Teclistamab Dosing Strategies in Relapsed/Refractory Myeloma: A Real-World Comparison of Weekly and Biweekly Versus Fixed Intervals
Simple Summary
Abstract
1. Communication
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajkumar, S.V. Multiple Myeloma: 2024 update on Diagnosis, Risk-stratification and Management. Am. J. Hematol. 2024, 99, 1802–1824. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.; Radpour, R.; Ochsenbein, A.F. Molecular and immunological mechanisms of clonal evolution in multiple myeloma. Front Immunol. 2023, 14, 1243997. [Google Scholar] [CrossRef] [PubMed]
- Management of Relapsed/Refractory Multiple Myeloma. 1 June 2023. Available online: https://jnccn.org/view/journals/jnccn/21/5.5/article-p552.xml (accessed on 23 October 2025).
- Moreau, P.; Garfall, A.L.; Donk, N.W.C.J.; van de Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Rees, M.; Abdallah, N.; Yohannan, B.; Gonsalves, W.I. Bispecific antibody targets and therapies in multiple myeloma. Front Immunol. 2024, 15, 1424925. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Karlin, L.; Benboubker, L.; Nahi, H.; San-Miguel, J.; Trancucci, D.; Qi, K.; Stephenson, T.; Perales-Puchalt, A.; Chastain, K.; et al. Durability of Responses with Biweekly Dosing of Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma Achieving a Clinical response in the majesTEC-1 Study. J. Clin. Oncol. 2023, 41, 8034. [Google Scholar] [CrossRef]
- Burgos, L.; Puig, N.; Cedena, M.T.; Mateos, M.V.; Lahuerta, J.J.; Paiva, B.; San-Miguel, J.F. Measurable residual disease in multiple myeloma: Ready for clinical practice? J. Hematol. Oncol. 2020, 13, 82. [Google Scholar] [CrossRef]
- Stork, M.; Radocha, J.; Mihalyova, J.; Spicka, I.; Pika, T.; Jungova, A.; Boichuk, I.; Mensikova, K.; Straub, J.; Sedlak, F.; et al. De-escalated Teclistamab dosing in relapsed/refractory multiple myeloma: Czech myeloma group real-world evidence analysis. Ann. Hematol. 2025, 104, 4141–4147. [Google Scholar] [CrossRef]
- Martin, T.G.; Moreau, P.; Usmani, S.Z.; Garfall, A.L.; Mateos, M.-V.; San-Miguel, J.F.; Rocafiguera, A.O.; Nooka, A.K.; Rosiñol, L.; Chari, A.; et al. Health-Related Quality of Life in Patients with Relapsed/Refractory Multiple Myeloma (RRMM) Treated with Teclistamab, a B-Cell Maturation Antigen (BCMA) × CD3 Bispecific Antibody: Patient-Reported Outcomes in MajesTEC-1. J. Clin. Oncol. 2022, 40, 8033. [Google Scholar] [CrossRef]
- Herrera, M.; Pretelli, G.; Desai, J.; Garralda, E.; Siu, L.L.; Steiner, T.M.; Au, L. Bispecific antibodies: Advancing precision oncology. Trends Cancer 2024, 10, 893–919. [Google Scholar] [CrossRef] [PubMed]
- Delforge, M.; Patel, K.; Eliason, L.; Dhanda, D.; Shi, L.; Guo, S.; Marshall, T.S.; Arnulf, B.; Cavo, M.; Nooka, A.; et al. Health-related quality of life in patients with triple-class exposed relapsed and refractory multiple myeloma treated with idecabtagene vicleucel or standard regimens: Patient-reported outcomes from the phase 3, randomised, open-label KarMMa-3 clinical trial. Lancet Haematol. 2024, 11, e216–e227. [Google Scholar] [PubMed]
- Janssen Research & Development, LLC. A Multi-Arm Phase 1b Study of Teclistamab with Other Anticancer Therapies in Participants with Multiple Myeloma [Internet]. Clinicaltrials.gov; 2025 Oct. Report No.: NCT04722146. Available online: https://clinicaltrials.gov/study/NCT04722146 (accessed on 23 October 2025).
- Rajkumar, S.V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 548–567. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Avet-Loiseau, H.; Lonial, S.; Usmani, S.; Siegel, D.; Anderson, K.C.; Chng, W.J.; Moreau, P.; Attal, M.; Kyle, R.A.; et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 2016, 127, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, K.A.; Verkleij, C.P.M.; Mateos, M.V.; Martin, T.G.; Rodriguez, C.; Nooka, A.; Banerjee, A.; Chastain, K.; Perales-Puchalt, A.; Stephenson, T.; et al. Teclistamab impairs humoral immunity in patients with heavily pretreated myeloma: Importance of immunoglobulin supplementation. Blood Adv. 2023, 8, 194–206. [Google Scholar] [CrossRef]
- Hammons, L.; Szabo, A.; Janardan, A.; Bhatlapenumarthi, V.; Annyapu, E.; Dhakal, B.; Al Hadidi, S.; Radhakrishnan, S.V.; Narra, R.; Bhutani, D.; et al. The changing spectrum of infection with BCMA and GPRC5D targeting bispecific antibody (bsAb) therapy in patients with relapsed refractory multiple myeloma. Haematologica 2023, 109, 906–914. [Google Scholar] [CrossRef]
- Chunara, F.; Lugo, C.; Osinski, K.; Shah, M.R.; Shah, N.; Kent, J.; Mohyuddin, G.R.; Radhakrishnan, S.V.; Kaur, G.; Chakraborty, R.; et al. Real-world treatment patterns for teclistamab and talquetamab in multiple myeloma (MM): Experience from 609 patients. Blood Cancer J. 2025, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Nooka, A.K.; Rodriguez, C.; Mateos, M.V.; Manier, S.; Chastain, K.; Banerjee, A.; Kobos, R.; Qi, K.; Verona, R.; Doyle, M.; et al. Incidence, timing, and management of infections in patients receiving teclistamab for the treatment of relapsed/refractory multiple myeloma in the MajesTEC-1 study. Cancer 2024, 130, 886–900. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Rasche, L.; Sidana, S.; Zweegman, S.; Garfall, A.L. T Cell–Redirecting Bispecific Antibodies in Multiple Myeloma: Optimal Dosing Schedule and Duration of Treatment. Blood Cancer Discov. 2024, 5, 388–399. [Google Scholar] [CrossRef]
- Reynolds, G.; Cliff, E.R.S.; Mohyuddin, G.R.; Popat, R.; Midha, S.; Liet Hing, M.N.; Harrison, S.J.; Kesselheim, A.S.; Teh, B.W. Infections following bispecific antibodies in myeloma: A systematic review and meta-analysis. Blood Adv. 2023, 7, 5898–5903. [Google Scholar] [CrossRef]
- Mohan, M.; Szabo, A.; Cheruvalath, H.; Clennon, A.; Bhatlapenumarthi, V.; Patwari, A.; Balev, M.; Bhutani, D.; Shrestha, A.; Thanendrarajan, S.; et al. Effect of Intravenous Immunoglobulin (IVIG) Supplementation on infection-free survival in recipients of BCMA-directed bispecific antibody therapy for multiple myeloma. Blood Cancer J. 2025, 15, 74. [Google Scholar] [CrossRef]
- FDA Approves Teclistamab-Cqyv for Relapsed or Refractory Multiple Myeloma|FDA [Internet]. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-teclistamab-cqyv-relapsed-or-refractory-multiple-myeloma (accessed on 23 October 2025).
- Derman, B.A. MRD-guided treatment cessation in multiple myeloma. Lancet Haematol. 2023, 10, e867–e868. [Google Scholar] [CrossRef]
- Tan, C.R.; Asoori, S.; Huang, C.Y.; Brunaldi, L.; Popat, R.; Kastritis, E.; Martinez-Lopez, J.; Bansal, R.; Silva Corraes, A.M.; Chhabra, S.; et al. Real-world evaluation of teclistamab for the treatment of relapsed/refractory multiple myeloma (RRMM): An International Myeloma Working Group Study. Blood Cancer J. 2025, 15, 53. [Google Scholar] [CrossRef]
- Derman, B.; Tan, C.; Steinfield, I.; Wilson, F.R.; Lin, D.; Wu, B.; Fernandez, M.; Fowler, J.; Paner-Straseviciute, A.; Kim, N.; et al. Real-World Evidence Evaluating Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: A Systematic Literature Review. Cancers 2025, 17, 1235. [Google Scholar] [CrossRef] [PubMed]
- Garfall, A.L.; Nooka, A.K.; van de Donk, N.W.C.J.; Moreau, P.; Bhutani, M.; Oriol, A.; Martin, T.G.; Rosiñol, L.; Mateos, M.-V.; Bahlis, N.J.; et al. Long-term follow-up from the phase 1/2 MajesTEC-1 trial of teclistamab in patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2024, 42 (Suppl. 16), 7540. [Google Scholar] [CrossRef]
- D’Souza, A.; Costa, L.J.; San-Miguel, J.F.; Berdeja, J.G.; Morillo Giles, D.; Touzeau, C.; McKay, J.; Martin, T.G.; Dholaria, B.; Perrot, A.; et al. Teclistamab, Daratumumab, and Pomalidomide in Patients with Relapsed/Refractory Multiple Myeloma: Results from the Majestec-2 Cohort a and Trimm-2 Studies. Blood 2024, 144 (Suppl. S1), 495. [Google Scholar] [CrossRef]
- Searle, E.; Quach, H.; Wong, S.W.; Costa, L.J.; Hulin, C.; Janowski, W.; Berdeja, J.; Anguille, S.; Matous, J.V.; Touzeau, C.; et al. Teclistamab in Combination with Subcutaneous Daratumumab and Lenalidomide in Patients with Multiple Myeloma: Results from One Cohort of MajesTEC-2, a Phase1b, Multicohort Study. Blood 2022, 140 (Suppl. 1), 394–396. [Google Scholar] [CrossRef]
- Cohen, Y.C.; Magen, H.; Gatt, M.; Sebag, M.; Kim, K.; Min, C.K.; Ocio, E.M.; Yoon, S.-S.; Chu, M.P.; Rodríguez-Otero, P.; et al. Talquetamab plus Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2025, 392, 138–149. [Google Scholar] [CrossRef] [PubMed]
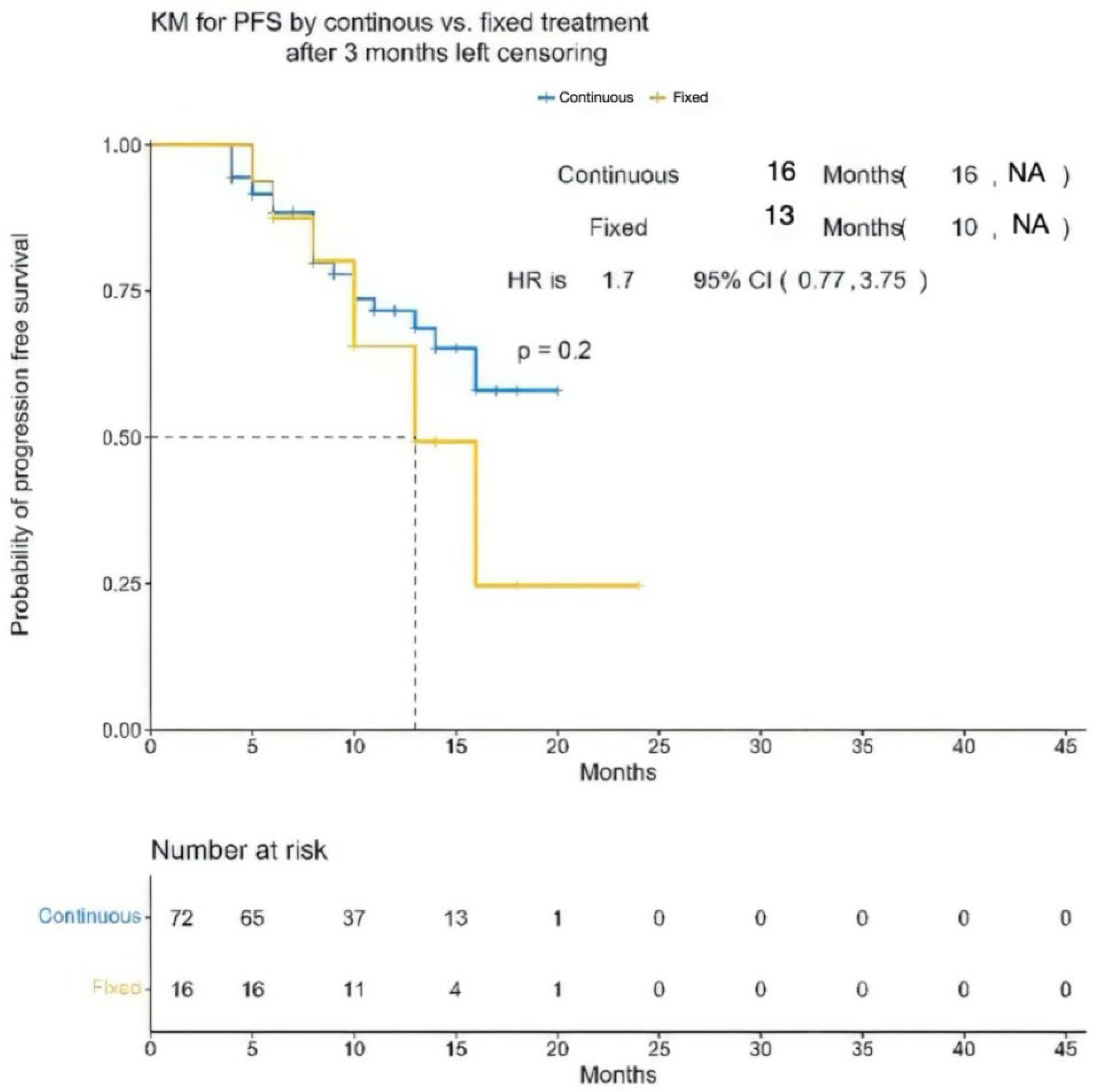
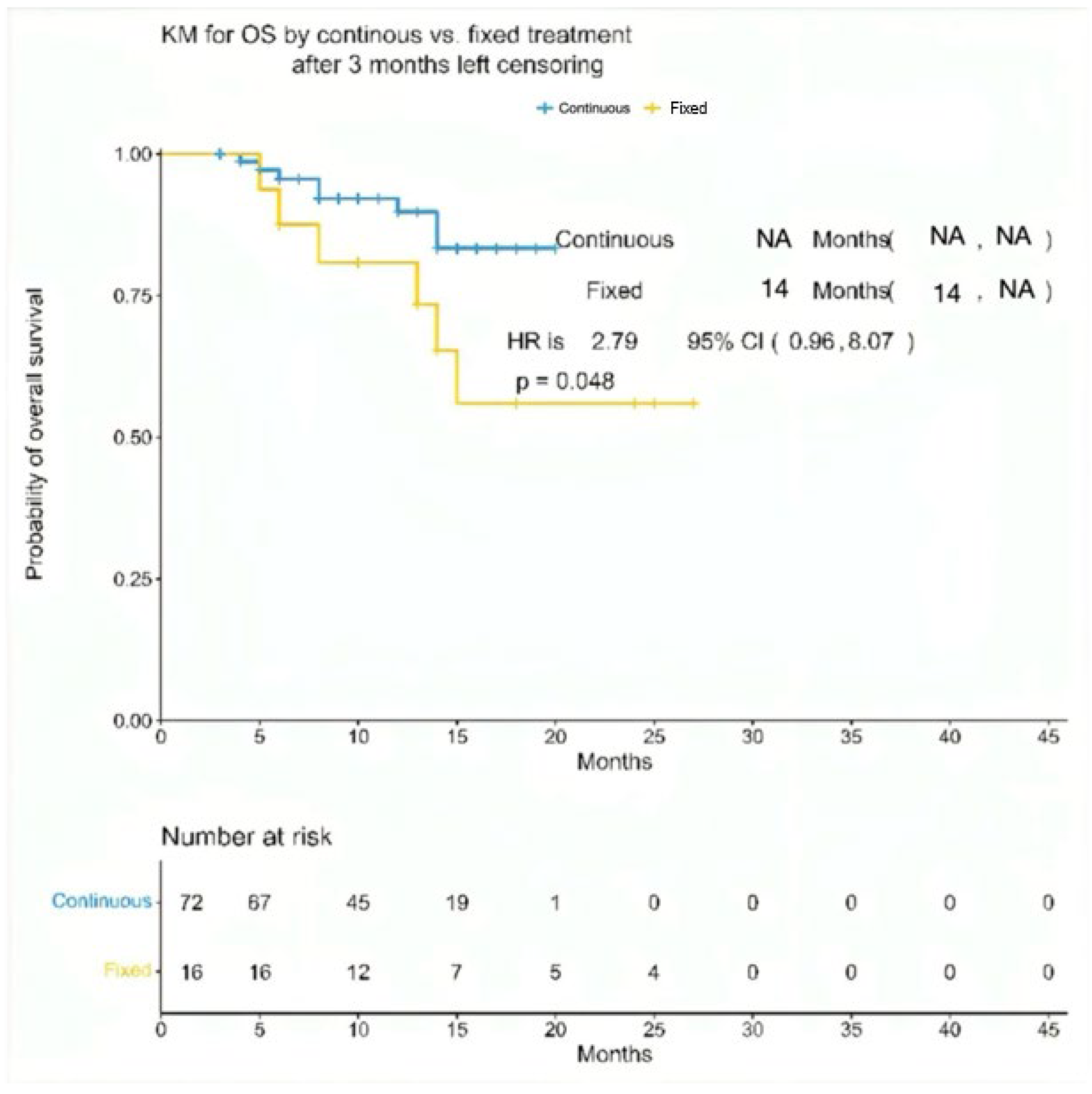
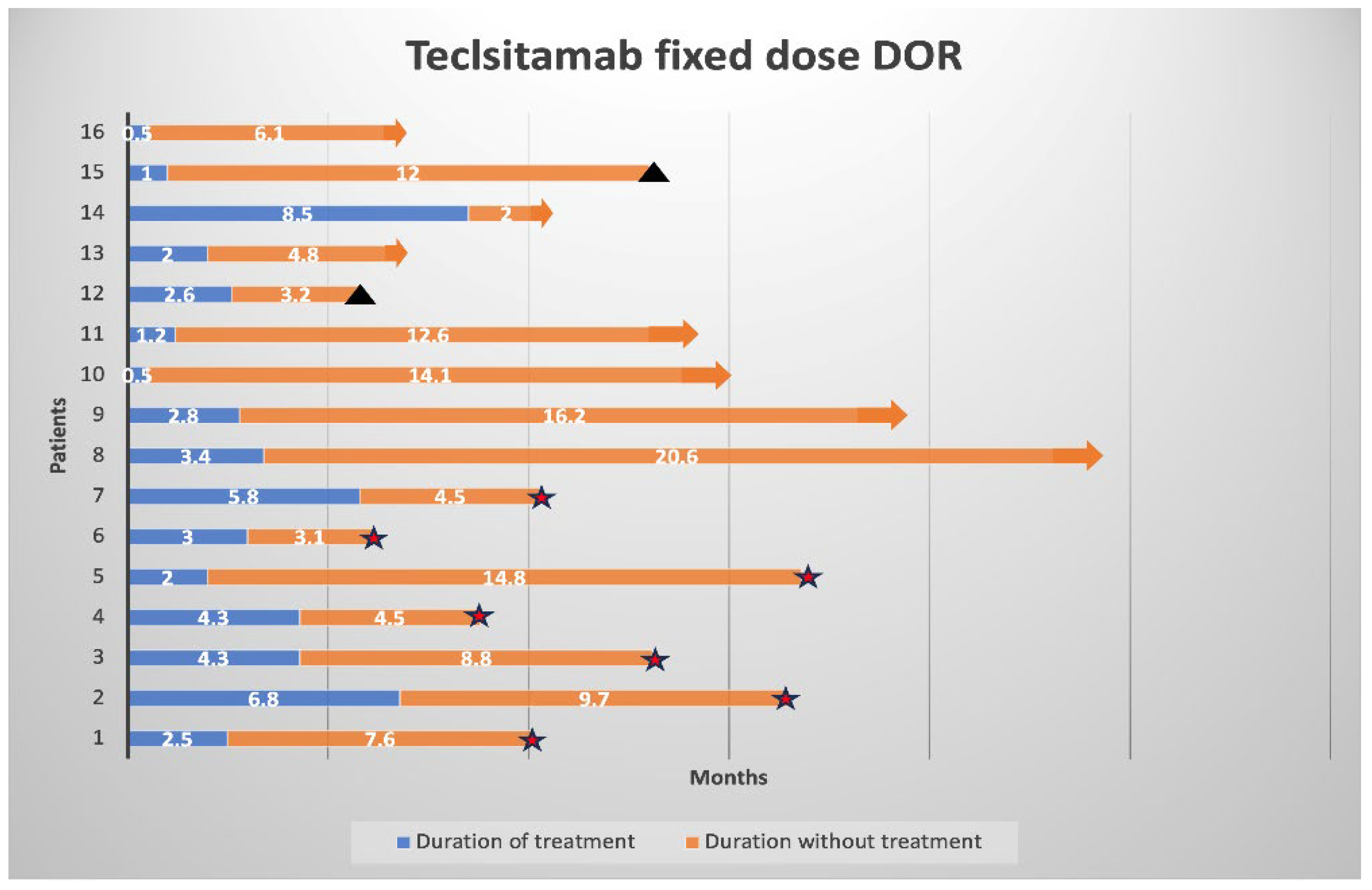
| Characteristic | Overall (N = 88) | Teclistamab (Continuous Cohort) (N = 72) | Teclistamab (Fixed Cohort) (N = 16) | p-Value |
|---|---|---|---|---|
| Age, Median (Range) | 71 (64–84) | 69 (64–73) | 73 (70–84) | 0.018 |
| Gender, n (%) | 0.7 | |||
| Female | 40 (45) | 32 (44) | 8 (50) | |
| Male | 48 (55) | 40 (56) | 8 (50) | |
| Race, n (%) | 0.7 | |||
| Caucasian | 73 (83) | 60 (83) | 13 (81) | |
| African American | 11 (13) | 9 (13) | 2 (13) | |
| Asian | 2 (2) | 2 (3) | 0 | |
| Hispanic | 2 (2) | 1 (1) | 1 (6) | |
| Isotype, n (%) | 0.3 | |||
| IgG | 49 (56) | 39 (54) | 10 (63) | |
| Non-IgG | 18 (20) | 16 (22) | 2 (13) | |
| Light chain only | 21 (24) | 17 (243) | 4 (25) | |
| R-ISS stage, n (%) | 0.6 | |||
| I | 18 (20) | 12 (17) | 5 (31) | |
| II | 41 (47) | 34 (47) | 7 (44) | |
| III | 18 (20) | 15 (21) | 3 (19) | |
| Unknown | 12 (14) | 11 (15) | 1 (6) | |
| * High-risk cytogenetics, n (%) | 38 (43) | 31(43) | 7 (44) | >0.9 |
| Extramedullary disease, n (%) | 18 (20) | 15 (21) | 3 (19) | >0.9 |
| Prior lines of therapy Median (range) | 5 (4–7) | 5.5 (4.5–7.5) | >0.9 | |
| ECOG PS, n (%) | 0.14 | |||
| 0–1 | 70 (80) | 59 (82) | 11 (69) | |
| ≥2 | 18 (20) | 13 (18) | 5 (31) | |
| Prior therapies exposed, n (%) | ||||
| Bortezomib | 86 (98) | 71 (99) | 15 (94) | 0.3 |
| Carfilzomib | 79 (90) | 64 (89) | 15 (94) | >0.9 |
| PI exposure | 88 (100) | 72 (100) | 16 (100) | >0.9 |
| Lenalidomide | 86 (98) | 70 (97) | 16 (100) | >0.9 |
| Pomalidomide | 82 (93) | 67 (93) | 15 (94) | >0.9 |
| IMiD exposure | 88 (100) | 72 (100) | 16 (100) | >0.9 |
| Anti-CD38 MoAb | 88 (100) | 72 (100) | 16 (100) | >0.9 |
| ASCT | 55 (63) | 46 (64) | 9 (56) | 0.6 |
| Refractory status, n (%) | ||||
| Bortezomib | 62 (70) | 50 (69) | 12 (75) | 0.8 |
| Carfilzomib | 71 (80) | 58 (81) | 13 (81) | >0.9 |
| PI refractory | 75 (85) | 61 (85) | 14 (88) | >0.9 |
| Lenalidomide | 68 (77) | 55 (76) | 13 (81) | >0.9 |
| Pomalidomide | 76 (86) | 61 (85) | 15 (94) | 0.7 |
| IMiD refractory | 78 (89) | 63 (88) | 15 (94) | 0.7 |
| Anti-CD38 MoAb | 84 (95) | 68 (94% | 16 (100) | >0.9 |
| Double refractory | 75 (85) | 62 (86) | 13 (81) | 0.7 |
| Triple Exposed | 87 (99) | 71 (99) | 16 (100) | >0.9 |
| Triple Refractory | 71 (81) | 58 (81) | 13 (81) | >0.9 |
| Penta Exposed | 71 (81) | 57 (79) | 14 (88) | 0.7 |
| Penta Refractory | 46 (52) | 37 (51) | 9 (56) | 0.7 |
| BCMA ADC (Belantamab) | 15 (17) | 11 (15) | 4 (25) | 0.5 |
| * BCMA CAR-T cell | 24 (27) | 17 (26) | 7 (43) | 0.2 |
| BDT exposed | 35 (40) | 26 (36) | 9 (56) | 0.14 |
| BDT refractory | 30 (34) | 21 (29) | 9 (56) | 0.039 |
| Adverse Events (AEs) n (%) | Teclistamab Continuous Cohort (N = 72) | Teclistamab Fixed Cohort (N = 16) | p-Value | ||
|---|---|---|---|---|---|
| Any Grades | Grade 3/4 | Any Grades | Grade 3/4 | ||
| CRS | 39 (54) | 2 (3) | 9 (57) | 0 | 0.8 |
| ICANS | |||||
| Elevated Liver function enzymes | 11 (15) | 1 (1) | 4 (25) | 0 | 0.4 |
| Neutropenic fever | 4 (6) | 4 (6) | 1 (6) | 1 (6) | >0.9 |
| Infection | 42 (59) | 16 (22) | 12 (75) | 11 (69) | <0.001 |
| * Hematological AE | |||||
| Leukopenia | 26 (36) | 4 (6) | 5 (32) | 3 (19) | 0.14 |
| Neutropenia | 18 (25) | 8 (11) | 5 (32) | 4 (26) | 0.2 |
| Anemia | 36 (49) | 3 (4) | 9 (56) | 0 | 0.5 |
| Thrombocytopenia | 29 (40) | 11 (15) | 6 (38) | 2 (13) | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snyder, J.; Atrash, S.; Paul, B.; Khan, A.M.; Habib, A.; Shaikh, H.; Strouse, C.; Alkharabsheh, O.; Mazloom, A.; Ahmed, N.; et al. Teclistamab Dosing Strategies in Relapsed/Refractory Myeloma: A Real-World Comparison of Weekly and Biweekly Versus Fixed Intervals. Cancers 2025, 17, 3569. https://doi.org/10.3390/cancers17213569
Snyder J, Atrash S, Paul B, Khan AM, Habib A, Shaikh H, Strouse C, Alkharabsheh O, Mazloom A, Ahmed N, et al. Teclistamab Dosing Strategies in Relapsed/Refractory Myeloma: A Real-World Comparison of Weekly and Biweekly Versus Fixed Intervals. Cancers. 2025; 17(21):3569. https://doi.org/10.3390/cancers17213569
Chicago/Turabian StyleSnyder, Jordan, Shebli Atrash, Barry Paul, Abdullah Mohammad Khan, Alma Habib, Hira Shaikh, Christopher Strouse, Omar Alkharabsheh, Anita Mazloom, Nausheen Ahmed, and et al. 2025. "Teclistamab Dosing Strategies in Relapsed/Refractory Myeloma: A Real-World Comparison of Weekly and Biweekly Versus Fixed Intervals" Cancers 17, no. 21: 3569. https://doi.org/10.3390/cancers17213569
APA StyleSnyder, J., Atrash, S., Paul, B., Khan, A. M., Habib, A., Shaikh, H., Strouse, C., Alkharabsheh, O., Mazloom, A., Ahmed, N., Mahmoudjafari, Z., Mushtaq, M. U., Zayad, A., McGuirk, J., Tiger, Y. K., Shah, M. R., & Abdallah, A.-O. (2025). Teclistamab Dosing Strategies in Relapsed/Refractory Myeloma: A Real-World Comparison of Weekly and Biweekly Versus Fixed Intervals. Cancers, 17(21), 3569. https://doi.org/10.3390/cancers17213569







