic Modifications in Acute Myeloid Leukemia: Prognosis, Treatment, and Heterogeneity. Front. Genet. 2019, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.P.; Shvedunova, M.; Akhtar, A. Epigenetic Regulators as the Gatekeepers of Hematopoiesis. Trends Genet. 2021, 37, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Lo-Coco, F.; Avvisati, G.; Vignetti, M.; Thiede, C.; Orlando, S.M.; Iacobelli, S.; Ferrara, F.; Fazi, P.; Cicconi, L.; Di Bona, E. Retinoic Acid and Arsenic Trioxide for Acute Promyelocytic Leukemia. N. Engl. J. Med. 2013, 369, 111–121. [Google Scholar] [CrossRef]
- Avgerinou, G.; Solomou, E.; Filippidou, M.; Perganti, F.; Roka, K.; Rigatou, E.; Katsibardi, K.; Glentis, S.; Vlachou, A.; Binenbaum, I.; et al. Chemotherapy-Free Approach with Arsenic Trioxide and All-Trans Retinoic Acid in Children with Acute Promyelocytic Leukemia. Leuk. Lymphoma 2024, 1–4. [Google Scholar] [CrossRef]
- Stahl, M.; Tallman, M.S. Acute Promyelocytic Leukemia (APL): Remaining Challenges towards a Cure for All. Leuk. Lymphoma 2019, 60, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-P.; Yang, L.-C.; Chen, Y.-Q.; Wan, W.-Q.; Zhou, D.-H.; Mai, H.-R.; Li, W.-L.; Yang, L.-H.; Lan, H.-K.; Chen, H.-Q. Long-Term Outcome of Children with Acute Promyelocytic Leukemia: A Randomized Study of Oral versus Intravenous Arsenic by SCCLG-APL Group. Blood Cancer J. 2023, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-F.; Lu, Y.; Wu, Q.; Lou, Y.-J.; Yang, M.; Xu, J.-Y.; Sun, C.-H.; Mao, L.-P.; Xu, G.-X.; Li, L. Oral Arsenic and Retinoic Acid for High-Risk Acute Promyelocytic Leukemia. J. Hematol. Oncol. 2022, 15, 148. [Google Scholar] [CrossRef]
- Ravandi, F.; Koumenis, I.; Johri, A.; Tallman, M.; Roboz, G.J.; Strickland, S.; Garcia-Manero, G.; Borthakur, G.; Naqvi, K.; Meyer, M. Oral Arsenic Trioxide ORH-2014 Pharmacokinetic and Safety Profile in Patients with Advanced Hematologic Disorders. Haematologica 2019, 105, 1567. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qian, J.; Yang, Y.; Gu, C. Novel Insights into the Impact of the SUMOylation Pathway in Hematological Malignancies (Review). Int. J. Oncol. 2021, 59, 1–16. [Google Scholar] [CrossRef]
- de Thé, H.; Lavau, C.; Marchio, A.; Chomienne, C.; Degos, L.; Dejean, A. The PML-RARα Fusion MRNA Generated by the t (15; 17) Translocation in Acute Promyelocytic Leukemia Encodes a Functionally Altered RAR. Cell 1991, 66, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Boddy, M.N.; Howe, K.; Etkin, L.D.; Solomon, E.; Freemont, P.S. PIC 1, a Novel Ubiquitin-like Protein Which Interacts with the PML Component of a Multiprotein Complex That Is Disrupted in Acute Promyelocytic Leukaemia. Oncogene 1996, 13, 971–982. [Google Scholar]
- Weis, K.; Rambaud, S.; Lavau, C.; Jansen, J.; Carvalho, T.; Carmo-Fonseca, M.; Lamond, A.; Dejean, A. Retinoic Acid Regulates Aberrant Nuclear Localization of PML-RARα in Acute Promyelocytic Leukemia Cells. Cell 1994, 76, 345–356. [Google Scholar] [CrossRef]
- Koken, M.H.; Puvion-Dutilleul, F.; Guillemin, M.C.; Viron, A.; Linares-Cruz, G.; Stuurman, N.; De Jong, L.; Szostecki, C.; Calvo, F.; Chomienne, C. The t (15; 17) Translocation Alters a Nuclear Body in a Retinoic Acid-reversible Fashion. EMBO J. 1994, 13, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.T.; Koken, M.; Romagne, O.; Barbey, S.; Bazarbachi, A.; Stadler, M.; Guillemin, M.C.; Degos, L.; Chomienne, C.; de The, H. PML Protein Expression in Hematopoietic and Acute Promyelocytic Leukemia Cells. Blood 1993, 82, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Müller, S.; Ronchetti, S.; Freemont, P.S.; Dejean, A.; Pandolfi, P.P. Role of SUMO-1–Modified PML in Nuclear Body Formation. Blood, J. Am. Soc. Hematol. 2000, 95, 2748–2752. [Google Scholar] [CrossRef]
- Sahin, U.; Ferhi, O.; Jeanne, M.; Benhenda, S.; Berthier, C.; Jollivet, F.; Niwa-Kawakita, M.; Faklaris, O.; Setterblad, N.; de Thé, H. Oxidative Stress–Induced Assembly of PML Nuclear Bodies Controls Sumoylation of Partner Proteins. J. Cell Biol. 2014, 204, 931–945. [Google Scholar] [CrossRef]
- Shen, T.H.; Lin, H.K.; Scaglioni, P.P.; Yung, T.M.; Pandolfi, P.P. The Mechanisms of PML-Nuclear Body Formation. Mol. Cell 2006, 24, 805. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Lee, C.-C.; Yao, Y.-L.; Lai, C.-C.; Schmitz, M.L.; Yang, W.-M. SUMO5, a Novel Poly-SUMO Isoform, Regulates PML Nuclear Bodies. Sci. Rep. 2016, 6, 26509. [Google Scholar] [CrossRef] [PubMed]
- Matunis, M.J.; Zhang, X.-D.; Ellis, N.A. SUMO: The Glue That Binds. Dev. Cell 2006, 11, 596–597. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhou, J.; Peres, L.; Riaucoux, F.; Honoré, N.; Kogan, S. A Sumoylation Site in PML/RARA Is Essential for Leukemic Transformation. Cancer Cell 2005, 7, 143–153. [Google Scholar] [CrossRef]
- Wojiski, S.; Guibal, F.C.; Kindler, T.; Lee, B.H.; Jesneck, J.L.; Fabian, A.; Tenen, D.G.; Gilliland, D.G. PML–RARα Initiates Leukemia by Conferring Properties of Self-Renewal to Committed Promyelocytic Progenitors. Leukemia 2009, 23, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Jeanne, M.; Lallemand-Breitenbach, V.; Ferhi, O.; Koken, M.; Le Bras, M.; Duffort, S.; Peres, L.; Berthier, C.; Soilihi, H.; Raught, B. PML/RARA Oxidation and Arsenic Binding Initiate the Antileukemia Response of As2O3. Cancer Cell 2010, 18, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Lallemand-Breitenbach, V.; Jeanne, M.; Benhenda, S.; Nasr, R.; Lei, M.; Peres, L.; Zhou, J.; Zhu, J.; Raught, B.; de Thé, H. Arsenic Degrades PML or PML–RARα through a SUMO-Triggered RNF4/Ubiquitin-Mediated Pathway. Nat. Cell Biol. 2008, 10, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Ablain, J.; Rice, K.; Soilihi, H.; De Reynies, A.; Minucci, S.; de Thé, H. Activation of a Promyelocytic Leukemia–Tumor Protein 53 Axis Underlies Acute Promyelocytic Leukemia Cure. Nat. Med. 2014, 20, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Breitman, T.R.; Collins, S.J.; Keene, B.R. Terminal Differentiation of Human Promyelocytic Leukemic Cells in Primary Culture in Response to Retinoic Acid. Blood 1981, 57, 1000–1004. [Google Scholar] [CrossRef]
- Ablain, J.; Leiva, M.; Peres, L.; Fonsart, J.; Anthony, E.; de Thé, H. Uncoupling RARA Transcriptional Activation and Degradation Clarifies the Bases for APL Response to Therapies. J. Exp. Med. 2013, 210, 647–653. [Google Scholar] [CrossRef]
- Fasci, D.; Anania, V.G.; Lill, J.R.; Salvesen, G.S. SUMO Deconjugation Is Required for Arsenic-Triggered Ubiquitylation of PML. Sci. Signal. 2015, 8, 56. [Google Scholar] [CrossRef]
- Ohlsson, E.; Schuster, M.B.; Hasemann, M.; Porse, B.T. The Multifaceted Functions of C/EBPα in Normal and Malignant Haematopoiesis. Leukemia 2016, 30, 767–775. [Google Scholar] [CrossRef]
- Geletu, M.; Balkhi, M.Y.; Peer Zada, A.A.; Christopeit, M.; Pulikkan, J.A.; Trivedi, A.K.; Tenen, D.G.; Behre, G. Target Proteins of C/EBPαp30 in AML: C/EBPαp30 Enhances Sumoylation of C/EBPαp42 via up-Regulation of Ubc9. Blood J. Am. Soc. Hematol. 2007, 110, 3301–3309. [Google Scholar] [CrossRef]
- Hankey, W.; Silver, M.; Sun, H.; Zibello, T.; Berliner, N.; Khanna-Gupta, A. Differential Effects of Sumoylation on the Activities of CCAAT Enhancer Binding Protein Alpha (C/EBPα) P42 versus P30 May Contribute in Part, to Aberrant C/EBPα Activity in Acute Leukemias. Hematol. Rep. 2011, 3, 5. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, F.-F.; Wu, D.-S.; Li, W.-J.; Zhan, H.-E.; Peng, M.-Y.; Fang, P.; Cao, P.-F.; Zhang, M.-M.; Zeng, H. SUMOylation of Insulin-like Growth Factor 1 Receptor, Promotes Proliferation in Acute Myeloid Leukemia. Cancer Lett. 2015, 357, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Chen, J. SUMOylation of SPRDM16 Promotes the Progression of Acute Myeloid Leukemia. BMC Cancer 2015, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nishikata, I.; Nakahata, S.; Saito, Y.; Kaneda, K.; Ichihara, E.; Yamakawa, N.; Morishita, K. Sumoylation of MEL1S at Lysine 568 and Its Interaction with CtBP Facilitates Its Repressor Activity and the Blockade of G-CSF-Induced Myeloid Differentiation. Oncogene 2011, 30, 4194–4207. [Google Scholar] [CrossRef]
- Li, X.L.; Arai, Y.; Harada, H.; Shima, Y.; Yoshida, H.; Rokudai, S.; Aikawa, Y.; Kimura, A.; Kitabayashi, I. Mutations of the HIPK2 Gene in Acute Myeloid Leukemia and Myelodysplastic Syndrome Impair AML1-and P53-Mediated Transcription. Oncogene 2007, 26, 7231–7239. [Google Scholar] [CrossRef] [PubMed]
- Sæther, T.; Pattabiraman, D.R.; Alm-Kristiansen, A.H.; Vogt-Kielland, L.T.; Gonda, T.J.; Gabrielsen, O.S. A Functional SUMO-Interacting Motif in the Transactivation Domain of c-Myb Regulates Its Myeloid Transforming Ability. Oncogene 2011, 30, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yu, S.; Zhu, D.; Huang, X.; Xu, Y.; Lao, Y.; Tian, Y.; Zhang, J.; Tang, Z.; Zhang, Z. HCINAP Regulates the DNA-Damage Response and Mediates the Resistance of Acute Myelocytic Leukemia Cells to Therapy. Nat. Commun. 2019, 10, 3812. [Google Scholar] [CrossRef]
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef]
- Mendez, L.M.; Posey, R.R.; Pandolfi, P.P. The Interplay between the Genetic and Immune Landscapes of AML: Mechanisms and Implications for Risk Stratification and Therapy. Front. Oncol. 2019, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Haindl, M.; Harasim, T.; Eick, D.; Muller, S. The Nucleolar SUMO-specific Protease SENP3 Reverses SUMO Modification of Nucleophosmin and Is Required for RRNA Processing. EMBO Rep. 2008, 9, 273–279. [Google Scholar] [CrossRef]
- Bossis, G.; Sarry, J.-E.; Kifagi, C.; Ristic, M.; Saland, E.; Vergez, F.; Salem, T.; Boutzen, H.; Baik, H.; Brockly, F. The ROS/SUMO Axis Contributes to the Response of Acute Myeloid Leukemia Cells to Chemotherapeutic Drugs. Cell Rep. 2014, 7, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, A.; Del Gaudio, N.; Conte, L.; Dell’Aversana, C.; Vermeulen, M.; de Thé, H.; Migliaccio, A.; Nebbioso, A.; Altucci, L. The HDAC Inhibitor SAHA Regulates CBX2 Stability via a SUMO-Triggered Ubiquitin-Mediated Pathway in Leukemia. Oncogene 2018, 37, 2559–2572. [Google Scholar] [CrossRef]
- Baik, H.; Boulanger, M.; Hosseini, M.; Kowalczyk, J.; Zaghdoudi, S.; Salem, T.; Sarry, J.-E.; Hicheri, Y.; Cartron, G.; Piechaczyk, M. Targeting the SUMO Pathway Primes All-Trans Retinoic Acid–Induced Differentiation of Nonpromyelocytic Acute Myeloid Leukemias. Cancer Res. 2018, 78, 2601–2613. [Google Scholar] [CrossRef]
- Kim, Y.S.; Keyser, S.G.L.; Schneekloth, J.S. Synthesis of 2′,3′,4′-Trihydroxyflavone (2-D08), an Inhibitor of Protein Sumoylation. Bioorg. Med. Chem. Lett. 2014, 24, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, B.-R.; Dao, T.T.P.; Kim, J.-M.; Kim, Y.-J.; Son, H.; Jo, S.; Kim, D.; Kim, J.; Suh, Y.J.; et al. TAK-981, a SUMOylation Inhibitor, Suppresses AML Growth Immune-Independently. Blood Adv. 2023, 7, 3155–3168. [Google Scholar] [CrossRef]
- Lightcap, E.S.; Yu, P.; Grossman, S.; Song, K.; Khattar, M.; Xega, K.; He, X.; Gavin, J.M.; Imaichi, H.; Garnsey, J.J. A Small-Molecule SUMOylation Inhibitor Activates Antitumor Immune Responses and Potentiates Immune Therapies in Preclinical Models. Sci. Transl. Med. 2021, 13, 7791. [Google Scholar] [CrossRef] [PubMed]
- Mohrbacher, A.M.; Yang, A.S.; Groshen, S.; Kummar, S.; Gutierrez, M.E.; Kang, M.H.; Tsao-Wei, D.; Reynolds, C.P.; Newman, E.M.; Maurer, B.J. Phase I Study of Fenretinide Delivered Intravenously in Patients with Relapsed or Refractory Hematologic Malignancies: A California Cancer Consortium Trial. Clin. Cancer Res. 2017, 23, 4550–4555. [Google Scholar] [CrossRef] [PubMed]
- Morad, S.A.F.; Davis, T.S.; Kester, M.; Loughran, T.P.; Cabot, M.C. Dynamics of Ceramide Generation and Metabolism in Response to Fenretinide—Diversity within and among Leukemia. Leuk. Res. 2015, 39, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Kroonen, J.S.; Wouters, A.K.; de Graaf, I.J.; Remst, D.F.G.; Kumar, S.; Wachsmann, T.L.A.; Teunisse, A.F.A.S.; Roelands, J.P.; de Miranda, N.F.C.C.; Griffioen, M.; et al. Targeting Epigenetic Regulation and Post-Translational Modification with 5-Aza-2’ Deoxycytidine and SUMO E1 Inhibition Augments T-Cell Receptor Therapy. J. Immunother. Cancer 2024, 12, e008654. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Chen, X.; Li, M.; Tan, J.; Zhang, Y.; Yuan, W.; Zhou, J.; Wang, G. 2-D08 as a SUMOylation Inhibitor Induced ROS Accumulation Mediates Apoptosis of Acute Myeloid Leukemia Cells Possibly through the DeSUMOylation of NOX2. Biochem. Biophys. Res. Commun. 2019, 513, 1063–1069. [Google Scholar] [CrossRef]
- He, X.; Riceberg, J.; Soucy, T.; Koenig, E.; Minissale, J.; Gallery, M.; Bernard, H.; Yang, X.; Liao, H.; Rabino, C.; et al. Probing the Roles of SUMOylation in Cancer Cell Biology by Using a Selective SAE Inhibitor. Nat. Chem. Biol. 2017, 13, 1164–1171. [Google Scholar] [CrossRef]
- Gabellier, L.; De Toledo, M.; Chakraborty, M.; Akl, D.; Hallal, R.; Aqrouq, M.; Buonocore, G.; Recasens-Zorzo, C.; Cartron, G.; Delort, A.; et al. SUMOylation Inhibitor TAK-981 (Subasumstat) Synergizes with 5-Azacytidine in Preclinical Models of Acute Myeloid Leukemia. Haematologica 2024, 109, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Minguez, P.; Parca, L.; Diella, F.; Mende, D.R.; Kumar, R.; Helmer-Citterich, M.; Gavin, A.C.; Van Noort, V.; Bork, P. Deciphering a Global Network of Functionally Associated Post-Translational Modifications. Mol. Syst. Biol. 2012, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fennell, K.A.; Vassiliadis, D.; Lam, E.Y.N.; Martelotto, L.G.; Balic, J.J.; Hollizeck, S.; Weber, T.S.; Semple, T.; Wang, Q.; Miles, D.C.; et al. Non-Genetic Determinants of Malignant Clonal Fitness at Single-Cell Resolution. Nature 2022, 601, 125–131. [Google Scholar] [CrossRef]
- Chatzikalil, E.; Stergiou, I.E.; Papadakos, S.P.; Konstantinidis, I.; Theocharis, S. The Clinical Relevance of the EPH/Ephrin Signaling Pathway in Pediatric Solid and Hematologic Malignancies. Int. J. Mol. Sci. 2024, 25, 3834. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Z.; Wang, X.; Zhao, X.; Sheng, Z.; Ye, Y.; He, G.; Zhou, L.; Zhu, H.; Xu, N. Small-Molecule Inhibitors Targeting Protein SUMOylation as Novel Anticancer Compounds. Mol. Pharmacol. 2018, 94, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Wild, N.; Kaiser, C.S.; Wunderlich, G.; Liebau, E.; Wrenger, C. Protein SUMOylation and Its Functional Role in Nuclear Receptor Control. Receptors 2024, 3, 408–424. [Google Scholar] [CrossRef]
- Kukkula, A.; Ojala, V.K.; Mendez, L.M.; Sistonen, L.; Elenius, K.; Sundvall, M. Therapeutic Potential of Targeting the SUMO Pathway in Cancer. Cancers 2021, 13, 4402. [Google Scholar] [CrossRef]
- Meulmeester, E.; Kunze, M.; Hsiao, H.H.; Urlaub, H.; Melchior, F. Mechanism and Consequences for Paralog-Specific Sumoylation of Ubiquitin-Specific Protease 25. Mol. Cell 2008, 30, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Fang, Y.; Wu, X.; Xu, T.; Hu, T.; Xu, Y.; Ma, P.; Wang, Q.; Shu, Y. The Emerging Roles of SUMOylation in the Tumor Microenvironment and Therapeutic Implications. Exp. Hematol. Oncol. 2023, 12, 58. [Google Scholar] [CrossRef]
- Gómez, S.; Castellano, G.; Mayol, G.; Suñol, M.; Queiros, A.; Bibikova, M.; Nazor, K.L.; Loring, J.F.; Lemos, I.; Rodríguez, E.; et al. DNA Methylation Fingerprint of Neuroblastoma Reveals New Biological and Clinical Insights. Epigenomics 2015, 7, 1137–1153. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, K.; Dowling, P.; Bazou, D.; O’Gorman, P. Current Methods of Post-Translational Modification Analysis and Their Applications in Blood Cancers. Cancers 2021, 13, 1930. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Chen, Y.; Xu, A.; Xiang, D.; Wang, W.; Du, W.; Huang, Y.; Zhang, X.; Cai, M.; Xia, Z.; et al. Deneddylation of PML/RARα Reconstructs Functional PML Nuclear Bodies via Orchestrating Phase Separation to Eradicate APL. Cell Death Differ. 2022, 29, 1654–1668. [Google Scholar] [CrossRef]
- Voisset, E.; Moravcsik, E.; Stratford, E.W.; Jaye, A.; Palgrave, C.J.; Hills, R.K.; Salomoni, P.; Kogan, S.C.; Solomon, E.; Grimwade, D. Pml Nuclear Body Disruption Cooperates in APL Pathogenesis and Impairs DNA Damage Repair Pathways in Mice. Blood 2018, 131, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, X.; Wu, W.; Chen, Z.; Meng, G. PML Nuclear Body Biogenesis, Carcinogenesis, and Targeted Therapy. Trends Cancer 2020, 6, 889–906. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric Molecules That Target Proteins to the Skp1–Cullin–F Box Complex for Ubiquitination and Degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.-C. Fusion Protein Linkers: Property, Design and Functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzikalil, E.; Arvanitakis, K.; Filippatos, F.; Diamantopoulos, P.T.; Koufakis, T.; Solomou, E.E. Diagnostic and Therapeutic Implications of the SUMOylation Pathway in Acute Myeloid Leukemia. Cancers 2025, 17, 631. https://doi.org/10.3390/cancers17040631
Chatzikalil E, Arvanitakis K, Filippatos F, Diamantopoulos PT, Koufakis T, Solomou EE. Diagnostic and Therapeutic Implications of the SUMOylation Pathway in Acute Myeloid Leukemia. Cancers. 2025; 17(4):631. https://doi.org/10.3390/cancers17040631
Chicago/Turabian StyleChatzikalil, Elena, Konstantinos Arvanitakis, Filippos Filippatos, Panagiotis T. Diamantopoulos, Theocharis Koufakis, and Elena E. Solomou. 2025. "Diagnostic and Therapeutic Implications of the SUMOylation Pathway in Acute Myeloid Leukemia" Cancers 17, no. 4: 631. https://doi.org/10.3390/cancers17040631
APA StyleChatzikalil, E., Arvanitakis, K., Filippatos, F., Diamantopoulos, P. T., Koufakis, T., & Solomou, E. E. (2025). Diagnostic and Therapeutic Implications of the SUMOylation Pathway in Acute Myeloid Leukemia. Cancers, 17(4), 631. https://doi.org/10.3390/cancers17040631







