1. Introduction
Brain metastases occur in approximately 20–30% of patients with advanced malignancies, representing one of the most common neurological complications in oncology [
1,
2,
3]. Despite major advances in diagnostics and systemic therapies, both the incidence and mortality associated with cancer continue to rise, with global projections indicating a further substantial increase in the coming decades [
4].
Surgical resection remains an important treatment option for carefully selected patients, particularly those with a solitary lesion, significant mass effect, or elevated intracranial pressure unresponsive to corticosteroids. In contrast, patients with multiple brain metastases rarely benefit from surgery, and their management often relies on radiotherapy or systemic treatments [
5]. Historically, whole-brain radiotherapy (WBRT) has been the standard of care for such patients, providing modest survival benefit but frequently resulting in neurocognitive decline—especially in memory and executive functions—as demonstrated in randomized trials [
6,
7,
8,
9]. Consequently, there has been a growing shift toward focal, less toxic approaches.
Stereotactic radiosurgery (SRS) has become a cornerstone of modern neuro-oncology, offering excellent local control with limited toxicity in appropriately selected patients [
10,
11]. However, treating multiple lesions remains technically challenging due to prolonged treatment times and increasing planning complexity, particularly in patients with limited neurological reserve.
Recent technological progress has enabled single-isocenter, multi-target (SIMT) radiosurgery using dynamic conformal arcs (DCA) on modern linear accelerators such as TrueBeam. This approach allows for simultaneous irradiation of multiple lesions in a single session, often completing treatment of ten or more metastases within thirty minutes while maintaining submillimeter accuracy through image-guided stereotactic immobilization [
12,
13]. Nevertheless, concerns persist regarding potential geometric uncertainties for lesions located farther from the isocenter, which may affect dose conformity and local control [
14].
In parallel with these technical innovations, the therapeutic landscape for brain metastases has expanded substantially. The introduction of molecularly targeted therapies and immune checkpoint inhibitors has markedly improved intracranial control in selected tumor subtypes such as non-small-cell lung cancer, breast cancer, and melanoma [
15,
16]. The synergistic potential between systemic therapy and focal irradiation has become an area of growing clinical and biological interest, as combined strategies may enhance both local and distant tumor response.
Moreover, several novel locoregional modalities are under active investigation. The phase III METIS trial (Mehta et al.) evaluated tumor treating fields (TTF) in patients with brain metastases from non-small-cell lung cancer, demonstrating feasibility and a potential delay in intracranial progression following SRS [
17]. Similarly, laser interstitial thermal therapy (LITT) has shown promising results for achieving local control in deep-seated or recurrent metastases not amenable to resection or re-irradiation [
18]. Furthermore, amplitude-modulated radiofrequency electromagnetic fields (AM RF EMF) represent a novel, noninvasive approach currently being explored for potential antiproliferative and signaling-modulating effects in cancer cells [
19].
These advances reflect a broader paradigm shift toward multimodal and biologically individualized management of brain metastases, where precision radiosurgery serves as both a definitive and complementary component of care. Against this background, the present retrospective study was designed to evaluate local control outcomes following single-isocenter dynamic conformal arc (DCA-SIMT) radiosurgery in patients with multiple brain metastases. In addition, we investigated the potential synergistic effect of concurrent or sequential immunotherapy and targeted therapy and identified treatment-planning parameters—such as conformity index (CI) thresholds and margin expansion—that may optimize plan quality, efficiency, and clinical effectiveness in contemporary SRS practice.
2. Materials and Methods
This study retrospectively evaluated patients with a total of 195 metastatic CNS lesions were treated at the Department of Neuro-Oncology and Radiosurgery, Franciszek Lukaszczyk Oncology Center in Bydgoszcz, Poland. All participants provided written informed consent for data collection prior to treatment, in accordance with the Central Nervous System Cancer Registry at the Oncology Center in Bydgoszcz. The study was approved by the Bioethics Committee of the Nicolaus Copernicus University in Toruń, Collegium Medicum in Bydgoszcz, Poland (KB720/2018, Approval Date: 27 November 2018). All procedures were performed in compliance with relevant ethical guidelines and regulations.
Patients were eligible for inclusion if they received stereotactic radiosurgery (SRS) using the Dynamic Conformal Arc—Single-Isocenter (DCA-SIMT) technique between August 2018 and September 2020. All consecutive patients treated within this time period who had follow-up MRI available for evaluation were included in the analysis. Patients for whom post-treatment MRI was not available were not considered for inclusion. Exclusion criteria included prior whole-brain radiotherapy (WBRT) or SRS to the same anatomical region, as well as cases involving postoperative irradiation of resected lesions. All patients had a baseline Karnofsky Performance Status (KPS) of at least 70.
Treatments were delivered using a Varian TrueBeam linear accelerator (LINAC), with patient positioning monitored by the ExacTrac system (Brainlab AG, Munich, Germany). Treatment planning was conducted using Brainlab Elements MultiMets SRS software [
20,
21].
Assessment of local control was performed based on contrast-enhanced MRI scans obtained 6 months after treatment, using a 1.5 Tesla MRI scanner (Siemens Healthineers, Erlangen, Germany). Lesion response was evaluated according to the Brain Metastases Response Assessment in Neuro-Oncology (BM-RANO) criteria by multidisciplinary tumor board. Local control was defined as a complete response, partial response, or stable disease. Radiation-Induced Contrast Enhancements (RICE) was diagnosed based on multiparametric magnetic resonance imaging, including diffusion-weighted imaging, perfusion sequences, and MR spectroscopy, as well as lesion dynamics consistent with the typical course of post-radiation necrosis.
Statistical analysis was performed using Statistica (version 13, TIBCO Software Inc., Palo Alto, CA, USA) and PQStat software (version 1.8.4, PQStat Software, Poznań, Poland). The Shapiro–Wilk test was used to assess normality of data distribution. For variables meeting assumptions of normality and equal variances, comparisons were made using the independent samples t-test. If assumptions were not met, the Mann–Whitney U test was used. Associations between categorical variables were examined using the chi-squared test, Fisher’s exact test, or the Fisher–Freeman–Halton test with the Benjamini–Hochberg correction for multiple comparisons. The odds ratio (OR) with 95% confidence intervals was calculated. A p-value < 0.05 was considered statistically significant.
3. Results
The patient characteristics are detailed in
Table 1. Local control was achieved in 93% of lesions treated with SRS. In 82% of cases, the response was classified as either complete or partial. A detailed description of treatment responses is presented in
Table 2. The median overall survival was 13.1 months, and the median follow-up duration was 12 months.
3.1. Clinical Factors
In the analysis of clinical factors influencing local control of metastatic lesions, no statistically significant association was found between age and lesion control at 6 months post-treatment (p = 0.640). Similarly, sex was not significantly associated with local control (Pearson’s chi-squared test, p = 0.878). However, histopathological subtype showed a significant relationship with treatment response (Fisher–Freeman–Halton test, p = 0.024), with better local control observed in patients with non-small-cell lung cancer.
3.2. GTV and PTV
The potential impact of GTV and PTV (expressed in cm3) on local control at 6 months post-treatment was analyzed. No statistically significant association was found for either PTV volume (p = 0.616) or GTV volume (p = 0.612), as assessed by the Mann–Whitney U test.
3.3. Conformity Index (CI)
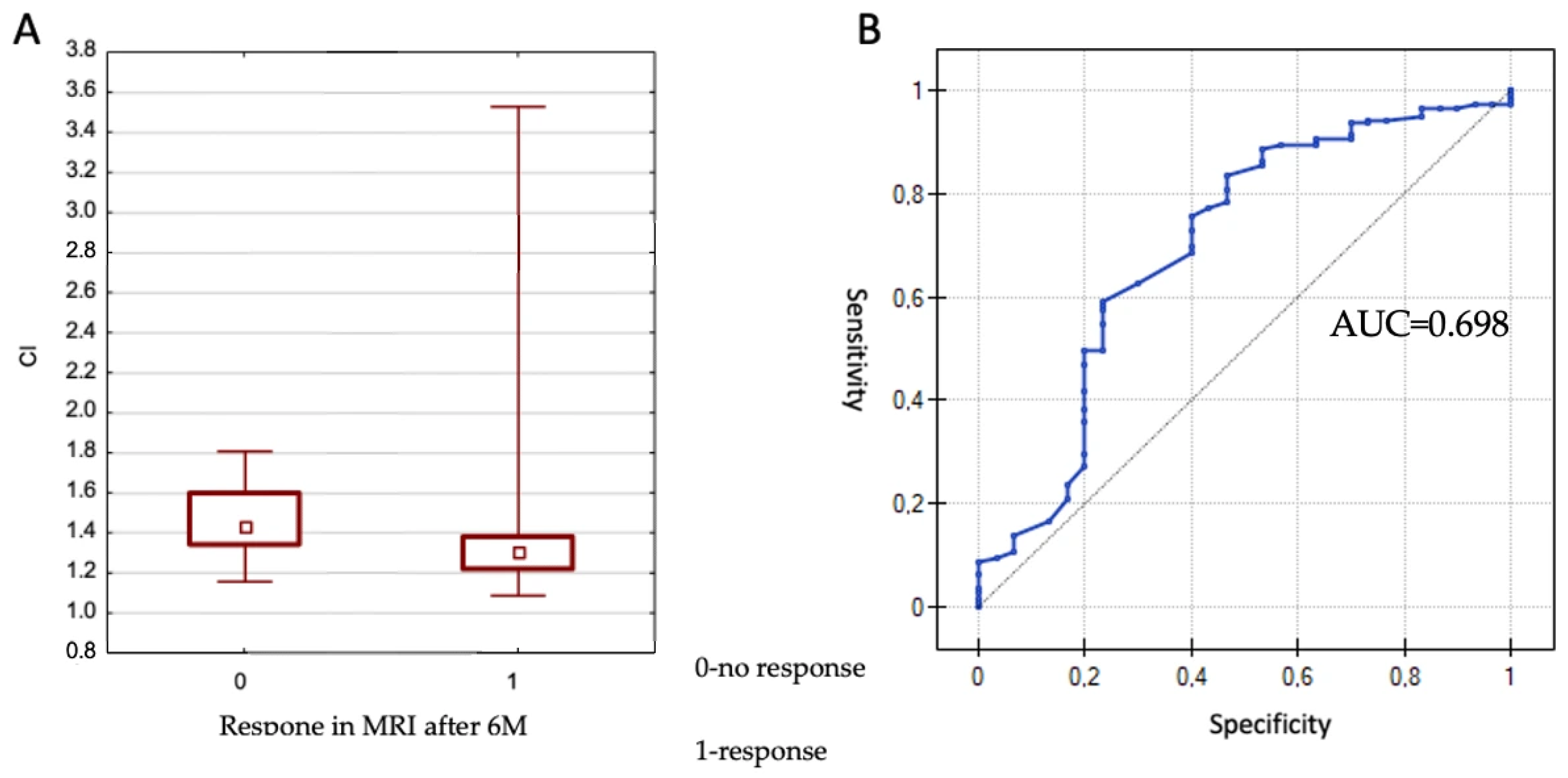
The CI (Conformity Index) values ranged from 1.09 to 2.97. The effect of CI on lesion control at 6 months was evaluated using the Mann–Whitney U test. No significant association was observed between CI and local control (
p = 0.697). However, a statistically significant association was found between CI and treatment response (partial or complete response only) at 6 months (
p = 0.001), as illustrated in
Figure 1.
Given the statistical significance, a receiver operating characteristic (ROC) curve was constructed to determine the optimal CI cut-off value differentiating between response and non-response. The area under the curve (AUC) was 0.698, which differed significantly from 0.5 (p = 0.00069). The proposed cut-off value was 1.42, corresponding to a sensitivity of 83.5% and a specificity of 53.3%. In summary, better treatment responses were observed in lesions with a CI < 1.42.
3.4. Gradient Index (GI)
The GI (Gradient Index) values ranged from 2.43 to 5.78. The influence of GI on lesion control at 6 months was analyzed using the independent samples t-test. No statistically significant association was found (p = 0.599).
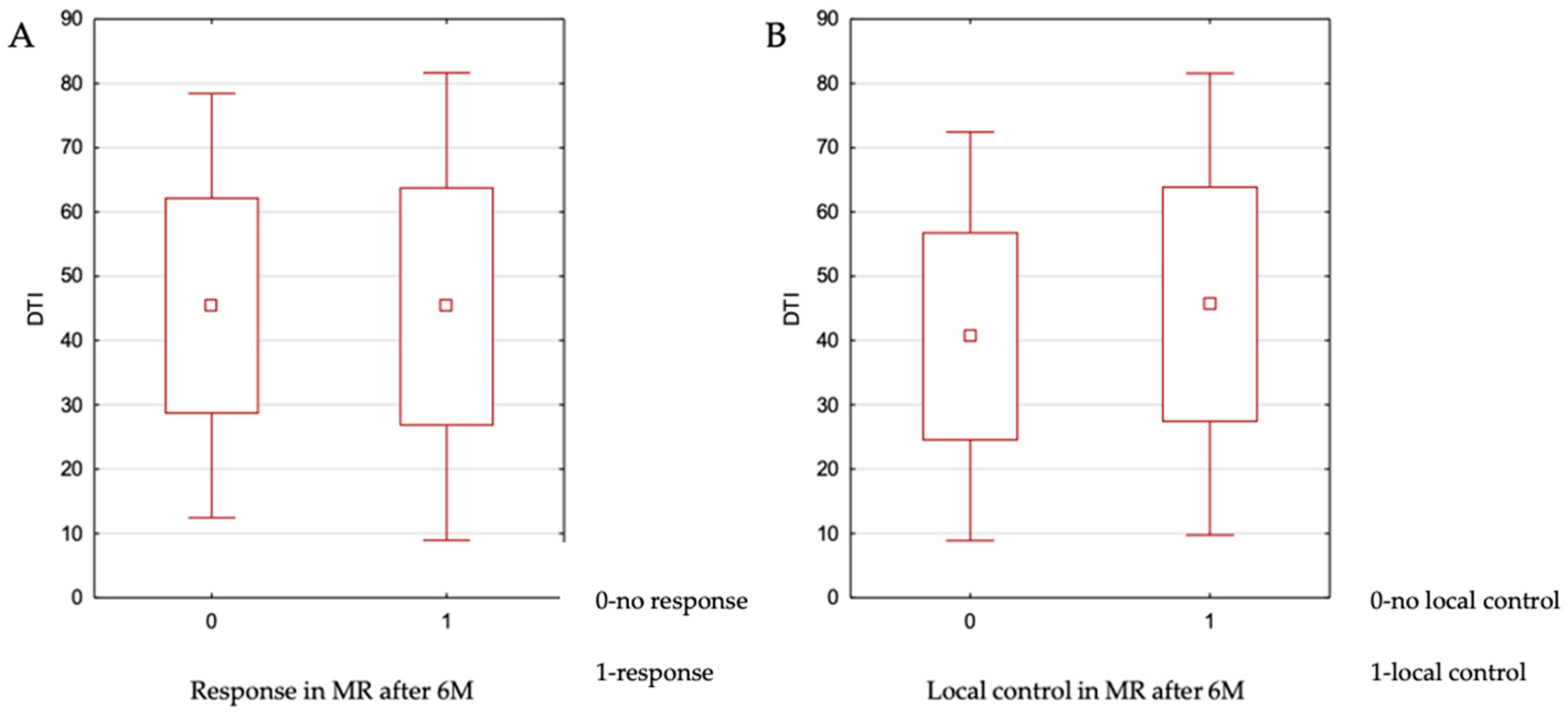
3.5. Distance to Isocenter (DTI)
The DTI parameter for the analyzed metastatic lesions ranged from 7 to 85 mm, with a median value of 45 mm. The impact of DTI on local control at 6 months was assessed using the independent samples
t-test. No statistically significant association was found (
p = 0.967). The relationship between these variables is illustrated in
Figure 2. Additionally, no significant relationship was observed between DTI and achieving a complete response versus partial response (
p = 0.704) or complete response versus stable disease (
p = 0.419).
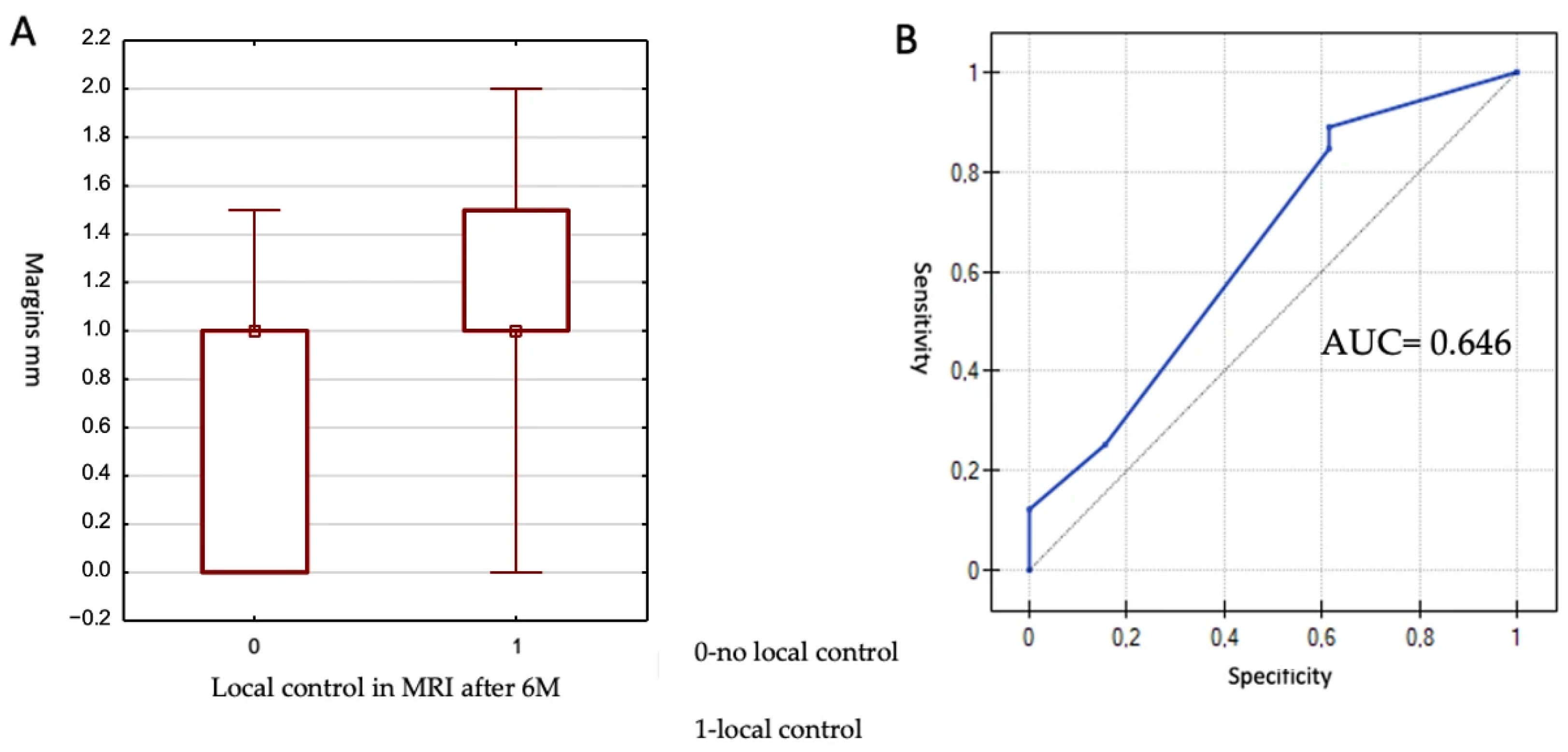
3.6. Margins
The smallest margin was 0 mm, and the largest was 2 mm, with a median of 1 mm. The Mann–Whitney U test was used for statistical analysis. A statistically significant impact of margin size on lesion control at 6 months was demonstrated (
p = 0.049). The results are presented in
Figure 3.
Due to the achievement of statistical significance, an attempt was made to determine a cut-off value for margin size associated with local control at 6 months. To this end, a receiver operating characteristic (ROC) curve was generated. The area under the ROC curve (AUC) was 0.646 and did not differ significantly from 0.5 (p = 0.079), indicating a borderline statistical significance. The proposed cut-off value was 0.5 mm, yielding a sensitivity of 89.0% and a specificity of 38.5%. In summary, lesions with a margin of at least 0.5 mm demonstrated better local control at 6 months post-treatment.
3.7. Dose
Doses ranging from 15 to 24 Gy in a single fraction were applied to the lesions. The specific dose used depended on the size and location of the tumor. The median dose administered was 20 Gy. The effect of the applied dose on lesion control at 6 months was analyzed. No statistically significant difference was observed (p = 0.360).
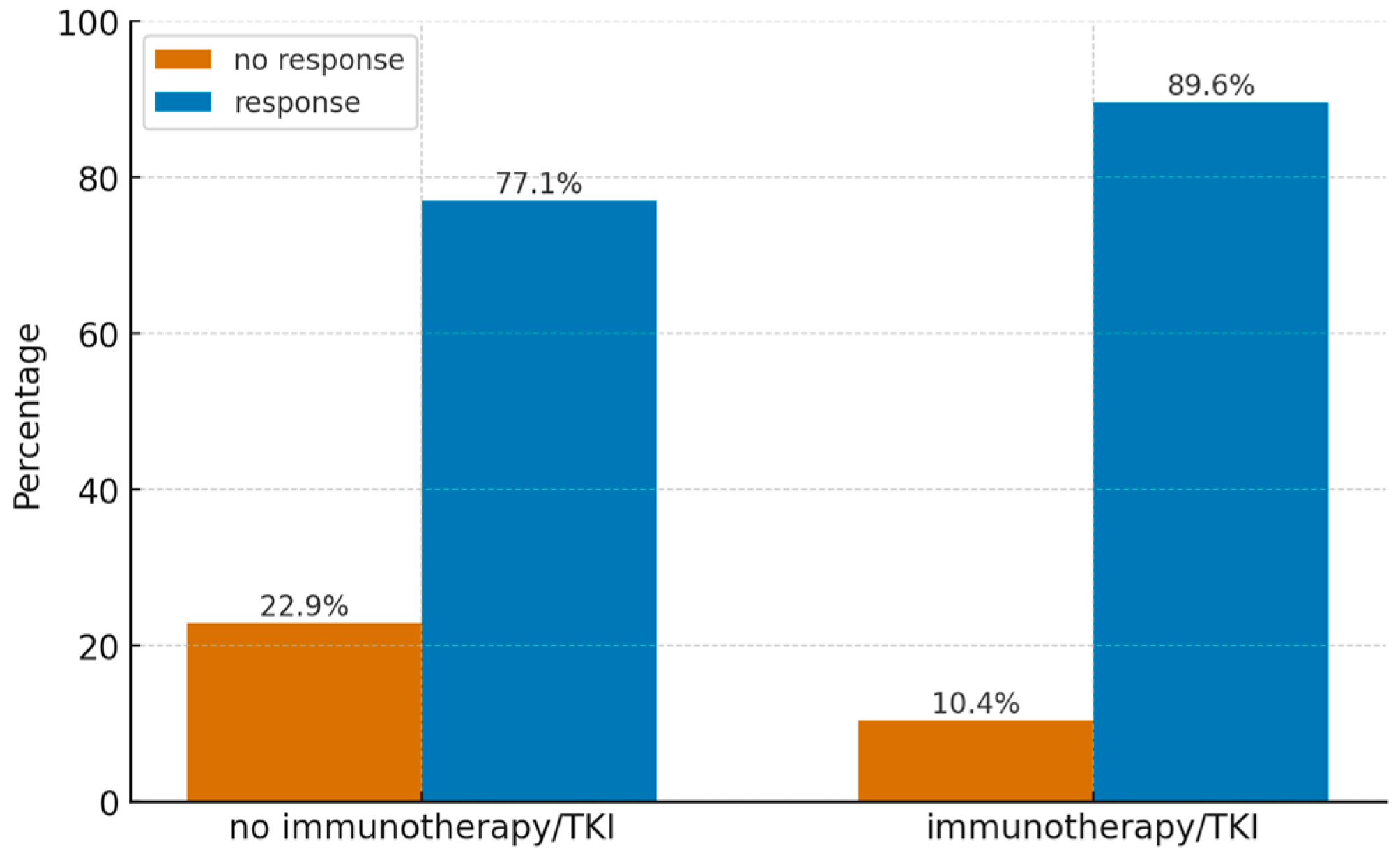
3.8. Effect of Immunotherapy or Targeted Therapy
In 13 patients (36%), immunotherapy or targeted therapy was administered within the first 4 months following stereotactic radiosurgery (SRS) for central nervous system (CNS) metastatic lesions. In all cases, immunotherapy or targeted therapy was administered sequentially, with systemic treatment initiated more than 2 weeks after SRS. The impact of systemic treatment on local control (defined as stabilization, partial response, or complete response) of the treated lesions was assessed. While no statistically significant association was confirmed, the result approached significance (
p = 0.066). A similar analysis was performed for lesion response at 6 months (partial or complete response only), which revealed a statistically significant difference (
p = 0.026). Data on the distribution of treatment response in patients with and without immunotherapy are presented in
Figure 4.
An asymptotic odds ratio was calculated, yielding a statistically significant value of p = 0.030 for an odds ratio of 2.55 (95%CI 1.10; 5.98) indicating that patients who received immunotherapy or targeted therapy had a 2.55-fold greater likelihood of achieving a response compared to those who did not.
The influence of histopathological diagnosis on this relationship was also examined, with a statistically significant result observed (p = 0.0001). Based on prior analyses, a statistically significant association was observed (p < 0.001), with lung adenocarcinoma demonstrating better local control compared to other histopathological categories.
4. Discussion
The rising incidence of brain metastases and the increasing number of lesions per patient underscore the need for efficient and accurate treatment strategies. The DCA-SIMT approach enables simultaneous irradiation of multiple targets within a short treatment time but raises concerns regarding geometric accuracy, particularly for lesions located far from the isocenter. In a retrospective study, Kraft et al. [
14] found that distance-to-isocenter (DTI) was not associated with local control, with one-year control rates exceeding 90% regardless of technique, while Aoki et al. [
22] similarly reported no significant relationship between DTI and local failure in patients treated with single-isocenter, multi-target radiosurgery. Consistent with these findings, our results showed no correlation between DTI and either local control or radiographic response in the present cohort; however, the safety of treating lesions with very high DTI values remains uncertain and warrants further clinical validation.
The conformity index (CI) emerged as a more relevant predictor. Similarly to the reports of Aiyama and Yamamoto [
23], we observed that lower CI values were associated with better local control. This is not surprising, as in clinical practice we strive to achieve lower CI values. However, when treating multiple small lesions with SIMT, high CI values are often encountered and are not optimal. An important practical implication of our analysis is the ability to define, within a large and representative cohort of lesions, a threshold CI value below which local control begins to decline. This finding may support treatment planning and plan acceptance processes, as it provides the opportunity to aim for a CI below the identified threshold in order to maintain therapeutic effectiveness. In contrast, the gradient index (GI) showed no association with local outcomes, consistent with previous studies.
Another modifiable factor is the margin expansion from GTV to PTV. While some centers omit margins altogether, particularly with Gamma Knife radiosurgery, linear accelerator-based approaches often incorporate 1–2 mm to account for intrafractional motion and image uncertainty. In our series, a margin of ≥0.5 mm appeared necessary to maintain local control, whereas larger margins (up to 2 mm) did not further improve outcomes and were not associated with an increased risk of radionecrosis. Kirkpatrick et al. [
24], however, evaluated margins beyond 3 mm and reported a clear correlation with higher radionecrosis rates. These findings suggest that a minimal but non-zero margin may offer the optimal balance between efficacy and safety. Importantly, this information may be particularly valuable for treatment-planning decisions, allowing clinicians to select a minimal margin while maintaining adequate tumor coverage and treatment effectiveness. This issue is particularly relevant in the context of DTI, as the absence of a margin may be associated with poorer local control. Further studies are needed to determine whether the margin should be a function of DTI, since such an approach is increasingly used in clinical practice. Our results, however, do not provide evidence that margins larger than 0.5 mm are required. Some planning systems offer automatic margin selection based on DTI, but it remains to be investigated whether escalation is justified or whether a range of 0–0.5 mm in relation to DTI is sufficient.
Dose prescription remains a cornerstone of brain metastasis radiosurgery. Several studies have consistently reported improved outcomes with doses ≥ 18–24 Gy [
25,
26,
27,
28]. In our analysis, however, dose did not significantly affect local control. This likely reflects the relatively narrow dose range applied (15–24 Gy, median 20 Gy) and the small number of lesions treated with <16 Gy, which may have limited the ability to detect a dose–response relationship. The lack of an observed dose–effect relationship may also be related to the timing of assessment, as local failures typically occur later than six months after treatment. Given the short survival in patients with multiple metastases, however, evaluation at this time point remains clinically relevant.
The integration of stereotactic radiosurgery (SRS) with systemic therapy, particularly immunotherapy and targeted agents, is of increasing clinical relevance. In our cohort, systemic treatment administered within four months of SRS was associated with improved radiographic response, consistent with previous reports [
29,
30]. This effect may reflect enhanced drug delivery due to blood–brain barrier disruption during radiosurgery. While the optimal sequencing of SRS and systemic therapy remains uncertain, current data favor concurrent or post-SRS administration. Importantly, no excess toxicity was observed in patients receiving immunotherapy within four months after SRS. Future treatment strategies and clinical research should be based on the synergistic interaction between SRS and systemic therapy. Further studies are needed to determine the optimal timing of drug administration relative to SRS, potential dose–response relationships, and how these factors influence both local and systemic disease control.
This study has several limitations that should be acknowledged. First, its retrospective design and lack of randomization may introduce selection bias. Second, the relatively short follow-up period may limit the detection of late local failures. Additionally, the results may be influenced by confounding factors such as histopathological subtype and the availability of modern systemic therapies. Finally, the cohort size did not allow a separate evaluation of individual immunotherapy and targeted therapy agents, which should be further investigated in larger future studies.
5. Conclusions
Our findings confirm the efficacy of SIMT-based stereotactic radiosurgery for patients with multiple brain metastases, while underscoring the importance of conformity, minimal but sufficient margin expansion, and integration with systemic therapy as key determinants of outcome. Further studies with longer follow up and larger lesion cohort may provide us further insights on DTI and margin correlation as well as concomitant immunotherapy and SRS details.
Author Contributions
Conceptualization, M.B.; methodology, M.B., I.Z.; validation, I.Z., M.H. and T.W.; formal analysis, M.B.; investigation, I.Z.; resources, T.W.; data curation, I.M.; writing—original draft preparation, M.B.; writing—review and editing, S.Z., M.H.; visualization, I.M.; supervision, M.H.; project administration, M.B.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Bioethics Committee of the Nicolaus Copernicus University in Toruń, Collegium Medicum in Bydgoszcz, Poland (protocol code KB720/2018, Approval Date: 27 November 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data supporting the findings of this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barnholtz-Sloan, J.S.; Sloan, A.E.; Davis, F.G.; Vigneau, F.D.; Lai, P.; Sawaya, R.E. Incidence Proportions of Brain Metastases in Patients Diagnosed (1973–2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004, 22, 2865–2872. [Google Scholar] [CrossRef]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of Brain Metastases. Curr. Oncol. Rep. 2011, 14, 48–54. [Google Scholar] [CrossRef]
- Sacks, P.; Rahman, M. Epidemiology of Brain Metastases. Neurosurg. Clin. N. Am. 2020, 31, 481–488. [Google Scholar] [CrossRef]
- Luo, Q.; Smith, D.P. Global Cancer Burden: Progress, Projections, and Challenges. Lancet 2025, 406, 1536–1537. [Google Scholar] [CrossRef]
- Ene, C.I.; Ferguson, S.D. Surgical Management of Brain Metastasis: Challenges and Nuances. Front. Oncol. 2022, 12, 847110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tong, X.; Wang, J. Management of Brain Metastases: History and the Present. Chin. Neurosurg. J. 2019, 5, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Trikhirhisthit, K.; Setakornnukul, J.; Thephamongkhol, K. Added Survival Benefit of Whole Brain Radiotherapy in Brain Metastatic Non-Small Cell Lung Cancer: Development and External Validation of an Individual Prediction Model. Front. Oncol. 2022, 12, 911835. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery with Whole Brain Radiation Therapy on Cognitive Function. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in Patients with Brain Metastases Treated with Radiosurgery or Radiosurgery plus WBRT. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- de la Peña, C.; Guajardo, J.H.; Gonzalez, M.F.; González, C.; Cruz, B. CyberKnife Stereotactic Radiosurgery in brain metastases: A report from Latin America with literature review. Rep. Pract. Oncol. Radiother. 2018, 23, 161–167. [Google Scholar] [CrossRef]
- Chen, W.C.; Baal, U.H.; Baal, J.D.; Pai, J.S.; Boreta, L.; Braunstein, S.E.; Raleigh, D.R. Efficacy and Safety of Stereotactic Radiosurgery for Brainstem Metastases: A Systematic Review and Meta-analysis. JAMA Oncol. 2021, 7, 1033–1040. [Google Scholar] [CrossRef]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.F.; Trasimeni, G.; Bozzao, A.; Romano, A.; Enrici, R.M. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011, 6, 48. [Google Scholar] [CrossRef]
- Huang, Y.; Chin, K.; Robbins, J.R.; Kim, J.; Li, H.; Amro, H.; Chetty, I.J.; Gordon, J.; Ryu, S. SIDCA for Multiple Brain Metastases. Radiother. Oncol. 2014, 112, 128–132. [Google Scholar] [CrossRef]
- Kraft, J.; van Timmeren, J.E.; Mayinger, M.; Frei, S.; Borsky, K.; Stark, L.S.; Krayenbuehl, J.; Zamburlini, M.; Guckenberger, M.; Tanadini-Lang, S.; et al. Distance to isocenter is not associated with an increased risk for local failure in LINAC-based single-isocenter SRS or SRT for multiple brain metastases. Radiother. Oncol. 2021, 159, 168–175. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Yang, T.J.; Beal, K.; Pan, H.; Brown, P.D.; Bangdiwala, A.; Shanley, R.; Yeh, N.; Gaspar, L.E.; Braunstein, S.; et al. Estimating Survival in Patients With Lung Cancer and Brain Metastases. JAMA Oncol. 2017, 3, 827–831. [Google Scholar] [CrossRef]
- Hendriks, L.E.L.; Henon, C.; Auclin, E.; Mezquita, L.; Ferrara, R.; Audigier-Valette, C.; Mazieres, J.; Lefebvre, C.; Rabeau, A.; Le Moulec, S.; et al. Outcome of Patients with Non–Small Cell Lung Cancer and Brain Metastases Treated with Checkpoint Inhib-itors. J. Thorac. Oncol. 2019, 14, 1244–1254. [Google Scholar] [CrossRef]
- Mehta, M.P.; Gondi, V.; Ahluwalia, M.S.; Roberge, D.; Ilic, R.; Sio, T.T.W.; Trifiletti, D.M.; Muanza, T.; Krpan, A.M.; Ramakrishna, N.R.; et al. Results from METIS (EF-25), an international, multicenter phase III randomized study evaluating the efficacy and safety of tumor treating fields (TTFields) therapy in NSCLC patients with brain metastases. J. Clin. Oncol. 2024, 42, 2008. [Google Scholar] [CrossRef]
- Srinivasan, E.S.; Grabowski, M.M.; Nahed, B.V.; Barnett, G.H.; Fecci, P.E. Laser interstitial thermal therapy for brain metastases. Neurooncol. Adv. 2021, 3, v16–v25. [Google Scholar] [CrossRef]
- Barbault, A.; Costa, F.P.; Bottger, B.; Munden, R.F.; Bomholt, F.; Kuster, N.; Pasche, B. Amplitude-modulated electromagnetic fields for the treatment of cancer: Discovery of tumor-specific frequencies and assessment of a novel therapeutic approach. J. Exp. Clin. Cancer Res. 2009, 28, 51. [Google Scholar] [CrossRef]
- Chea, M.; Fezzani, K.; Jacob, J.; Cuttat, M.; Croisé, M.; Simon, J.M.; Feuvret, L.; Valery, C.A.; Maingon, P.; Benadjaoud, M.A.; et al. Dosimetric study between a single isocenter dynamic conformal arc therapy technique and Gamma Knife radiosurgery for multiple brain metastases treatment: Impact of target volume geometrical characteristics. Radiat Oncol. 2021, 16, 45. [Google Scholar] [CrossRef]
- Minniti, G.; Capone, L.; Alongi, F.; Figlia, V.; Nardiello, B.; El Gawhary, R.; Scaringi, C.; Bianciardi, F.; Tolu, B.; Gentile, P.; et al. Initial Experience With Single-Isocenter Radiosurgery to Target Multiple Brain Metastases Using an Automated Treatment Planning Software: Clinical Outcomes and Optimal Target Volume Margins Strategy. Adv. Radiat. Oncol. 2020, 5, 856–864. [Google Scholar] [CrossRef]
- Aoki, K.; Nagatani, Y.; Noma, K.; Tsugawa, T.; Kono, N.; Kida, Y.; Harada, N.; Tanoue, Y.; Watanabe, Y. Effective Control of Brain Metastases Irrespective of Distance from Isocenter in Single-isocenter Multitarget Stereotactic Radiosurgery. Anticancer. Res. 2021, 41, 2575–2581. [Google Scholar] [CrossRef]
- Aiyama, H.; Yamamoto, M.; Kawabe, T.; Watanabe, S.; Koiso, T.; Sato, Y.; Higuchi, Y.; Ishikawa, E.; Yamamoto, T.; Matsumura, A.; et al. Clinical significance of conformity index and gradient index in patients undergoing stereotactic radiosurgery for a single metastatic tumor. J. Neurosurg. 2018, 129, 103–110. [Google Scholar] [CrossRef]
- Kirkpatrick, J.P.; Wang, Z.; Sampson, J.H.; McSherry, F.; Herndon, J.E.; Allen, K.J.; Duffy, E.; Hoang, J.K.; Chang, Z.; Yoo, D.S.; et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: Results of a randomized trial. Int. J. Radiat. Oncol. 2015, 91, 100–108. [Google Scholar] [CrossRef]
- Shiau, C.-Y.; Sneed, P.K.; Shu, H.-K.G.; Lamborn, K.R.; McDermott, M.W.; Chang, S.; Nowak, P.; Petti, P.L.; Smith, V.; Verhey, L.J.; et al. Radiosurgery for brain metastases: Relationship of dose and pattern of enhancement to local control. Int. J. Radiat. Oncol. 1997, 37, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, R.; Wiggenraad, R.; van der Kanter, A.; Walchenbach, R.; Vecht, C. Relationship between volume, dose and local control in stereotactic radiosurgery of brain metastasis. Br. J. Neurosurg. 2009, 23, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.M.; Schroeder, J.L.; Angelov, L.; Chao, S.T.; Murphy, E.S.; Yu, J.S.; Neyman, G.; Jia, X.; Suh, J.H.; Barnett, G.H.; et al. Impact of the radiosurgery prescription dose on the local control of small (2 cm or smaller) brain metastases. J. Neurosurg. 2017, 126, 735–743. [Google Scholar] [CrossRef]
- Abdulhaleem, M.; Johnston, H.; D’Agostino, R.; Lanier, C.; LeCompte, M.; Cramer, C.K.; Ruiz, J.; Lycan, T.; Lo, H.-W.; Watabe, K.; et al. Local control outcomes for combination of stereotactic radiosurgery and immunotherapy for non-small cell lung cancer brain metastases. J. Neurooncol. 2022, 157, 101–107. [Google Scholar] [CrossRef]
- Chen, L.; Douglass, J.; Kleinberg, L.; Ye, X.; Marciscano, A.E.; Forde, P.M.; Brahmer, J.; Lipson, E.; Sharfman, W.; Hammers, H.; et al. Concurrent Immune Checkpoint Inhibitors and Stereotactic Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer, Melanoma, and Renal Cell Carcinoma. Int. J. Radiat. Oncol. 2018, 100, 916–925. [Google Scholar] [CrossRef]
- Yomo, S.; Serizawa, T.; Yamamoto, M.; Higuchi, Y.; Sato, Y.; Shuto, T.; Akabane, A.; Jokura, H.; Kawagishi, J.; Aoyama, H. The impact of EGFR-TKI use on clinical outcomes of lung adenocarcinoma patients with brain metastases after Gamma Knife radiosurgery: A propensity score-matched analysis based on extended JLGK0901 dataset (JLGK0901-EGFR-TKI). J. Neurooncol. 2019, 145, 151–157. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).