Photon-Counting Computed Tomography in Thoracic Surgery: A Narrative Review of Current and Future Applications
Simple Summary
Abstract
1. Introduction
2. Technical Overview of PCCT
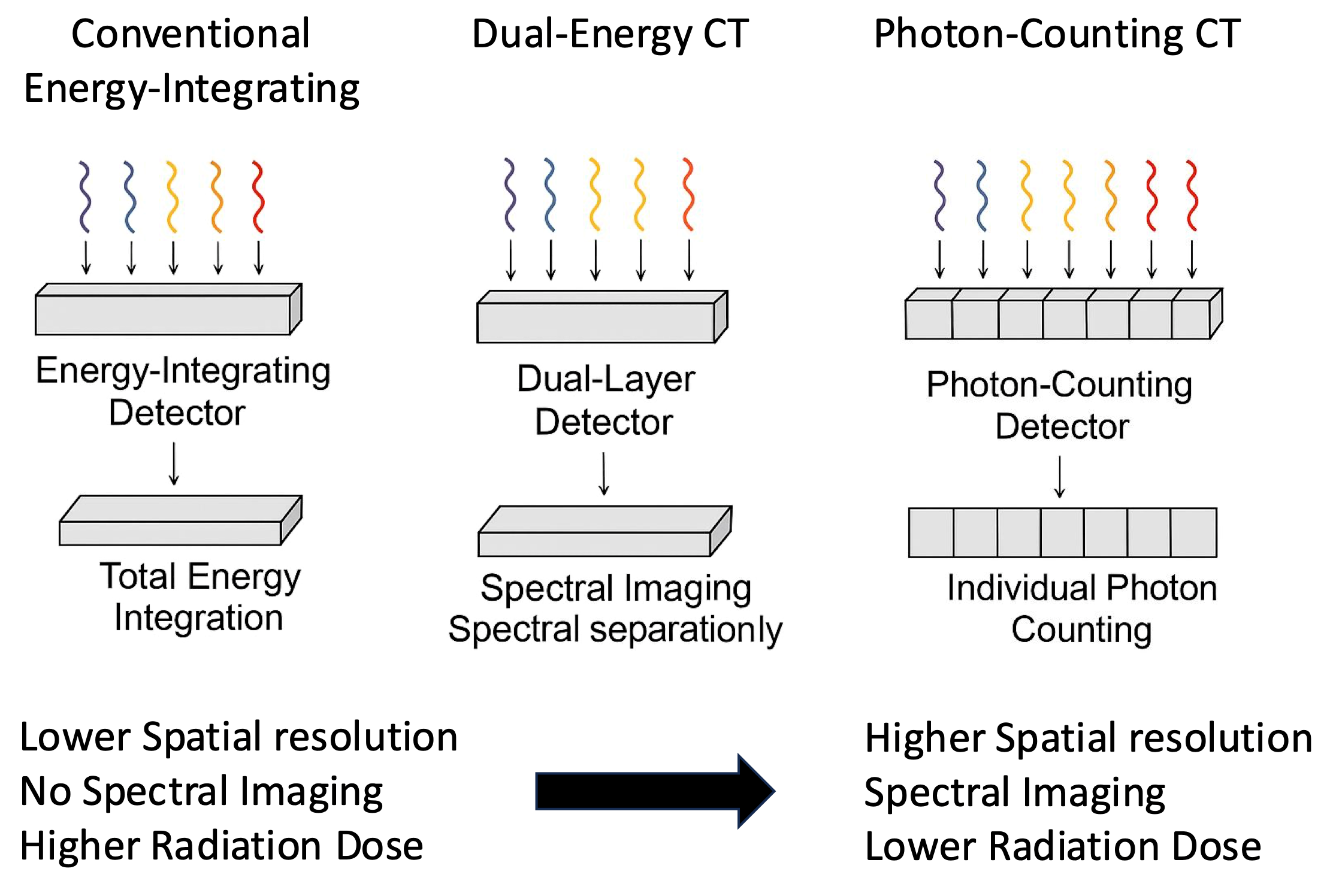
2.1. Improved Spatial Resolution
2.2. Enhanced Contrast-to-Noise Ratio (CNR)
2.3. Spectral Imaging and Material Decomposition
- (i)
- Iodine maps provide quantitative surrogates of tissue vascularity and regional perfusion within a single acquisition, aiding in the differentiation between viable tumor and post-treatment fibrosis—a common diagnostic dilemma in surgical follow-up [11].
- (ii)
- Virtual monoenergetic images (VMIs)—low-keV VMIs (≈40–60 keV) maximize iodine conspicuity and are helpful for detecting subtle enhancing foci (e.g., early recurrence or active inflammation) [11].
- (iii)
2.4. Lower Radiation Exposure
2.5. Metal Artifact Reduction
3. Clinical Applications in Thoracic Surgery
3.1. PCCT: From Lung Nodule Detection to Nodal Staging
3.2. Preoperative Surgical Planning: Functional and Structural Planning with PCCT
3.3. Preoperative Surgical Planning: Functional Planning with PCCT
3.4. Redefining Postoperative Imaging in Thoracic Surgery: The Role of PCCT
3.5. Non-Oncological Thoracic Diseases
3.5.1. Interstitial Lung Diseases
3.5.2. Pulmonary Infections and Empyema
3.5.3. Pulmonary Embolism and Vascular Anomalies
3.5.4. Tracheobronchial Diseases
3.5.5. Thoracic Trauma
3.5.6. Transplant Imaging
4. Limitations and Barriers to Implementation
5. Conclusions
Funding
Conflicts of Interest
References
- Krass, S.; Lassen-Schmidt, B.; Schenk, A. Computer-assisted image-based risk analysis and planning in lung surgery—A review. Front. Surg. 2022, 9, 920457. [Google Scholar] [CrossRef]
- Azlan, C.A.; Wong, J.H.D.; Tan, L.K.; Huri, M.S.N.A.; Ung, N.M.; Pallath, V.; Tan, C.P.L.; Yeong, C.H.; Ng, K.H. Teaching and learning of postgraduate medical physics using Internet-based e-learning during the COVID-19 pandemic—A case study from Malaysia. Phys. Medica 2020, 80, 10–16. [Google Scholar] [CrossRef]
- Griffey, R.T.; Sodickson, A. Cumulative Radiation Exposure and Cancer Risk Estimates in Emergency Department Patients Undergoing Repeat or Multiple CT. Am. J. Roentgenol. 2009, 192, 887–892. [Google Scholar] [CrossRef]
- Mascalchi, M.; Sali, L. Lung cancer screening with low dose CT and radiation harm—From prediction models to cancer incidence data. Ann. Transl. Med. 2017, 5, 360. [Google Scholar] [CrossRef]
- van der Bie, J.; van der Laan, T.; van Straten, M.; Booij, R.; Bos, D.; Dijkshoorn, M.L.; Hirsch, A.; Oei, E.H.G.; Budde, R.P.J. Photon-counting CT: An Updated Review of Clinical Results. Eur. J. Radiol. 2025, 190, 112189. [Google Scholar] [CrossRef]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-Counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- Rajendran, K.; Petersilka, M.; Henning, A.; Shanblatt, E.R.; Schmidt, B.; Flohr, T.G.; Ferrero, A.; Baffour, F.; Diehn, F.E.; Yu, L.; et al. First Clinical Photon-Counting Detector CT System: Technical Evaluation. Radiology 2021, 303, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Cork, T.E.; Sahbaee, P.; Fuld, M.K.; Kappler, S.; Folio, L.R.; A Bluemke, D.; Pourmorteza, A. Low-dose lung cancer screening with photon-counting CT: A feasibility study. Phys. Med. Biol. 2018, 63, 145009. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.J.; Koo, C.W.; Bartholmai, B.J.; Rajendran, K.; Weaver, J.M.; Halaweish, A.F.; Leng, S.; McCollough, C.H.; Fletcher, J.G. High-Resolution Chest Computed Tomography Imaging of the Lungs. Investig. Radiol. 2019, 54, 129–137. [Google Scholar] [CrossRef]
- Decker, J.A.; Bette, S.; Lubina, N.; Rippel, K.; Braun, F.; Risch, F.; Woźnicki, P.; Wollny, C.; Scheurig-Muenkler, C.; Kroencke, T.J.; et al. Low-dose CT of the abdomen: Initial experience on a novel photon-counting detector CT and comparison with energy-integrating detector CT. Eur. J. Radiol. 2022, 148, 110181. [Google Scholar] [CrossRef]
- Douek, P.C.; Boccalini, S.; Oei, E.H.G.; Cormode, D.P.; Pourmorteza, A.; Boussel, L.; Si-Mohamed, S.A.; Budde, R.P.J. Clinical Applications of Photon-Counting CT: A Review of Pioneer Studies and a Glimpse into the Future. Radiology 2023, 309, e222432. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Johnson, T.F.; Walkoff, L.A.; Levin, D.L.; Hartman, T.E.; Burke, K.A.; Rajendran, K.; Yu, L.; McCollough, C.H.; Fletcher, J.G. Lung Cancer Screening Using Clinical Photon-Counting Detector Computed Tomography and Energy-Integrating-Detector Computed Tomography: A Prospective Patient Study. J. Comput. Assist. Tomogr. 2022, 47, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, S.; Mohebbi, A.; Kiani, I.; Mohammadi, A. Direct comparison of photon counting-CT and conventional CT in image quality of lung nodules: A systematic review and meta-analysis. Eur. J. Radiol. 2024, 183, 111859. [Google Scholar] [CrossRef] [PubMed]
- Hop, J.F.; Walstra, A.N.H.; Pelgrim, G.-J.; Xie, X.; Panneman, N.A.; Schurink, N.W.; Faby, S.; van Straten, M.; de Bock, G.H.; Vliegenthart, R.; et al. Detectability and Volumetric Accuracy of Pulmonary Nodules in Low-Dose Photon-Counting Detector Computed Tomography: An Anthropomorphic Phantom Study. Diagnostics 2023, 13, 3448. [Google Scholar] [CrossRef]
- Kopp, F.K.; Daerr, H.; Si-Mohamed, S.; Sauter, A.P.; Ehn, S.; Fingerle, A.A.; Brendel, B.; Pfeiffer, F.; Roessl, E.; Rummeny, E.J.; et al. Evaluation of a preclinical photon-counting CT prototype for pulmonary imaging. Sci. Rep. 2018, 8, 17386. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Greffier, J.; Miailhes, J.; Boccalini, S.; Rodesch, P.-A.; Vuillod, A.; van der Werf, N.; Dabli, D.; Racine, D.; Rotzinger, D.; et al. Comparison of image quality between spectral photon-counting CT and dual-layer CT for the evaluation of lung nodules: A phantom study. Eur. Radiol. 2021, 32, 524–532. [Google Scholar] [CrossRef]
- Symons, R.; Reich, D.S.; Bagheri, M.; Cork, T.E.; Krauss, B.; Ulzheimer, S.; Kappler, S.; Bluemke, D.A.; Pourmorteza, A. Photon-Counting Computed Tomography for Vascular Imaging of the Head and Neck. Investig. Radiol. 2018, 53, 135–142. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, R.; Niu, X.; Hein, I.; Budden, B.; Wu, S.; Markov, N.; Clarke, C.; Qiang, Y.; Taguchi, H.; et al. Comprehensive evaluations of a prototype full field-of-view photon counting CT system through phantom studies. Phys. Med. Biol. 2023, 68, 175007. [Google Scholar] [CrossRef]
- Yalon, M.; Sae-Kho, T.; Khanna, A.; Chang, S.; Andrist, B.R.; Weber, N.M.; Hoodeshenas, S.; Ferrero, A.; Glazebrook, K.N.; McCollough, C.H.; et al. Staging of breast cancer in the breast and regional lymph nodes using contrast-enhanced photon-counting detector CT: Accuracy and potential impact on patient management. Br. J. Radiol. 2023, 97, 93–97. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, Y.; Zhou, X.; Wu, D.; Xie, Q. Dual-Energy Computed Tomography in Detecting and Predicting Lymph Node Metastasis in Malignant Tumor Patients: A Comprehensive Review. Diagnostics 2024, 14, 377. [Google Scholar] [CrossRef]
- Hojski, A.; Hassan, M.; Mallaev, M.; Tsvetkov, N.; Gahl, B.; Lardinois, D. Planning thoracoscopic segmentectomies with 3-dimensional reconstruction software improves outcomes. Interdiscip. Cardiovasc. Thorac. Surg. 2025, 40, ivaf043. [Google Scholar] [CrossRef]
- Niu, Z.; Chen, K.; Jin, R.; Zheng, B.; Gong, X.; Nie, Q.; Jiang, B.; Zhong, W.; Chen, C.; Li, H. Three-dimensional computed tomography reconstruction in video-assisted thoracoscopic segmentectomy (DRIVATS): A prospective, multicenter randomized controlled trial. Front. Surg. 2022, 9, 941582. [Google Scholar] [CrossRef]
- Kerber, B.; Hüllner, M.; Maurer, A.; Flohr, T.; Ulrich, S.; Lichtblau, M.; Frauenfelder, T.; Franckenberg, S. Photon-Counting Detector CT Iodine Maps Versus SPECT/CT. Investig. Radiol. 2025, 60, 569–576. [Google Scholar] [CrossRef]
- Remy-Jardin, M.; Guiffault, L.; Oufriche, I.; Duhamel, A.; Flohr, T.; Schmidt, B.; Remy, J. Image quality of lung perfusion with photon-counting-detector CT: Comparison with dual-source, dual-energy CT. Eur. Radiol. 2024, 34, 7831–7844. [Google Scholar] [CrossRef]
- Opitz, M.; Funke, F.; Darwiche, K.; Zensen, S.; Frings, M.; Salhöfer, L.; Haubold, J.; Forsting, M.; Doerr, F.; Bölükbas, S.; et al. First assessment of photon-counting CT for virtual bronchoscopic navigation. Eur. Respir. J. 2025, 65, 2402476. [Google Scholar] [CrossRef]
- Li, C.; Ji, A.; Jian, Z.; Zheng, Y.; Feng, X.; Guo, W.; Lerut, T.; Lin, J.; Li, H. Augmented reality navigation-guided in-traoperative pulmonary nodule localization: A pilot study. Transl. Lung Cancer Res. 2023, 12, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh-Paima, S.; Segars, W.P.; Ghosh, D.; Luo, S.; Samei, E.; Abadi, E. A systematic assessment and optimization of photon-counting CT for lung density quantifications. Med. Phys. 2024, 51, 2893–2904. [Google Scholar] [CrossRef]
- Van Ballaer, V.; Dubbeldam, A.; Muscogiuri, E.; Cockmartin, L.; Bosmans, H.; Coudyzer, W.; Coolen, J.; de Wever, W. Impact of ultra-high-resolution imaging of the lungs on perceived diagnostic image quality using photon-counting CT. Eur. Radiol. 2024, 34, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Leivaditis, V.; Skevis, K.; Mulita, F.; Tsalikidis, C.; Mitsala, A.; Dahm, M.; Grapatsas, K.; Papatriantafyllou, A.; Markakis, K.; Kefaloyannis, E.; et al. Advancements in the Management of Postoperative Air Leak following Thoracic Surgery: From Traditional Practices to Innovative Therapies. Medicina 2024, 60, 802. [Google Scholar] [CrossRef]
- Meloni, A.; Frijia, F.; Panetta, D.; Degiorgi, G.; De Gori, C.; Maffei, E.; Clemente, A.; Positano, V.; Cademartiri, F. Pho-ton-Counting Computed Tomography (PCCT): Technical Background and Cardio-Vascular Applications. Diagnostics 2023, 13, 645. [Google Scholar] [CrossRef] [PubMed]
- Skornitzke, S.; Mergen, V.; Biederer, J.; Alkadhi, H.; Do, T.D.; Stiller, W.; Frauenfelder, T.; Kauczor, H.U.; Euler, A. Metal artifact reduction in photon-counting detector CT: Quantitative evaluation of artifact reduction techniques. Investig. Radiol. 2024, 59, 442–449. [Google Scholar] [CrossRef]
- Pallasch, F.B.; Rau, A.; Reisert, M.; Rau, S.; Diallo, T.; Stein, T.; Faby, S.; Bamberg, F.; Weiss, J. Photon-counting detector computed tomography for metal artifact reduction: A comparative study in orthopedic patients. Radiol. Med. 2024, 129, 890–900. [Google Scholar] [CrossRef]
- Becker, B.V.; Kaatsch, H.L.; Nestler, K.; Overhoff, D.; Schneider, J.; Dillinger, D.; Piechotka, J.; Brockmann, M.A.; Ullmann, R.; Port, M.; et al. Initial experience on abdominal photon-counting computed tomog-raphy in clinical routine: General image quality and dose exposure. Eur. Radiol. 2023, 33, 2461–2468. [Google Scholar] [CrossRef]
- Lim, W.; Sodemann, E.B.; Büttner, L.; Jonczyk, M.; Lüdemann, W.M.; Kahn, J.; Geisel, D.; Jann, H.; Aigner, A.; Böning, G. Spectral Computed Tomography-Derived Iodine Content and Tumor Response in the Follow-Up of Neuroendocrine Tumors-A Single-Center Experience. Curr. Oncol. 2023, 30, 1502–1515. [Google Scholar] [CrossRef] [PubMed]
- Woeltjen, M.M.; Niehoff, J.H.; Michael, A.E.; Horstmeier, S.; Moenninghoff, C.; Borggrefe, J.; Kroeger, J.R. Low-Dose High-Resolution Photon-Counting CT of the Lung: Radiation Dose and Image Quality in the Clinical Routine. Diagnostics 2022, 12, 1441. [Google Scholar] [CrossRef] [PubMed]
- Scharm, S.C.; Schaefer-Prokop, C.; Winther, H.B.; Huisinga, C.; Werncke, T.; Vogel-Claussen, J.; Wacker, F.K.; Shin, H.-O. Regional Pulmonary Morphology and Function: Photon-Counting CT Assessment. Radiology 2023, 308, e230318. [Google Scholar] [CrossRef]
- Gaillandre, Y.; Duhamel, A.; Flohr, T.; Faivre, J.-B.; Khung, S.; Hutt, A.; Felloni, P.; Remy, J.; Remy-Jardin, M. Ultra-high resolution CT imaging of interstitial lung disease: Impact of photon-counting CT in 112 patients. Eur. Radiol. 2023, 33, 5528–5539. [Google Scholar] [CrossRef]
- Koo, C.W.; Huls, S.J.; Baffour, F.; McCollough, C.H.; Yu, L.; Bartholmai, B.J.; Zhou, Z. Impact of photon-counting detector CT on a quantitative interstitial lung disease machine learning model. J. Thorac. Imaging 2025, 40, e0807. [Google Scholar] [CrossRef]
- Fletcher, J.G.; Inoue, A.; Bratt, A.; Horst, K.K.; Koo, C.W.; Rajiah, P.S.; Baffour, F.I.; Ko, J.P.; Remy-Jardin, M.; McCollough, C.H.; et al. Photon-counting CT in Thoracic Imaging: Early Clinical Evidence and Incorporation into Clinical Practice. Radiology 2024, 310, e231986. [Google Scholar] [CrossRef]
- Jungblut, L.; Abel, F.; Nakhostin, D.; Mergen, V.; Sartoretti, T.; Euler, A.; Frauenfelder, T.; Martini, K. Impact of photon count-ing detector CT derived virtual monoenergetic images and iodine maps on the diagnosis of pleural empyema. Diagn. Interv. Imaging 2023, 104, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Pannenbecker, P.; Heidenreich, J.F.; Huflage, H.; Gruschwitz, P.; Patzer, T.S.; Weng, A.M.; Grunz, J.P.; Kunz, A.S.; Bley, T.A.; Petritsch, B. The best of both worlds: Ultra high pitch pulmonary angiography with free breathing technique by means of photon counting detector CT for diagnosis of acute pulmonary embolism. Acad. Radiol. 2024, 31, 5280–5288. [Google Scholar] [CrossRef] [PubMed]
- Kerber, B.; Flohr, T.; Ulrich, S.; Lichtblau, M.; Frauenfelder, T.; Franckenberg, S. Photon-counting CT iodine maps for diagnosing chronic pulmonary thromboembolism: A pilot study. Investig. Radiol. 2025, 60, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Milos, R.I.; Röhrich, S.; Prayer, F.; Strassl, A.; Beer, L.; Heidinger, B.H.; Weber, M.; Watzenboeck, M.L.; Kifjak, D.; Tamandl, D.; et al. Ultrahigh-Resolution Photon-Counting Detector CT of the Lungs: Association of Reconstruction Kernel and Slice Thickness with Image Quality. AJR Am. J. Roentgenol. 2023, 220, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Kaatsch, H.L.; Völlmecke, M.F.; Becker, B.V.; Dillinger, D.; Kubitscheck, L.; Wöhler, A.; Schaaf, S.; Piechotka, J.; Schreyer, C.; Schwab, R.; et al. Improved Discriminability of Severe Lung Injury and Atelectasis in Thoracic Trauma at Low keV Virtual Monoenergetic Images from Photon-Counting Detector CT. Diagnostics 2024, 14, 2231. [Google Scholar] [CrossRef]
- Milos, R.I.; Lechner, L.; Korajac, A.; Kifjak, D.; Watzenböck, M.L.; Tamandl, D.; Strassl, A.; Stuempflen, M.; Beer, L.; Weber, M.; et al. Accuracy of ultralow dose photon-counting CT in the detection of lung changes after lung transplant. Radiology 2024, 312, e240271. [Google Scholar] [CrossRef]
- Vecsey-Nagy, M.; Emrich, T.; Tremamunno, G.; Kravchenko, D.; Taha Hagar, M.; Laux, G.S.; Schoepf, U.J.; O’Doherty, J.; Boussoussou, M.; Szilveszter, B.; et al. Cost effectiveness of ultrahigh resolution photon counting detector coronary CT angiography for the evaluation of stable chest pain. J. Cardiovasc. Comput. Tomogr. 2024, 19, 106–112. [Google Scholar] [CrossRef]
- Varga-Szemes, A.; Emrich, T. Photon-counting detector CT: A disrupting innovation in medical imaging. Eur. Radiol. Exp. 2025, 9, 38. [Google Scholar] [CrossRef]
- Remy-Jardin, M.; Flohr, T.; Remy, J. Thoracic applications of photon-counting CT: Where are WE after three years of clinical implementation? Br. J. Radiol. 2025, tqaf026. [Google Scholar] [CrossRef]
- Chen-Yoshikawa, T.F. Evolution of Three-Dimensional Computed Tomography Imaging in Thoracic Surgery. Cancers 2024, 16, 2161. [Google Scholar] [CrossRef]
| Clinical Domain | PCCT Advantage | Surgical Relevance |
|---|---|---|
| Diagnosis | ||
| Lung nodule evaluation | High-resolution morphology, material decomposition | Accurate malignancy prediction |
| Tumor staging | Precise lymph node assessment, vascular invasion detection | Improved resectability evaluation |
| Preoperative planning | ||
| Segmentectomy planning | 3D visualization of vessels and bronchi | Safe and tailored resections |
| Postoperative assessment | ||
| Postoperative follow-up | Artifact reduction, detection of small collections or air leaks | Early detection of complications |
| Non-oncologic thoracic conditions | ||
| Interstitial lung disease (ILD) | Better parenchymal detail, quantitative analysis | Pre-op risk stratification, especially in CPFE |
| Metallic implants/sutures | Reduced blooming artifacts | Clear visualization in re-operations |
| Radiation dose | Up to 40% reduction vs. conventional CT | Safer follow-up protocols, especially in oncology |
| Parameter | Conventional EID-CT | Dual-Energy CT (DECT) | Photon-Counting CT (PCCT) |
|---|---|---|---|
| Detector pixel size | ~0.5–0.625 mm | ~0.4–0.6 mm | ~0.25–0.3 mm |
| Spatial resolution (line pairs/cm) | 20–25 | 25–30 | 35–40 |
| Energy discrimination | None | Dual spectra (dual-source or kVp-switching) | True multi-energy (per-photon energy binning) |
| Contrast-to-noise ratio (CNR) | Baseline | ~1.2 × EID | ~1.5–2 × EID |
| Noise characteristics | Electronic noise included | Reduced | Minimized (direct conversion) |
| Metal artifact reduction | Limited | Moderate | Enhanced |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangiameli, G.; Brascia, D.; Lococo, F.; Marulli, G. Photon-Counting Computed Tomography in Thoracic Surgery: A Narrative Review of Current and Future Applications. Cancers 2025, 17, 3656. https://doi.org/10.3390/cancers17223656
Mangiameli G, Brascia D, Lococo F, Marulli G. Photon-Counting Computed Tomography in Thoracic Surgery: A Narrative Review of Current and Future Applications. Cancers. 2025; 17(22):3656. https://doi.org/10.3390/cancers17223656
Chicago/Turabian StyleMangiameli, Giuseppe, Debora Brascia, Filippo Lococo, and Giuseppe Marulli. 2025. "Photon-Counting Computed Tomography in Thoracic Surgery: A Narrative Review of Current and Future Applications" Cancers 17, no. 22: 3656. https://doi.org/10.3390/cancers17223656
APA StyleMangiameli, G., Brascia, D., Lococo, F., & Marulli, G. (2025). Photon-Counting Computed Tomography in Thoracic Surgery: A Narrative Review of Current and Future Applications. Cancers, 17(22), 3656. https://doi.org/10.3390/cancers17223656






