A Comparative Study of Clinical and Molecular Features of Microsatellite Stable Colorectal Cancer With and Without Liver Metastases
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population and Data Collection
2.2. Statistical Analysis
2.3. Molecular Testing
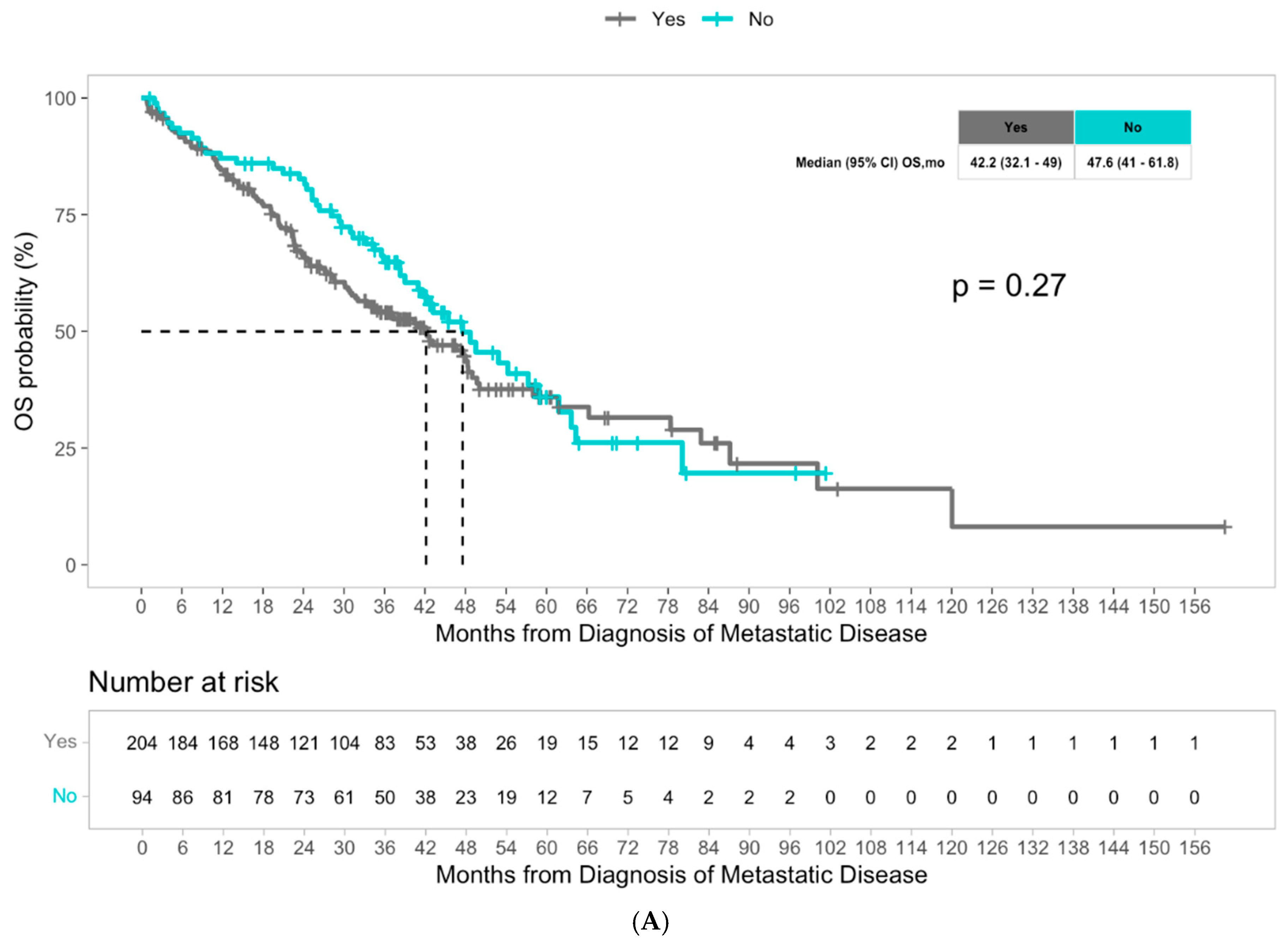
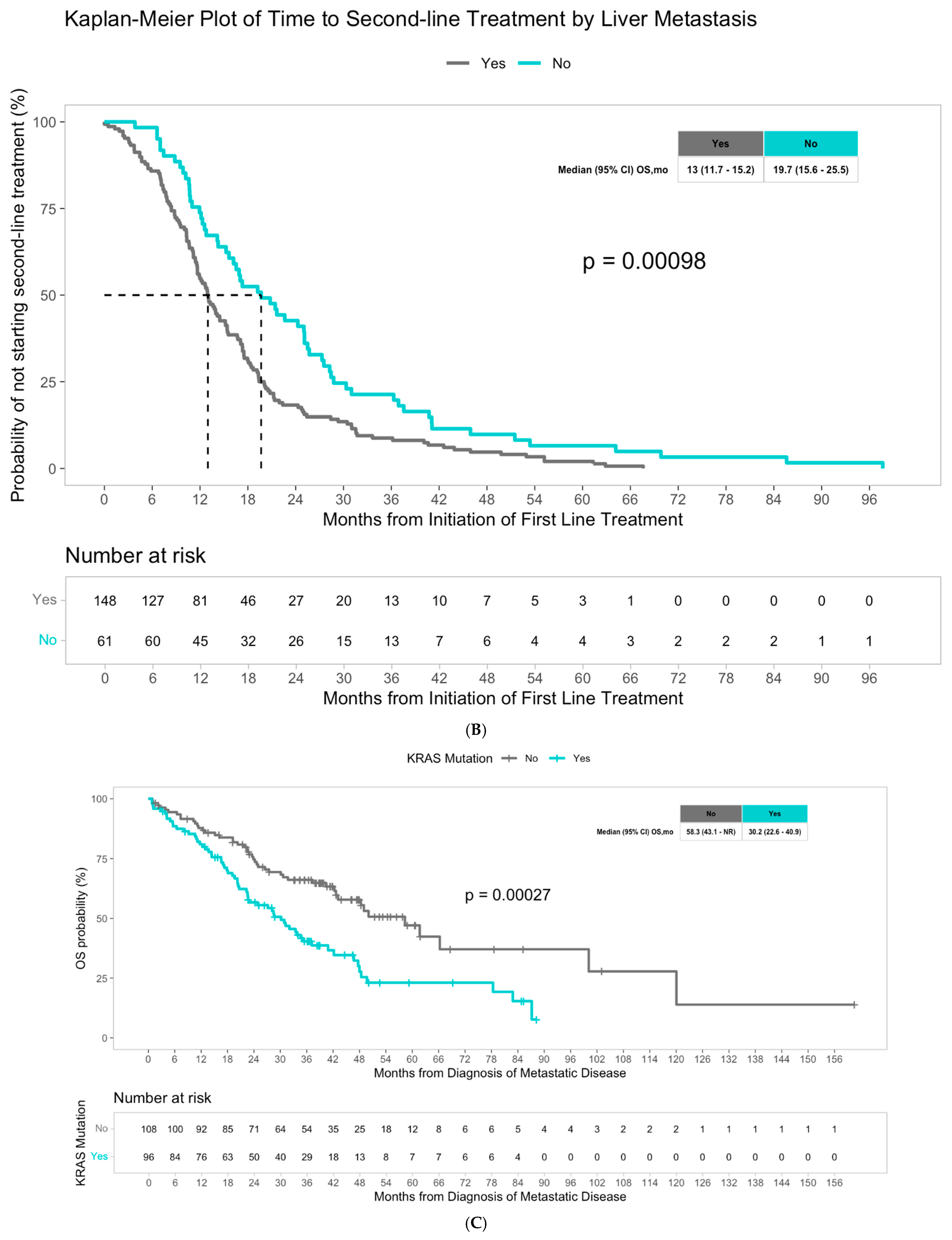
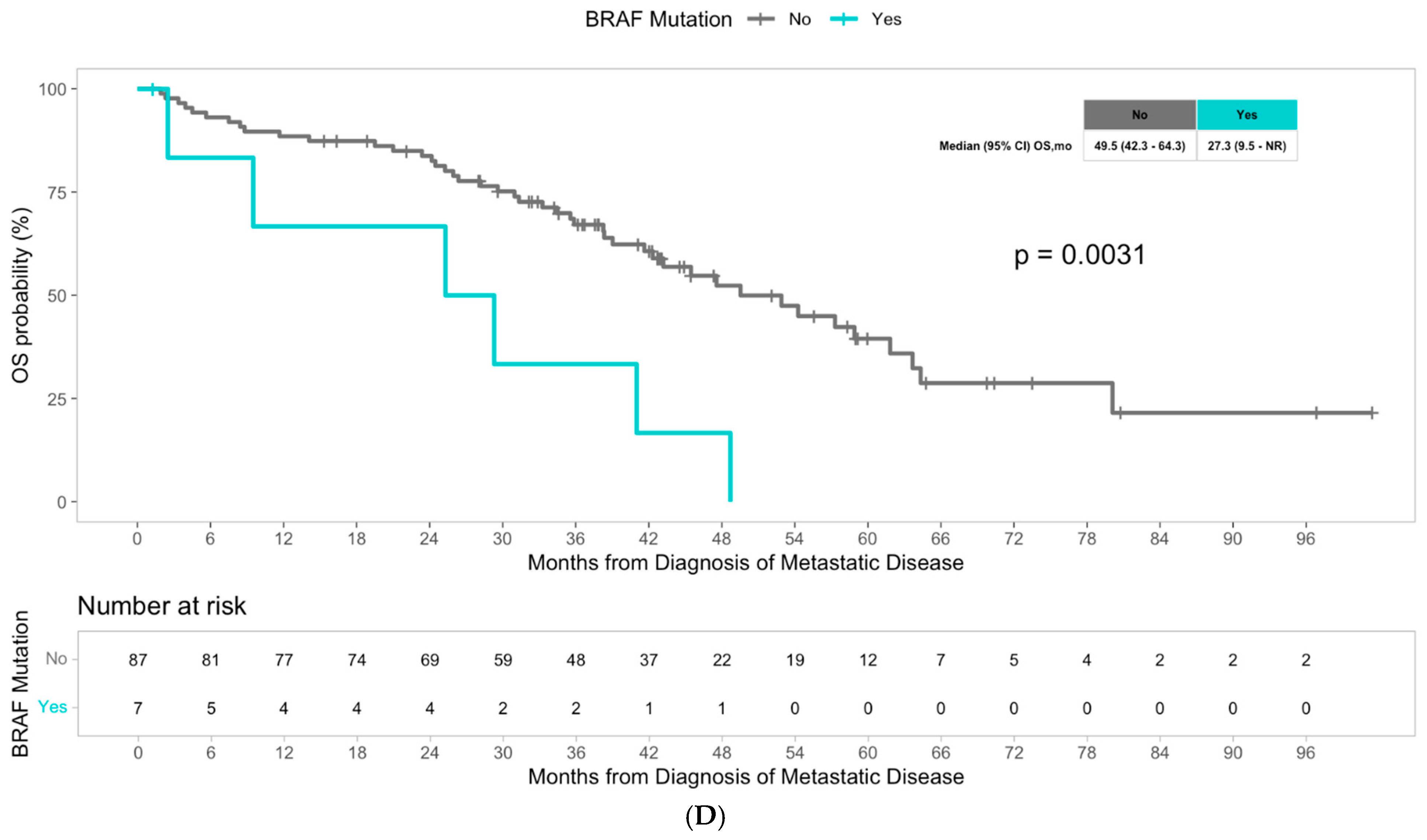
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Surveillance Research Program, National Cancer Institute. SEER*Explorer: An Interactive Website for SEER Cancer Statistics. National Cancer Institute. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 1 June 2025).
- Eng, C.; Kim, T.W.; Bendell, J.; Argilés, G.; Tebbutt, N.C.; Di Bartolomeo, M.; Falcone, A.; Fakih, M.; Kozloff, M.; Segal, N.H.; et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019, 20, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.-Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Randomized, Phase III Trial of Panitumumab With Infusional Fluorouracil, Leucovorin, and Oxaliplatin (FOLFOX4) Versus FOLFOX4 Alone As First-Line Treatment in Patients With Previously Untreated Metastatic Colorectal Cancer: The PRIME Study. J. Clin. Oncol. 2010, 28, 4697–4705. [Google Scholar] [CrossRef] [PubMed]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.-J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A randomized clinical trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef]
- Kawazoe, A.; Xu, R.; Passhak, M.; Teng, H.; Shergill, A.; Gumus, M.; Qvortrup, C.; Stintzing, S.; Towns, K.; Kim, T.; et al. LBA-5 Lenvatinib plus pembrolizumab versus standard of care for previously treated metastatic colorectal cancer (mCRC): The phase 3 LEAP-017 study. Ann. Oncol. 2023, 34, S179. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Guler, I.; Askan, G.; Klostergaard, J.; Sahin, I.H. Precision medicine for metastatic colorectal cancer: An evolving era. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 919–931. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability–High/Mismatch Repair–Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef]
- Chen, E.X.; Jonker, D.J.; Loree, J.M.; Kennecke, H.F.; Berry, S.R.; Couture, F.; Ahmad, C.E.; Goffin, J.R.; Kavan, P.; Harb, M.; et al. Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients With Advanced Colorectal Cancer. JAMA Oncol. 2020, 6, 831–838. [Google Scholar] [CrossRef]
- Wang, C.; Chevalier, D.; Saluja, J.; Sandhu, J.; Lau, C.; Fakih, M. Regorafenib and Nivolumab or Pembrolizumab Combination and Circulating Tumor DNA Response Assessment in Refractory Microsatellite Stable Colorectal Cancer. Oncologist 2020, 25, e1188–e1194. [Google Scholar] [CrossRef]
- Nie, C.; Lv, H.; Chen, B.; Xu, W.; Wang, J.; Liu, Y.; Wang, S.; Zhao, J.; He, Y.; Chen, X. Microsatellite stable metastatic colorectal cancer without liver metastasis may be preferred population for regorafenib or fruquintinib plus sintilimab as third-line or above therapy:A real-world study. Front. Oncol. 2022, 12, 917353. [Google Scholar] [CrossRef]
- Wang, C.; Sandhu, J.; Ouyang, C.; Ye, J.; Lee, P.P.; Fakih, M. Clinical Response to Immunotherapy Targeting Programmed Cell Death Receptor 1/Programmed Cell Death Ligand 1 in Patients With Treatment-Resistant Microsatellite Stable Colorectal Cancer With and Without Liver Metastases. JAMA Netw. Open 2021, 4, e2118416. [Google Scholar] [CrossRef] [PubMed]
- Saberzadeh-Ardestani, B.; Jones, J.C.; Hubbard, J.M.; McWilliams, R.R.; Halfdanarson, T.R.; Shi, Q.; Sonbol, M.B.; Ticku, J.; Jin, Z.; Sinicrope, F.A. Association Between Survival and Metastatic Site in Mismatch Repair–Deficient Metastatic Colorectal Cancer Treated With First-line Pembrolizumab. JAMA Netw. Open 2023, 6, e230400. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.X.; Loree, J.M.; Titmuss, E.; Jonker, D.J.; Kennecke, H.F.; Berry, S.; Couture, F.; Ahmad, C.E.; Goffin, J.R.; Kavan, P.; et al. Liver Metastases and Immune Checkpoint Inhibitor Efficacy in Patients With Refractory Metastatic Colorectal Cancer. JAMA Netw. Open 2023, 6, e2346094. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.; Raghav, K.P.S.; Chang, D.Z.; Bendell, J.C.; Larson, T.; Cohn, A.L.; Huyck, T.K.; Cosgrove, D.; Fiorillo, J.A.; Garbo, L.E.; et al. Single-arm, phase 2 study of regorafenib plus nivolumab in patients with mismatch repair-proficient (pMMR)/microsatellite stable (MSS) colorectal cancer (CRC). J. Clin. Oncol. 2021, 39, 3560. [Google Scholar] [CrossRef]
- Cohen, R.; Raeisi, M.; Shi, Q.; Chibaudel, B.; Yoshino, T.; Zalcberg, J.R.; Adams, R.; Cremolini, C.; Van Cutsem, E.; Heinemann, V.; et al. Prognostic value of liver metastases in colorectal cancer treated by systemic therapy: An ARCAD pooled analysis. J. Clin. Oncol. 2023, 41, 3554. [Google Scholar] [CrossRef]
- Sahin, I.H.; Ciombor, K.K.; Diaz, L.A.; Yu, J.; Kim, R. Immunotherapy for Microsatellite Stable Colorectal Cancers: Challenges and Novel Therapeutic Avenues. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 242–253. [Google Scholar] [CrossRef]
- Guven, D.C.; Kavgaci, G.; Erul, E.; Syed, M.P.; Magge, T.; Saeed, A.; Yalcin, S.; Sahin, I.H. The Efficacy of Immune Checkpoint Inhibitors in Microsatellite Stable Colorectal Cancer: A Systematic Review. Oncologist 2024, 29, e580–e600. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Fakih, M.; Gordon, M.S.; Tsimberidou, A.M.; Bullock, A.J.; Wilky, B.A.; Trent, J.C.; Margolin, K.A.; Mahadevan, D.; Balmanoukian, A.S.; et al. Results from a phase 1a/1b study of botensilimab (BOT), a novel innate/adaptive immune activator, plus balstilimab (BAL; anti-PD-1 antibody) in metastatic heavily pretreated microsatellite stable colorectal cancer (MSS CRC). J. Clin. Oncol. 2023, 41, LBA8. [Google Scholar] [CrossRef]
- Paniccia, A.; Polanco, P.M.; Boone, B.A.; Wald, A.I.; McGrath, K.; Brand, R.E.; Khalid, A.; Kubiliun, N.; O’BRoin-Lennon, A.M.; Park, W.G.; et al. Prospective, Multi-Institutional, Real-Time Next-Generation Sequencing of Pancreatic Cyst Fluid Reveals Diverse Genomic Alterations That Improve the Clinical Management of Pancreatic Cysts. Gastroenterology 2022, 164, 117–133.e7. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.H.; Goyal, S.; Pumpalova, Y.; Sonbol, M.B.; Das, S.; Haraldsdottir, S.; Ahn, D.; Ciombor, K.K.; Chen, Z.; Draper, A.; et al. Mismatch Repair (MMR) Gene Alteration and BRAF V600E Mutation Are Potential Predictive Biomarkers of Immune Checkpoint Inhibitors in MMR-Deficient Colorectal Cancer. Oncologist 2021, 26, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Kovari, B.P.; Martinez, M.; Xie, H.; Sahin, I.H.; Mehta, R.; Strosberg, J.; Imanirad, I.; Ghayouri, M.; Kim, Y.-C.; et al. A phase I/Ib study of regorafenib and nivolumab in mismatch repair proficient advanced refractory colorectal cancer. Eur. J. Cancer 2022, 169, 93–102. [Google Scholar] [CrossRef]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Cañellas, A.; Hernando-Momblona, X.; et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef]
- Yu, J.; Green, M.D.; Li, S.; Sun, Y.; Journey, S.N.; Choi, J.E.; Rizvi, S.M.; Qin, A.; Waninger, J.J.; Lang, X.; et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 2021, 27, 152–164. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van Den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef]
- Kasi, P.M.; Hidalgo, M.; Jafari, M.D.; Yeo, H.; Lowenfeld, L.; Khan, U.; Nguyen, A.T.H.; Siolas, D.; Swed, B.; Hyun, J.; et al. Neoadjuvant botensilimab plus balstilimab response pattern in locally advanced mismatch repair proficient colorectal cancer. Oncogene 2023, 42, 3252–3259. [Google Scholar] [CrossRef]
- Hissong, E.; Jafari, M.D.; Khan, S.; Kasi, P.M.; Guniganti, P.; Ocean, A.J.; Siolas, D.; Yeo, H.; Khan, U.; Pigazzi, A.; et al. Neoadjuvant botensilimab (BOT) plus balstilimab (BAL) in resectable mismatch repair proficient (pMMR) and deficient (dMMR) colorectal cancer (CRC): NEST clinical trial update. J. Clin. Oncol. 2025, 43, 207. [Google Scholar] [CrossRef]
- Sahin, I.H.; Saridogan, T.; Kim, R. Predictive biomarkers for immune checkpoint inhibition for patients with colorectal cancer: A comprehensive review. Immunotherapy 2025, 17, 727–734. [Google Scholar] [CrossRef]
- Magge, T.; Wang, S.; Syed, M.P.; Nasrollahi, E.; McFarquhar, A.; Overacre-Delgoffe, A.E.; Singhi, A.D.; Saeed, A.; Sahin, I.H. A comprehensive study of clinical and molecular features in patients with metastatic colorectal cancer with and without liver mets. J. Clin. Oncol. 2025, 43, 242. [Google Scholar] [CrossRef]
| Characteristic | Patients with Advanced CRC (n = 299) | |
|---|---|---|
| Age: mean [SD] | 62.9 [11.3] | |
| Gender | Female | 126 (42%) |
| Male | 173 (58%) | |
| Primary Site | Colon | 233 (78%) |
| Rectum | 64 (21%) | |
| Unknown | 2 (0.7%) | |
| Liver Metastases | Yes | 205 (69%) |
| No | 94 (31%) | |
| Molecular Alteration | Liver Metastasis (n = 205) | Non-Liver Metastasis (n = 94) | Overall (n = 299) | p-Value | ||
|---|---|---|---|---|---|---|
| KRAS | Overall | 96 (47%) | 51 (54%) | 147 (49%) | 0.263 | |
| Exon 2 mutation | 83/96 (86%) | 44/51 (86%) | 127/147 (86%) | 1 | ||
| Non-exon 2 mutation | 13/96 (14%) | 7/51 (14%) | 20/147 (14%) | 1 | ||
| NRAS | 7 (3.4%) | 4. (4.3%) | 11 (3.7%) | 0.746 | ||
| BRAF | 13 (6.3%) | 7 (7.4%) | 20 (6.7%) | 0.804 | ||
| TP53 | 170 (83%) | 71 (76%) | 241 (81%) | 0.156 | ||
| BRCA2 | 10 (4.9%) | 4 (4.3%) | 14 (4.7%) | 1 | ||
| PIK3CA | 29 (14%) | 15 (16%) | 44 (15%) | 0.726 | ||
| ERRB2 | 2 (1.0%) | 1 (1.1%) | 3 (1.0%) | 1 | ||
| TMB (muts/Mb) | 8.86 | 8.86 | 8.86 | 0.834 | ||
| TMB (>10 muts/Mb) | 80 (39%) | 35 (37%) | 115 (38%) | 0.798 | ||
| Gender | Male (n = 173) | 121 (70%) | 52 (30%) | 173 (100%) | 0.614 | |
| Female (n = 126) | 84 (67%) | 42 (33%) | 126 (100%) | |||
| Primary tumor site | Colon (n = 233) | 173 (74%) | 60 (26%) | 233 (100%) | 0.00013 | |
| Rectum (n = 64) | 31 (48%) | 33 (52%) | 64 (100%) | |||
| Variable | Multivariate Cox Regression Analysis | |
|---|---|---|
| HR | p-Value | |
| Liver metastases | 1.28 [0.91, 1.79] | 0.2 |
| KRAS mutation | 2.12 [1.50, 2.99] | <0.001 |
| NRAS mutation | 1.37 [0.61, 3.04] | 0.4 |
| BRAF mutation | 3.37 [1.81, 6.25] | <0.001 |
| TP53 mutation | 1.24 [0.83, 1.86] | 0.3 |
| PIK3CA mutation | 0.88 [0.56, 1.40] | 0.6 |
| BRCA2 mutation | 1.13 [0.52, 2.44] | 0.8 |
| Liver | |||||
|---|---|---|---|---|---|
| Variable | Cox Regression Analysis | Multivariate Cox Regression Analysis | Kaplan–Meier Survival Analysis | ||
| HR | p-Value | HR | p-Value | p-Value | |
| Age at analysis | 1.01 [0.99, 1.03] | 0.3 | N/A | N/A | N/A |
| Site of primary tumor (rectum/colon) | 1.36 [0.81, 2.28] | 0.3 | N/A | N/A | 0.25 |
| KRAS | 2.01 [1.37, 2.95] | <0.001 | 2.29 [1.52, 3.43] | <0.001 | 0.00027 |
| NRAS | 1.10 [0.43, 2.79] | 0.8 | 1.43 [0.54, 3.81] | 0.5 | 0.84 |
| BRAF | 1.59 [0.74, 3.43] | 0.2 | 2.48 [1.11, 5.54] | 0.027 | 0.23 |
| TP53 | 1.23 [0.73, 2.07] | 0.4 | 1.49 [0.87, 2.55] | 0.15 | 0.43 |
| BRCA2 | 1.03 [0.42, 2.54] | >0.9 | 0.94 [0.38, 2.35] | 0.9 | 0.94 |
| PIK3CA | 1.06 [0.62, 1.84] | 0.8 | 0.95 [0.54, 1.67] | 0.9 | 0.82 |
| TMB (categorical) | 1.00 [0.68, 1.46] | >0.9 | N/A | N/A | 1 |
| TMB (continuous) | 1.00 [0.97, 1.03] | >0.9 | N/A | N/A | N/A |
| Non-Liver Metastasis Cohort | |||||
| Variable | Cox Regression Analysis | Multivariate Cox Regression Analysis | Kaplan–Meier Survival Analysis | ||
| HR | p-Value | HR | p-Value | p-Value | |
| Age at analysis | 1.40 [1.07, 1.85] | 0.0148 | N/A | N/A | N/A |
| Site of primary tumor (rectum/colon) | 0.59 [0.32, 1.08] | 0.087 | N/A | N/A | 0.084 |
| KRAS | 1.31 [0.75, 2.30] | 0.3 | 1.89 [0.95, 3.77] | 0.069 | 0.35 |
| NRAS | 0.78 [0.19, 3.27] | 0.7 | 1.16 [0.26, 5.20] | 0.8 | 0.74 |
| BRAF | 3.42 [1.44, 8.16] | 0.006 | 6.42 [2.12, 19.5] | 0.001 | 0.0031 |
| TP53 | 0.73 [0.40, 1.33] | 0.3 | 0.87 [0.45, 1.69] | 0.7 | 0.3 |
| BRCA2 | 1.76 [0.42, 7.38] | 0.4 | 2.65 [0.60, 11.70] | 0.2 | 0.43 |
| PIK3CA | 0.95 [0.44, 2.02] | 0.9 | 0.59 [0.25, 1.38] | 0.2 | 0.89 |
| TMB (categorical) | 0.68 [0.37, 1.24] | 0.2 | N/A | N/A | 0.2 |
| TMB (continuous) | 0.96 [0.92, 1.01] | 0.1 | N/A | N/A | N/A |
| Variable | Multivariate Cox Regression Analysis | |
|---|---|---|
| HR | p-Value | |
| Liver metastasis | 1.75 | <0.001 |
| KRAS mutation | 1.53 | 0.005 |
| NRAS mutation | 1.02 | >0.9 |
| BRAF mutation | 1.45 | 0.2 |
| TP53 mutation | 0.97 | 0.9 |
| PIK3CA mutation | 1.08 | 0.7 |
| BRCA2 mutation | 1.10 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magge, T.; Cheng, S.; Wang, S.; Syed, M.P.; Jambunathan, B.; Mcfarquhar, A.; Zinser Peniche, P.; Kahramangil Baytar, D.; Singhi, A.; Saeed, A.; et al. A Comparative Study of Clinical and Molecular Features of Microsatellite Stable Colorectal Cancer With and Without Liver Metastases. Cancers 2025, 17, 3677. https://doi.org/10.3390/cancers17223677
Magge T, Cheng S, Wang S, Syed MP, Jambunathan B, Mcfarquhar A, Zinser Peniche P, Kahramangil Baytar D, Singhi A, Saeed A, et al. A Comparative Study of Clinical and Molecular Features of Microsatellite Stable Colorectal Cancer With and Without Liver Metastases. Cancers. 2025; 17(22):3677. https://doi.org/10.3390/cancers17223677
Chicago/Turabian StyleMagge, Tara, Svea Cheng, Shuaichao Wang, Masood Pasha Syed, Bhaghyasree Jambunathan, Ashley Mcfarquhar, Paola Zinser Peniche, Doga Kahramangil Baytar, Aatur Singhi, Anwaar Saeed, and et al. 2025. "A Comparative Study of Clinical and Molecular Features of Microsatellite Stable Colorectal Cancer With and Without Liver Metastases" Cancers 17, no. 22: 3677. https://doi.org/10.3390/cancers17223677
APA StyleMagge, T., Cheng, S., Wang, S., Syed, M. P., Jambunathan, B., Mcfarquhar, A., Zinser Peniche, P., Kahramangil Baytar, D., Singhi, A., Saeed, A., & Sahin, I. H. (2025). A Comparative Study of Clinical and Molecular Features of Microsatellite Stable Colorectal Cancer With and Without Liver Metastases. Cancers, 17(22), 3677. https://doi.org/10.3390/cancers17223677







