Association Between the Use of DPP4 Inhibitors and Metformin and the Risk of Cancer in Patients with Type 2 Diabetes: A Multicenter Retrospective Cohort Study Using the OMOP CDM Database
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Network and Tools
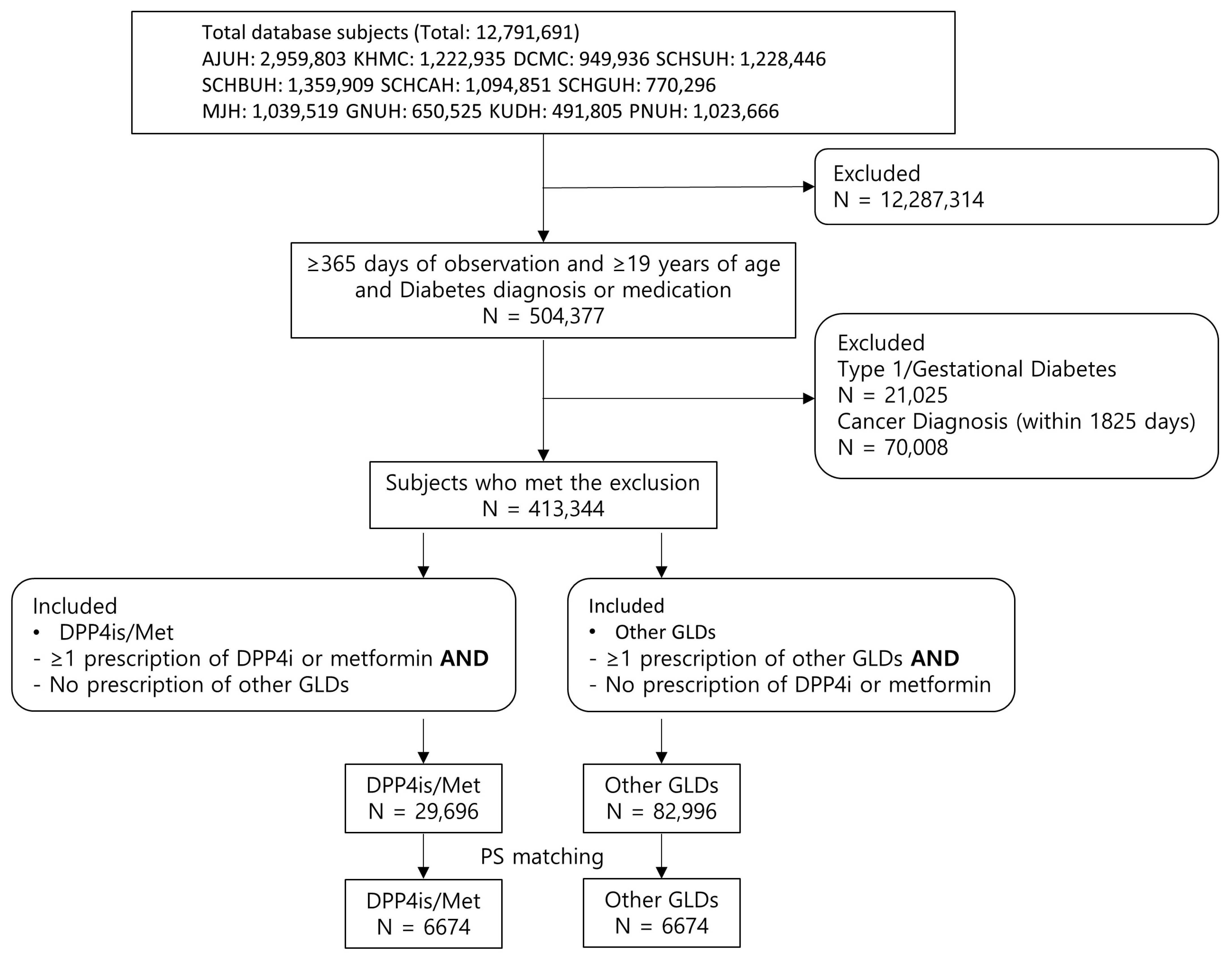
2.2. Data Source and Study Population
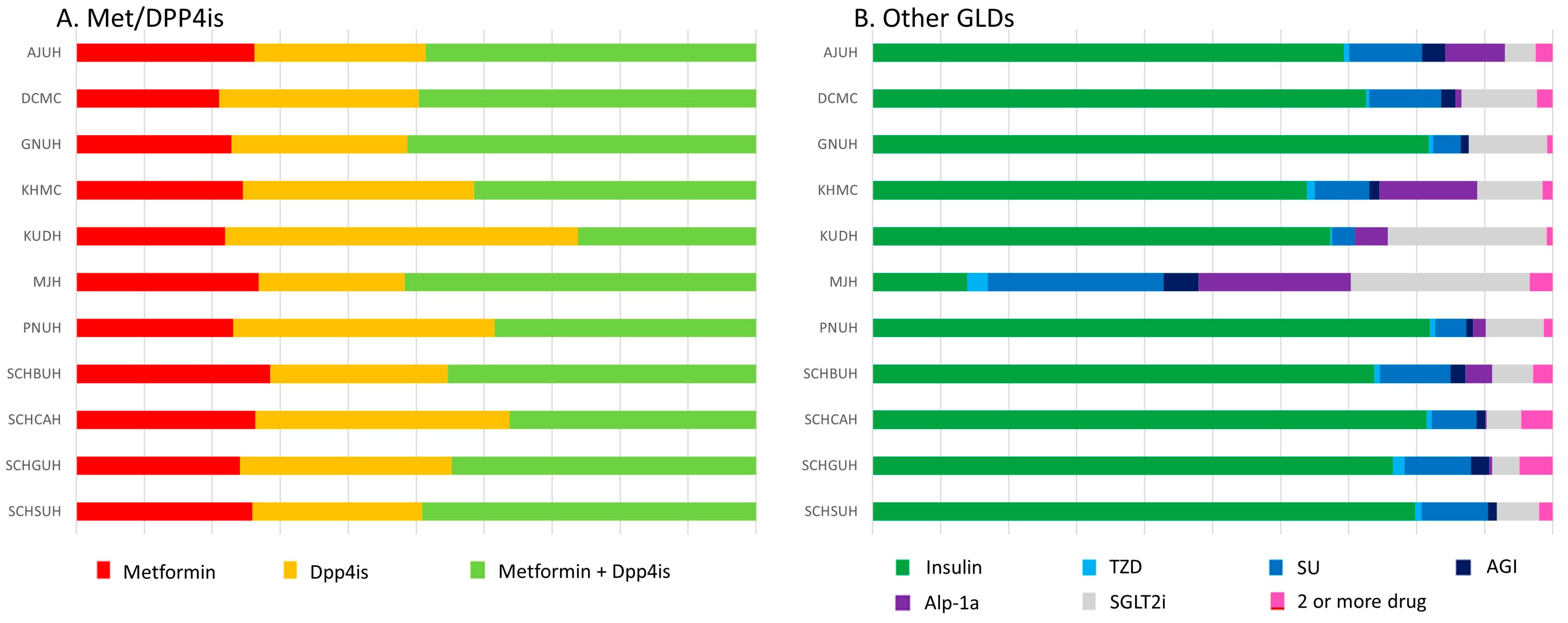
2.3. Exposure
2.4. Outcomes
2.5. Statistical Analysis
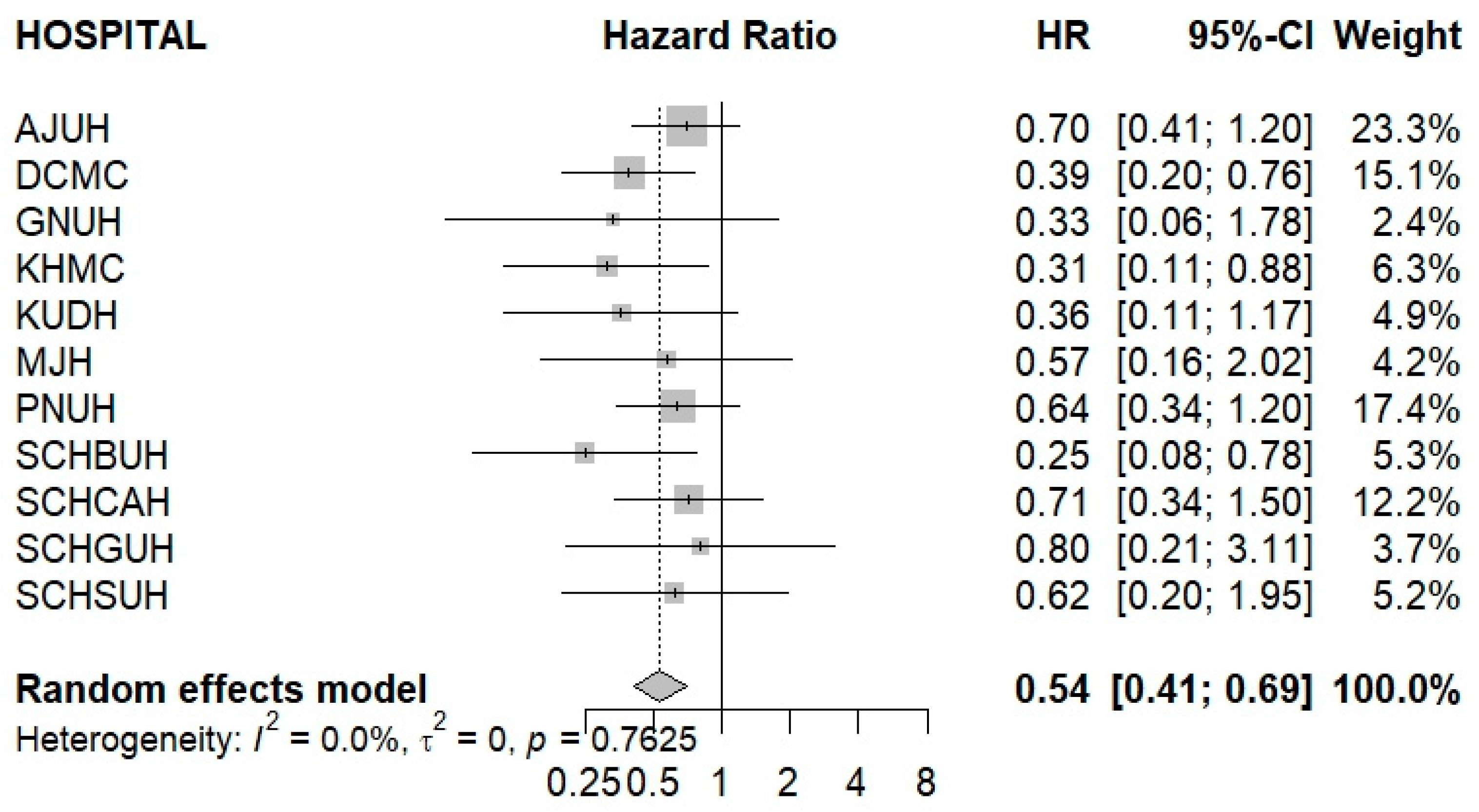
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, N.D.; Sattar, N. Cardiovascular risk in diabetes mellitus: Epidemiology, assessment and prevention. Nat. Rev. Cardiol. 2023, 20, 685–695. [Google Scholar] [CrossRef]
- Sacerdote, C.; Ricceri, F. Epidemiological dimensions of the association between type 2 diabetes and cancer: A review of observational studies. Diabetes Res. Clin. Pract. 2018, 143, 369–377. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, S.R.; Han, K.D.; Jung, J.H.; Lee, J.; Lee, B.W.; Cha, B.S.; Kim, D.J.; Ahn, C.W.; Kim, J.H. Role of SGLT2 inhibitors, DPP-4 inhibitors, and metformin in the development of pancreatic cancer in patients with diabetes. Diabetes Metab. J. 2023, 47, 250–262. [Google Scholar]
- Chen, Y.; Mushashi, F.; Son, S.; Bhatti, P.; Dummer, T.; Murphy, R.A. Diabetes medications and cancer risk associations: A systematic review and meta-analysis of evidence over the past 10 years. Sci. Rep. 2023, 13, 11844. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.; Bailey-Whyte, M.; Bhattacharya, M.; Butera, G.; Lewis Hardell, K.N.; Seidenberg, A.B.; Castle, P.E.; Loomans-Kropp, H.A. Association of Metformin Use and Cancer Incidence: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2024, 116, 518–529. [Google Scholar] [CrossRef]
- Yang, J.; Yang, H.; Cao, L.; Yin, Y.; Shen, Y.; Zhu, W. Prognostic Value of Metformin in Cancers: An Updated Meta-Analysis Based on 80 Cohort Studies. Medicine 2022, 101, e31799. [Google Scholar] [CrossRef]
- Wang, T.; Chai, B.; Chen, W.Y.; Holmes, M.D.; Erdrich, J.; Hu, F.B.; Rosner, B.A.; Tamimi, R.M.; Willett, W.C.; Kang, J.H.; et al. Metformin and Other Anti-Diabetic Medication Use and Breast Cancer Incidence in the Nurses’ Health Studies. Int. J. Cancer 2024, 155, 211–225. [Google Scholar] [CrossRef]
- Heckman-Stoddard, B.M.; Crandall, J.P.; Edelstein, S.L.; Prorok, P.C.; Dabelea, D.; Hamman, R.; Hazuda, H.P.; Horton, E.; Hoskin, M.A.; Perloff, M.; et al. Randomized Study of Metformin and Intensive Lifestyle Intervention on Cancer Incidence Over 21 Years of Follow-Up in the Diabetes Prevention Program. Cancer Prev. Res. 2025, 18, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; He, X.; Sun, Y. Hypoglycemic agents and incidence of pancreatic cancer in diabetic patients: A meta-analysis. Front. Pharmacol. 2023, 14, 1193610. [Google Scholar] [CrossRef]
- Na, Y.; Kim, S.W.; Park, I.B.; Choi, S.J.; Nam, S.; Jung, J.; Lee, D.H. Association Between DPP4 Inhibitor Use and the Incidence of Cirrhosis, ESRD, and Some Cancers in Patients with Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 3022–3034. [Google Scholar] [CrossRef]
- Hidayat, K.; Zhou, Y.Y.; Du, H.Z.; Qin, L.; Shi, B.; Li, Z. A Systematic Review and Meta-Analysis of Observational Studies of the Association Between the Use of Incretin-Based Therapies and the Risk of Pancreatic Cancer. Pharmacoepidemiol. Drug Saf. 2023, 32, 107–125. [Google Scholar] [CrossRef]
- Suchard, M.A.; Schuemie, M.J.; Krumholz, H.M.; You, S.C.; Chen, R.; Pratt, N.; Reich, C.G.; Duke, J.; Madigan, D.; Hripcsak, G.; et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: A multinational cohort study. Lancet 2019, 394, 1816–1826. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Miao, S.; Xu, H.; Yin, Y.; Zhu, Y.; Dai, Z.; Shan, T.; Jing, S.; Wang, J.; et al. Analysis of treatment pathways for three chronic diseases using OMOP CDM. J. Med. Syst. 2018, 42, 260. [Google Scholar] [CrossRef]
- Fortin, S.P.; Johnston, S.S.; Schuemie, M.J. Applied comparison of large-scale propensity score matching and cardinality matching for causal inference in observational research. BMC Med. Res. Methodol. 2021, 21, 70. [Google Scholar]
- Abd El Aziz, M.; Cahyadi, O.; Meier, J.J.; Schmidt, W.E.; Nauck, M.A. Incretin-based glucose-lowering medications and the risk of acute pancreatitis and malignancies: A meta-analysis based on cardiovascular outcomes trials. Diabetes Obes. Metab. 2020, 22, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Bea, S.; Son, H.; Bae, J.H.; Cho, S.W.; Shin, J.Y.; Cho, Y.M. Risk of Thyroid Cancer Associated with Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors in Patients with Type 2 Diabetes: A Population-Based Cohort Study. Diabetes Obes. Metab. 2024, 26, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Ueda, P.; Wintzell, V.; Melbye, M.; Eliasson, B.; Svensson, A.M.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Svanström, H.; et al. Use of incretin-based drugs and risk of cholangiocarcinoma: Scandinavian cohort study. Diabetologia 2021, 64, 2204–2214. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.C.; Rados, D.V.; Barkan, S.S.; Leitão, C.B.; Gross, J.L. Dipeptidyl peptidase-4 inhibitors, pancreatic cancer and acute pancreatitis: A meta-analysis with trial sequential analysis. Sci. Rep. 2018, 8, 782. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yin, H.; Yu, O.H.Y.; Azoulay, L. Incretin-Based Drugs and the Incidence of Prostate Cancer Among Patients with Type 2 Diabetes. Epidemiology 2022, 33, 563–571. [Google Scholar] [CrossRef]
- Li, Z.; Lin, C.; Zhou, J.; Cai, X.; Zhu, X.; Hu, S.; Lv, F.; Yang, W.; Ji, L. Dipeptidyl peptidase 4-inhibitor treatment was associated with a reduced incidence of neoplasm in patients with type 2 diabetes: A meta-analysis of 115 randomized controlled trials with 121961 participants. Expert Opin. Investig. Drugs 2022, 31, 957–964. [Google Scholar] [CrossRef]
- Busek, P.; Duke-Cohan, J.S.; Sedo, A. Does DPP-IV inhibition offer new avenues for therapeutic intervention in malignant disease? Cancers 2022, 14, 2072. [Google Scholar] [CrossRef]
- Bishnoi, R.; Hong, Y.R.; Shah, C.; Ali, A.; Skelton, W.P., IV; Huo, J.; Dang, N.H.; Dang, L.H. Dipeptidyl peptidase 4 inhibitors as novel agents in improving survival in diabetic patients with colorectal cancer and lung cancer: A Surveillance Epidemiology and Endpoint Research Medicare study. Cancer Med. 2019, 8, 3918–3927. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Luo, J.; Yu, T.; Zhou, L.; Lv, H.; Shang, P. Anticancer mechanisms of metformin: A review of the current evidence. Life Sci. 2020, 254, 117717. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shi, M. Effect of Metformin Use on the Risk and Prognosis of Colorectal Cancer in Diabetes Mellitus: A Meta-Analysis. Anti-Cancer Drugs 2022, 33, 191–199. [Google Scholar] [CrossRef]
- Myung, S.; Park, Y.Y.; Kim, M.S. Metformin in Colorectal Cancer: Epidemiological Evidence, Predictive Biomarkers, and Implications for Prevention and Treatment. Int. J. Mol. Sci. 2025, 26, 6040. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kaneko, H.; Okada, A.; Ko, T.; Jimba, T.; Fujiu, K.; Morita, H.; Komuro, J.; Ieda, M.; Node, K.; et al. Association of SGLT2 inhibitors with incident cancer. Diabetes Metab. 2024, 50, 101585. [Google Scholar] [CrossRef]
- Benedetti, R.; Benincasa, G.; Glass, K.; Chianese, U.; Vietri, M.T.; Congi, R.; Altucci, L.; Napoli, C. Effects of novel SGLT2 inhibitors on cancer incidence in hyperglycemic patients: A meta-analysis of randomized clinical trials. Pharmacol. Res. 2022, 175, 106039. [Google Scholar] [CrossRef]
- Noh, Y.; Jeon, S.M.; Shin, S. Association between glucose-lowering treatment and cancer metastasis among patients with preexisting type 2 diabetes and incident malignancy. Int. J. Cancer 2019, 144, 1530–1539. [Google Scholar] [CrossRef]
- Søndergaard, C.S.; Esquivel, P.N.; Dalamaga, M.; Magkos, F. Use of antihyperglycemic drugs and risk of cancer in patients with diabetes. Curr. Oncol. Rep. 2023, 25, 29–40. [Google Scholar] [CrossRef]
- Kawakita, E.; Yang, F.; Kumagai, A.; Takagaki, Y.; Kitada, M.; Yoshitomi, Y.; Ikeda, T.; Nakamura, Y.; Ishigaki, Y.; Kanasaki, K.; et al. Metformin mitigates DPP-4 inhibitor–induced breast cancer metastasis via suppression of mTOR signaling. Mol. Cancer Res. 2021, 19, 61–73. [Google Scholar] [CrossRef]


| Before PS Adjustment | After PS Adjustment | ||||||
|---|---|---|---|---|---|---|---|
| DPP4is/Met | Other GLDs | Std_Diff | DPP4is/Met | Other GLDs | Std_Diff | ||
| Age (years) | AJUH | 59.7 | 58.4 | −0.01 | 60.3 | 60.8 | −0.005 |
| DCMC | 64.3 | 63.9 | −0.011 | 65.3 | 65.1 | 0.006 | |
| GNUH | 66.5 | 69.1 | −0.009 | 61.1 | 61.8 | −0.011 | |
| KHMC | 66.5 | 64.5 | −0.036 | 66.1 | 66.7 | <0.001 | |
| KUDH | 66.5 | 65.9 | −0.014 | 66.9 | 66.7 | −0.004 | |
| MJH | 70.2 | 65.7 | −0.045 | 68.9 | 68.6 | −0.003 | |
| PNUH | 65.3 | 66.1 | −0.028 | 65.8 | 65.2 | 0.002 | |
| SCHBUH | 61.7 | 63.3 | −0.034 | 62.5 | 62.3 | −0.003 | |
| SCHCAH | 63.2 | 66.3 | −0.034 | 65.8 | 64.9 | −0.01 | |
| SCHGUH | 62.2 | 63.6 | 0.014 | 60.2 | 60.4 | <0.001 | |
| SCHSUH | 62.5 | 63.2 | 0.002 | 63.4 | 63.0 | −0.003 | |
| Male (%) | AJUH | 60.4 | 53.0 | 0.15 | 56.3 | 56.2 | 0.003 |
| DCMC | 54.4 | 53.3 | 0.022 | 52.3 | 53.1 | −0.016 | |
| GNUH | 56.2 | 58.6 | −0.048 | 61.0 | 54.8 | 0.126 | |
| KHMC | 47.8 | 45.8 | 0.041 | 45.7 | 43.9 | 0.036 | |
| KUDH | 51.9 | 51.1 | 0.016 | 55.8 | 54.2 | 0.03 | |
| MJH | 47.2 | 45.3 | 0.039 | 48.3 | 48.6 | −0.005 | |
| PNUH | 48.7 | 54.4 | −0.115 | 52.7 | 50.6 | 0.041 | |
| SCHBUH | 54.7 | 51.0 | 0.074 | 54.3 | 50.4 | 0.079 | |
| SCHCAH | 54.6 | 53.0 | 0.031 | 51.5 | 53.7 | −0.044 | |
| SCHGUH | 54.9 | 55.5 | −0.011 | 61.6 | 55.0 | 0.135 | |
| SCHSUH | 54.4 | 49.4 | 0.099 | 49.8 | 49.5 | 0.007 | |
| Charlson index | AJUH | 1.801 | 1.674 | 0.086 | 1.801 | 1.836 | −0.029 |
| DCMC | 2.177 | 2.143 | 0.023 | 2.396 | 2.389 | 0.005 | |
| GNUH | 1.304 | 1.489 | −0.132 | 2.224 | 2.046 | 0.152 | |
| KHMC | 2.216 | 2.138 | 0.049 | 2.494 | 2.505 | −0.008 | |
| KUDH | 2.048 | 1.407 | 0.482 | 2.076 | 2.255 | −0.144 | |
| MJH | 2.139 | 2.134 | 0.003 | 2.445 | 2.421 | 0.016 | |
| PNUH * | |||||||
| SCHBUH | 2.014 | 2.093 | −0.051 | 2.009 | 2.052 | −0.033 | |
| SCHCAH | 1.742 | 2.031 | −0.18 | 1.926 | 1.901 | 0.019 | |
| SCHGUH | 1.837 | 1.814 | 0.015 | 1.815 | 1.848 | −0.026 | |
| SCHSUH | 2.362 | 2.656 | −0.173 | 2.595 | 2.608 | −0.008 | |
| 1:1 Matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DPP4is/Met | Other GLDs | ||||||||
| Patients | Person-Years | Events | IR | Patients | Person-Years | Events | IR | MDRR | |
| AJUH | 1339 | 2758 | 27 | 9.79 | 1339 | 2651 | 40 | 15.09 | 1.98 |
| KHMC | 788 | 1703 | 12 | 7.04 | 788 | 1626 | 39 | 23.98 | 2.19 |
| DCMC | 868 | 1913 | 14 | 7.32 | 868 | 1779 | 38 | 21.36 | 2.17 |
| SCHSUH | 592 | 1222 | 12 | 9.82 | 592 | 1131 | 14 | 12.37 | 3.00 |
| SCHBUH | 762 | 1607 | 7 | 4.35 | 762 | 1511 | 20 | 13.23 | 2.94 |
| SCHCAH | 635 | 1251 | 14 | 11.19 | 635 | 1172 | 22 | 18.76 | 2.54 |
| SCHGUH | 211 | 452 | 5 | 11.05 | 211 | 408 | 7 | 17.16 | 5.04 |
| MJH | 420 | 810 | 5 | 6.17 | 420 | 770 | 8 | 10.39 | 4.73 |
| GNUH | 241 | 488 | 5 | 10.23 | 241 | 474 | 8 | 16.86 | 4.73 |
| KUDH | 330 | 613 | 7 | 11.42 | 330 | 671 | 12 | 17.86 | 3.62 |
| PNUH | 488 | 1018 | 21 | 20.62 | 488 | 969 | 43 | 44.37 | 2.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.L.; Yi, Y.H.; Lee, J.G.; Tak, Y.J.; Lee, S.H.; Ra, Y.J.; Choi, B.K.; Lee, S.Y.; Cho, Y.H.; Park, E.J.; et al. Association Between the Use of DPP4 Inhibitors and Metformin and the Risk of Cancer in Patients with Type 2 Diabetes: A Multicenter Retrospective Cohort Study Using the OMOP CDM Database. Cancers 2025, 17, 3620. https://doi.org/10.3390/cancers17223620
Kim GL, Yi YH, Lee JG, Tak YJ, Lee SH, Ra YJ, Choi BK, Lee SY, Cho YH, Park EJ, et al. Association Between the Use of DPP4 Inhibitors and Metformin and the Risk of Cancer in Patients with Type 2 Diabetes: A Multicenter Retrospective Cohort Study Using the OMOP CDM Database. Cancers. 2025; 17(22):3620. https://doi.org/10.3390/cancers17223620
Chicago/Turabian StyleKim, Gyu Lee, Yu Hyeon Yi, Jeong Gyu Lee, Young Jin Tak, Seung Hun Lee, Young Jin Ra, Byung Kwan Choi, Sang Yeoup Lee, Young Hye Cho, Eun Ju Park, and et al. 2025. "Association Between the Use of DPP4 Inhibitors and Metformin and the Risk of Cancer in Patients with Type 2 Diabetes: A Multicenter Retrospective Cohort Study Using the OMOP CDM Database" Cancers 17, no. 22: 3620. https://doi.org/10.3390/cancers17223620
APA StyleKim, G. L., Yi, Y. H., Lee, J. G., Tak, Y. J., Lee, S. H., Ra, Y. J., Choi, B. K., Lee, S. Y., Cho, Y. H., Park, E. J., Lee, Y., Choi, J. I., Lee, S. R., Kwon, R. J., & Son, S. M. (2025). Association Between the Use of DPP4 Inhibitors and Metformin and the Risk of Cancer in Patients with Type 2 Diabetes: A Multicenter Retrospective Cohort Study Using the OMOP CDM Database. Cancers, 17(22), 3620. https://doi.org/10.3390/cancers17223620









