Is Nutritional Ultrasound as Useful and Accurate as Computed Tomography to Assess Sarcopenia in Cancer Patients? A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Selection Criteria
2.3. Search Strategy
2.4. Study Selection and Data Collection
2.5. Assessment of Risk of Bias in Every Selected Study
3. Results
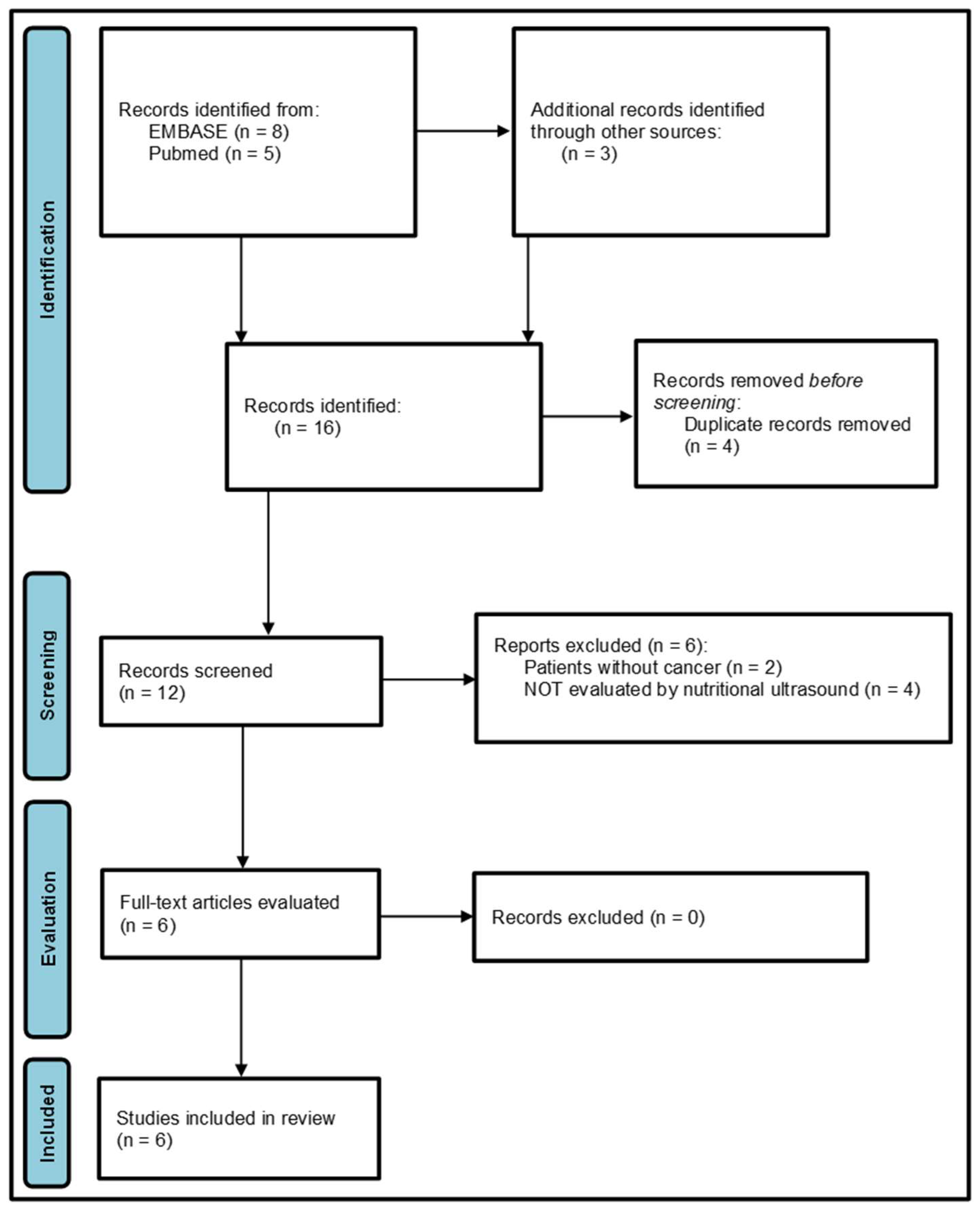
3.1. Study Selection
3.2. Study Characteristics
| Reference (Country) | Study Design | Method | Sample Size (F/M, Age, BMI, Stage/Type) | Sarcopenia (Low Muscle Mass) | Correlation US vs. CT | Risk of Bias 1 |
|---|---|---|---|---|---|---|
| Sousa IM et al., 2025 (Brazil) [22] | Secondary cross-sectional analysis of cohort studies with prospective data collection | CT-CSA vs. US BMT &/or TMT | 120 hospitalized patients with cancer (53.3% female, age 62 (55–70), 40.9% overweight, 65.8% digestive, 85.8% stages III-IV) | CT: low CSA 49.2% US: low BMT 30.8%, low TMT 18.3%, low BMT + TMT in 48.3% (34% sarcopenia) Female, all lower predictive accuracy (AUC = 0.50). Male, TMT highest accuracy (AUC = 0.78), combined BMT + TMT (AUC = 0.76) | TMT and BMT + TMT vs. CSA (R2 = 0.35) *; +accuracy (AUC > 0.70); moderate agreement w. CSA (k = 0.48) | Moderate |
| Jiménez-Sánchez A et al., 2024 (Spain) [8] | Cross-sectional | CT-SMA at L3 vs. RF-CSA/RF-MT/QMT | 156 consecutive colorectal outputs (51.9% male, age 65.2 (SD 13.6), overweight 62.9%, IIIB 20.8%) | CT: 1 vs. 4/156 (Van Vogt vs. Dolan)/US: 2 vs. 0/156 (RF-CSA vs. RF-MT)//Muscle atrophy CT: 10 vs. 76/156/US: 13 vs. 16/156, respect. | Muscle atrophy. CT (Van Vogt) vs. US-MT/CSA k = 0.165 */k = 0.109 (ns) | Moderate |
| de Lellis J et al., 2025 (Brazil) [13] | Prospective cohort study | QMT (2/3, VALIDUM method, +/− compression) vs. L3 CT-SMMI | 88 patients from oncological intensive unit (male 54.5%, age 60.6 (SD 13.0), BMI 25.1 (SD 6.3), 47.7% digestive, TNM NR) | CT (Toledo threshold): 63.6% vs. US. QMT AUC 0.706 (p < 0.001)/0.667 (p = 0.005) (2/3 +/− compression) Similar at 1/2 | CT vs. US-QMT (2/3 compression) ≤1.29 cm: PPV 85.4%, NPV 62.5%, agreement 75% QMT ≤ 1.29 cm more likely lower CT-SMMI (OR 0.96, 95% CI: 0.92–0.99) * | Low |
| Guirado-Peláez P et al., 2024 (Spain) [31] | Retrospective cross-sectional study | CT-SMI (L3) vs. US-RF-CSA | 267 colorectal cancer patients (male 61.8%, age 68.2 (SD 10.9), BMI 26.8 (SD 4.93), III-IV 39.7%) | Low SMI: Martin’s criteria, 43.8%/Prado’s criteria, 49.8% low SMI” | CT-SMI (L3) vs. US-RF-CSA: r = 0.56 (p < 0.001) | Moderate |
| González-Bollos M et al., 2024 (Spain) [28] | Retrospective cross-sectional study | CT-SMM/SMI and ASMM vs. US-RF-CSA | 43 oncological surgery patients (post-surgery 65.1%), (male 72.1%, age 64.7 (SD 6.7), BMI 23.7 (SD 4.31), 67.5% digestive, TNM NR) | 14/32 (CT) (11 patients without HGS) | CT-SMI vs. US-RF-CSA (cutoff 3.6 cm2): AUC 0.770, sensibility 70%, specificity 100%, r = 0.700 * CT-ASMM vs. US-RF-CSA (cutoff 3.29 cm2), AUC 0.609, sensibility 42.55%, specificity 100%, r = 0.548 * | High |
| López-Gómez et al., 2025 (Spain) [23] | Cross-sectional observational | US: CSA, Mi and FATi vs. CT: SMA, LMA and SM-HU | 337 oncology patients on treatment (58.8% male, age 69.7 (SD 10.9), BMI 23.69 (SD 4.62), 77.4% digestive, TNM NR) | Sarcopenia 8%, low muscle mass 23.7%, dynapenia 34.7%, malnutrition 78.3% | US RF-CSA vs. CT SMA and LMA r = 0.44 and 0.47 (p < 0.01) US RFT vs. CT SMA & LMA r = 0.43 and 0.43 (p < 0.01) | Moderate |
3.3. Risk of Bias Assessment
3.4. Synthesis of Results
3.4.1. Low Muscle Mass Prevalence and Cutoff Values
- BMT: 16 cm male, 12 cm female. Prevalence: 30.8%;
- TMT: 20 cm male, 15 cm female. Prevalence: 18.3%;
- TMT + BMT: 36 cm male, 43 cm female. Prevalence 48.3%. Closest to CT-CSA index.
3.4.2. Correlation Nutritional Ultrasound vs. Computed Tomography
4. Discussion
4.1. Summary of Evidence
4.2. Strengths and Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| * | Statistically significant |
| ASMM | Appendicular skeletal muscle mass |
| AUC | Area under the curve |
| BIA | Bioelectrical impedance analysis |
| BMT | Biceps muscle thickness |
| CSA | Cross-sectional area |
| CT | Computed tomography |
| DXA | Dual X-ray absorptiometry |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| FATi | ROI fat percentage |
| LMA | Lean muscle area |
| Mi | ROI muscle percentage |
| MT | Muscle thickness |
| NIH | National Institutes of Health |
| NPA | Negative percent agreement |
| NPV | Negative predictive value |
| NR | Not reported |
| n.s. | Not significant |
| NU | Nutritional ultrasound |
| OSF | Open Science Framework |
| PPV | Positive predictive value |
| PRISMA | Preferred reporting items for systematic reviews and meta-analyses |
| QMT | Quadriceps muscle thickness |
| RF | Rectus femoris |
| RFT | Rectus femoris thickness |
| ROI | Region of interest |
| SD | Standard deviation |
| SMA | Skeletal muscle area |
| SM-HU | Skeletal muscle-Hounsfield units |
| SMI | Skeletal muscle index |
| SMM | Skeletal muscle mass |
| SMMI | Skeletal muscle mass index |
| TMT | Thigh muscle thickness |
| US | Ultrasound |
References
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition: A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Laviano, A.; Koverech, A.; Seelaender, M. Assessing pathophysiology of cancer anorexia. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31, Erratum in Age Ageing 2019, 48, 601. https://doi.org/10.1093/ageing/afz046. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, A.; Soriano-Redondo, M.E.; Roque-Cuéllar, M.D.C.; García-Rey, S.; Valladares-Ayerbes, M.; Pereira-Cunill, J.L.; García-Luna, P.P. Muscle biomarkers in colorectal cancer outpatients: Agreement between computed tomography, bioelectrical impedance analysis, and nutritional ultrasound. Nutrients 2024, 16, 4312. [Google Scholar] [CrossRef]
- Barazzoni, R.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- van Vugt, J.L.A.; Buettner, S.; Levolger, S.; Coebergh-van den Braak, R.R.J.; Suker, M.; Gaspersz, M.P.; de Bruin, R.W.F.; Verhoef, C.; van Eijck, C.H.C.; Bossche, N.; et al. Low skeletal muscle mass is associated with increased hospital expenditure in patients undergoing cancer surgery of the alimentary tract. PLoS ONE 2017, 12, e0186547. [Google Scholar] [CrossRef]
- de Lellis, J.Z.; Stamato-Oliveira, F.J.; Toloi, J.M.; de Oliveira-Medeiros, I.A.; Silva, J.M., Jr. Screening for low muscularity and sarcopenia risk using thigh ultrasound in critically ill oncology patients: A prospective cohort study. Clin. Nutr. ESPEN 2025, 68, 160–167. [Google Scholar] [CrossRef]
- Sánchez-Torralvo, F.J.; Alonso-Gallardo, S.P.; García-Olivares, M. La masa muscular: Métodos de valoración e importancia actual. Nutr. Clín. Med. 2021, 15, 100–108. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Jiang, Y.; Su, N.; Li, J.; Kang, L.; Zhang, Y.; Yang, M. Evaluation of Appendicular Muscle Mass in Sarcopenia in Older Adults Using Ultrasonography: A Systematic Review and Meta-Analysis. Gerontology 2022, 68, 1174–1198. [Google Scholar] [CrossRef]
- Nies, I.; Ackermans, L.L.G.C.; Poeze, M.; Blokhuis, T.J.; Ten-Bosch, J.A. The Diagnostic Value of Ultrasound of the Rectus Femoris for the diagnosis of Sarcopenia in adults: A systematic review. Injury 2022, 53 (Suppl. S3), S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Rustani, K.; Kundisova, L.; Capecchi, P.L.; Nante, N.; Bicchi, M. Ultrasound measurement of rectus femoris muscle thickness as a quick screening test for sarcopenia assessment. Arch. Gerontol. Geriatr. 2019, 83, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Wang, L.; Zhang, W.; Lu, J.; Yang, M. Diagnostic test accuracy of ultrasound for sarcopenia diagnosis: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 57–70. [Google Scholar] [CrossRef] [PubMed]
- de Luis-Roman, D.; García-Almeida, J.M.; Bellido-Guerrero, D.; Guzmán-Rolo, G.; Martín, A.; Primo-Martín, D.; García-Delgado, Y.; Guirado-Peláez, P.; Palmas, F.; Tejera-Pérez, C.; et al. Ultrasound Cut-Off Values for Rectus Femoris for Detecting Sarcopenia in Patients with Nutritional Risk. Nutrients 2024, 16, 1552. [Google Scholar] [CrossRef]
- Vongchaiudomchoke, W.; Cho, A.R.; Mahmoud, I.; Carli, F. Ultrasound for skeletal muscle assessment in surgical oncology: A scoping review. Eur. J. Surg. Oncol. 2025, 51, 109676. [Google Scholar] [CrossRef]
- Casey, P.; Alasmar, M.; McLaughlin, J.; Ang, Y.; McPhee, J.; Heire, P.; Sultan, J. The current use of ultrasound to measure skeletal muscle and its ability to predict clinical outcomes: A systematic review. J. Cachexia Sarcopenia Muscle 2022, 13, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Sousa, I.M.; Pereira, J.P.D.C.; Rüegg, R.A.B.; Calado, G.C.F.; Xavier, J.G.; Bennemann, N.A.; do Nascimento, M.K.; Fayh, A.P.T. Comparing A-mode ultrasound and computed tomography for assessing cancer-related sarcopenia: A cross-sectional study. Nutr. Clin. Pract. 2025, 40, 699–708. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, J.J.; Sánchez-Lite, I.; Fernández-Velasco, P.; Izaola-Jauregui, O.; Cebriá, Á.; Pérez-López, P.; González-Gutiérrez, J.; Estévez-Asensio, L.; Primo-Martín, D.; Gómez-Hoyos, E.; et al. Artificial intelligence–assisted rectus femoris ultrasound vs. L3 computed tomography for sarcopenia assessment in oncology patients: Establishing diagnostic cut-offs for muscle mass and quality. Front. Nutr. 2025, 12, 1678989. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Messina, C.; Vitale, J.; Sconfienza, L.M. Imaging of sarcopenia: Old evidence and new insights. Eur. Radiol. 2020, 30, 2199–2208. [Google Scholar] [CrossRef]
- Vangelov, B.; Bauer, J.; Kotevski, D.; Smee, R.I. The use of alternate vertebral levels to L3 in computed tomography scans for skeletal muscle mass evaluation and sarcopenia assessment in patients with cancer: A systematic review. Br. J. Nutr. 2022, 127, 722–735. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Gonzalez-Boillos, M.; Padilla-Segura, M.; Castro-De la Vega, I.; Martinez-Chiva, M.; Abellan-Galiana, P.; Maldonado-Castro, G.F.; Merchante-Alfaro, A.A.; Garcia-Almeida, J.M. Study of the Relationship Between the Area of the Rectus Femoris Measured by Ultrasound and Psoas Muscle Index (PMI) and Skeletal Muscle Index (SMI) Measured by Opportunistic CT in Oncological Surgical Patients. preprints 2024. [Google Scholar]
- National Institutes of Health. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 5 September 2025).
- Campani, F.; Li-Cavoli, T.V.; Arena, U.; Marra, F.; Lynch, E.N.; Campani, C. Quick and easy assessment of sarcopenia in cirrhosis: Can ultrasound be the solution? World J. Gastroenterol. 2024, 30, 2287–2293. [Google Scholar] [CrossRef]
- Guirado-Peláez, P.; Fernández-Jiménez, R.; Sánchez-Torralvo, F.J.; Mucarzel-Suárez-Arana, F.; Palmas-Candia, F.X.; Vegas-Aguilar, I.; Amaya-Campos, M.D.M.; Martínez-Tamés, G.; Soria-Utrilla, V.; Tinahones-Madueño, F.; et al. Multiparametric Approach to the Colorectal Cancer Phenotypes Integrating Morphofunctional Assessment and Computer Tomography. Cancers 2024, 16, 3493. [Google Scholar] [CrossRef]
- Elkrief, L.; Houssel-Debry, P.; Ackermann, O.; Franchi-Abella, S.; Branchereau, S.; Valla, D.; Hillaire, S.; Dutheil, D.; Plessier, A.; Hernandez-Gea, V.; et al. Portal cavernoma or chronic non cirrhotic extrahepatic portal vein obstruction. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 491–496. [Google Scholar] [CrossRef]
- Jalal, M.; Campbell, J.A.; Wadsley, J.; Hopper, A.D. Computed Tomographic Sarcopenia in Pancreatic Cancer: Further Utilization to Plan Patient Management. J. Gastrointest. Cancer 2021, 52, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.; Kloth, C.; Theis, M.; Marinova, M.; Attenberger, U.I.; Sprinkart, A.M.; Luetkens, J.A. Deep learning-based assessment of CT markers of sarcopenia and myosteatosis for outcome assessment in patients with advanced pancreatic cancer after high-intensity focused ultrasound treatment. Eur. Radiol. 2024, 34, 279–286. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Kariyama, K.; Tada, T.; Tani, J.; Fukunishi, S.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; et al. Clinical importance of muscle volume in lenvatinib treatment for hepatocellular carcinoma: Analysis adjusted with inverse probability weighting. J. Gastroenterol. Hepatol. 2021, 36, 1812–1819. [Google Scholar] [CrossRef]
- Schulze-Hagen, M.; Truhn, D.; Duong, F.; Keil, S.; Pedersoli, F.; Kuhl, C.K.; Lurje, G.; Neumann, U.; Isfort, P.; Bruners, P.; et al. Correlation Between Sarcopenia and Growth Rate of the Future Liver Remnant After Portal Vein Embolization in Patients with Colorectal Liver Metastases. Cardiovasc. Intervent. Radiol. 2020, 43, 875–881. [Google Scholar] [CrossRef]
- Pedro da Costa-Pereira, J.; Santana-Costa, J.H.; Gomes de Miranda, B.L.; de Sousa-Rebouças, A.; Denise de Lima-Bezerra, A.; Gomes-Dantas-Lopes, M.M.; Trussardi-Fayh, A.P. Prognostic value of overhydration and bioelectrical impedance vector on short- and long-term outcomes in hospitalized patients with cancer. Clin. Nutr. 2024, 43, 756–764. [Google Scholar] [CrossRef]
- do Nascimento, M.K.; Costa-Pereira, J.P.D.; de Araújo, J.O.; Gonzalez, M.C.; Fayh, A.P.T. Exploring the role of body mass index-adjusted calf circumference within the SARC-CalF screening tool among older patients with cancer. J. Nutr. Health Aging 2024, 28, 100251. [Google Scholar] [CrossRef]
- Van Vugt, J.L.A.; Van Putten, Y.; Van Der Kall, I.M.; Buettner, S.; D’Ancona, F.C.H.; Dekker, H.M.; Kimenai, H.J.A.N.; De Bruin, R.W.F.; Warlé, M.C.; IJzermans, J.N.M. Estimated Skeletal Muscle Mass and Density Values Measured on Computed Tomography Examinations in over 1000 Living Kidney Donors. Eur. J. Clin. Nutr. 2019, 73, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.D.; Almasaudi, A.S.; Dieu, L.B.; Horgan, P.G.; McSorley, S.T.; McMillan, D.C. The Relationship between Computed Tomography-derived Body Composition, Systemic Inflammatory Response, and Survival in Patients Undergoing Surgery for Colorectal Cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Maeder, Y.; Kobayashi, K.; Schneider, M.; Koerfer, J.; Melloul, E.; Halkic, N.; Hübner, M.; Demartines, N.; Becce, F.; et al. Association between CT-Based Preoperative Sarcopenia and Outcomes in Patients That Underwent Liver Resections. Cancers 2022, 14, 261. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Toledo, D.O.; Carvalho, A.M.; Oliveira, A.M.R.R.; Toloi, J.M.; Silva, A.C.; Francisco de Mattos-Farah, J.; Prado, C.M.; Silva, J.M., Jr. The use of computed tomography images as a prognostic marker in critically ill cancer patients. Clin. Nutr. ESPEN 2018, 25, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Hertwig, A.; Hahn, R.; Anwar, M.; Siebenrock, T.; Pesta, M.; Liebau, K.; Timmermann, I.; Brugger, J.; Posch, M.; et al. Validation of Bedside Ultrasound to Predict Lumbar Muscle Area in the Computed Tomography in 200 Non-Critically Ill Patients: The USVALID Prospective Study. Clin. Nutr. 2022, 41, 829–837. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Bellido-Castañeda, V.; Bellido-Guerrero, D. Morphofunctional assessment of patient´s nutritional status: A global approach. Nutr. Hosp. 2021, 38, 592–600. (In English) [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Ballesteros-Pomar, M.D.; Cornejo-Pareja, I.M.; Fernández-Medina, B.; de Luis-Román, D.A.; Bellido-Guerrero, D.; Bretón-Lesmes, I.; Tinahones-Madueño, F.J. Nutritional ultrasound®: Conceptualisation, technical considerations and standardisation. Endocrinol. Diabetes Nutr. 2023, 70 (Suppl. S1), 74–84. [Google Scholar] [CrossRef]
- Prasad, M. Introduction to the GRADE tool for rating certainty in evidence and recommendations. Clin. Epidemiol. Glob. Health 2024, 25, 101484. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luengo-Pérez, L.M.; García-Lobato, C.; Lázaro-Martín, L.; Gallardo-Sánchez, J.D.; Guijarro-Chacón, M.M. Is Nutritional Ultrasound as Useful and Accurate as Computed Tomography to Assess Sarcopenia in Cancer Patients? A Systematic Review. Cancers 2025, 17, 3683. https://doi.org/10.3390/cancers17223683
Luengo-Pérez LM, García-Lobato C, Lázaro-Martín L, Gallardo-Sánchez JD, Guijarro-Chacón MM. Is Nutritional Ultrasound as Useful and Accurate as Computed Tomography to Assess Sarcopenia in Cancer Patients? A Systematic Review. Cancers. 2025; 17(22):3683. https://doi.org/10.3390/cancers17223683
Chicago/Turabian StyleLuengo-Pérez, Luis M., Claudia García-Lobato, Lucía Lázaro-Martín, Juan D. Gallardo-Sánchez, and Marta M. Guijarro-Chacón. 2025. "Is Nutritional Ultrasound as Useful and Accurate as Computed Tomography to Assess Sarcopenia in Cancer Patients? A Systematic Review" Cancers 17, no. 22: 3683. https://doi.org/10.3390/cancers17223683
APA StyleLuengo-Pérez, L. M., García-Lobato, C., Lázaro-Martín, L., Gallardo-Sánchez, J. D., & Guijarro-Chacón, M. M. (2025). Is Nutritional Ultrasound as Useful and Accurate as Computed Tomography to Assess Sarcopenia in Cancer Patients? A Systematic Review. Cancers, 17(22), 3683. https://doi.org/10.3390/cancers17223683






