Investigating the Trajectories of Association Between Biomarkers and Cancer-Related Cognitive Impairment in Patients with Breast Cancer: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources, Search, and Selection Criteria
2.2. Study Selection
2.3. Data Extraction and Synthesis
2.4. Risk of Bias and Methodological Quality Assessment
3. Results and Characteristics of Included Studies
3.1. Selected Studies
3.2. Assessment Timepoints
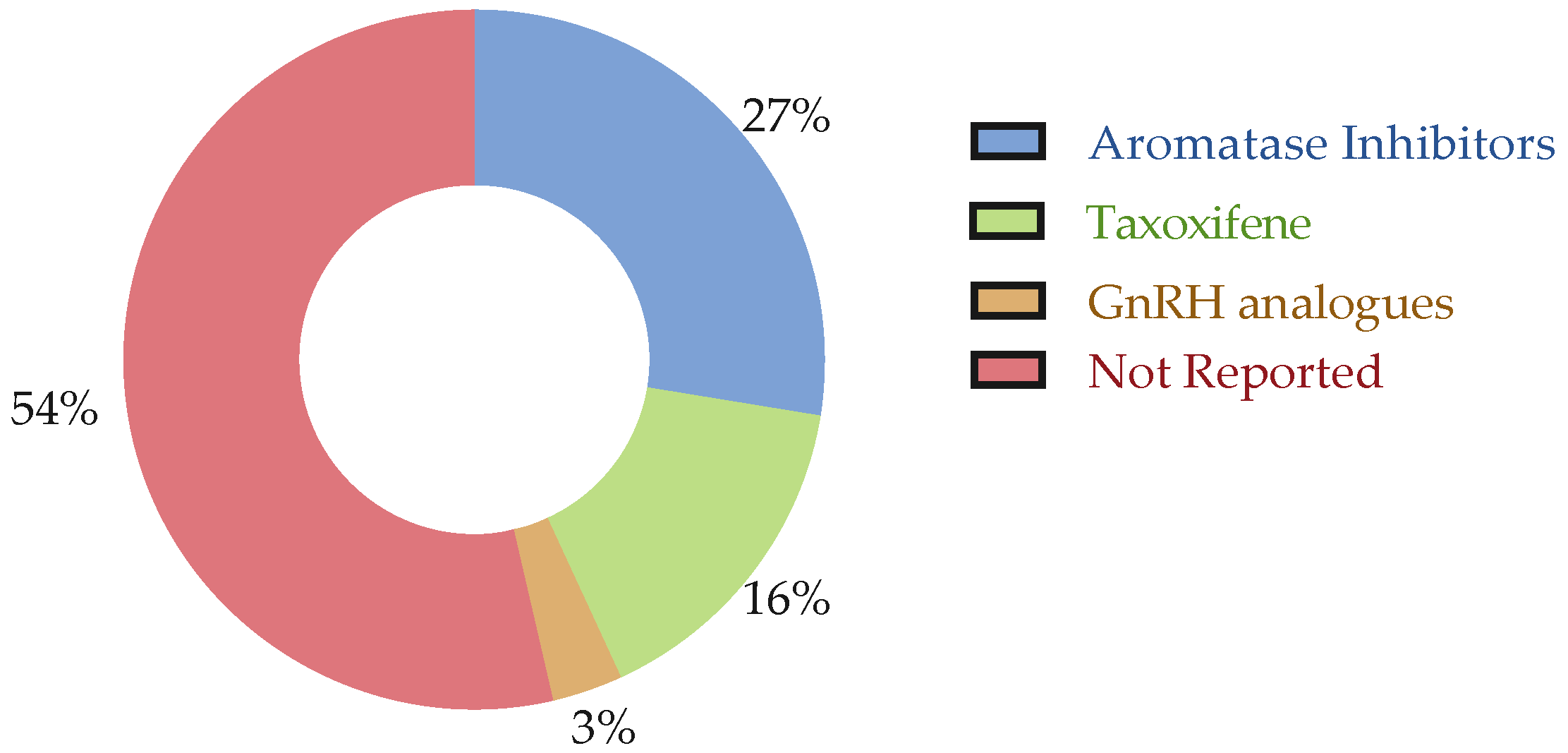
3.3. Treatment Regimes
3.4. Outcome Measures
4. Risk of Bias Assessment and Quality Appraisal
5. Relationship Between Biochemical Biomarkers and CRCI
5.1. Stress-Axis Biomarkers
5.2. Pro-Inflammatory Cytokines
5.3. Anti-Inflammatory Cytokines
5.4. TNF Pathway Markers
5.5. Other Inflammatory Markers
5.6. Growth Hormones
5.7. Cluster of Differentiation (CD) Markers
5.8. Tumor-Associated Antigens
5.9. BDNF
5.10. Null Findings
6. Genetic Biomarkers
6.1. DNA Repair and Oxidative Stress Pathways
6.2. Telomere Biology
6.3. Cytokine Genotypes
6.4. Epigenetics
6.5. APOE
6.6. COMT
6.7. BDNF (Val66Met)
6.8. DRD2
6.9. Genome-Wide Association Findings (GWAS)
6.10. Other Polymorphisms
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cognitive Tests/Scales/Questionnaires | |
| ANAM | Automated Neuropsychological Assessment Metric |
| BCPT | Breast Cancer Prevention Trial Symptom Checklist |
| BCT | Brief Cognitive Test |
| BRIEF-A | Behavior Rating Inventory of Executive Function-Adult Version |
| BTACT | Telephone Brief Test of Adult Cognition |
| BVMT-DR | Brief Visuospatial Memory Test—Delayed Recall |
| BVMT-R | Brief Visuospatial Memory Test Revised |
| BVMT-TR | Brief Visuospatial Memory Test—Total Recall |
| CANTAB | Cambridge Neuropsychological Test Automated Battery |
| CNSVS | Central Nervous System Vital Signs™ |
| COWA/COWAT | Controlled Oral Word Association |
| CPT-3 | Continuous Performance Test—Third Edition |
| CTT | Color Trails Test |
| CVLT-2 | California Verbal Learning Test 2nd Edition |
| D-KEFS | Delis-Kaplan Executive Function System |
| DLCT | Double Letter Cancelation Task |
| DMS | Delayed Matching-to-Sample |
| DST | Digit Span Test |
| EBPM | Event-Based Prospective Memory Task |
| FACT-Cog | Functional Assessment of Cancer Therapy-Cognitive Function |
| FACT-PCA | Functional Assessment of Cancer Therapy—Perceived Cognitive Abilities |
| FACT-PCI | Functional Assessment of Cancer Therapy—Perceived Cognitive Impairments |
| HVLT-D | Hopkins Verbal Learning Test—Delayed Recall |
| HVLT-I | Hopkins Verbal Learning Test—Immediate Recall |
| HVLT-R | Hopkins Verbal Learning Test-Revised |
| LNS | Letter–Number Sequencing |
| MASQ | Multiple Ability Self-Report Questionnaire |
| MMQ | Multifactorial Memory Questionnaire Ability Scale |
| MMSE | Mini Mental State Examination |
| MOCA | Montreal Cognitive Assessment |
| NAB | Neuropsychological Assessment Battery |
| NABC | Neuropsychological Assessment Battery—Cognition |
| NART | National Adult Reading Test |
| NART-R | National Adult Reading Test-Revised |
| NIHTB-CB | NIH Toolbox Cognition Battery |
| OFT | Orthographical Fluency |
| OTS | One-Touch Stockings of Cambridge |
| PAOFI | Patient Assessment of Own Functioning Inventory |
| PASAT | Rao Paced Auditory Serial Addition Test |
| PMRQ | Penn Memory-Related Questionnaire |
| PRMQ | Prospective and Retrospective Memory Questionnaire |
| PROMIS | Patient-Reported Outcomes Measurement Information System |
| PVT | Psychomotor Vigilance Test |
| RAVLT | Rey Auditory-Verbal Learning Test |
| ROCF | Rey Osterreith Complex Figure |
| RVP | Rapid Visual Information Processing |
| SDMT | Symbol Digit Modalities Test |
| SMQ | Squire Memory Questionnaire |
| STAI-S | State-Trait Anxiety Inventory-State Scale |
| TBPM | Time-Based Prospective Memory Task |
| TMT A & B | Trail Making Test A & B |
| VFT | Verbal Fluency Test |
| VPA | Verbal Paired Associates |
| VRM | Verbal Recognition Memory |
| WAIS | Wechsler Adult Intelligence Scale |
| WASI | Wechsler Abbreviated Scale of Intelligence |
| WRAT | Wide Range Achievement Test |
| WTAR | Wechsler Test of Adult Reading |
| Cognitive domains | |
| APE | Attention—Processing Speed—Executive function |
| GDS | Global Deficit Score |
| LM | Logical Memory |
| PM | Prospective Memory |
| RM | Recognition Memory |
| RM | Retrospective Memory |
| Biomarkers | |
| ACTH | Adrenocorticotropic Hormone |
| AKAP6 | A-Kinase Anchoring Protein 6 |
| ALDH2 | Aldehyde Dehydrogenase 2 |
| ANKK1 | Ankyrin Repeat and Kinase Domain Containing 1 |
| APBA1 | Amyloid Beta Precursor Protein-Binding Family A Member 1 |
| APOE | Apolipoproteina E |
| ARPP21 | cAMP-Regulated Phosphoprotein 21 |
| AURKA | Aurora Kinase A |
| Aβ | Amyloid-Beta |
| BAG1 | BCL-2-Associated Thanogene 1 |
| BCL | B-Cell Lymphoma |
| BDNF | Brain-Derived Neurotrophic Factor |
| BIRC5 | Baculoviral IAP Repeat Containing 5 |
| BRCA | Breast Cancer Susceptibility Gene |
| CA153 | Carbohydrate Antigen 153 |
| CAT | Catalase |
| CCDC170 | Coiled-Coil Domain Containing 170 |
| CCNB1 | Cyclin B |
| CD | Cluster of Differentiation |
| CDKN2B | Cyclin-Dependent Kinase Inhibitor 2B |
| CEA | Carcinoembryonic Antigen |
| CENPA | Centromere Protein A |
| CHD13 | Chromodomain Helicase DNA-Binding Protein 13 |
| CMC2 | C-X9-C Motif Containing 2 |
| COMT | Catechol-O-Methyltransferase |
| CpGs | Cytosine-Phosphate-Guanine Sites |
| CRP | C Reactive Protein |
| CTSL2 | Cathepsin L2 |
| CXCL12 | C-X-C Motif Chemokine Ligand 12 |
| CYP2D6 | Cytochrome P450 Family 2 Subfamily D Member 6 |
| DDHD1 | DDHD Domain Containing 1 |
| DGKA | Diacylglycerol Kinase Alpha |
| DHEA | Dehydroepiandrosterone |
| DIAPH3 | Diaphanous-Related Formin 3 |
| DNMT1 gene | DNA Methyltransferase 1 Gene |
| DRD2 | Dopamine Receptor D2 |
| ECE2 | Endothelin-Converting Enzyme 2 |
| ERBB2 | Human Epidermal Growth Factor Receptor 2 |
| ERCC | Excision Repair Cross-Complementation |
| ESR1 | Estrogen Receptor 1 |
| FGF-2 | Fibroblast Growth Factor 2 |
| FSH | Follicle-Stimulating Hormone |
| G/A | Guanine/Adenine |
| G-CSF | Granulocyte-Colony Stimulating Factor |
| GLR | Granulocyte-to-Lymphocyte Ratio |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| GnRH | Gonadotropin-Releasing Hormone |
| GPX1 | Glutathione Peroxidase 1 |
| GRB7 | Growth Factor Receptor-Bound Protein 7 |
| GST | Glutathione S-Transferase Genes |
| GSTM1 | Glutathione S-Transferase Mu 1 |
| HDL-C | High-Density Lipoprotein Cholesterol |
| HSD17B3 | Hydroxysteroid 17-Beta Dehydrogenase 3 |
| HTR2A | 5-Hydroxytryptamine (Serotonin) Receptor 2A |
| IFNGR1 | Interferon Gamma Receptor 1 |
| IFN-γ | Interferon-γ |
| IGF-1 | Insulin Growth Factor-1 |
| IL1R1 | IL1 Receptor Type 1 |
| IL-1ra | IL-1 Receptor Antagonist |
| ILs | Interleukins |
| KLF5 | Krüppel-Like Factor 5 |
| LBP | Lipopolysaccharide-Binding Protein |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| LH | Luteinizing Hormone |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MDR1 | Multidrug Resistance 1 |
| MELK | Maternal Embryonic Leucine Zipper Kinase |
| MIP-1β | Macrophage Inflammatory Protein-1β |
| MIR125A | MicroRNA-125a |
| MLR | Monocyte-to-Lymphocyte Ratio |
| MMP11 | Matrix Metallopeptidase 11 |
| mtDNA | Mitochondrial DNA |
| MTHFR | Methylenetetrahydrofolate Reductase |
| MYBL2 | MYB Proto-Oncogene-Like 2 |
| NDC80 | Nuclear Division Cycle 80 |
| NDE | Neuron-Derived Exosomes |
| NDST3 | N-Deacetylase/N-Sulfotransferase 3 |
| NFE2L2 | Nuclear Factor Erythroid 2 Like 2 |
| NF-KB | Nuclear Factor Kappa B |
| NGF | Nerve Growth Factor |
| NLR | Neutrophil-Lymphocyte Ratio |
| ORC6 | Origin Recognition Complex Subunit 6 |
| PARP1 | Poly (ADP-ribose) Polymerase 1 |
| PBMC | Peripheral Blood Mononuclear Cell |
| PGR | Progesterone Receptor |
| PIV | Pan-Immune-Inflammation Value |
| PLR | Platelet-to-Lymphocyte Ratio |
| POC5 | Proteome Of Centriole 5 |
| PPFIBP2 | Interacting Protein Binding Protein 2 |
| RACGAP1 | Rac GTPase Activating Protein 1 |
| RASA2 gene | RAS p21 Protein Activator 2 Gene |
| RFC4 | Replication Factor C Subunit 4 |
| RIPOR2 | Rho Family-Interacting Cell Polarization Regulator 2 |
| RPS6KA1 | Ribosomal Protein S6 Kinase A1 |
| RRM2 | Ribonucleotide Reductase Regulatory Subunit M2 |
| rScO or rcSO2 | Regional Cerebral Oxygen Saturation |
| SCUBE2 | Signal Peptide-CUB-EGF Domain-Containing Protein 2 |
| SEPP1 | Selenoprotein P Plasma 1 |
| SII | Systemic Immune-Inflammation Index |
| SLC6A4 | Solute Carrier Family 6 Member 4 |
| SNPs | Single Nucleotide Polymorphisms |
| SOD | Superoxide Dismutase |
| sTNFRI | TNF Receptor Type I |
| sTNFRII | Soluble Form of the TNF Receptor Type II |
| Tau | Tubulin-Associated Unit |
| TMEM161B | Transmembrane Protein 161B |
| TNF | Tumor Necrosis Factor |
| TNF-α | Tumor Necrosis Factor-Alpha |
| TOMM40 | Translocase of Outer Mitochondrial Membrane 40 |
| UBE2V1 | Ubiquitin-Conjugating Enzyme E2 Variant 1 |
| USP6NL | Ubiquitin Specific Peptidase 6 N-Terminal Like |
| VEGF-A | Vascular Endothelial Growth Factor |
| VLDL-C | Very-Low-Density Lipoprotein Cholesterol |
| Other | |
| AI | Aromatase Inhibitor |
| AIC | Akaike Information Criterion |
| BC | Breast Cancer |
| BMI | Body Mass Index |
| CALM | Managing Cancer and Living Meaningfully |
| ChT | Chemotherapy |
| Chr | Chromosome |
| CI | Confidence Interval |
| CMDE | Cyclophosphamide—Methotrexate—Doxorubicin—Etoposide |
| CMF | Cyclophosphamide—Methotrexate—5-FU |
| CogPCA | Cognitive Principal Component Analysis |
| CRCI | Cancer-Related Cognitive Impairment |
| EDTA | Ethylenediaminetetraacetic Acid |
| ER | Estrogen Receptor |
| ET | Endocrine Therapy |
| FAC | Fluorouracil-Adriamycin-Cyclophosphamid |
| GED | General Educational Development |
| GEE | Generalized Estimating Equation |
| GMM | Growth Mixture Modeling |
| HC | Healthy Controls |
| HER-2 | Human Epidermal Growth Factor Receptor 2 |
| MCID | Minimal Clinically Important Difference |
| NR | Not Reported |
| OR | Odds Ratio |
| PR | Progesterone Receptor |
| RCTs | Randomized Controlled Trials |
| RT | Radiotherapy |
| SD | Standard Deviation |
| TT | Targeted Therapy |
Appendix A
| Non-Genetic Biomarkers and Associationa with CRCI | Positive | Negative | Mixed | Null | Overall | ||
|---|---|---|---|---|---|---|---|
| Inflammation-related markers | |||||||
| Immune-related markers (cytokines, including pro-inflammatory markers and anti-inflammatory markers) | |||||||
| IL-1β | [17,18,49,53,60,68,69,72] | [55,81] | [44,52,63,73,79] | Mixed | |||
| IL-2 | [17,52] | [44,48,60,63,72,73] | Mixed | ||||
| IL-4 | [17,49,68,69] | [16,44,60,81] | [48,52,72,73,79] | Mixed | |||
| IL-5 | [44] | [60] | [17,52] | Mixed | |||
| IL-6 | [18,41,48,62,72] | [16,60] | [17,38,39,50,52,53,54,55,63,71,73,79,82] | Mixed | |||
| IL-7 | [17] | [52] | Mixed | ||||
| IL-8 | [16,18,52,72,73] | [17,48,55,60] | [53,62,79] | Mixed | |||
| IL-10 | [18,44,52,62] | [16,17] | [48,54,60,72,73,79] | Mixed | |||
| IL-12 | [18] | [60] | [17,44,73] | Mixed | |||
| IL-13 | [44,52] | [17,60] | Mixed | ||||
| TNF-α | [17,18,42,49,60,62,68,69] | [48,55,81] | [72] | [16,39,44,52,54,61,63,73,79] | Mixed | ||
| IFN-γ | [18] | [60] | [17,44,52,62,63,72,73,79] | Mixed | |||
| GM-CSF | [17,52] | [60] | [72,73,79] | Mixed | |||
| IL-1ra | [50,54,82] | Null | |||||
| CRP | [38,48] | [39,50,61,63,71,82] | Mixed | ||||
| Inflammatory indexes | |||||||
| NLR | [49,80] | [76] | Mixed | ||||
| Stress-axis markers | |||||||
| Cortisol | [24] | [23] | [36] | Mixed | |||
| Neurotrophin | |||||||
| BDNF | [65] | [78] | [54,61,63,74,79] | Mixed | |||
| Genetic Biomarkers and Association with CRCI | Positive | Negative | Mixed | Null | Overall | ||
|---|---|---|---|---|---|---|---|
| Genetic (polymorphism) | |||||||
| ANKKI | |||||||
| rs1800497 | [70] | Negative | |||||
| ALDH2 | |||||||
| rs671 | [49] | Positive | |||||
| rs886205 | [77] | Positive | |||||
| rs4648328 | [58] | Positive | |||||
| rs4767944 | [58] | Positive | |||||
| APOE | |||||||
| rs429358 | [56] | [45,59,70] | Mixed | ||||
| rs7412 | [56] | [45,59] | Mixed | ||||
| SNP in ε4 allele (not specified) | [51] | [73] | Mixed | ||||
| BDNF | |||||||
| rs6265 | [78] | [65] | [45,59,64,70] | Mixed | |||
| CAT | |||||||
| rs511895 | [58] | Negative | |||||
| rs769214 | [58] | Negative | |||||
| CCNBI | |||||||
| rs164390 (SNP 102 GT in the promoter) | [57] | Positive | |||||
| rs350099 (SNP 957 CT in the promoter) | [58] | Positive | |||||
| rs350104 (SNP 457 CT in the promoter) | [58] | Positive | |||||
| COMT | |||||||
| rs165599 | [45] | [59] | Mixed | ||||
| rs4680 | [70] | [45,59,70] | Mixed | ||||
| rs737865 | [59] | [45] | Mixed | ||||
| DIAPH3 | |||||||
| rs1337652 | [57] | Negative | |||||
| rs4547237 | [57] | Negative | |||||
| DNMT1 | |||||||
| rs2162560 | [42] | Negative | |||||
| DRD2 | |||||||
| rs6277 | [70] | Negative | |||||
| ERCC2 | |||||||
| rs13181 | [58] | Positive | |||||
| rs3916874 | [58] | Negative | |||||
| rs50872 | [58] | Negative | |||||
| ERCC3 | |||||||
| rs4150407 | [35,58] | Negative | |||||
| rs4150477 | [58] | Negative | |||||
| rs2134794 | [58] | Positive | |||||
| ERCC5 | |||||||
| rs751402 | [58] | [35] | Mixed | ||||
| rs873601 | [58] | Positive | |||||
| rs11069498 | [58] | Positive | |||||
| rs2296147 | [58] | Negative | |||||
| ESR1 | |||||||
| rs488133 | [57] | Mixed | |||||
| rs2234693 | [58] | Null | |||||
| rs9340799 | [58] | Null | |||||
| GPX1 | |||||||
| rs1050450 | [35] | Negative | |||||
| GSTM1 | |||||||
| rs412543 (SNP 498 CG in the promoter) | [57] | Mixed | |||||
| HMCN1 | |||||||
| rs76859653 (intronic region) | [66] | Positive | |||||
| rs78786199 (intergenic region) | [66] | Positive | |||||
| ILB | |||||||
| rs16944 (SNP 511 CT in the promoter) | [37] | Null | |||||
| IL6 | |||||||
| rs1800795 (SNP 174 GC in the promoter) | [37] | [41] | Null | ||||
| MYBL2 | |||||||
| rs11556379 | [57] | Mixed | |||||
| rs2070235 | [57] | Mixed | |||||
| PARP1 | |||||||
| rs2271347 | [38,58] | Negative | |||||
| PGR | |||||||
| rs1042838 | [57] | Negative | |||||
| rs474320 | [57] | Negative | |||||
| rs484389 | [57] | Negative | |||||
| rs608995 | [57] | Negative | |||||
| SEPP1 | |||||||
| rs3877899 | [58] | Positive | |||||
| rs230819 | [58] | Negative | |||||
| SOD1 | |||||||
| rs1041740 | [58] | Negative | |||||
| SOD2 | |||||||
| rs4880 | [58] | Positive | |||||
| rs5746136 | [58] | Positive | |||||
| rs8031 | [58] | Positive | |||||
| TNF | |||||||
| rs1800629 (SNP 308 GA in the promoter) | [37] | [41] | Null | ||||
| Leukocyte DNA Damage | [83] | Positive | |||||
| PBMC Telomerase Activity | [58] | Negative | |||||
| PBMC Telomere Length | [58] | Null | |||||
| Mitochondrial DNA | [41] | Null | |||||
| Direct and Oxidative DNA Damage—Comet Assay | [47] | Null | |||||
| DNA Methylation | |||||||
| BDNF | [46] | Positive | |||||
| RASA2 | [46] | Positive | |||||
| ECE2 | [75] | Positive | |||||
| USP6NL | [75] | Positive | |||||
| PPFIBP2 | [75] | Positive | |||||
| DDHD1 | [75] | Null | |||||
| RIPOR2 | [75] | Positive | |||||
| KLF5 | [75] | Positive | |||||
| UBE2V1 | [75] | Positive | |||||
| HSD17B3 | [75] | Null | |||||
| DGKA | [75] | Positive | |||||
| RPS6KA1 | [75] | Positive | |||||
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Weigel, M.T.; Dowsett, M. Current and Emerging Biomarkers in Breast Cancer: Prognosis and Prediction. Endocr.-Relat. Cancer 2010, 17, R245–R262. [Google Scholar] [CrossRef]
- Shien, T.; Iwata, H. Adjuvant and Neoadjuvant Therapy for Breast Cancer. Jpn. J. Clin. Oncol. 2020, 50, 225–229. [Google Scholar] [CrossRef]
- Stone, J.B.; DeAngelis, L.M. Cancer-Treatment-Induced Neurotoxicity—Focus on Newer Treatments. Nat. Rev. Clin. Oncol. 2016, 13, 92–105. [Google Scholar] [CrossRef]
- McDonald, B.C.; Conroy, S.K.; Ahles, T.A.; West, J.D.; Saykin, A.J. Gray Matter Reduction Associated with Systemic Chemotherapy for Breast Cancer: A Prospective MRI Study. Breast Cancer Res. Treat. 2010, 123, 819–828. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Kesler, S.R.; Ahles, T.A.; Morrow, G.R. Prevalence, Mechanisms, and Management of Cancer-Related Cognitive Impairment. Int. Rev. Psychiatry 2014, 26, 102–113. [Google Scholar] [CrossRef]
- Bernstein, L.J.; McCreath, G.A.; Komeylian, Z.; Rich, J.B. Cognitive Impairment in Breast Cancer Survivors Treated with Chemotherapy Depends on Control Group Type and Cognitive Domains Assessed: A Multilevel Meta-Analysis. Neurosci. Biobehav. Rev. 2017, 83, 417–428. [Google Scholar] [CrossRef]
- Falleti, M.G.; Sanfilippo, A.; Maruff, P.; Weih, L.; Phillips, K.-A. The Nature and Severity of Cognitive Impairment Associated with Adjuvant Chemotherapy in Women with Breast Cancer: A Meta-Analysis of the Current Literature. Brain Cogn. 2005, 59, 60–70. [Google Scholar] [CrossRef]
- Stewart, A.; Bielajew, C.; Collins, B.; Parkinson, M.; Tomiak, E. A Meta-Analysis of the Neuropsychological Effects of Adjuvant Chemotherapy Treatment in Women Treated for Breast Cancer. Clin. Neuropsychol. 2006, 20, 76–89. [Google Scholar] [CrossRef]
- Bakoyiannis, I.; Tsigka, E.-A.; Perrea, D.; Pergialiotis, V. The Impact of Endocrine Therapy on Cognitive Functions of Breast Cancer Patients: A Systematic Review. Clin. Drug Investig. 2016, 36, 109–118. [Google Scholar] [CrossRef]
- Lee, P.E.; Tierney, M.C.; Wu, W.; Pritchard, K.I.; Rochon, P.A. Endocrine Treatment-Associated Cognitive Impairment in Breast Cancer Survivors: Evidence from Published Studies. Breast Cancer Res. Treat. 2016, 158, 407–420. [Google Scholar] [CrossRef]
- Noal, S.; Levy, C.; Hardouin, A.; Rieux, C.; Heutte, N.; Ségura, C.; Collet, F.; Allouache, D.; Switsers, O.; Delcambre, C.; et al. One-Year Longitudinal Study of Fatigue, Cognitive Functions, and Quality of Life After Adjuvant Radiotherapy for Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 795–803. [Google Scholar] [CrossRef]
- Shibayama, O.; Yoshiuchi, K.; Inagaki, M.; Matsuoka, Y.; Yoshikawa, E.; Sugawara, Y.; Akechi, T.; Wada, N.; Imoto, S.; Murakami, K.; et al. Association between Adjuvant Regional Radiotherapy and Cognitive Function in Breast Cancer Patients Treated with Conservation Therapy. Cancer Med. 2014, 3, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Wefel, J.S.; Saleeba, A.K.; Buzdar, A.U.; Meyers, C.A. Acute and Late Onset Cognitive Dysfunction Associated with Chemotherapy in Women with Breast Cancer. Cancer 2010, 116, 3348–3356. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, A.B.C.; van Stralen, H.E.; Sloots, M.; Schagen, S.B.; Visser-Meily, J.M.A.; Schepers, V.P.M. Prevalence of Cognitive Impairment and Change in Patients with Breast Cancer: A Systematic Review of Longitudinal Studies. Psycho-Oncol. 2021, 30, 635–648. [Google Scholar] [CrossRef]
- Belcher, E.K.; Culakova, E.; Gilmore, N.J.; Hardy, S.J.; Kleckner, A.S.; Kleckner, I.R.; Lei, L.; Heckler, C.; Sohn, M.B.; Thompson, B.D.; et al. Inflammation, Attention, and Processing Speed in Patients with Breast Cancer before and after Chemotherapy. J. Natl. Cancer Inst. 2022, 114, 712–721. [Google Scholar] [CrossRef]
- Henneghan, A.M.; Palesh, O.; Harrison, M.; Kesler, S.R. Identifying Cytokine Predictors of Cognitive Functioning in Breast Cancer Survivors up to 10 years Post Chemotherapy Using Machine Learning. J. Neuroimmunol. 2018, 320, 38–47. [Google Scholar] [CrossRef]
- Kesler, S.; Janelsins, M.; Koovakkattu, D.; Palesh, O.; Mustian, K.; Morrow, G.; Dhabhar, F.S. Reduced Hippocampal Volume and Verbal Memory Performance Associated with Interleukin-6 and Tumor Necrosis Factor-Alpha Levels in Chemotherapy-Treated Breast Cancer Survivors. Brain Behav. Immun. 2013, 30, S109–S116. [Google Scholar] [CrossRef]
- Loke, S.Y.; Lee, A.S.G. The Future of Blood-Based Biomarkers for the Early Detection of Breast Cancer. Eur. J. Cancer 2018, 92, 54–68. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Carreras-Presas, C.M.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T. Saliva Diagnostics–Current Views and Directions. Exp. Biol. Med. 2017, 242, 459–472. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C.; Kudielka, B.M.; Adam, E.K.; Pruessner, J.C.; Wüst, S.; Dockray, S.; Smyth, N.; Evans, P.; Hellhammer, D.H.; et al. Assessment of the Cortisol Awakening Response: Expert Consensus Guidelines. Psychoneuroendocrinology 2016, 63, 414–432. [Google Scholar] [CrossRef] [PubMed]
- Koopaie, M.; Kolahdooz, S.; Fatahzadeh, M.; Manifar, S. Salivary Biomarkers in Breast Cancer Diagnosis: A Systematic Review and Diagnostic Meta-Analysis. Cancer Med. 2022, 11, 2644–2661. [Google Scholar] [CrossRef] [PubMed]
- Andreano, J.M.; Waisman, J.; Donley, L.; Cahill, L. Effects of Breast Cancer Treatment on the Hormonal and Cognitive Consequences of Acute Stress. Psycho-Oncology 2012, 21, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, S.G.; Halldorsdottir, T.; Agustsson, G.; Tobin, H.R.S.; Wu, L.M.; Amidi, A.; Johannsdottir, K.R.; Lutgendorf, S.K.; Telles, R.; Daly, H.F.; et al. Biological and Psychological Predictors of Cognitive Function in Breast Cancer Patients before Surgery. Support. Care Cancer 2024, 32, 88. [Google Scholar] [CrossRef]
- Castel, H.; Denouel, A.; Lange, M.; Tonon, M.-C.; Dubois, M.; Joly, F. Biomarkers Associated with Cognitive Impairment in Treated Cancer Patients: Potential Predisposition and Risk Factors. Front. Pharmacol. 2017, 8, 138. [Google Scholar] [CrossRef]
- Buskbjerg, C.D.R.; Amidi, A.; Demontis, D.; Nissen, E.R.; Zachariae, R. Genetic Risk Factors for Cancer-Related Cognitive Impairment: A Systematic Review. Acta Oncol. 2019, 58, 537–547. [Google Scholar] [CrossRef]
- Oppegaard, K.R.; Armstrong, T.S.; Anguera, J.A.; Kober, K.M.; Kelly, D.L.; Laister, R.C.; Saligan, L.N.; Ayala, A.P.; Kuruvilla, J.; Alm, M.W.; et al. Blood-Based Biomarkers of Cancer-Related Cognitive Impairment in Non-Central Nervous System Cancer: A Scoping Review. Crit. Rev. Oncol. Hematol. 2022, 180, 103822. [Google Scholar] [CrossRef]
- Yang, G.S.; Kumar, S.; Dorsey, S.G.; Starkweather, A.R.; Kelly, D.L.; Lyon, D.E. Systematic Review of Genetic Polymorphisms Associated with Psychoneurological Symptoms in Breast Cancer Survivors. Support. Care Cancer 2019, 27, 351–371. [Google Scholar] [CrossRef]
- Schroyen, G.; Vissers, J.; Smeets, A.; Gillebert, C.R.; Lemiere, J.; Sunaert, S.; Deprez, S.; Sleurs, C. Blood and Neuroimaging Biomarkers of Cognitive Sequelae in Breast Cancer Patients throughout Chemotherapy: A Systematic Review. Transl. Oncol. 2022, 16, 101297. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; Brennan, S.; Akl, E.A.; Hultcrantz, M.; Alonso-Coello, P.; Xia, J.; Davoli, M.; Rojas, M.X.; Meerpohl, J.J.; Flottorp, S.; et al. The Development Methods of Official GRADE Articles and Requirements for Claiming the Use of GRADE–A Statement by the GRADE Guidance Group. J. Clin. Epidemiol. 2023, 159, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Mustafa, R.A.; Schünemann, H.J.; Sultan, S.; Santesso, N. Rating the Certainty in Evidence in the Absence of a Single Estimate of Effect. Evid. Based Med. 2017, 22, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Habibi, N.; Aromataris, E.; Stone, J.C.; Leonardi-Bee, J.; Sears, K.; Hasanoff, S.; Klugar, M.; Tufanaru, C.; Moola, S.; et al. The Revised JBI Critical Appraisal Tool for the Assessment of Risk of Bias for Quasi-Experimental Studies. JBI Evid. Synth. 2024, 22, 378. [Google Scholar] [CrossRef]
- Bender, C.M.; Merriman, J.D.; Sereika, S.M.; Gentry, A.L.; Casillo, F.E.; Koleck, T.A.; Rosenzweig, M.Q.; Brufsky, A.M.; McAuliffe, P.; Zhu, Y.; et al. Trajectories of Cognitive Function and Associated Phenotypic and Genotypic Factors in Breast Cancer. Oncol. Nurs. Forum 2018, 45, 308–326. [Google Scholar] [CrossRef]
- Boivin, M.J.; Aaron, G.P.; Felt, N.G.; Shamoun, L. Preliminary Study on the Effects of Treatment for Breast Cancer: Immunological Markers as They Relate to Quality of Life and Neuropsychological Performance. BMC Women’s Health 2020, 20, 109. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Castellon, S.; Arevalo, J.; Cole, S.W. Cytokine Genetic Variations and Fatigue among Patients with Breast Cancer. J. Clin. Oncol. 2013, 31, 1656–1661. [Google Scholar] [CrossRef]
- Boyle, C.C.; Ganz, P.A.; Van Dyk, K.M.; Bower, J.E. Inflammation and Attentional Bias in Breast Cancer Survivors. Brain Behav. Immun. 2017, 66, 85–88. [Google Scholar] [CrossRef]
- Carlson, B.W.; Craft, M.A.; Carlson, J.R.; Razaq, W.; Deardeuff, K.K.; Benbrook, D.M. Accelerated Vascular Aging and Persistent Cognitive Impairment in Older Female Breast Cancer Survivors. Geroscience 2018, 40, 325–336. [Google Scholar] [CrossRef]
- Carroll, J.E.; Van Dyk, K.; Bower, J.E.; Scuric, Z.; Petersen, L.; Schiestl, R.; Irwin, M.R.; Ganz, P.A. Cognitive Performance in Survivors of Breast Cancer and Markers of Biological Aging. Cancer 2019, 125, 298–306. [Google Scholar] [CrossRef]
- Chae, J.-W.; Ng, T.; Yeo, H.L.; Shwe, M.; Gan, Y.X.; Ho, H.K.; Chan, A. Impact of TNF-α (Rs1800629) and IL-6 (Rs1800795) Polymorphisms on Cognitive Impairment in Asian Breast Cancer Patients. PLoS ONE 2016, 11, e0164204. [Google Scholar] [CrossRef]
- Chae, J.-W.; Chua, P.S.; Ng, T.; Yeo, A.H.L.; Shwe, M.; Gan, Y.X.; Dorajoo, S.; Foo, K.M.; Loh, K.W.-J.; Koo, S.-L.; et al. Association of Mitochondrial DNA Content in Peripheral Blood with Cancer-Related Fatigue and Chemotherapy-Related Cognitive Impairment in Early-Stage Breast Cancer Patients: A Prospective Cohort Study. Breast Cancer Res. Treat. 2018, 168, 713–721. [Google Scholar] [CrossRef]
- Chan, A.; Yeo, A.; Shwe, M.; Tan, C.J.; Foo, K.M.; Chu, P.; Khor, C.C.; Ho, H.K. An Evaluation of DNA Methyltransferase 1 (DNMT1) Single Nucleotide Polymorphisms and Chemotherapy-Associated Cognitive Impairment: A Prospective, Longitudinal Study. Sci. Rep. 2019, 9, 14570. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.C.-H.; Lin, C.-K.; Hsiao, H.-P.; Tzang, B.-S.; Hsu, Y.-H.; Wu, S.-I.; Stewart, R. Effects of Cancer, Chemotherapy and Cytokines on Subjective and Objective Cognitive Functioning among Patients with Breast Cancer. Cancers 2021, 13, 2576. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Li, W.; Gan, C.; Zhang, B.; Jia, Q.; Wang, K. The COMT (Rs165599) Gene Polymorphism Contributes to Chemotherapy-Induced Cognitive Impairment in Breast Cancer Patients. Am. J. Transl. Res. 2016, 8, 5087–5097. [Google Scholar] [PubMed]
- Cho, M.; Sereika, S.M.; Cummings, M.; Erickson, K.I.; Bender, C.M.; Conley, Y.P. DNA Methylation of BDNF and RASA2 Genes Is Associated With Cognitive Function in Postmenopausal Women With Breast Cancer. Oncol. Nurs. Forum 2024, 51, 349. [Google Scholar] [CrossRef]
- Conroy, S.K.; McDonald, B.C.; Smith, D.J.; Moser, L.R.; West, J.D.; Kamendulis, L.M.; Klaunig, J.E.; Champion, V.L.; Unverzagt, F.W.; Saykin, A.J. Alterations in Brain Structure and Function in Breast Cancer Survivors: Effect of Post-Chemotherapy Interval and Relation to Oxidative DNA Damage. Breast Cancer Res. Treat. 2013, 137, 493–502. [Google Scholar] [CrossRef]
- Duivon, M.; Lequesne, J.; Di Meglio, A.; Pradon, C.; Vaz-Luis, I.; Martin, A.-L.; Everhard, S.; Broutin, S.; Rigal, O.; Bousrih, C.; et al. Inflammation at Diagnosis and Cognitive Impairment Two Years Later in Breast Cancer Patients from the Canto-Cog Study. Breast Cancer Res. 2024, 26, 93. [Google Scholar] [CrossRef]
- Gan, C.; Yao, S.; Zhao, J.; Shi, H.; Xu, J.; Zhang, M.; Cheng, H. Expression of Inflammatory States in Response to Psychological Distress in Breast Cancer Survivors and Its Relationship to Subjective Memory Function Complaints. BMC Women’s Health 2025, 25, 1–12. [Google Scholar] [CrossRef]
- Ganz, P.A.; Bower, J.; Kwan, L.; Castellon, S.; Silverman, D.; Geist, C.; Breen, E.; Irwin, M.; Cole, S. Does Tumor Necrosis Factor-Alpha (TNF-α) Play a Role in Post-Chemotherapy Cerebral Dysfunction? Brain Behav. Immun. 2013, 30, S99–S108. [Google Scholar] [CrossRef]
- Harrison, R.A.; Rao, V.; Kesler, S.R. The Association of Genetic Polymorphisms with Neuroconnectivity in Breast Cancer Patients. Sci. Rep. 2021, 11, 6169. [Google Scholar] [CrossRef]
- Henneghan, A.; Wright, M.L.; Bourne, G.; Sales, A.C. A Cross-Sectional Exploration of Cytokine-Symptom Networks in Breast Cancer Survivors Using Network Analysis. Can. J. Nurs. Res. 2021, 53, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Lei, L.; Netherby-Winslow, C.; Kleckner, A.S.; Kerns, S.; Gilmore, N.; Belcher, E.; Thompson, B.D.; Werner, Z.A.; Hopkins, J.O.; et al. Relationships between Cytokines and Cognitive Function from Pre- to Post-Chemotherapy in Patients with Breast Cancer. J. Neuroimmunol. 2022, 362, 577769. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, V.; Thwaites, R.; Cercignani, M.; Sacre, S.; Harrison, N.; Whiteley-Jones, H.; Mullen, L.; Chamberlain, G.; Davies, K.; Zammit, C.; et al. A Feasibility Study Exploring the Role of Pre-Operative Assessment When Examining the Mechanism of ‘chemo-Brain’ in Breast Cancer Patients. SpringerPlus 2016, 5, 390. [Google Scholar] [CrossRef] [PubMed]
- Keetile, N.; Osuch, E.; Lentoor, A.G.; Rasakanya, T. Association of Circulating Levels of Inflammatory Cytokines and Chemotherapy-Associated Subjective Cognitive Impairment in a South African Cohort of Breast Cancer Patients. NeuroSci 2023, 4, 296–304. [Google Scholar] [CrossRef]
- Koleck, T.A.; Bender, C.M.; Sereika, S.M.; Ahrendt, G.; Jankowitz, R.C.; McGuire, K.P.; Ryan, C.M.; Conley, Y.P. Apolipoprotein E Genotype and Cognitive Function in Postmenopausal Women With Early-Stage Breast Cancer. Oncol. Nurs. Forum 2014, 41, E313–E325. [Google Scholar] [CrossRef]
- Koleck, T.; Bender, C.M.; Clark, B.Z.; Ryan, C.M.; Ghotkar, P.; Brufsky, A.; McAuliffe, P.F.; Rastogi, P.; Sereika, S.M.; Conley, Y.P. An Exploratory Study of Host Polymorphisms in Genes That Clinically Characterize Breast Cancer Tumors and Pretreatment Cognitive Performance in Breast Cancer Survivors. Breast Cancer Targets Ther. 2017, 9, 95–110. [Google Scholar] [CrossRef]
- Koleck, T.A.; Bender, C.M.; Sereika, S.M.; Brufsky, A.M.; Lembersky, B.C.; McAuliffe, P.F.; Puhalla, S.L.; Rastogi, P.; Conley, Y.P. Polymorphisms in DNA Repair and Oxidative Stress Genes Associated with Pre-Treatment Cognitive Function in Breast Cancer Survivors: An Exploratory Study. SpringerPlus 2016, 5, 422. [Google Scholar] [CrossRef]
- Li, W.; Zhao, J.; Ding, K.; Chao, H.H.; Li, C.-S.R.; Cheng, H.; Shen, L. Catechol-O-Methyltransferase Gene Polymorphisms and the Risk of Chemotherapy-Induced Prospective Memory Impairment in Breast Cancer Patients with Varying Tumor Hormonal Receptor Expression. Med. Sci. Monit. 2020, 26, e923567. [Google Scholar] [CrossRef]
- Lyon, D.E.; Cohen, R.; Chen, H.; Kelly, D.L.; McCain, N.L.; Starkweather, A.; Ahn, H.; Sturgill, J.; Jackson-Cook, C.K. Relationship of Systemic Cytokine Concentrations to Cognitive Function over Two Years in Women with Early Stage Breast Cancer. J. Neuroimmunol. 2016, 301, 74–82. [Google Scholar] [CrossRef]
- Madison, A.A.; Andridge, R.; Renna, M.E.; Sheridan, J.F.; Lustberg, M.; Ramaswamy, B.; Wesolowski, R.; Williams, N.O.; Sardesai, S.D.; Noonan, A.M.; et al. Inflamed but Not Impulsive: Acute Inflammatory Cytokine Response Does Not Impact Prepotent Response Inhibition. J. Affect. Disord. 2023, 342, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mandelblatt, J.S.; Small, B.J.; Zhou, X.; Nakamura, Z.M.; Cohen, H.J.; Ahles, T.A.; Ahn, J.; Bethea, T.N.; Extermann, M.; Graham, D.; et al. Plasma Levels of Interleukin-6 Mediate Neurocognitive Performance in Older Breast Cancer Survivors: The Thinking and Living with Cancer Study. Cancer 2023, 129, 2409–2421. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.S.; Pathak, H.B.; He, J.; Ghosh, A.; Puri, R.V.; Asakura, Y.; Miyashita, M. Combined Exercise and Game-Based Cognitive Training Intervention: Correlative Pilot Study of Neurotrophic and Inflammatory Biomarkers for Women with Breast Cancer. Cancer Nurs. 2022, 47, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.; Teo, S.M.; Yeo, H.L.; Shwe, M.; Gan, Y.X.; Cheung, Y.T.; Foo, K.M.; Cham, M.T.; Lee, J.A.; Tan, Y.P.; et al. Brain-Derived Neurotrophic Factor. Genetic Polymorphism (Rs6265) Is Protective against Chemotherapy-Associated Cognitive Impairment in Patients with Early-Stage Breast Cancer. Neuro-Oncology 2016, 18, 244–251. [Google Scholar] [CrossRef]
- Ng, T.; Lee, Y.Y.; Chae, J.-W.; Yeo, A.H.L.; Shwe, M.; Gan, Y.X.; Ng, R.C.H.; Chu, P.P.Y.; Khor, C.C.; Ho, H.K.; et al. Evaluation of Plasma Brain-Derived Neurotrophic Factor Levels and Self-Perceived Cognitive Impairment Post-Chemotherapy: A Longitudinal Study. BMC Cancer 2017, 17, 867. [Google Scholar] [CrossRef]
- Nudelman, K.; Nho, K.; Zhang, M.; McDonald, B.C.; Zhai, W.; Small, B.J.; Wegel, C.E.; Jacobsen, P.B.; Jim, H.S.L.; Patel, S.K.; et al. Genetic Variants Associated with Longitudinal Cognitive Performance in Older Breast Cancer Patients and Controls. Cancers 2023, 15, 2877. [Google Scholar] [CrossRef]
- Palesh, O.; Braun, S.E.; Truong, T.; Hong, S.; Mitsuhashi, M.; Nyagaka, R.; Lee, S.; Gandhi, A.; Schutz, A.D.L.T.; Kesler, S.R. Natural Trajectory Subclasses of Cognitive Impairment in Breast Cancer Patients Experiencing Insomnia. Cancer 2025, 131, e35816. [Google Scholar] [CrossRef]
- Pang, L.; Bi, Z.; Jing, Y.; Yin, X.; Zhang, X.; Yao, S.; Zhao, J.; Cheng, H. Changes in Cytokine Levels in Breast Cancer Patients with CRCI before or after CALM Intervention. Am. J. Cancer Res. 2021, 11, 5415–5427. [Google Scholar]
- Pang, L.; Li, W.; Yao, S.; Jing, Y.; Yin, X.; Cheng, H. Psychological Distress Is Involved in CRCI in Breast Cancer Survivors via Mediating Cytokine Levels. Cancer Med. 2023, 12, 11806–11815. [Google Scholar] [CrossRef]
- Park, J.Y.; Lengacher, C.A.; Rodriguez, C.S.; Meng, H.; Kip, K.E.; Morgan, S.; Joshi, A.; Hueluer, G.; Wang, J.R.; Tinsley, S.; et al. The Moderating Role of Genetics on the Effectiveness of the Mindfulness-Based Stress Reduction for Breast Cancer (MBSR(BC)) Program on Cognitive Impairment. Biol. Res. Nurs. 2025, 27, 216–228. [Google Scholar] [CrossRef]
- Patel, S.K.; Breen, E.C.; Paz, I.B.; Kruper, L.; Mortimer, J.; Wong, F.L.; Bhatia, S.; Irwin, M.R.; Behrendt, C.E. Inflammation-Related Proteins as Biomarkers of Treatment-Related Behavioral Symptoms: A Longitudinal Study of Breast Cancer Patients and Age-Matched Controls. Brain Behav. Immun.-Health 2023, 32, 100670. [Google Scholar] [CrossRef]
- Toh, Y.L.; Wang, C.; Ho, H.K.; Chan, A. Distinct Cytokine Profiles across Trajectories of Self-Perceived Cognitive Impairment among Early-Stage Breast Cancer Survivors. J. Neuroimmunol. 2020, 342, 577196. [Google Scholar] [CrossRef]
- Vardy, J.L.; Stouten-Kemperman, M.M.; Pond, G.; Booth, C.M.; Rourke, S.B.; Dhillon, H.M.; Dodd, A.; Crawley, A.; Tannock, I.F. A Mechanistic Cohort Study Evaluating Cognitive Impairment in Women Treated for Breast Cancer. Brain Imaging Behav. 2019, 13, 15–26. [Google Scholar] [CrossRef]
- Von Ah, D.; McDonald, B.C.; Crouch, A.D.; Ofner, S.; Perkins, S.; Storey, S.; Considine, R.; Unverzagt, F. Randomized Double-Masked Controlled Trial of Cognitive Training in Breast Cancer Survivors: A Preliminary Study. Support. Care Cancer 2022, 30, 7457–7467. [Google Scholar] [CrossRef]
- Yang, G.S.; Mi, X.; Jackson-Cook, C.K.; Starkweather, A.R.; Kelly, D.L.; Archer, K.J.; Zou, F.; Lyon, D.E. Differential DNA Methylation Following Chemotherapy for Breast Cancer Is Associated with Lack of Memory Improvement at One Year. Epigenetics 2020, 15, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Ding, K.; Liu, S.; Zhang, Q.; Li, W.; Tang, L.; Yu, S.; Pang, L.; Yin, X.; Cheng, H. The Managing Cancer and Living Meaningfully (CALM) Intervention Alleviates Chemotherapy-Related Cognitive Impairment in Patients with Breast Cancer by Modulating Pan-Immune-Inflammation Values. Integr. Cancer Ther. 2022, 21, 15347354221140498. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Li, W.; Liu, S.; Cai, Y.; Zhang, Q.; Tang, L.; Yu, S.; Jing, Y.; Yin, X.; Cheng, H. Aldehyde Dehydrogenase 2 Polymorphism Is Associated with Chemotherapy-Related Cognitive Impairment in Patients with Breast Cancer Who Receive Chemotherapy. Cancer Med. 2023, 12, 5209–5221. [Google Scholar] [CrossRef] [PubMed]
- Yap, N.Y.; Tan, N.Y.T.; Tan, C.J.; Loh, K.W.-J.; Ng, R.C.H.; Ho, H.K.; Chan, A. Associations of Plasma Brain-Derived Neurotrophic Factor (BDNF) and Val66Met Polymorphism (Rs6265) with Long-Term Cancer-Related Cognitive Impairment in Survivors of Breast Cancer. Breast Cancer Res. Treat. 2020, 183, 683–696. [Google Scholar] [CrossRef]
- Yap, N.Y.; Toh, Y.L.; Tan, C.J.; Acharya, M.M.; Chan, A. Relationship between Cytokines and Brain-Derived Neurotrophic Factor (BDNF) in Trajectories of Cancer-Related Cognitive Impairment. Cytokine 2021, 144, 155556. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, J.; Wang, M.; Cheng, G.; Li, W.; Tang, L.; Yao, S.; Pang, L.; Yin, X.; Jing, Y.; et al. The Correlation between Neutrophil-to-Lymphocyte Ratio, Carcinoembryonic Antigen, and Carbohydrate Antigen 153 Levels with Chemotherapy-Related Cognitive Impairment in Early-Stage Breast Cancer Patients. Front. Med. 2022, 9, 945433. [Google Scholar] [CrossRef]
- Zhao, J.; Zuo, H.; Ding, K.; Zhang, X.; Bi, Z.; Cheng, H. Changes in Plasma IL-1β, TNF-α and IL-4 Levels Are Involved in Chemotherapy-Related Cognitive Impairment in Early-Stage Breast Cancer Patients. Am. J. Transl. Res. 2020, 12, 3046–3056. [Google Scholar] [PubMed]
- Zuniga, K.E.; Moran, N.E. Low Serum Carotenoids Are Associated with Self-Reported Cognitive Dysfunction and Inflammatory Markers in Breast Cancer Survivors. Nutrients 2018, 10, 1111. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.E.; Small, B.J.; Tometich, D.B.; Zhai, W.; Zhou, X.; Luta, G.; Ahles, T.A.; Saykin, A.J.; Nudelman, K.N.H.; Clapp, J.D.; et al. Sleep Disturbance and Neurocognitive Outcomes in Older Patients with Breast Cancer: Interaction with Genotype. Cancer 2019, 125, 4516–4524. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Dent, R.; McArthur, H.; Pusztai, L.; Kümmel, S.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Overall Survival with Pembrolizumab in Early-Stage Triple-Negative Breast Cancer. N. Engl. J. Med. 2024, 391, 1981–1991. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Chen, X.; Ye, M.; Ai, R.; Shan, C.; Lai, M.; Hong, W.; Yang, Y.; Wang, H.; Li, J.; Zhen, J.; et al. PD-1-Induced Encephalopathy: A Report of 2 Cases on Neurological Toxicities with Immune Checkpoint Inhibitors. Transl. Cancer Res. 2024, 13, 1196–1207. [Google Scholar] [CrossRef]
- Otto, F.; Seiberl, M.; Bieler, L.; Moser, T.; Kleindienst, W.; Wallner-Essl, W.; Koelblinger, P.; Wipfler, P.; Harrer, A. Beyond T Cell Toxicity-Intrathecal Chemokine CXCL13 Indicating B Cell Involvement in Immune-Related Adverse Events Following Checkpoint Inhibition: A Two-Case Series and Literature Review. Eur. J. Neurol. 2024, 31, e16279. [Google Scholar] [CrossRef]
- Oliva, G.; Giustiniani, A.; Danesin, L.; Burgio, F.; Arcara, G.; Conte, P. Cognitive Impairment Following Breast Cancer Treatments: An Umbrella Review. Oncologist 2024, 29, e848–e863. [Google Scholar] [CrossRef]
- Giustiniani, A.; Danesin, L.; Pezzetta, R.; Masina, F.; Oliva, G.; Arcara, G.; Burgio, F.; Conte, P. Use of Telemedicine to Improve Cognitive Functions and Psychological Well-Being in Patients with Breast Cancer: A Systematic Review of the Current Literature. Cancers 2023, 15, 1353. [Google Scholar] [CrossRef]

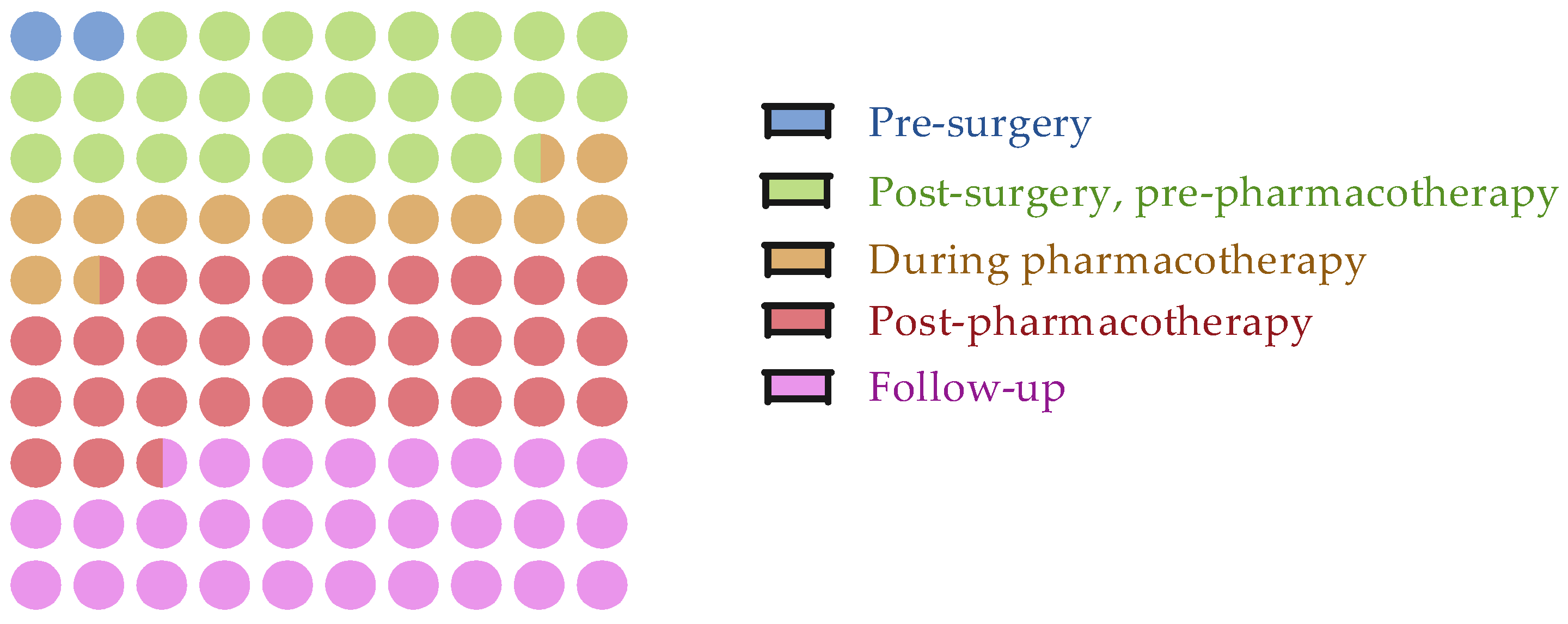



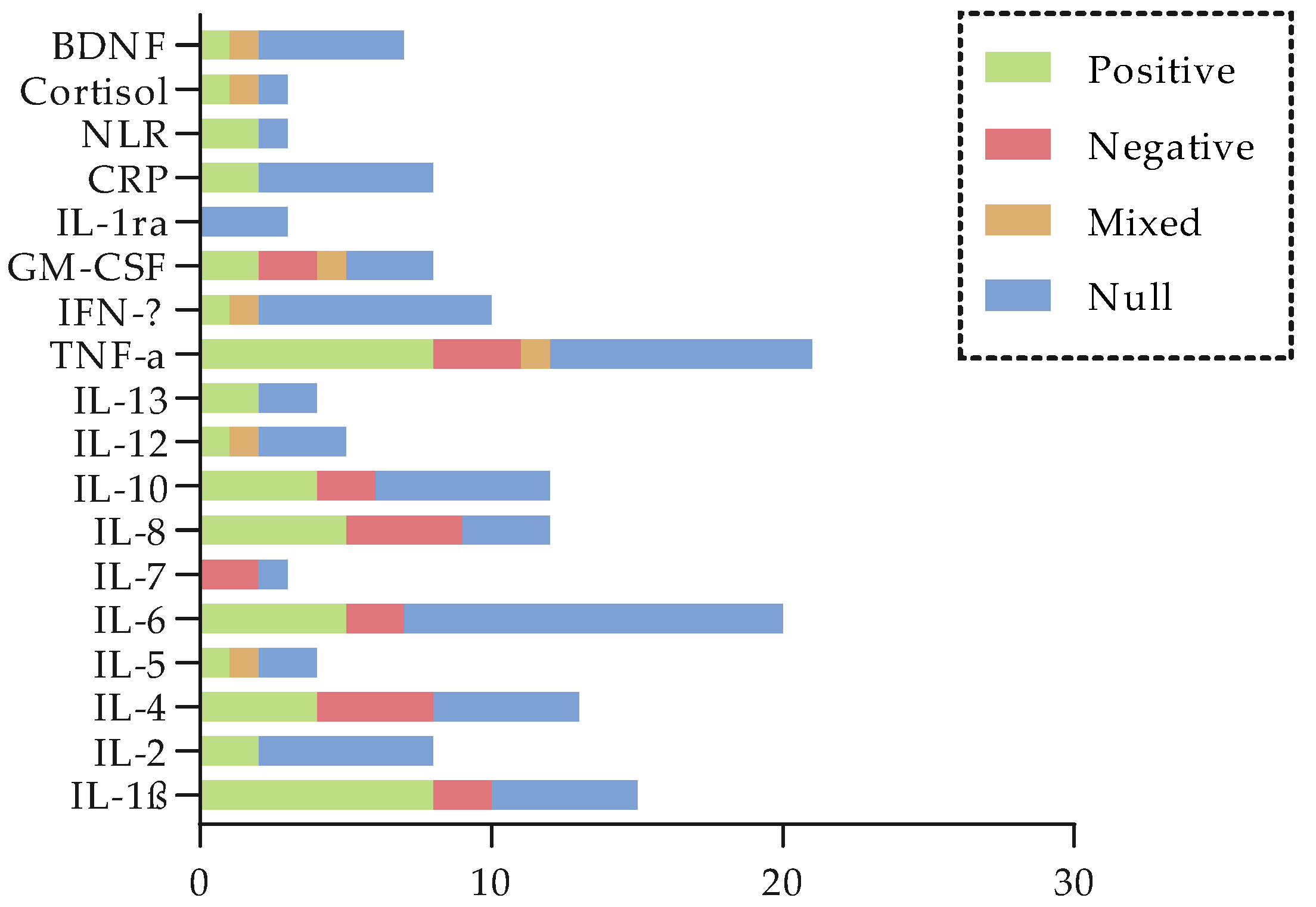
| Search Strategy | Details |
|---|---|
| Search query (title/abstract/keywords) | (“Breast Neoplasms” OR “Neoplasm Staging” OR “Breast cancer” OR “Mammary cancer” OR “Breast carcinoma” OR “breast neoplasm *” OR “breast tumor *” OR “breast malignan *”) AND (“Biomarkers” OR “Interleukins” OR “Oxidative Stress” OR “Brain-Derived Neurotrophic Factor” OR “BRCA *” OR “Human Epidermal Growth Factor Receptor 2” OR “HER2” OR “Hormone receptor *” OR “blood biomarker *” OR “serum biomarker *” OR “plasma biomarker *” OR “saliva biomarker *” OR “salivary biomarker *” OR “inflammatory marker *” OR “immune marker *” OR “cytokine *” OR “chemokine *” OR “tumor necrosis factor *” OR “Cortisol” OR “glucocorticoid *” OR “neurotransmitter *” OR “ILs” OR “TNF-alpha” OR “tnfr *” OR “BDNF” OR “SNP”) AND (“cognitive impair *” OR “cognitive deficit *” OR “cognitive difficult *” OR “cognitive dysfunction” OR “Chemo-brain” OR “cancer related cognitive impair *” OR “CRCI” OR “Neurocognitive dysfunction” OR “neuropsychological test *” OR “cognitive test *” OR “cognitive assessment *” OR “cognitive evaluation *” OR “neuropsychology *” OR “neuropsychological assessment *” OR “neuropsychological evaluation *” OR “Memory” OR “Attention” OR “Executive” OR “processing speed *” OR “Fatigue” OR “Cancer-related Fatigue” OR “CRF”)) NOT (“Neurological disorder *” OR “Brain disease *” OR “Neurodegenerative disease *” OR “Dementia” OR “Stroke” OR “Traumatic brain injury” OR “Epilepsy” OR “Multiple sclerosis” OR “Parkinson*” OR “Alzheimer *” OR “Schizophrenia” OR “Bipolar disorder” OR “Psychosis” OR “PTSD” OR “Personality disorder *” OR “Substance abuse” OR “Alcohol abuse” OR “Drug addiction” OR “Opioid use disorder”) |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Language filter | English |
| Time filter | 4–25 April 2025 |
| Author(s), Year | Study Design | Study Aim(s) |
|---|---|---|
| Andreano et al., 2012 [23] | Cross-sectional case–control study | To investigate whether BC-related alterations in the ovarian and glucocorticoid systems interact to influence cognitive function |
| Aspelund et al., 2024 [24] | Cross-sectional case–control study | To investigate whether pre-treatment cortisol, α-amylase, and stress-related psychiatric symptoms predict CRCI in BC patients |
| Belcher et al., 2022 [16] | Prospective longitudinal cohort study | To compare serum cytokine and receptor concentrations in BC patients and cancer-free controls, and to examine how these immune markers relate to patients’ cognitive performance |
| Bender et al., 2018 [35] | Prospective longitudinal cohort study | To describe different patterns of change in cognition from before to during adjuvant therapy in BC patients, and to determine the phenotypic and genetic factors that predict subgroup classification |
| Boivin et al., 2020 [36] | Prospective longitudinal cohort study | To determine how emotional psychological variables relate to circulating immune cells and to explore whether these immunological markers are associated with neuropsychological performance |
| Bower et al., 2013 [37] | Cross-sectional study | To test whether promoter polymorphisms are associated with self-reported fatigue in BC survivors and to explore whether these same variants also relate to depressive symptoms, subjective CRCI, and sleep disturbance |
| Boyle et al., 2017 [38] | Cross-sectional study | To examine whether heightened inflammation in BC survivors is linked to attentional bias towards sad faces |
| Carlson et al., 2018 [39] | Cross-sectional study | To assess whether biomarkers of vascular aging and ChT-related changes in cerebral blood flow are associated with neuropsychological symptoms in BC survivors |
| Carrol et al., 2019 * [40] | Cross-sectional study | To investigate whether cellular indicators of biological aging and inflammation are linked to more severe CRCI in BC survivors |
| Chae et al., 2016 * [41] | Prospective longitudinal cohort study | To determine whether pro-inflammatory cytokine gene variants IL6-174 and TNF-308 are associated with CRCI in early-stage BC patients |
| Chae et al., 2018 [42] | Prospective longitudinal cohort study | To investigate whether ChT-linked declines in peripheral-blood mitochondrial DNA are associated with the onset and severity of cancer-related fatigue and CRCI in early-stage BC patients |
| Chan et al., 2019 [43] | Prospective longitudinal cohort study | To investigate whether the DNMT1 gene polymorphism rs2162560 is associated with CRCI in BC patients |
| Chen et al., 2021 [44] | Cross-sectional study | To explore links between cancer status, ChT exposure, and peripheral cytokine levels with both objective and self-reported CRCI in women with BC, comparing three cross-sectional cohorts |
| Cheng et al., 2016 [45] | Prospective longitudinal cohort study | To examine whether genetic polymorphisms in COMT, APOE, and BDNF influence susceptibility to CRCI in BC patients |
| Cho et al., 2024 [46] | Cross-sectional study | To assess whether DNA methylation of the BDNF and RASA2 genes is associated with processing speed and self-reported cognitive function in BC patients |
| Conroy et al., 2013 [47] | Cross-sectional study | To investigate whether BC patients exhibit greater cognitive dysfunction, neuroimaging abnormalities, and DNA damage compared to than HC, especially with shorter post-ChT intervals |
| Duivon et al., 2024 [48] | Prospective longitudinal cohort study | To investigate whether higher pre-treatment levels of pro-inflammatory markers predict greater CRCI two years after BC diagnosis |
| Gan et al., 2025 [49] | Cross-sectional study | To test whether inflammation mediates the link between psychological distress and self-reported CRCI in BC survivors |
| Ganz et al., 2013 [50] | Prospective longitudinal cohort study | To evaluate whether recent ChT exposure in women with early-stage BC is associated with higher pro-inflammatory cytokine levels and to explore how these inflammatory markers relate to cerebral function and behavioral symptoms |
| Harrison et al., 2021 [51] | Cross-sectional study | To evaluate how genetic variants relate to brain health in BC patients, as measured by neurocognitive performance and functional connectome analysis |
| Henneghan et al., 2018 [17] | Cross-sectional study | To investigate whether cytokines predict CRCI in BC survivors 6 months to 10 years after completing ChT, using multivariate non-parametric analyses |
| Henneghan et al., 2021 [52] | Cross-sectional study | To explore the symptom-cytokine networks and their principal metrics in BC survivors post-ChT |
| Janelsins et al., 2022 [53] | Prospective longitudinal cohort study | To investigate the inflammatory pathways that underlie CRCI in BC survivors |
| Jenkins et al., 2016 [54] | Prospective longitudinal cohort study | To evaluate the feasibility and relevance of pre-surgical assessments for CRCI and to determine whether inflammatory markers mediate ChT-related neuropsychological impairments in women with BC |
| Keetile et al., 2023 [55] | Prospective longitudinal cohort study | To investigate whether neuroinflammation is linked to self-reported CRCI in BC patients |
| Kesler et al., 2013 [18] | Cross-sectional case–control study | To determine whether ChT-treated BC survivors exhibit hippocampal atrophy and verbal-memory deficits and whether these impairments correlate with circulating levels of pro-inflammatory cytokines |
| Koleck et al., 2014 [56] | Prospective longitudinal cohort study | To investigate the influence of APOE genotype on cognitive function in postmenopausal women with early-stage BC, both before the start of adjuvant therapy and longitudinally throughout treatment |
| Koleck et al., 2017 [57] | Cross-sectional study | The current study investigated associations between SNPs in 25 breast cancer-related candidate genes and cognitive performance prior to treatment initiation |
| Koleck, et al., 2016 [58] | Cross-sectional study | To investigate the associations between genetic polymorphisms and cognitive function in postmenopausal women with BC prior to the initiation of adjuvant therapy |
| Li et al., 2020 [59] | Prospective longitudinal cohort study | To examine how BDNF, APOE, and COMT polymorphisms influence ChT-induced prospective memory impairments in BC patients, considering varying levels of estrogen and progesterone receptor expression |
| Lyon et al., 2016 [60] | Prospective longitudinal cohort study | To prospectively assess whether systemic cytokine levels predict CRCI over the two years following diagnosis in women with early-stage BC |
| Madison et al., 2023 [61] | Prospective longitudinal cohort study (secondary analysis from four distinct studies) | To investigate whether depression accompanied by elevated inflammation or increased intestinal permeability predicts worse subjective and objective CRCI in BC survivors |
| Mandelblatt et al., 2023 [62] | Cross-sectional study | To assess whether immune activation and inflammatory markers account for CRCI differences between older BC survivors and cancer-free controls |
| Myers et al., 2022 [63] | Prospective longitudinal sub study nested within a randomized, wait-list controlled trial | To characterize neurotrophic/growth factors and inflammatory biomarkers at baseline, 4 weeks post-intervention, and 16 weeks post-intervention, as well as examine whether shifts in neuroprotective and inflammatory biomarkers track with changes in CRCI |
| Ng et al., 2016 [64] | Prospective longitudinal cohort study | To evaluate the impact of the BDNF Val66Met polymorphism on CRCI in patients with early-stage BC |
| Ng et al., 2017 * [65] | Prospective longitudinal cohort study | To investigate how plasma BDNF levels and self-reported CRCI change over the course of ChT in early-stage BC patients and to determine whether plasma BDNF trajectories differ according to BDNF Val66Met genotype |
| Nudelman et al., 2023 [66] | Prospective longitudinal cohort study | To identify genetic factors that contribute to the risk of cognitive decline in older female BC survivors |
| Palesh et al., 2025 [67] | Prospective longitudinal cohort study | To investigate the mechanisms driving CRCI in BC survivors, focusing on neuron-derived exosomes as a novel biomarker of neurocognitive decline |
| Pang et al., 2021 [68] | Prospective longitudinal quasi-experimental cohort study | To assess whether a psychosocial intervention alters serum and buffy-coat biomarkers and cognitive performance in early-stage BC patients with CRCI |
| Pang et al., 2023 [69] | Cross-sectional study | To determine whether psychological distress contributes to CRCI by modulating circulating levels of IL-1β, TNF-α, and IL-4 |
| Park et al., 2025 [70] | Prospective longitudinal randomized controlled trial | To assess whether genetic variants moderate the effects of Mindfulness-Based Stress Reduction for BC on improvements in cognitive functioning and related symptoms among BC survivors |
| Patel et al., 2023 [71] | Prospective longitudinal cohort study | To quantify how inflammatory-marker levels shift across specific BC treatment modalities and to test whether treatment-related rises in these markers coincide with worse symptoms, such as greater fatigue, poorer physical functioning, both objective and self-reported CRCI, amplified pain, disrupted sleep, and depressed mood |
| Toh et al., 2020 [72] | Prospective longitudinal cohort study | To identify distinct cytokine profiles that correspond to differing self-perceived CRCI trajectories in BC survivors |
| Vardy et al., 2019 * [73] | Cross-sectional study | To examine how neuropsychological performance measured via clinical tests, CANTAB, and FACT-Cog relates to laboratory biomarkers and fMRI findings across three groups |
| Von Ah et al., 2022 [74] | Prospective longitudinal randomized controlled trial | Exploratory outcomes encompassed objective performance on neuropsychological tests and plasma BDNF concentrations |
| Yang et al., 2020 [75] | Prospective longitudinal quasi-experimental observational study | To determine whether early-stage BC ChT-induced changes in DNA methylation patterns that persist one year post-treatment initiation are associated with cognitive function |
| Yao et al., 2022 [76] | Prospective longitudinal randomized controlled trial | To test whether CALM psychotherapy lowers systemic inflammation and, in turn, lessens CRCI in BC patients undergoing ChT |
| Yao et al., 2023 [77] | Prospective longitudinal cohort study | To determine whether ALDH2 genotyping can identify subgroups of BC patients who are more susceptible to cognitive impairment during ChT |
| Yap et al., 2020 * [78] | Prospective longitudinal cohort study | To evaluate whether plasma BDNF concentrations and the BDNF Val66Met genotype are linked to CRCI at ChT completion and to persistent or delayed subjective CRCI up to 24 months afterwards in early-stage BC survivors |
| Yap et al., 2021 [79] | Prospective longitudinal cohort study | To investigate how cytokine and BDNF levels vary across distinct CRCI trajectories in early-stage BC patients |
| Yu et al., 2022 [80] | Cross-sectional study | To determine whether peripheral blood biomarkers NLR, carcinoembryonic antigen CEA and CA153 are associated with CRCI in early-stage BC patients pre- and post-ChT |
| Zhao et al., 2020 [81] | Cross-sectional study | To assess pre- versus post-ChT shifts in plasma cytokines and determine how those changes relate to CRCI and quality of life in early-stage BC patients |
| Zuniga et al., 2018 [82] | Cross-sectional study | To determine whether BC survivors with higher serum total carotenoid levels exhibit better cognitive function and to explore whether elevated carotenoid concentrations correspond to lower systemic inflammatory markers, thereby implicating reduced inflammation as a potential pathway for carotenoid-related cognitive benefits |
| Author(s), Year, Country | Group of Interest | Group of Interest: Demographics | Group of Interest: Cancer-Related Information | Control Group | Control Group: Demographics | Control Group: Cancer-Related Information |
|---|---|---|---|---|---|---|
| Andreano et al., 2012 [23] Country: USA | 18 BC patients | Age (years, mean): 41.9 | 20 HC | Age (years, mean): 39.9 | / | |
| Aspelund et al., 2024 [24] Country: Iceland | 112 BC patients | Age (years, mean ± SD): 61.8 ± 10.7 Education level (%): Primary 16.1; Secondary 32.1; University 47.3 | Cancer stage (%): 0 3.6; I 50.9; II 36.6; III 8.9 Molecular type (%): HER-2 positive 7.1; ER positive 90.2; PR positive 71.4 | 67 HC | Age (years, mean ± SD): 60.9 ± 9.5 Education level (%): Primary 14.9; Secondary 25.4; University 55.2 | / |
| Belcher et al., 2022 [16] Country: USA | 519 BC patients | Age (years, mean ± SD): 53.3 ± 10.6 Education level (%): <High school 2.1; High school or GED 22.7; >Some college 75.1; Ethnicity (%): American Indian or Alaska Native 1.2; Asian or Asian American 1.7; Black or African American 8.3; White 88.8 | Cancer stage (%): I 26.8; II 49.9; III 18.9; Unknown 4.4 | 338 HC | Age (years, mean ± SD): 52.8 ± 10.3 Education level (%): <High school 0; High school or GED 11.8; >Some college 88.2; Ethnicity (%): Asian or Asian American 0.9; Black or African American 4.1; White 95 | / |
| Bender et al., 2018 [35] Country: USA | 288 early-stage BC (one cohort received ChT followed by anastrozole and the other received anastrozole alone) | BC cohort only (n = 261) Cancer stage (%): I 68; IIa 21; IIb 7; IIIa 4 | 111 HC | / | / | |
| Boivin et al., 2020 [36] Country: USA | 20 BC patients in remission | Age (years, mean ± SD): 55.2 ± 12.26 Education level (%): High school or less 40; Some college 20; College degree 30; Post-graduate work or degree 10 | In remission | 26 BC patients in active treatment | Age (years, mean ± SD): 55.7 ± 10.21 Education level (%): High school or less 23; Some college 31; College degree 19; Post-graduate work or degree 27 | Cancer stage (%): 0 4; I 25; II 55; III 15 |
| Bower et al., 2013 [37] Country: USA | 171 BC patients | Age (years, mean, range): 51.5 (31–66) Education level (%): High school/some college 15.20; Associate degree/college graduate 54.39; Graduate degree 30.41 Ethnicity (%): White 79.53; Other 20.47 | Cancer stage: 0–IIIA | / | / | / |
| Boyle et al., 2017 [38] Country: USA | 91 BC patients | Age (years, mean ± SD): 57 ± 7.85 | Cancer stage (%): I 48; II 30; III 22 | / | / | / |
| Carlson et al., 2018 [39] Country: USA | 15 BC patients | Age (years, mean ± SD): 64.4 ± 12.3 Education level (n): Post-secondary education 7 Ethnicity (n): Caucasian 13 | Cancer stage (%): I 6.67; II 53.33; III 40 Biological type: mostly invasive ductal carcinoma | / | / | / |
| Carrol et al., 2019 * [40] Country: USA | 94 BC patients | Age (years, mean ± SD): 56.5 ± 8.1 Education level (%): After college 50; College degree 31; No college degree 19 Ethnicity (%): White, non-Hispanic 80; Hispanic 9; Black 4; Asian 3; Other 4 | Cancer stage: 0–IIIA | / | / | / |
| Chae et al., 2016 * [41] Country: Singapore | 125 BC patients | Age (years, mean ± SD): 50.26 ± 8.82 Education level (%): Primary school 16; Secondary school 46.40; Pre-university 20; Graduate/postgraduate 17.60 Ethnicity (%): Chinese 80.80; Malay 10.40; Indian 5.60; Others 3.20 | Cancer stage (%): I 17.60; II 52.80; III 29.60 | / | / | / |
| Chae et al., 2018 [42] Country: Singapore | 108 BC patients | Age (years, mean ± SD): 52.0 ± 9.2 Ethnicity (%): Chinese 82.4; Malay 9.3; Indian 4.6; Others 3.7 | Cancer stage (%): I 12.0; II 66.7; III 21.3 | / | / | / |
| Chan et al., 2019 [43] Country: Singapore | 351 BC patients pre-ChT | Age (years, mean ± SD): 51.2 ± 9.1 Education level (%): No education 1.1; Grade school 13.7; High school 45.6; Pre-university college 20.5; College/graduate degree 19.1 Ethnicity (%): Chinese 81.2; Malay 9.7; Indian 5.7; Other 3.4 | Cancer stage (%): I 17.4; II 59.8; III 22.8 | / | / | / |
| Chen et al., 2021 [44] Country: Taiwan | 106 BC patients divided into two groups: (1) Pre-ChT (70) (2) Post-ChT (36) | Pre-ChT Age (years, mean ± SD): 51.74 ± 11.39 Education (years, mean ± SD): 12.50 ± 3.97 Post-ChT Age (years, mean ± SD): 49.97 ± 10.04 Education (years, mean ± SD): 11.97 ± 3.82 | Pre-ChT Cancer stage (%): I 30; II 51.4; III 17.1 Biological type: Invasive breast carcinoma Post-ChT Cancer stage (%): I 30; II 44.4; III 19.4 Biological type: Invasive breast carcinoma | 30 HC | Age (years, mean ± SD): 49.97 ± 10.04 Education (years, mean ± SD): 12.50 ± 3.97 | / |
| Cheng et al., 2016 [45] Country: China | 80 triple-negative BC patients | Age (years, mean ± SD): 48.48 ± 10.57 Education (years, mean ± SD): 10.09 ± 3.37 | Molecular type (%): triple-negative 100 Biological type (%): Non-special type invasive carcinoma 92.5; Special type invasive carcinoma 3.75; Carcinoma in situ 3.75 | 165 non-triple negative BC patients | Age (years, mean ± SD): 49.39 ± 10.61 Education (years, mean ± SD): 10.08 ± 3.63 | Molecular type (%): Non-triple-negative 100 Biological type (%): Non-special type invasive carcinoma 94.55; Carcinoma in situ 4.85; Micro invasive carcinoma 0.61 |
| Cho et al., 2024 [46] Country: USA | 102 BC patients | Age (years, mean ± SD): 62.7 ± 7.99 Education (years, mean ± SD): 16.3 ± 2.46 Ethnicity (%): White 88.2; Black 7.8; Other 4 | Cancer stage (%): 0 13.7; I 62.7; IIA 14.7; IIB 4.9; IIIA 3.9 Biological type (%): Ductal carcinoma in situ 13.7 | / | / | / |
| Conroy et al., 2013 [47] Country: USA | 24 BC patients who received ChT | Age (years, mean ± SD): 57.8 ± 9.6 Education (years, mean ± SD): 15.7 ± 2.1 | Cancer stage (%): I 29; IIa 33; IIb 25; IIIa 8; IIIb 4 | 23 HC | Age (years, mean ± SD): 61.2 ± 9.9 Education (years, mean ± SD): 16.0 ± 2.3 | / |
| Duivon et al., 2024 [48] Country: France | 200 BC patients | Age (years, mean ± SD): 54 ± 11 Education (years, mean ± SD): 13.2 ± 2.8 | Cancer stage (%): I 43; II 42; III 15; Missing 1 Molecular type (%): HER-2 positive 13; HER-2 negative 87; Missing 0.5 | / | / | / |
| Gan et al., 2025 [49] Country: China | 53 BC patients with psychological distress | Age (years, mean ± SD): 51.00 ± 8.06 Education level (%): Primary school 30.19; Junior high school 45.28; University and above 24.53 | Cancer stage (%): I 15.09; II 47.17; III 37.74 Molecular type (%): Luminal A 7.55; Luminal B 67.92; HER-2 overexpression 16.98; Triple-negative 7.55 Pathological type (%): Non-invasive carcinoma 3.77; Invasive carcinoma no special type 96.23 | 51 BC patients without psychological distress | Age (years, mean ± SD): 50.94 ± 6.78 Education level (%): Primary school 21.57; Junior high school 50.98; University and above 27.45 | Cancer stage (%): I 7.84; II 54.90; III 37.26 Molecular type (%): Luminal A 3.92; Luminal B 54.90; HER-2 overexpression 31.37; Triple-negative 9.81 Pathological type (%): Non-invasive carcinoma 1.96; Invasive carcinoma no special type 98.04 |
| Ganz et al., 2013 [50] Country: USA | 49 BC treated with ChT | Age (years, mean ± SD): 49.9 ± 8.5 Education level (%): Post-high school 16; College 35; Post-college 49 Ethnicity (%): White 84 | Cancer stage (%): I 35; II 57; III 8 | 44 BC not treated with ChT | Age (years, mean ± SD): 52.8 ± 6.7 Education level (%): Post-high school 18; College 32; Post-college 50 Ethnicity (%): White 86 | Cancer stage: 0 37; I 56; II 7; |
| Harrison et al., 2021 [51] Country: USA | 83 BC patients divided into two groups: (1) Group treated with ChT (42) (2) Group ChT naïve (41) | Group treated with ChT Age (years, mean ± SD): 55 ± 7 Education (years, mean ± SD): 16 ± 3 Group ChT naïve Age (years, mean ± SD): 59 ± 7 Education (years, mean ± SD): 17 ± 2 | Group treated with ChT Cancer stage (%): I 26; II 55; III 19 Group ChT naïve Cancer stage (%): 0 37; I 50; II 13; III 0 | 53 HC | Age (years, mean ± SD): 55 ± 9 Education (years, mean ± SD): 17 ± 3 | / |
| Henneghan et al., 2018 [17] Country: USA | 66 BC patients | Age (years, mean ± SD): 49 ± 8.77 Education (years, mean ± SD): 16.7 ± 2.16 Ethnicity (%): White 93.4; Other 6.6 | Cancer stage (%): II-III 81.8 Molecular type (%): ER positive/PR positive 84.8 Biological type (%): Invasive ductal carcinoma 69.7 | / | / | / |
| Henneghan et al., 2021 [52] Country: USA | 66 BC patients | Age (years, mean ± SD): 48.44 ± 8.73 Education (years, mean ± SD): 16.7 ± 2.16 Ethnicity (%): White 93.4; African American 1.5; Asian 4.5 | Cancer stage (%): I 18.2; II 62.1; III 19.7 Molecular type (%): ER positive/PR positive 84.8; HER-2 positive 39.4 Biological type (%): Invasive ductal carcinoma 69.7; Ductal carcinoma in situ 15.2; Invasive lobular carcinoma 7.6; Multiple 7.6 | / | / | / |
| Janelsins et al., 2022 [53] Country: USA | 78 BC patients | Age (years, mean, range): 53.1 (30–73) Education level (%): <High-school diploma or General Educational Development certificate 23.1; > High school 76.9 Ethnicity (%): White 89.7; Other 10.3 | Cancer stage (%): I 16.7; II 28.2; III 16.7; Unknown 38.5 | 78 HC | Age (years, mean, range): 54.4 (28–81) Education level (%): <High-school diploma or General Educational Development certificate 20.5; >High school 79.5 Ethnicity (%): White 98.7; Other 1.3 | |
| Jenkins et al., 2016 [54] Country: UK | 8 BC patients receiving ChT | Age (years, mean ± SD): 52.6 ± 3.9 Education level (%): Higher 37.5; Further 25; Secondary 37.5 | Cancer stage (n): II 12.5; III 87.5 | 6 BC patients not receiving ChT | Age (years, mean ± SD): 50.2 ± 2.3 Education level (n): Higher 66.67; Further 16.67; Secondary 16.67 | Molecular type: NR Cancer stage (n): I 80; II 20 |
| Keetile et al., 2023 [55] Country: South Africa | 113 BC patients divided into two groups: (1) CMF group (53) (2) FAC group (60) | CMF group Age (years, mean): 48.2 Educational level (%): Primary 17; Middle school 47.2; High school 34; Tertiary 1.9 Ethnicity (%): Black 100 FAC group Age (years, mean): 47.1 Educational level (%): Primary 13.3; Middle school 46.7; High school 38.3; Tertiary 1.7 Ethnicity (%): Black 100 | CMF group Cancer stage (%): II 41.5; III 58.5 Molecular type: Mostly hormone-independent FAC group Cancer stage (%): II 53.3; III 46.7 Molecular type: Mostly hormone-independent | / | / | / |
| Kesler et al., 2013 [18] Country: USA | 42 BC patients | Age (years, mean ± SD): 54.6 ± 6.5 Education (years, mean ± SD): 16.3 ± 2.6 | Cancer stage: I–IIIa | 35 HC | Age (years, mean ± SD): 55.5 ± 9.3 Education (years, mean ± SD): 17.0 ± 2.7 | / |
| Koleck et al., 2014 [56] Country: USA | 78 BC patients divided into two groups: (1) ChT + Anastrozole (37)–(11) e4 carriers and (26) non-e4 carriers (2) Anastrozole alone (41)–(9) e4 carriers and (32) non-e4 carriers | ChT + Anastrozole Age (years, mean ± SD): e4: 58.64 ± 4.61; non-e4: 58.5 ± 5.67 Education (years, mean ± SD): e4: 16.27 ± 3.35; non-e4: 15.42 ± 2.59 Ethnicity (n): e4: 10 Caucasian; non-e4: 25 Caucasian Anastrozole alone Age (years, mean ± SD): e4: 61.56 ± 4.61; non-e4: 61.03 ± 5.61 Education (years, mean ± SD): e4: 15.67 ± 2.96; non-e4: 14.97 ± 3.61 Ethnicity: e4: 9 Caucasian; non-e4 32 Caucasian | ChT + Anastrozole Cancer stage (%): e4: I 72.73; II 27.27; non-e4: I 42.31; IIa 38.46; IIb 11.54; IIIa 7.69 Anastrozole alone Cancer stage: e4: I 88.89; IIa 11.11; non-e4: I 78.13; IIa 21.88 | 50 HC—16 e4 carriers, 34 non-e4 carriers | Age (years, mean ± SD): e4: 60.25 ± 6.56; non-e4: 57.5 ± 5.59 Education (years, mean ± SD): e4: 15 ± 2.63; non-e4: 14.94 ± 3.43 Ethnicity: e4: 15 Caucasian; non-e4: 33 Caucasian | / |
| Koleck et al., 2017 [57] Country: USA | 138 BC patients divided into two groups: (1) ChT + Anastrozole (55) (2) Anastrozole only (83) | ChT + Anastrozole Age (years, mean ± SD): 58.76 ± 5.46 Education (years, mean ± SD): 15.67 ± 2.78 Ethnicity (%): Caucasian 94.5 Anastrozole alone Age (years, mean ± SD): 62.47 ± 5.96 Education (years, mean ± SD): 14.95 ± 3.05 Ethnicity (%): Caucasian 97.6 | ChT + Anastrozole Cancer stage (%): I 44; IIa 34; IIb 12; IIIa 10 Molecular type (%): ER status: Positive 96, Negative 4; PR status: Positive 76, Negative 24; HER-2 status: Positive 19.1, Negative 80.9 Biological type (%): Lymph-node status: Positive 38, Negative 62; Invasive type: Ductal 90, Lobular 10 Anastrozole alone Cancer stage (%): I 81.3; IIa 16.3; IIb 2.5 Molecular type (%): ER status: Positive 100; PR status: Positive 88.8; Negative 11.3; HER-2 status: Positive 5.1; Negative 94.9 Biological type (%): Lymph-node status: Positive 6.3; Negative 93.7; Invasive type: Ductal 79.7; Lobular 17.7; Ductal and lobular 2.5 | 82 HC | Age (years, mean ± SD): 58.39 ± 5.85 Education (years, mean ± SD): 14.93 ± 2.99 Ethnicity (%): Caucasian 92.7 | / |
| Koleck, et al., 2016 [58] Country: USA | 138 BC patients divided into two groups: (1) ChT followe + Anastrozole (55) (2) Anastrozole only (83) | ChT + Anastrozole Age (years, mean ± SD): 58.76 ± 5.47 Education (years, mean ± SD): 15.67 ± 2.78 Ethnicity (%): Caucasian 94.5 Anastrozole alone Age (years, mean ± SD): 62.47 ± 5.96 Education (years, mean ± SD): 14.95 ± 3.06 Ethnicity (%): Caucasian 97.6 | ChT + Anastrozole Cancer stage (%): I 45.5; IIa 34.5; IIb 9.1; IIIa 10.9 Anastrozole alone Cancer stage (%): I 83.1; IIa 14.5; IIb 2.4 | 82 HC | Age (years, mean ± SD): 60.06 ± 6.08 Education (years, mean ± SD): 14.84 ± 2.91 Ethnicity (%): Caucasian 92.6 | / |
| Li et al., 2020 [59] Country: China | 232 BC patients divided into two groups: (1) ER negative/PR negative group (113) (2) ER positive/PR positive group (119) | ER negative/PR negative group Age (years, mean ± SD): 48.50 ± 10.70 Education (years, mean ± SD): 9.98 ± 3.66 ER positive/PR positive group Age (years, mean ± SD): 48.92 ± 10.14 Education (years, mean ± SD): 9.74 ± 4.07 | ER negative/PR negative group Biological type (%): Non-special type invasive carcinoma of breast 92.04; Special type invasive carcinoma of breast 2.65; Carcinoma in situ 5.31 ER positive/PR positive group Biological type (%): Non-special type invasive carcinoma of breast 94.96; Carcinoma in situ 4.20; Micro invasive carcinoma 0.84 | / | ||
| Lyon et al., 2016 [60] Country: USA | 75 BC patients | Age (years, mean ± SD): 51.52 ± 10.34 Educational level (%): <high school 9; High school 12; >high school 79 Ethnicity (%): Caucasian 71; African American 29 | Cancer stage (%): I 27; IIa 41; IIb 21; IIIa 11 Molecular type (%): Luminal A 51; Luminal B 11; Triple negative 29; HER2 positive, ER negative/PR negative 9 Biological type (%): Grade 1 7; Grade 2 37; Grade 3 56 | / | / | / |
| Madison et al., 2023 [61] Country: US | 613 BC patients who | Age (years, mean ± SD): 54.4 ± 10.2 Education level (%): High school or less 17; Some college 21; College degree 31; Graduate or professional training 31 Ethnicity (%): White 86; Black 10; Asian 2; Native American 1; Mixed 1 | Cancer stage (%): 0 8; I 46; II 39; III 7 | / | / | / |
| Mandelblatt et al., 2023 [62] Country: USA | 400 BC patients | Age (years, mean ± SD): 67.8 ± 5.3 Education (years, mean ± SD): 15.5 ± 2.1 Ethnicity (%): Non-White (Black, Hispanic, Asian, other) 17.3; White, non-Hispanic: 82.8 | Cancer stage (%): 0 17.4; I 60.9; II 18.2; III 3.6 Molecular type (%): ER positive 18.2; HER-2 positive 3.6 | 329 HC | Age (years, mean ± SD): 67.6 ± 6.2 Education (years, mean ± SD): 15.6 ± 2.2 Ethnicity (%): Non-White (Black, Hispanic, Asian, other) 16.4; White, non-Hispanic 83.6 | / |
| Myers et al., 2022 [63] Country: USA | 15 BC patients undergoing combined exercise and game-based cognitive training | Age (years, mean, range): 54.6 (42–66) Education level (%): High school 7; College 60; Graduate School 33 Ethnicity (%): Black or African American 20; White 80 | Cancer stage (%): I 33; II 67; III 7 | 15 BC wait-list controls | Age (years, mean, range): 55.3 (37–66) Education (%): High School 7; College 80; Graduate School 13 Ethnicity (%): Black or African American 6.7; White 86.7; Two or more races 6.7 | Cancer stage (%):I 46; II 46; III 7 |
| Ng et al., 2016 [64] Country: Singapore | 145 BC patients | Age (years, mean ± SD): 50.8 + 8.8 Education level (%): Primary school 15.2; Secondary school 48.3; Pre-university 20; Graduate/postgraduate 16.6 Ethnicity (%): Chinese 82.1; Malay 10.3; Indian 4.8; Others 2.8 | Cancer stage (%): I 22.1; II 49.7; III 28.3 | / | / | / |
| Ng et al., 2017 * [65] Country: Singapore | 51 BC patients | Age (years, mean ± SD): 52.6 ± 9.5 Education level (%): None 2; Primary school 11.8; Secondary school 43.1; Pre-university 13.7; Graduate/postgraduate 29.4 Ethnicity (%): Chinese 78.4; Malay 7.8; Indian 11.8; Other 2% | Cancer stage (%): I 13.7; II 62.8; III 23.5 | / | / | / |
| Nudelman et al., 2023 [66] Country: USA | 325 BC patients | Age (years, mean ± SD): 68.2 ± 5.7 Education (years, mean ± SD): 15.3 ± 2.1 Ethnicity (%): White, non-Hispanic 100% | Cancer stage: 0–III | 340 HC | Age (years, mean ± SD): 67.9 ± 6.6 Education (years, mean ± SD): 15.7 ± 2.2 Ethnicity (%): White, non-Hispanic 100% | |
| Palesh et al., 2025 [67] Country: USA | 73 BC (behavioral therapy for cancer-related insomnia) | Age (years, mean ± SD): 51 ± 12 Education (years, mean ± SD): 16 ± 3 | Cancer stage (%): 0 1; I 32; II 49; III 18 Molecular type (%): ER positive 74; PR positive 53; HER-2 positive 37 | 55 BC patients (healthy eating education for healthy sleep) | Age (years, mean ± SD): 49 ± 10 Education (years, mean ± SD): 16 ± 3 | Cancer stage (%): 0 3; I 32; II 47; III 18 Molecular type: ER positive 72; PR positive 63; HER-2 positive 50 |
| Pang et al., 2021 [68] Country: China | 50 BC undergoing the CALM intervention | Age (years, mean ± SD): 52.04 ± 8.55 Education level (%): Primary school 44; Secondary school 52; Technical school 2; University 2 | Molecular type (%): HER-2 positive 78; HER-2 negative 22 Biological type (%): Infiltrative 52; Invasive ductal 36; Other 12 | 78 BC undergoing care as usual | Age (years, mean ± SD): 50.60 ± 6.72 Education level (%): Primary school 44.8; Secondary school 50; Technical school 2.6; University 2.6 | Molecular type (%): HER-2 positive 79.5; HER-2 negative 20.5 Biological type (%) Infiltrative 47.4; Invasive ductal 50; Other 2.6 |
| Pang et al., 2023 [69] Country: China | 62 BC with psychological distress | Age (years, mean ± SD): 51.37 ± 6.63 Education (years, mean ± SD): 8.58 ± 1.97 | Cancer stage (%): I 9.7; II 51.6; III 38.7 Molecular type (%): HER-2 positive 85.5; HER-2 negative 14.5 Biological type (%): Infiltrative 48.4; Invasive ductal 51.6 | 60 BC without psychological distress | Age (years, mean ± SD): 49.48 ± 7 Education (years, mean ± SD): 9.12 ± 2.28 | Cancer stage (%): I 13.3; II 45; III 41.7 Molecular type (%): HER-2 positive 78.3; HER-2 negative 21.7 Biological type (%): Infiltrative 60; Invasive ductal 40 |
| Park et al., 2025 [70] Country: USA | 69 BC (Mindfulness-Based Stress Reduction program) | Age (years, mean ± SD): 58.0 ± 11.5 Education level (%): Less than college 23.2; Some college or AA degree 26.1; College degree 24.6; Graduate or professional school 26.1 Ethnicity (%): White 100 | Cancer stage: I–III Biological type (%): Lobular carcinoma 4.4; Ductal carcinoma 26.1; Invasive lobular 2.9; Invasive ductal 42; Other/unknown 24.6 | 59 BC (BC Education Support Program) | Age (years, mean ± SD): 57.2 ± 9.9 Education level (%): Less than college 11.9; Some college or AA degree 32.2; College degree 25.4; Graduate or professional school 30.5 Ethnicity (%): White 100 | Cancer stage: I–III Biological type (%): Lobular carcinoma 10.2; Ductal carcinoma 35.6; Invasive Lobular 8.5; Invasive ductal 28.8; Other/Unknown 17 |
| Patel et al., 2023 [71] Country: USA | 173 BC patients | Age (years, mean, range): 60 (45–84) Education level (%): Less than High School 6.4; High School Diploma 22; Some College 38.2; Bachelor’s Degree 20.2; Graduate Degree 13.3 Ethnicity (%): White, Non-Hispanic 56.1; White, Hispanic 22; Asian 8.1; Black 6.9; Other 6.9 | Cancer Stage (%): 0 15.6; I 43.4; II 31.8; III 9.2 Molecular type (%): Hormone positive, HER-2 negative 63.6; Hormone positive, HER-2 positive 8.7; Hormone positive, HER-2 unknown 10.4; Hormone negative, HER-2 negative (Triple Negative) 8.7; Hormone negative, HER-2 positive 5.2; Hormone negative, HER-2 unknown 1.2; Not tested 2.3 | 77 HC | Age (years, range): 61 (45–86) Education level (%): Less than High School 1.3; High School Diploma 9.1; Some College 33.8; Bachelor’s Degree 20.8; Graduate Degree 35.1 Ethnicity (%): White, Non-Hispanic 81.8; White, Hispanic 5.2; Asian 2.6; Black 2.6; Other 7.8 | |
| Toh et al., 2020 [72] Country: Singapore | 128 BC patients | Age (years, mean ± SD): 51.8 ± 8.9 Education (years, mean ± SD): 10.8 ± 3.4 Education level (%): None 2.3; Primary 11.7; Secondary 48.4; Pre-University 18.8; Graduate and above 18.8 Ethnicity (%): Chinese 82.8; Malay 9.4; Indian 3.9; Others 3.9 | Cancer stage (%): I 10.9; II 68.7; III 20.3 | / | / | / |
| Vardy et al., 2019 * [73] Country: Canada | BC patients divided into three groups: (1) ChT + CS + (n = 44) (2) ChT + CS − (n = 52) (3) ChT − (n = 30) | ChT + CS+ Age (years, mean, range): 48.39 (30–60) Education (years, mean, range): 15.5 (9–20) ChT + CS− Age (years, mean, range): 48.39 (29–60) Education (years, mean, range): 15.1 (8–20) ChT− Age (years, mean, range): 54.10 (30–59) Education (years, mean, range): 15.37 (12–20) | / | / | / | |
| Von Ah et al., 2022 [74] Country: USA | 19 BC (cognitive training) | Age (years, mean ± SD): 56.3 ± 9.3 Education (years, mean ± SD): 15.2 ± 1.9 Ethnicity (%): Black 36.8; White 63.2 | Cancer stage (%): I 26.3; II 47.4; III 21.1; Unsure 5.3 | 17 BC (active control activities) | Age (years, mean ± SD): 58.8 ± 6.7 Education (years, mean ± SD): 16.5 ± 1.9 Ethnicity (%): Black 41.2; White 58.8 | Cancer stage (%): I 23.5; II 58.8; III 11.8; Unsure 5.9 |
| Yang et al., 2020 [75] Country: USA | 58 BC patients | Age (years, mean, range): 51.48 ± 10.52 Ethnicity (%): African American 32.8; Caucasian 67.2 | Cancer stage (%): I 27.6; IIa 44.8; IIb 19; IIIa 8.6 Molecular type (%): Triple negative 32.8 | / | / | / |
| Yao et al., 2022 [76] Country: China | 31 BC (CALM intervention) | Age (years, mean ± SD): 51.65 ± 6.67 Education (years, mean ± SD): 8.36 ± 1.91 | Cancer stage (%): I 6.5; II 61.3; III 32.3 Molecular type: ER negative 48.4; ER positive 51.6; PR negative 45.2; PR positive 54.8; HER-2 negative 16.1; HER-2 positive 83.9 Pathological type: Invasive carcinoma no special type 58.1; Invasive carcinoma 41.9 | 38 BC (care as usual) | Age (years, mean ± SD): 51.65 ± 6.67 Education (years, mean ± SD): 8.36 ± 1.91 | Cancer stage (%): I 18.4; II 42.1; III 39.5 Molecular type: ER negative 39.5; ER positive 60.5; PR negative 44.7; PR positive 55.3; HER-2 negative 18.4; HER-2 positive 81.6 Biological type: Invasive carcinoma, no special type: 44.7; Invasive carcinoma: 52.6; Invasive carcinoma special type: 2.6 |
| Yao et al., 2023 [77] Country: China | 124 BC patients | Age (years, mean, range): 49.5 ± 10.50 Education (years, mean, range): 10.3 ± 3.86 | Molecular type (%): Luminal A 18.55; Luminal B 37.9; HER-2 overexpression 27.42; TNBC 16.13 Biological type (%): Invasive carcinoma no special type 92.74; Invasive Carcinoma Special type 0.81; Noninvasive carcinoma 5.65; Other type 0.81 | / | / | / |
| Yap et al., 2020 * [78] Country: Singapore | 174 BC patients | Age (years, mean ± SD): 51.8 ± 8.91 Education level (%): Primary school 11.5; Secondary school 46.6; Pre-university 18.4; Graduate/postgraduate 21.8; Not reported 1.7 Ethnicity (%): Chinese 83.3; Malay 9.8; Indian 4; Others 2.9 | Cancer stage (%): I 12.1; II 66.1; III 21.8 | / | / | / |
| Yap et al., 2021 [79] Country: Singapore | 136 BC patients | Age (years, mean ± SD): 52.0 ± 8.9 Education level (%): Primary school 12.5; Secondary school 49.3; Pre-university 15.4; Graduate/postgraduate 20.6 Ethnicity (%): Chinese 85.3; Malay 7.4; Indian 3.7; Others 3.7 | Cancer (%): I 11; II 66.9; III 22.1 | / | / | / |
| Yu et al., 2022 [80] Country: China | 122 BC post-ChT divided into two groups: (1) No cognitive impairment (44) (2) Cognitive impairment (78) | Age (years, mean ± SD): 50.6 ± 6.8 Education (years, mean ± SD): 8.8 ± 2.1 | Cancer stage (%): I 11.6; II 48.9; III 39.7 Molecular type (%): HER-2 positive 63.6; HER-2 negative 36.4 Biological type (%): Infiltrative non-specific cancer 52.1; Invasive ductal carcinoma 47.9 | 66 BC patients pre-ChT | Age (years, mean ± SD): 51.9 ± 7.6 Education (years, mean ± SD): 8.6 ± 2.1 | Cancer stage (%): I 15.2; II 48.5; III 36.4 Molecular type (%): HER-2 positive 59.1; HER-2 negative 40.1 Biological type (%): Infiltrative non-specific cancer 51.5; Invasive ductal carcinoma 48.5 |
| Zhao et al., 2020 [81] Country: China | 122 BC post-ChT divided into two groups: (1) No cognitive impairment (44) (2) Cognitive impairment (78) | No cognitive impairment Age (years, mean ± SD): 50.15 ± 7.15 Education (years, mean ± SD): 8.77 ± 2.11 Cognitive impairment Age (years, mean ± SD): 50.60 ± 6.71 Education (years, mean ± SD): 8.88 ± 2.16 | No cognitive impairment Cancer stage (%): I 11.36; II 47.73; III 40.91 Biological type (%): Infiltrative non-specific cancer 47.73; Invasive ductal carcinoma 52.27 Cognitive impairment Cancer stage (%): I 11.54; II 50; III 38.46 Biological type: Infiltrative non-specific cancer 47.44; Invasive ductal carcinoma 52.56 | 68 BC patients pre-ChT | Age (years, mean ± SD): 51.55 ± 7.69 Education (years, mean ± SD): 8.67 ± 2.07 | Cancer stage (%): I 14.71; II 50; III 35.29 Biological type (%): Infiltrative non-specific cancer 50; Invasive ductal carcinoma 50 |
| Zuniga et al., 2018 [82] Country: USA | 29 BC patients | Age (years, mean ± SD): 50.1 ± 10.1 Education level (%): ≥4-year college degree 69; Ethnicity (%): White 69; Black 10.3; Asian 6.9; More than one 13.8 | Cancer stage (%): I 27.6; II 37.9; III 20.7; Unknown 3.4 Biological type (%): Ductal carcinoma in situ 10.3 | 38 HC | Age (years, mean ± SD): 50.8 ± 10.0 Education level (%): ≥4-year college degree 84.2 Ethnicity (%): White 86.8; Asian 2.6; More than one 10.5 |
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | % of Yes | Overall Risk |
|---|---|---|---|---|---|---|---|---|---|---|
| Andreano et al. (2012) [23] | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | 75% | Moderate risk |
| Aspelund et al. (2024) [24] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Bower et al. (2013) [37] | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 87% | Low risk |
| Carlson et al. (2018) [39] | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | 75% | Moderate risk |
| Carroll et al. (2019) [40] | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 87% | Low risk |
| Chen et al. (2021) [44] | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 87% | Low risk |
| Cho et al. (2024) [46] | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 87% | Low risk |
| Conroy et al. (2013) [47] | ✗ | ✗ | ✓ | ✓ | ? | ? | ✓ | ✓ | 50% | Moderate risk |
| Gan et al. (2025) [49] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Harrison et al. (2021) [51] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Henneghan et al. (2018) [17] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Henneghan et al. (2021) [52] | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ | 87% | Low risk |
| Kesler et al. (2013) [18] | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 87% | Low risk |
| Koleck et al. (2016) [58] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Koleck et al. (2017) [57] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Mandelblatt et al. (2023) [62] | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 87% | Low risk |
| Pang et al. (2023) [69] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Vardy et al. (2019) [73] | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 87% | Low risk |
| Yu et al. (2022) [80] | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | 75% | Moderate risk |
| Zhao et al. (2020) [81] | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | 75% | Moderate risk |
| Zuniga et al. (2018) [82] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | % of Yes | Overall Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nudelman et al. (2023) [66] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Koleck et al. (2014) [56] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | % of Yes | Overall Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bender et al. (2018) [35] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | 100% | Low risk |
| Ng et al. (2016) [64] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | 100% | Low risk |
| Ng et al. (2017) [65] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | 100% | Low risk |
| Belcher et al. (2022) [16] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ? | ✓ | 82% | Low risk |
| Boivin et al. (2020) [36] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ? | ✓ | 82% | Low risk |
| Chae et al. (2016) [41] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ? | ✓ | 82% | Low risk |
| Chae et al. (2018) [42] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | 90% | Low risk |
| Duivon et al. (2024) [48] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Toh et al. (2020) [72] | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ? | ✗ | ✓ | 73% | Moderate Risk |
| Yap et al. (2020) [78] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Yap et al. (2021) [79] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Janelsins et al. (2022) [53] | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ? | ✓ | N/A | ✓ | 70% | Moderate Risk |
| Keetile et al. (2023) [55] | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | 80% | Low Risk |
| Li et al. (2020) [59] | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ? | ? | N/A | ✓ | 60% | Moderate Risk |
| Lyon et al. (2016) [60] | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ? | ? | N/A | ✓ | 64% | Moderate Risk |
| Madison et al. (2023) [61] | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | 80% | Low Risk |
| Palesh et al. (2025) [67] | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | 80% | Low Risk |
| Pang et al. (2021) [68] | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | 80% | Low Risk |
| Patel et al. (2023) [71] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Yao et al. (2023) [77] | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ? | N/A | ✓ | 50% | Moderate Risk |
| Jenkins et al. (2016) [54] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | 100% | Low risk |
| Ganz et al. (2013) [50] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 100% | Low risk |
| Cheng et al. (2016) [45] | ✓ | ✓ | ✓ | ? | ✗ | ✗ | ✓ | ✗ | ? | ? | ✓ | 45% | High risk |
| Chan et al. (2019) [43] | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | 91% | Low risk |
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | % of Yes | Overall Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yang et al. (2020) [75] | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✓ | ✗ | ✓ | 71% | Moderate risk |
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | % of Yes | Overall Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myers (2022) [63] | ✓ | ? | ✓ | ? | ? | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | 67% | Moderate risk |
| Park et al. (2025) [70] | ✓ | ? | ✓ | ✗ | ✗ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | 67% | Moderate risk |
| Von Ah et al. (2022) [74] | ✓ | ✓ | ✓ | ? | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 83% | Low risk |
| Yao et al. (2022) [76] | ✓ | ✓ | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | 75% | Moderate risk |
| Cytokine | Pre-Pharmacotherapy | During Pharmacotherapy | Post-Pharmacotherapy | Long-Term Survivorship |
|---|---|---|---|---|
| IL- β | Distress → IL-1β → ↑ perceived memory impairment (post-ChT) ↑ IL-1β → ↑ self-reported cognitive complaints; ↓ memory; ↓ cognitive flexibility; ↓ psychomotor speed; ↓ global cognition (post-ChT) | |||
| IL-2 | ↑ IL-2 → ↑ self-reported cognitive complaints; ↓ immediate/delayed recall; ↓ cognitive flexibility; ↓ psychomotor speed; ↓ global cognition (post-ChT) ↑ IL-2 → ↓ self-reported cognitive complaints (post-ChT) | |||
| IL-5 | ↑ IL-5 → ↑ self-reported cognitive complaints (pre-ChT) | ↑ IL-5 → ↑ psychomotor speed; ↑ memory (during ChT) | ↑ IL-5 → ↑ psychomotor speed; ↑ memory (post-ChT) | ↑ IL-5 → ↓ memory (2 years post-ChT) |
| IL-6 | ↑ IL-6 → ↑ self-reported cognitive complaints (during ChT) | ↑ IL-6 → ↑ self-reported cognitive complaints; ↑ objective CRCI (post-ChT) ↑ (baseline) IL-6 → ↓ executive functions; ↑ executive functions (post-ChT) | ↑ (baseline) IL-6 → ↓ processing speed; ↓ memory (2 years post-ChT) | |
| TNF-α | ↓ TNF-α → ↑ risk of later CRCI | ↑ TNF-α → ↑ self-reported cognitive complaints (during ChT) | ↑ TNF-α → ↓ psychomotor speed (2 y post-ChT); ↓ delayed recall; ↓ global cognition (6 months–10 year-survivors) | |
| Il-7 | IL-7 → ↑ memory (during ChT) | ↑ IL-7 → ↓ memory; ↑ reaction time; ↑ executive functions (6 mo post-ChT); ↓ memory (2 years post-ChT) | ||
| IL-8 | ↑ IL-8 → ↓ attention; ↓ working memory; ↓ processing speed (pre-ChT) | ↑ IL-8 → ↓ attention; ↓ working memory; ↓ processing speed (post-ChT) | ↑ IL-8 → ↑ executive functioning (1 year post-ChT); ↓ overall/global cognition (6 months–10 year-survivors) | |
| Il-17 | ↑ IL-17 → ↓ psychomotor speed (pre-ChT) | ↑ IL-17 → ↑ psychomotor speed (during ChT) | ↑ IL-17 → ↑ psychomotor speed (post-ChT) | ↑ IL-17 → ↑ psychomotor speed (6 months → 2 years post-ChT); ↓ reaction time (6 months post-ChT) |
| IFN-γ | ↑ IFN-γ → ↑ reaction time (6 months post-ChT) | |||
| IL-12p70 | IL-12p70 → ↓ psychomotor speed (during ChT) | ↑ IL-12p70 → ↑ composite memory (6 months post-ChT) | ||
| GM-CSF | ↑ GM-CSF → ↓ reaction time (pre-ChT) | ↑ GM-CSF → ↓ processing speed; ↓ self-reported cognitive complaints (post-ChT) | ↑ GM-CSF → ↓ composite memory (6 months post-ChT); ↑ psychomotor speed (2 years post-ChT) | |
| G-CSF | ↑ G-CSF → ↑ psychomotor speed; ↑ executive functions (pre-ChT) | ↑ G-CSF → ↑ reaction times (post-ChT) |
| Outcome | Effect (Summary of Evidence) | Number of Participants (Studies) | Certainty in the Evidence * |
|---|---|---|---|
| IL-1β | Overall, the direction of the effect is inconsistent but tends toward higher IL-1β being associated with cognitive impairment. | ~1600 participants (15 studies). | Low ⊕⊕OO (downgraded for serious inconsistency and imprecision. Biological coherence across studies provides minor upgrading). |
| IL-2 | Overall, the body of evidence is predominantly null with a few positive studies and no consistent direction across cognitive domains. | ~750 participants (8 studies). | Very low ⊕OOO (downgraded for serious inconsistency and serious imprecision; observational design starts at low and is further downgraded). |
| IL-4 | Evidence is highly mixed: No consistent cognitive domain pattern emerges. | ~1700 participants (13 studies). | Very low ⊕OOO (downgraded for serious inconsistency and serious imprecision. Observational designs start at low; no convincing upgrade). |
| IL-5 | Evidence is sparse and inconsistent: No coherent domain pattern. | ~250 participants (4 studies). | Very low ⊕OOO (downgraded for serious imprecision and inconsistency). |
| IL-6 | Across the body of evidence, findings are mixed: direction tends toward a relationship between higher IL-6 and worse cognition in several studies, but many analyses show no effect. | ~2500 participants (20 studies). | Low ⊕⊕OO (downgraded for inconsistency and imprecision; modest upgrade consideration for biological coherence and replication, but not enough to exceed low). |
| IL-7 | Evidence is very limited and largely non-supportive: no positive studies identified | ~66 participants (2 studies). | Very low ⊕OOO (downgraded for serious imprecision and inconsistency/absence of effect; no upgrading factors). |
| IL-8 | Findings are mixed: several studies link higher IL-8 to worse cognition (executive, attention, global), but many analyses are null or opposite. | ~1900 (12 studies). | Low ⊕⊕OO (downgraded for inconsistency and imprecision; observational designs). |
| IL-10 | Evidence is inconsistent: direction varies by study (some report worse, others better performance with higher IL-10), and many results are null. | ~1800 (12 studies). | Very low ⊕OOO (serious inconsistency and serious imprecision). |
| IL-12 | Evidence is sparse: no consistent domain pattern. | ~415 (5 studies). | Very low ⊕OOO (downgraded for serious imprecision and inconsistency; no upgrading factors). |
| IL-13 | Limited but somewhat consistent evidence: positive studies reported higher IL-13 associated with poorer cognition. Additional studies found null results. | ~250 (4 studies). | Low ⊕⊕OO (small but directionally consistent evidence of association; downgraded for imprecision but not for inconsistency). |
| TNF-α/sTNFRI/II | Broad evidence base, but mixed: effects generally suggest higher TNF-α or soluble receptor levels predict worse cognitive performance, though nulls are frequent. | ~3100 (21 studies) | Low ⊕⊕OO (downgraded for inconsistency and imprecision; biological coherence prevents downgrading to very low). |
| IFN-γ | Very limited evidence: no replication of direction across studies. | ~1110 (9 studies) | Very low ⊕OOO (downgraded for serious inconsistency and imprecision). |
| GM-CSF | Evidence is mixed but limited: associations were inconsistent across domains (attention, memory). | ~530 (6 studies) | Very low ⊕OOO (downgraded for imprecision and inconsistency; no upgrading factors). |
| IL-1ra | No study reported a significant or directional association with cognition. All identified studies found null results. | ~300 (3 studies) | Very low ⊕OOO (downgraded for serious imprecision; consistent null evidence, but too limited for confidence). |
| CRP | Evidence is inconsistent: some studies report higher CRP linked to lower attention or executive scores, but most analyses show no association. | ~1250 (8 studies) | Low ⊕⊕OO (downgraded for inconsistency and imprecision; modest biological plausibility supports low rating). |
| NLR | Evidence is mixed, but directionally tends toward higher NLR associated with poorer cognition | ~350 (3 studies) | Low ⊕⊕OO (downgraded for imprecision and risk of bias, but not for inconsistency). |
| Cortisol | Evidence is inconsistent across studies. | ~170 (3 studies) | Very low ⊕OOO (downgraded for serious imprecision and inconsistency). |
| BDNF | Evidence is mixed and inconsistent: the direction of effects (protective vs. detrimental) varied. | ~920 (6–7 studies) | Low ⊕⊕OO (downgraded for inconsistency and imprecision, but biological plausibility prevents rating as very low). |
| Outcome | Effect | Number of Participants (Studies) | Certainty in the Evidence * |
|---|---|---|---|
| ANKKI polymorphism | Variants associated with reduced odds of CRCI | ~130 participants (1 study) | Very low ⊕OOO (downgraded for concerns with risk of bias and imprecision) |
| ALDH2 polymorphism | Variants associated with increased odds of CRCI | ~230 participants (2 studies) | Low ⊕⊕OO (downgraded for concerns with risk of bias) |
| APOE polymorphism | Evidence is mixed | ~890 participants (6 studies) | Very low ⊕OOO (due to inconsistencies in the results and some concerns about the risk of bias) |
| BDNF polymorphism | Evidence is mixed | ~920 participants (6 studies) | Very low ⊕OOO (downgraded for serious inconsistency and concerns about the risk of bias) |
| CAT polymorphism | Variants associated with reduced odds of CRCI | ~140 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| CCNBI polymorphism | Variants associated with increased odds of CRCI | ~140 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| COMT polymorphism | Evidence is mixed | ~600 participants (3 studies) | Very low ⊕OOO (due to inconsistencies in the results and some concerns about the risk of bias) |
| DIAPH3 | Variants associated with reduced odds of CRCI | ~140 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| DNMT1 | Variants associated with reduced odds of CRCI | ~110 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| DRD2 polymorphism | Variants associated with reduced odds of CRCI | ~130 participants (1 study) | Very low ⊕OOO (downgraded due to some concerns in the risk of bias and imprecisions) |
| ERCC2 polymorphism | Mixed results, rs13181 variant associated with increased odds of CRCI, the others with decreased odds | ~140 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| ERCC3 polymorphism | Mixed results, rs2134794 variant associated with increased odds of CRCI, the others with decreased odds | ~430 participants (2 studies) | Very low ⊕OOO (downgraded due to inconsistencies in the results) |
| ERCC5 polymorphism | Mixed results, rs2296147 variant associated with reduced odds of CRCI, the rs751402 with mixed effects, and the others with increased odds of CRCI | ~430 participants (2 studies) | Very low ⊕OOO (downgraded due to inconsistencies in the results) |
| ESR1 | Mixed results on the rs488133 variant, the other yielded null results | ~140 participants (1 study) | Very low ⊕OOO (downgraded due to inconsistencies in the results and imprecision) |
| GPX1 polymorphism | Variants associated with preserved concentration | ~300 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| GSTM1 | Evidence is mixed | ~140 participants (1 study) | Very low ⊕OOO (downgraded due to inconsistencies in the results and imprecision) |
| HMCN1 | Variants associated with increased odds of CRCI | ~325 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| ILB | Null results | ~170 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| IL6 | Evidence is mixed | ~300 participants (2 studies) | Very low ⊕OOO (downgraded due to inconsistencies in the results) |
| MYBL2 | Evidence is mixed | ~140 participants (1 study) | Very low ⊕OOO (downgraded due to inconsistencies in the results and imprecision) |
| PARP1 | Variants associated with reduced odds of CRCI | ~430 participants (2 studies) | Moderate ⊕⊕⊕O (replicated, larger samples) |
| PGR | Variants associated with reduced odds of CRCI | ~140 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| SEPP1 | Mixed results, rs3877899 variant associated with increased odds of CRCI, while the rs230819 variant was associated with reduced odds | ~140 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| SOD1 | Variants associated with reduced odds of CRCI | ~140 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| SOD2 | Variants associated with increased odds of CRCI | ~140 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| TNF | Evidence is mixed | ~300 participants (2 studies) | Very low ⊕OOO (downgraded due to inconsistencies in the results) |
| Leukocyte DNA damage | Variants associated with increased odds of CRCI | ~95 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| PBMC Telomerase Activity | Variants associated with reduced odds of CRCI | ~95 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| PBMC Telomere Length | Null results | ~95 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| Mitochondrial DNA | Null results | ~125 participants (1 study) | Low ⊕⊕OO (downgraded for imprecision) |
| Direct and oxidative DNA Damage—Comet Assay | Null results | ~ 25 participants (1 study) | Very low ⊕OOO (downgraded for imprecision and some concerns about the risk of bias) |
| DNA methylation | Variants associated with increased odds of CRCI, except for DDHD1 and HSD17B3 | ~160 participants (2 studies) | Very low ⊕OOO (downgraded for imprecision and some concerns about the risk of bias) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschetti, A.; Danesin, L.; Bevilacqua, E.; Giada, R.; Gion, M.; Conte, P.; Burgio, F. Investigating the Trajectories of Association Between Biomarkers and Cancer-Related Cognitive Impairment in Patients with Breast Cancer: A Systematic Review. Cancers 2025, 17, 3522. https://doi.org/10.3390/cancers17213522
Boschetti A, Danesin L, Bevilacqua E, Giada R, Gion M, Conte P, Burgio F. Investigating the Trajectories of Association Between Biomarkers and Cancer-Related Cognitive Impairment in Patients with Breast Cancer: A Systematic Review. Cancers. 2025; 17(21):3522. https://doi.org/10.3390/cancers17213522
Chicago/Turabian StyleBoschetti, Angela, Laura Danesin, Elisa Bevilacqua, Riccardo Giada, Massimo Gion, Pierfranco Conte, and Francesca Burgio. 2025. "Investigating the Trajectories of Association Between Biomarkers and Cancer-Related Cognitive Impairment in Patients with Breast Cancer: A Systematic Review" Cancers 17, no. 21: 3522. https://doi.org/10.3390/cancers17213522
APA StyleBoschetti, A., Danesin, L., Bevilacqua, E., Giada, R., Gion, M., Conte, P., & Burgio, F. (2025). Investigating the Trajectories of Association Between Biomarkers and Cancer-Related Cognitive Impairment in Patients with Breast Cancer: A Systematic Review. Cancers, 17(21), 3522. https://doi.org/10.3390/cancers17213522








