Simple Summary
Skin cancer is one of the most common cancers worldwide, making its prevention a global priority. Historically, public health and dermatology were focused on primary and secondary prevention, respectively. Merging these efforts resulted in greater impacts. Multidisciplinary research combines the expertise of dermatologists, epidemiologists, behavioural scientists, health economists, geneticists, imaging, and artificial intelligence teams. New tools have helped to improve the accuracy and tailoring of prevention and early detection. However, there are ethical and data protection concerns surrounding their use, alongside unequal access and the need for more reliable long-term evidence. Future research should maintain and expand collaborative relationships, focusing on the fair and equal application of new technologies for use in real-life situations. The involvement of a multidisciplinary team shows potential in reducing the skin cancer burden, as well as contributing to prevention efforts for other health conditions.
Abstract
Background/objectives: The global incidence of skin cancer is rising, creating a need to strengthen prevention strategies. In this review, we examine the contributions of public health, dermatology, behavioural science, and emerging technologies such as artificial intelligence and bioinformatics, which have collectively shaped prevention in recent decades. Methods: Using a narrative scoping review approach guided by the PRISMA-ScR framework, we synthesised research across these disciplines to highlight their roles in enhancing skin cancer prevention. Results: Initial efforts focused on increasing public knowledge through sun protection campaigns and symptom recognition. Dermatologists enhanced early detection through refined techniques and clinical guidelines. Initiatives such as Euromelanoma enabled broader collaboration and population-level screening. As more disciplines joined, advances in risk stratification, digital imaging, artificial intelligence, molecular and genetic diagnostics and bioinformatics became possible. Beyond skin cancer prevention, these tools may have additional applications for systemic health issues. However, a number of challenges remain, particularly regarding data privacy concerns, cost-effectiveness, equitable access, and the validation of artificial intelligence tools in diverse populations. Conclusions: The prevention of skin cancer brings together knowledge spanning the fields of public health and dermatology to behavioural research and digital innovation. Working together, these disciplines have improved early detection and awareness. However, fragmented collaboration across regions throughout the world continue to limit their impact. Improved equity alongside stronger, more coordinated partnerships will be essential for the next phase of progress.
1. Introduction
Skin cancer prevention and early detection remain major public health challenges. The incidence of both melanoma and keratinocyte cancers (including basal cell carcinoma and squamous cell carcinoma) continue to rise globally with aging populations [1]. This trend has occurred due to a combination of increased disease burden from cumulative ultraviolet (UV) exposure, as well as improved diagnostic tools and reporting practices. Melanoma is still one of the leading causes of cancer-related deaths [2], and keratinocyte cancers impose substantial clinical and economic burdens on healthcare systems [3].
Over recent decades, approaches to skin cancer prevention have expanded beyond traditional education campaigns to include improved behavioural, clinical and technological strategies, collectively representing multidisciplinary collaboration, where dermatologists, public health professionals, behavioural scientists, engineers and policymakers work together to address skin cancer prevention. Unlike earlier siloed efforts, this integrated approach enables broader reach, improved messaging strategies, and scalability to effectively target both individuals and entire populations.
To understand how these strategies have evolved, this narrative scoping review examines the major methods used in skin cancer prevention, including population-based interventions, risk assessment models, imaging technologies and artificial intelligence (AI) tools. We explore both individual- and population-level contributions to skin cancer prevention and highlight how the contributions of many fields across both these domains have transformed prevention from simple awareness campaigns into data-informed, precision-based approaches.
2. Methods
This narrative scoping review followed the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) framework [4], integrating literature from a wide range of fields, including public health, dermatology and oncology, epidemiology and biostatistics, behavioural and social sciences, health economics, primary care and implementation research, pathology and genetics, and emerging fields such as AI, imaging, and bioinformatics. We conducted searches primarily through PubMed from inception through September 2025. Further literature was identified through the professional networks and disciplinary expertise of the co-authors. Studies were selected for their conceptual and illustrative relevance rather than formal inclusion or exclusion criteria, and no new data was generated.
3. Evolution of Skin Cancer Prevention
Following the scoping review outlined in Section 2, we identified three distinct phases in the evolution of skin cancer prevention: (1) public health initiatives, (2) dermatology-led interventions, and (3) integrated multidisciplinary models. This section synthesises key developments across these phases, highlighting how collaborative approaches have improved outcomes and addressed the limitations of earlier siloed efforts.
3.1. Primary Prevention: Public Health Initiatives
Since UV radiation was first recognised as a carcinogen [5], primary prevention strategies have focused on reducing sun exposure and promoting protective behaviours through public education and policy [6]. These efforts, demonstrated by Australian campaigns such as “Slip! Slop! Slap!” [7] and SunSmart [8] in the early 1980s, have demonstrably shifted cultural attitudes away from tanning, particularly in Australia and New Zealand. This also inspired similar campaigns across Europe [9] and the United States [10,11]. Long-term evaluations suggest that public health messaging programs have contributed to improvements in sun protective behaviours and reduced melanoma incidence for those under the age of 40 [12,13]. However, their impact on overall mortality and morbidity varies across populations and timeframes, influenced by factors such as age, unequal access to care, and cumulative lifetime UV exposure [14].
Public health efforts extended into policy, such as complete bans or stricter regulation of sunbed use [15], “No Hat, No Play” requirements in schools [16], and workplace standards mandating the use of sun protection for outdoor workers [6,17]. Health economic studies have since confirmed the cost-effectiveness of these strategies, which resulted in estimated savings of up to $8 for each $1 invested [18,19]. With aging populations and greater access to outdoor recreational activities, the incidence of skin cancer continued to rise, highlighting that early detection is necessary given the long-term effects of sun-exposed activities.
3.2. Secondary Prevention: Dermatology-Led Interventions
At the same time as primary prevention initiatives, dermatologists advanced secondary prevention by developing tools such as dermatoscopy [20] and the ABCDE criteria [21,22] to improve melanoma early detection [23]. Dermatoscopy transformed the assessment of pigmented and non-pigmented lesions [20,23]. The ABCDE criteria [21,22] gave patients a practical approach for monitoring their own moles, which strengthened the doctor-patient partnership in early detection. However, concerns regarding their use included variability in diagnostic accuracy [24], over-reliance on clinician judgement [25], and the lack of accessibility in under-resourced clinics [26], all which limited their potential impacts on population-level outcomes.
In 2008, Germany introduced a national skin cancer screening program, showing how dermatology-led strategies could be scaled to the population level. Early data suggested that such a program would reduce melanoma mortality [27]; however, participation rates, cost-effectiveness, and long-term benefits were inconsistent [28]. These limitations highlighted the need to combine clinical expertise and public health infrastructure to achieve sustainable population-level impact.
3.3. Integrated Multidisciplinary Models
By the late 1990s, it became clear that siloed approaches to skin cancer prevention were insufficient. Public health campaigns influenced prevention knowledge and attitudes, and dermatology advanced early detection, but neither could fully address the many biopsychosocial determinants of skin cancer risk [29,30]. For example, genetic susceptibility may be influenced by higher UV exposures in certain geographic areas [31]. Behavioural factors such as attitudes towards tanning or sunscreen use could be caused by differences in cultural norms, educational levels, and socioeconomic status [32]. Furthermore, variable access to diagnostic tools and treatment access is likely to cause greater disparities in outcomes [33]. These complex interactions factors highlight how the multifaceted nature of skin cancer raises a need for more integrated strategies that expand beyond isolated interventions.
Since 1999, Euromelanoma [34] has united dermatologists in providing free skin checks across 33 countries, raising awareness, and educating the public. Euromelanoma, and other global initiatives from the United States [35,36] and Australia [37] also generated population data, helping public health to refine messaging strategies for high-risk groups. This movement showed how community influence and clinical expertise together made prevention more effective.
4. Multidisciplinary Teams: Roles and Contributions
Although public health and dermatology laid essential foundations for early prevention efforts, broader progress has required input from many other specialties. The combination of diverse skillsets has strengthened prevention strategies, making them more responsive and equitable than traditional, single-discipline models.
4.1. Dermatology and Oncology
Joint efforts of dermatologists and oncologists have set the clinical standard for secondary prevention strategies, driven by their shared recognition that survival depends on timely diagnosis. Surgical management remains the cornerstone of early-stage melanoma, with five-year survival rates exceeding 98% [38], while late-stage disease is associated with poorer outcomes [39].
Dermatologists have integrated novel diagnostic imaging tools beyond dermatoscopy [20,23], including confocal microscopy [40] and total body photography (TBP) [41], while advances in dermatopathology led to greater accuracy in histological assessment [42]. Staging guidelines, excision margins, and sentinel lymph node biopsy techniques continue to be refined [43,44], allowing interventions to be both diagnostic and curative [45,46,47]. Mohs micrographic surgery has further enabled complete margin clearance with tissue conservation in selected cases [48].
The most dramatic progress has come from systemic therapy: collaboration between oncologists, basic scientists, immunologists, and the pharmaceutical industry has driven the development of immune checkpoint inhibitors and targeted BRAF/MEK inhibitors [49,50,51], which have transformed outcomes for advanced melanoma, achieving durable remissions and long-term survival in over half of the patients who previously had a dismal prognosis [52]. Despite being initially used only for advanced disease, these agents have rapidly expanded into curative-intent settings, now forming the basis of adjuvant, and more recently, neoadjuvant treatment strategies [53]. These systemic therapies also have implications for secondary prevention due to improved clinical benefits with early detection since timely diagnosis now has potential curative treatment. This may also influence screening strategies in high-risk populations by improving risk-benefit calculations. In addition, supramolecular methods have emerged in cancer therapy, which make use of non-covalent interactions to create drug delivery systems that can overcome biological barriers and deliver highly precise treatment [54,55]. Supramolecular methods may complement existing systemic therapies in the future.
More precise radiation techniques, such as intensity-modulated radiotherapy, stereotactic body radiotherapy, and proton therapy [56], alongside improved imaging-based radiotherapy planning [57], have helped maintain radiotherapy as a useful modality for selected patients. Whilst many of these advanced technologies and procedures across dermatology and oncology may be preferred by patients, the associated costs, concentration in metropolitan areas, and requirements for trained staff and infrastructure are barriers that prevent their widespread uptake [58,59,60]. These equitability and sustainability issues may restrict the ability of these developments to impact broader population-level outcomes.
4.2. Biostatistics and Epidemiology
Biostatistics and epidemiology have enabled the identification of individual risk factors by examining patterns and associations over entire populations. Large cohort studies, such as QSkin, which enrolled over 43,000 Queensland participants in its initial cohort, have demonstrated how UV exposure, genetic susceptibility, and behavioural factors interact to influence an individual’s skin cancer risk [61]. Prior to QSkin, prevention strategies were based on small observational studies [62,63,64], limiting their generalisability. The integration of epidemiological expertise has allowed for the development of risk prediction tools designed for more targeted prevention strategies, for example, identifying high-risk individuals for screening [65,66,67].
Although the roles of biostatisticians and epidemiologists may have been overlooked in the past, their contributions are clear. Analytical methods including multivariate modelling and survival analysis have refined clinical guidelines and enabled tracking of long-term incidence trends for subgroups of the population. For instance, multivariate modelling has helped to isolate the effectiveness of sunscreen from confounding variables [68], whilst survival analysis of melanoma registries has shown improvements in outcomes due to early detection [69]. However, limitations of biostatistics include overfitting risk prediction models and reduced accuracy in underrepresented populations [70]. For epidemiology, assumptions made regarding behaviours or access may not be accurate for those who are culturally diverse or socioeconomically disadvantaged [71]. Therefore, ongoing refinement in biostatistics and epidemiology is necessary to ensure equity across all demographic groups and ensure accuracy of population-level insights.
4.3. Behavioural Research, Social Sciences and Psychology
Behavioural and social science research has shown that awareness on its own does not always lead to sustained behavioural changes. Cultural norms around tanning, convenience, and perceived low risk all contribute to how much people will protect themselves in certain situations [72,73,74,75]. In response, prevention strategies are now increasingly targeted, for example, appearance-based messaging for adolescents [76,77], cancer-focused and situational alerts for older adults [78], and culturally adapted programs designed to engage Indigenous and migrant communities [79,80]. More recently, digital tools such as web-based programs, mobile applications, and social media, have helped to extend both the reach and personalisation of these approaches [80,81].
Psychological factors add another important layer. Fear of diagnosis [82], burden of treatment, or concerns about body image may lead to anxiety or depression, especially among adolescents [83], indicating a need for increased mental health support. The behavioural and social sciences, in combination with psychology, have helped strategies become more human-centred, adaptable, and culturally responsive.
4.4. Health Economics and Health Services Research
As prevention efforts expanded and showed success, health economists and health services researchers questioned whether and how these strategies could be delivered sustainably and fairly. Economic analyses consistently showed strong returns. For instance, the SunSmart program has an estimated return on investment of approximately $8.70 for every $1 spent and expected savings of $63.9 million for the Western Australia economy [19]. Similarly, sunscreen use in high-risk populations showed a 100% probability of being cost-effective compared to early melanoma detection [14]. These findings provided convincing evidence for policy action, such as sunbed bans and workplace safety standards. As advanced cancer treatment becomes more expensive with new targeted immunotherapies [84,85,86], health economic evaluations will likely become even more critical.
However, disparities by geographic location, socioeconomic status, and health system capacity influenced those who benefited most [87]. In response, health services researchers tested new delivery models, including screening in primary care, fly-in fly-out workers, training non-specialists to recognise suspicious lesions, and strengthening follow-up pathways [88,89]. Moreover, the allocation of resources between prevention and treatment raises important ethical considerations. Whilst prevention may yield more favourable cost-effectiveness ratios and population-level benefits [85], treatment on the other hand is medically necessary, time-sensitive and potentially lifesaving [90] for those already affected by skin cancer. Ethical trade-offs occur when either prevention or treatment is prioritised, especially in underserved communities where there is limited access to both [91]. There remains is a strong need for policymakers to balance cost-effectiveness with fairness, equity, and moral obligations, ensuring that all people, regardless of their background, can benefit from prevention and early detection programs.
4.5. Primary Care and Implementation Sciences
Primary care, nursing, other allied health professionals and professionals who see a lot of skin such as hairdressers, massage therapists and others working together is essential to prevention program success. General practitioners already provide opportunistic skin checks, risk counselling, and education, and often serve patients in areas where access to secondary or tertiary care is limited [92]. Nurses have expanded their roles to include patient education and follow-up [93], and pharmacists can reinforce sun protection in their daily interactions [94].
Implementation science has developed tools to improve the efficiency of integrating research-tested interventions into practice [95], including decision-support systems [96], teledermatology training [97], and risk-stratified care models [98]. Culturally tailored programs also promote equity, particularly for First Nations peoples, migrants and rural communities [99,100]. Together, these developments have embedded prevention more firmly into routine care.
4.6. Pathology and Genetics
Pathologists make important contributions to improving skin cancer outcomes and research into the value of primary and secondary prevention, as histopathology remains the gold standard for diagnosis. However, recent discoveries of immunohistochemical and molecular markers have further clarified how premalignant lesions progress to invasive disease [101,102]. Somatic mutations including BRAF [103], NRAS [104], and KIT [105] provide useful information regarding prognosis and management. Combined with epidemiological data, these findings support personalised prevention that can be uniquely tailored to inherited and acquired risk [106].
The identification of high-penetrance genes linked to hereditary melanoma such as CDKN2A [107] and POT1 [108] have enabled more opportunities for genetic counselling and improved risk stratification [109]. Furthermore, international collaborations such as GenoMEL [110] have linked genotype-phenotype data, providing greater support for precision prevention models [111]. Examples of genomic-informed programs involve individuals with pathogenic variants in CDKN2A [112] or POT1 [113], who are at elevated risk for melanoma. These expert guidelines recommend annual or more frequent full skin examinations as early as age 10 [112] or 20 [113], with adjunctive use of mole mapping or TBP [112] to monitor lesion changes over time. Behavioural counselling focuses on strict photoprotection, regular skin self-examination, and family risk communication [112,113].
Importantly, these genetic findings have limited use in general population screening due to their rarity and complexity in interpreting variants of uncertain significance [114]. In addition, genetic testing is typically only for individuals with a strong family history or suggestion of hereditary melanoma syndromes [115]. From an ethical perspective, genetic counselling is needed to support informed consent, insurance implications, employment, privacy, and testing in family relatives [115]. This is especially relevant in countries like Australia where comprehensive legal protections against genetic discrimination, including life insurance, is lacking [116]. Further ethical issues regarding autonomy, psychological impacts, and timing of disclosure are relevant for testing in minors [117]. As technologies become more accessible, genetic testing should remain clinically justifiable and ethically sound with adequate psychosocial support provided.
4.7. Computational Science, Imaging, and Artificial Intelligence
Imaging has become one of the most important new tools for skin cancer prevention and early detection. Dermatoscopy [20,23], confocal microscopy [40], and 3D-TBP [118], have revolutionised how lesion monitoring, risk assessment, preventive counselling and early detection are conducted.
These advances have been strengthened by integrating experts in computational science, imaging analysis, and AI methods [119]. Recent studies have shown that AI algorithms can match or exceed expert-level performance in skin cancer detection, particularly in reader studies using curated dermatoscopic datasets such as the International Skin Imaging Collaboration (ISIC) Archive [120]. However, results from clinical workflow studies have been more variable, and many models are limited due to the underrepresentation of skin of colour [121,122]. For example, AI models trained on predominantly light skin types have a higher rate of misclassified lesions on darker skin tones [123], emphasising these inequities.
Validation is also a challenge, given that many AI tools have not been tested in real-world clinical settings outside of controlled environments [124]. Regulatory standards are still evolving, and questions remain about how to ensure seamless clinical integration and how to track outcomes over time [124]. To help readers critically assess the readiness of AI tools for clinical use, we have included a checklist adapted from the TRIPOD+AI [125] and DECIDE-AI [126] reporting standards, which reflects the current best practices for reporting, validation, and implementation of AI in healthcare (Table 1).

Table 1.
AI readiness checklist for skin cancer detection tools, adapted from the TRIPOD+AI and DECIDE-AI reporting standards.
Clinically, AI tools can show the degree of sun damage on the skin, prioritise lesions for review, highlight lesion changes in sequential imaging, guide decisions on follow-up intervals, and extend equitable care to communities with limited specialist access [127,128,129,130,131]. At the same time, these innovations raise valid concerns. Protecting patient privacy, maintaining accountability, and clinical judgement are essential. Thus, the use of imaging and AI require care and transparency, guided by ethical and professional standards [132].
4.8. Bioinformatics and Data Integration
The rapid growth of registries, imaging archives, and genomic studies has highlighted the challenges of data integration. Bioinformatics, through linkage of these diverse resources, has made predictive modelling, risk stratification, and long-term surveillance possible.
The Australian Centre of Excellence in Melanoma Imaging and Diagnosis (ACEMID) provides a leading example (Figure 1) [133,134]. By combining 3D-TBP, clinical data, and genomic testing into a national platform, ACEMID has concurrently advanced AI algorithms, while seamlessly integrating them into multiple hospital and health care settings, learning important health services lessons along the way [135,136]. In addition to ACEMID, several global infrastructures have advanced research and innovation in skin cancer prevention and diagnosis. The ISIC Archive [103] provides open-access dermatoscopic image datasets supporting AI development for melanoma detection, while national registries such as the United States Surveillance, Epidemiology, and End Results (SEER) Program [137] supply epidemiological data for population-level surveillance. Together, these efforts reinforce the international framework for skin cancer control (Table 2).
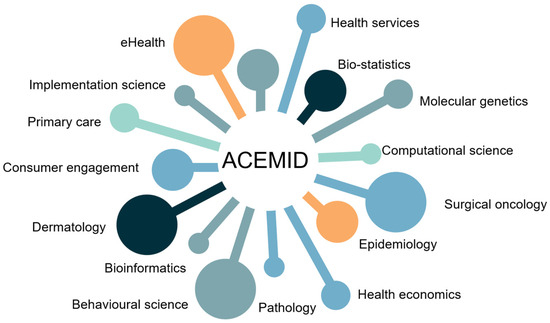
Figure 1.
ACEMID team capability framework, illustrating the central role of ACEMID and its multidisciplinary teams.

Table 2.
Global infrastructures supporting skin cancer prevention. This table highlights some key parallel infrastructures that operate alongside centre-specific initiatives such as ACEMID.
Similarly, international collaborations are being remodeled by bioinformatics. The use of federated learning, cloud-based platforms, and cross-border networks has allowed researchers to share findings with collaborators across the world and align their methods to accelerate scientific discovery [138]. This in turn has made bioinformatics the infrastructure that connects and sustains multidisciplinary research.
5. Technology and Innovation: Shaping the Future
The translational trajectory of skin cancer prevention has moved scientific discoveries from bench to bedside whilst maintaining scalability. This can be seen through the rise of specialist training programs with joint fellowships [139], preparing future health professionals who will have to work across disciplines and building the collaborative capacity required for this next phase.
Unfortunately, the path from bench to bedside is rarely straightforward. New technologies and models often create significant barriers. High set-up expenses are often associated with AI tools [140], and regulations surrounding data privacy and validation can cause further delays [141]. Some clinicians may be reluctant to accept these new tools if they use unfamiliar systems or alter established workflows [142]. Unless these barriers are addressed early, even the most promising technologies risk underuse. We have provided a forward-looking toolkit for stakeholders, summarising methods that could be used to model future prevention scenarios, arranged from lowest to highest fidelity (Table 3). At the lower end, expert consensus and epidemiological modelling offer broad projections of risk and intervention impacts. Mid-level approaches of behavioural interventions and implementation trials test their feasibility in real-world settings. Finally, high-fidelity computational tools, including microsimulation modelling, digital twins and AI-driven prediction platforms, create dynamic, increasingly personalised forecasts of individual skin health.

Table 3.
Examples of methods to model future prevention scenarios. From left to right, approaches are ordered from lowest fidelity to highest fidelity.
The concept of “cutaneous digital twins” [143], computational models representing an individual’s skin in real time, is an exciting progression in skin cancer research. Through a combination of imaging, demographic, behavioural, and molecular data, these models can simulate how the skin reacts under different exposures and interventions. In practice, measurable outputs include naevus evolution tracking and UV vulnerability mapping, with practical data sources such as serial dermatoscopic imaging from the ISIC Archive [120] and ACEMID [134]. An early version of this has already shown promise with encouraging preventive behaviours in high school students [144].
Dermatological imaging tools can even be repurposed for uses beyond the skin. The ACEMID study has demonstrated how 3D-TBP can be leveraged to derive anthropometric measures such as body mass index, waist circumference, and android/gynoid fat distribution [145,146]. These metrics, when linked to cardiometabolic risk scores, offer measurable outputs for systemic health prevention, with ACEMID’s longitudinal dataset serving as a practical data source [134]. Given the association between obesity measures and cardiometabolic risk, existing dermatology tools are likely to become important in systemic health prevention. Despite this, although early studies show promise, accuracy can be affected by factors such as body posture, lighting conditions, and image quality. These tools should therefore be used as adjuncts, rather than replacements for comprehensive clinical assessment, including medical history, laboratory testing, and broader cardiometabolic assessment [146]. Moreover, there is potential for misuse, especially if AI-derived metrics are interpreted in the absence of appropriate clinical context, such as in consumer-based applications or wellness platforms.
However, these advances are not always equitable. An example is the widespread underrepresentation of individuals with skin of colour in dermatology research [147], particularly in training datasets for machine learning algorithms [148]. Melanoma in skin of colour typically presents differently (e.g., in acral sites), and often at more advanced stages [149]. These disparities are worsened by limited access to dermatologists, underrepresentation in clinical trials, and the absence of inclusive design in technology development. If no deliberate correction is made, this risks the perpetuation of inequalities in skin cancer research [147]. Algorithm auditing, inclusive data collection, and culturally sensitive methodologies are to ensure that technological innovations benefit all groups equally.
Different fields contribute to unique measurable outputs. For epidemiology, this includes age-standardised incidence and mortality rates from practical data sources such as SEER [137] and the Australian Cancer Database [150]. Outputs in behavioural science include sun protection adherence scores that can be obtained from population surveys and wearable UV sensors [151]. Health economics provides measurable cost-effective ratios or return-on-investment metrics, which can be calculated through Medicare claims data or program-level expenditure reports [19,152]. Implementation science offers uptake and fidelity metrics sourced from clinic-level audits and electronic health records [91,153]. When integrated with digital twin technologies, these disciplines enable personalised prevention strategies. We have summarised these measurable outputs, practical data sources, and associated equity and diagnosis metrics (Table 4) to guide service leads and policy teams in evaluating and adapting prevention strategies for diverse populations.

Table 4.
Summary of impacts across different skin cancer prevention domains.
The current literature shows that technology is not necessarily replacing multidisciplinary research but instead extending its reach. The future of prevention requires collaboration between teams to effectively integrate and adapt new technologies to meet the needs of populations and individuals. As prevention strategies become increasingly complex and technology-driven, effective collaboration requires a diverse skillset from each discipline. Key skills are required within multidisciplinary teams to ensure that equitable and sustainable health outcomes are achieved (Table 5).

Table 5.
Examples of key skills required by multidisciplinary teams.
6. Gaps, Controversies, and Challenges
Multidisciplinary research has achieved renewed focus on skin cancer prevention; however, important gaps remain. The translation of precision prevention into practice depends on stronger, long-term evidence. Randomised trials and health economic assessments are necessary to confirm the value of technologies like AI diagnostics, genomic risk mapping, and risk-adapted screening [135,154]. At present, small sample sizes, limited follow-up, and variable study methods restrict both interpretability and reproducibility across populations [128].
Furthermore, increased diagnostic sensitivity also raises issues of overdiagnosis and overtreatment, which have uncertain benefit for long-term patient outcomes. To address these concerns, we have also incorporated equity and overdiagnosis metrics into our summary framework (Table 4), and advocate for future studies to prioritise rigorous methodologies, longer follow-up periods, and more inclusive recruitment strategies to optimise the effectiveness and equity of precision prevention strategies.
Even well-established prevention components, such as sunscreens, remain subject to debate. Although decades of evidence support their role in skin cancer risk reduction [155,156,157], uncertainties around the effectiveness, long-term safety, and adequacy of current regulatory testing have recently been raised. These issues prompted the creation of international workshops that brought together experts from dermatology, epidemiology, policy, pharmaceutical formulation, and public health [158]. Their conclusions emphasise the importance of ongoing assessment, and the search for new and more precise laboratory methods, to ensure they can be used by the population with certainty.
The unequal distribution of new technologies continues to limit timely access to skin cancer prevention. Metropolitan centres often benefit from a higher concentration of dermatologists and imaging specialists, whereas rural and disadvantaged areas struggle with limited services [159]. These inequities apparent in under-resourced settings are reinforced by inadequate health policies and the absence of stable funding for teledermatology services [160,161], leading these systemic issues to potentially limit progress and delay early diagnosis.
Another barrier is the lack of reliable biomarkers to guide individualised risk assessment [162]. In response, input from social sciences, primary care, and policy input are needed to design strategies that ensure equitable and cost-effective access. This may include expanding training programs for rural clinicians, integrating teledermatology into national screening programs, and focusing on funding models that prioritise inclusive technology development.
The use of AI in healthcare requires the collaborative efforts of ethicists, legal experts, and patient advocates, to instill public trust and responsible implementation [163]. If not handled appropriately, data breaches have the potential to expose sensitive patient information [164]. Algorithmic bias shows potential to widen existing health disparities [165]. As legal frameworks are still in development, it leaves many clinician and patient questions surrounding accountability, consent, and liability unanswered [166]. Therefore, there is a need for transparent, consistent, and inclusive governance.
Climate change adds a further layer of complexity to skin cancer prevention. For example, Australia has seen an average temperature rise of 1.44 °C since 1910 [167], which has contributed to people’s behaviour when outdoors, leading to an increased intensity of sun exposure [168]. Combined with well-established ozone depletion and atmospheric changes [169], elevated cumulative UV exposure will likely impact future skin cancer trends. In addition, urban heat islands may amplify the intensity of local UV radiation, most pronounced for dense populations with limited shade access [170]. Public health communication should undergo updates to better reflect the dynamic nature of UV risk, and access to real-time data obtained through the free SunSmart Global UV app [171] could help individuals improve tailoring of their sun protection. Similarly, outdoor workers could benefit from shade, protective clothing, flexible scheduling, and rest breaks during peak UV periods [172]. Considering climate change in prevention strategies is necessary for their effectiveness amidst the ongoing challenges of global warming.
Debates also continue around population versus targeted screening. Germany’s national program has shown mixed mortality outcomes [28] in contrast to opportunistic screening in countries such as Australia, the United States, and the United Kingdom, offering flexibility at the expense of inequity, inconsistency, and lack of quality assurance. Future prospective studies are needed to define the optimal frequency and modality of screening for high-risk groups.
From the patient’s perspective, skin cancer prevention has undergone substantial changes. Historically, dermatologist visits used to be for individuals who noticed visible or progressive lesions on their skin, and delayed diagnoses were common. Today, current population-level campaigns [34,35,36], digital risk assessment tools [30], and TBP [41] have all facilitated earlier patient engagement, with skin checks viewed as a more preventive measure. Genetic testing [109] and AI tools [124] raise questions regarding resource allocation, specifically how individuals should be identified as high-risk, enrolled in registries, or included in behavioural studies. Importantly, psychosocial therapies [173] have helped address anxiety surrounding surveillance, provide reassurance after benign results, and even manage distress related to overdiagnosis. By incorporating psychological therapies into patient management, it has the potential to improve adherence to sun protective behaviours, reduce potential barriers to avoidance, and overall contribute to lower skin cancer incidence on both an individual- and population-level in the future.
7. Conclusions
This narrative scoping review shows that a much wider range of disciplines are now involved in skin cancer prevention. While partnerships between public health and dermatology have expanded the scope of prevention, the impact of these collaborations on accuracy, accessibility, and effectiveness remains promising but still inconclusive.
The development of new technologies also raises challenges in how they should be effectively integrated into routine patient care. Algorithmic bias, regulatory uncertainty, and clinical validation pose issues towards the use of AI lesion detection algorithms. Despite the promise of imaging, substantial infrastructure and workforce changes are necessary before equitable access can be achieved. Issues of cost, interpretation, and ethical considerations for genetic testing are especially relevant in the context of population-level screening.
Equity-focused strategies are required to actively address these health disparities, including inclusive data collection protocols, culturally sensitive public health messaging, and workforce development focused on underserved areas. Skin cancer prevention is therefore not only a global priority, but it also can be a model for progress in modern medicine, demonstrating how diverse disciplines can work together to transform innovation into inclusive real-world impacts.
Author Contributions
Conceptualisation, H.P.S. and M.J.; methodology, M.J., C.P. and H.P.S.; writing—original draft preparation, A.S.; writing—review and editing, A.S., C.P., S.M.G., M.J. and H.P.S.; visualisation, M.J.; supervision, C.P. and H.P.S.; project administration, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Health and Medical Research Council Centre for Research Excellence Grant 2021/GNT2006551 and Synergy Grant 2021/GNT2009923.
Data Availability Statement
No new data were generated or analysed in this narrative review. The findings are based on the synthesis of previously published literature, which can be accessed by readers through the citations provided within the manuscript.
Conflicts of Interest
HPS is a shareholder of MoleMap NZ Limited and e-derm consult GmbH and undertakes regular teledermatological reporting for both companies. HPS is a Medical Consultant for Canfield Scientific Inc and a Medical Advisor for First Derm. All other authors (AK, CP, SMG, and MJ) have no conflicts. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| 3D-TBP | Three-Dimensional Total Body Photography |
| ACEMID | Australian Centre of Excellence in Melanoma Imaging and Diagnosis |
| AI | Artificial intelligence |
| ISIC | International Skin Imaging Collaboration |
| SEER | Surveillance, Epidemiology, and End Results |
| TBP | Total Body Photography |
| UV | Ultraviolet |
References
- World Health Organisation: Ultraviolet Radiation and Skin Cancer. Available online: https://www.who.int/uv/faq/skincancer/en/index1.html (accessed on 2 September 2025).
- Arnold, M.; Singh, D.; Laversanne, M. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Pandeya, N.; Green, A.C.; Ragaini, B.S.; Venn, A.J.; Whiteman, D.C. Keratinocyte cancer incidence in Australia: A review of population-based incidence trends and estimates of lifetime risk. Public Health Res. Pract. 2022, 32, 3212203. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Lancaster, H.O.; Nelson, J. Sunlight as a cause of malignant melanoma: A clinical survey. Med. J. Aust. 1957, 1, 452–456. [Google Scholar] [CrossRef]
- Iannacone, M.R.; Green, A.C. Towards skin cancer prevention and early detection: Evolution of skin cancer awareness campaigns in Australia. Melanoma Manag. 2014, 1, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Montague, M.; Borland, R.; Sinclair, C. Slip! Slop! Slap! And SunSmart, 1980–2000: Skin Cancer Control and 20 Years of Population-Based Campaigning. Health Educ. Behav. 2001, 28, 290–305. [Google Scholar] [CrossRef]
- SunSmart. Available online: https://www.sunsmart.com.au/about-sunsmart (accessed on 2 September 2025).
- Aulbert, W.; Parpart, C.; Schulz-Hornbostel, R.; Hinrichs, B.; Krüger-Corcoran, D.; Stockfleth, E. Certification of sun protection practices in a German child day-care centre improves children’s sun protection: The ‘SunPass’ pilot study. Br. J. Dermatol. 2009, 161, 5–12. [Google Scholar] [CrossRef]
- Hewitt, M.; Denman, S.; Hayes, L.; Pearson, J.; Wallbanks, C. Evaluation of ‘Sun-safe’: A health education resource for primary schools. Health Educ. Res. 2001, 16, 623–633. [Google Scholar] [CrossRef][Green Version]
- Miller, D.R.; Geller, A.C.; Wood, M.C.; Lew, R.A.; Koh, H.K. The Falmouth Safe Skin Project: Evaluation of a community program to promote sun protection in youth. Health Educ. Behav. 1999, 26, 369–384. [Google Scholar] [CrossRef]
- Aitken, J.F.; Youlden, J.R.; Baade, P.D.; Soyer, H.P.; Green, A.C.; Smithers, B.M. Generational shift in melanoma incidence and mortality in Queensland, Australia, 1995–2014. Int. J. Cancer 2018, 142, 1528–1535. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef]
- Gordon, L.; Olsen, C.; Whiteman, D.C.; Elliot, T.M.; Janda, M.; Green, A. Prevention versus early detection for long-term control of melanoma and keratinocyte carcinomas: A cost-effectiveness modelling study. BMJ Open 2020, 10, e034388. [Google Scholar] [CrossRef]
- Janda, M.; Sinclair, C. Experience from an outright ban of commercial sunbeds in the Australian context. Br. J. Dermatol. 2022, 187, 7. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Harrison, S.L.; Buettner, P.; Nowak, M. School sun-protection policies—Does being SunSmart make a difference? Health Educ. Res. 2014, 29, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Horsham, C.; Auster, J.; Sendall, M.C.; Stoneham, M.; Youl, P.; Crane, P.; Tenkate, T.; Janda, M.; Kimlin, M. Interventions to decrease skin cancer risk in outdoor workers: Update to a 2007 systematic review. BMC Res. Notes 2014, 7, 10. [Google Scholar] [CrossRef]
- Gordon, L.G.; Rowell, D. Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: A systematic review. Eur. J. Cancer Prev. 2015, 24, 141–149. [Google Scholar] [CrossRef]
- Collins, L.G.; Minto, C.; Ledger, M.; Blane, S.; Hendrie, D. Cost-effectiveness analysis and return on investment of SunSmart Western Australia to prevent skin cancer. Health Promot. Int. 2024, 39, daae091. [Google Scholar] [CrossRef] [PubMed]
- Kittler, H.; Pehamberger, H.; Wolff, K.; Binder, M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002, 3, 159–165. [Google Scholar] [CrossRef]
- Friedman, R.J.; Rigel, D.S.; Kopf, A.W. Early detection of malignant melanoma: The role of physician examination and self-examination of the skin. CA Cancer J. Clin. 1985, 35, 130–151. [Google Scholar] [CrossRef]
- Abbasi, N.R.; Shaw, H.M.; Rigel, D.S.; Friedman, R.J.; McCarthy, W.H.; Osman, I.; Kopf, A.W.; Polsky, D. Early Diagnosis of Cutaneous Melanoma: Revisiting the ABCD Criteria. JAMA 2004, 292, 2771–2776. [Google Scholar] [CrossRef]
- Venugopal, S.S.; Soyer, H.P.; Menzies, S.W. Results of a nationwide dermoscopy survey investigating the prevalence, advantages and disadvantages of dermoscopy use among Australian dermatologists. Aust. J. Dermatol. 2011, 52, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Ahnlinde, I.; Bjellerup, M.; Nilsson, F.; Nielsen, K. Validity of ABCD Rule of Dermoscopy in Clinical Practice. Acta Derm. Venerol. 2016, 96, 367–372. [Google Scholar] [CrossRef]
- Tsao, H.; Olazagasti, J.M.; Cordoro, K.M.; Brewer, J.D.; Taylor, S.C.; Bordeaux, J.S.; Chren, M.M.; Sober, A.J.; Tegeler, C.; Bhushan, R.; et al. Early detection of melanoma: Reviewing the ABCDEs. J. Am. Acad. Dermatol. 2015, 72, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Staples, M.P.; Elwood, M.; Burton, R.C.; Williams, J.L.; Marks, R.; Giles, G.G. Non-melanoma skin cancer in Australia: The 2002 national survey and trends since 1985. Med. J. Aust. 2006, 184, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, A.; Waldmann, A.; Weinstock, M.A.; Geller, A.C.; Eisemann, N.; Greinert, R.; Volkmer, B.; Breitbart, E. Does skin cancer screening save lives?: An observational study comparing trends in melanoma mortality in regions with and without screening. Cancer 2012, 118, 5395–5402. [Google Scholar] [CrossRef]
- Stang, A.; Garbe, C.; Autier, P.; Jöckel, K.H. The many unanswered questions related to the German skin cancer screening programme. Eur. J. Cancer 2016, 64, 83–88. [Google Scholar] [CrossRef]
- Braveman, P.; Gottlieb, L. The Social Determinants of Health: It’s Time to Consider the Causes of the Causes. Public Health Rep. 2014, 129, 19–31. [Google Scholar] [CrossRef]
- Wunderlich, K.; Suppa, M.; Gandini, S.; Lipski, J.; White, J.M.; Marmol, V.D. Risk Factors and Innovations in Risk Assessment for Melanoma, Basal Cell Carcinoma, and Squamous Cell Carcinoma. Cancers 2024, 16, 1016. [Google Scholar] [CrossRef]
- Urbach, F. Environmental Risk Factors for Skin Cancer. In Skin Carcinogenesis in Man and in Experimental Models; Hecker, E., Jung, E.G., Marks, F., Tilgen, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 243–262. [Google Scholar] [CrossRef]
- Mawn, V.B.; Fleischer, A.B., Jr. A survey of attitudes, beliefs, and behavior regarding tanning bed use, sunbathing, and sunscreen use. J. Am. Acad. Dermatol. 1993, 29, 959–962. [Google Scholar] [CrossRef]
- Australasian College of Dermatologists. Accessible and Quality Dermatology Care for All Australians. ACD White Paper; November 2022. Available online: https://www.dermcoll.edu.au/wp-content/uploads/2022/12/ACD_Accessible-and-quality-dermatology-care-for-all-Australians_White-Paper_Nov-2022.pdf (accessed on 15 October 2025).
- Del Marmol, V. Prevention and screening of melanoma in Europe: 20 years of the Euromelanoma campaign. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 5–11. [Google Scholar] [CrossRef]
- Okhovat, J.P.; Beaulieu, D.; Tsao, H.; Halpern, A.C.; Michaud, D.S.; Shaykevich, S.; Geller, A.C. The first 30 years of the American Academy of Dermatology skin cancer screening program: 1985–2014. J. Am. Acad. Dermatol. 2018, 79, 884–891. [Google Scholar] [CrossRef]
- Skin Cancer Foundation. Destination: Healthy Skin. Available online: https://www.skincancer.org/press/2019-destination-healthy-skin-the-skin-cancer-foundations-mobile-education-and-screening-program-concludes-another-trip-around-the-country (accessed on 4 September 2025).
- Cancer Council. National Skin Cancer Action Week: Combatting Australia’s ‘National Cancer’. Available online: https://www.cancer.org.au/cancer-information/causes-and-prevention/sun-safety/campaigns-and-events/national-skin-cancer-action-week (accessed on 4 September 2025).
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Sundararajan, S.; Thida, A.M.; Yadlapti, S.; Mukkamalla, S.K.R.; Koya, S. Metastatic Melanoma. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, CA, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470358/ (accessed on 2 September 2025).
- González, S.; Swindells, K.; Rajadhyaksha, M.; Torres, A. Changing paradigms in dermatology: Confocal microscopy in clinical and surgical dermatology. Clin. Dermatol. 2003, 21, 359–369. [Google Scholar] [CrossRef]
- Hornung, A.; Steeb, T.; Wessely, A.; Brinker, T.J.; Breakell, T.; Erdmann, M.; Berking, C.; Heppt, M.V. The Value of Total Body Photography for the Early Detection of Melanoma: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1726. [Google Scholar] [CrossRef]
- Bhawan, J. The Evolution of Dermatopathology—The American Experience. Am. J. Dermatopathol. 2006, 28, 67–71. [Google Scholar] [CrossRef]
- Maurichi, A.; Barretta, F.; Patuzzo, R.; Gallino, G.; Mattavelli, I.; Shimonovitz-Moore, M.; Nizri, E.; Matteucci, M.; Summo, V.; Cossa, M.; et al. Local Recurrence and Survival in Patients with Melanoma >2 mm in Thickness at Difficult Sites Treated with 1-cm Versus 2-cm Margins. J. Natl. Compr. Cancer Netw. 2024, 22, 687–693. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Senguin, N.; Bastholt, L.; Bataille, V.; Brochez, L.; Del Marmol, V.; et al. European Association of Dermato-Oncology (EADO), the European Dermatology Forum (EDF), and the European Organization for Research and Treatment of Cancer (EORTC). European consensus-based interdisciplinary guideline for maelanoma. Part 1: Diagnostics—Update 2024. Eur. J. Cancer 2025, 215, 115152. [Google Scholar] [CrossRef]
- Diegel, D.M.; Ellis, J.I. Excision of skin cancer. In Skin Cancer, 2nd ed.; Schwartz, R.A., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2008; pp. 385–402. [Google Scholar]
- Lideikaitė, A.; Mozūraitienė, J.; Letautienė, S. Analysis of prognostic factors for melanoma patients. Acta Med. Litu. 2017, 24, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.; Newton-Bishop, J.; A’Hern, R.; Coombes, G.; Timmons, M.; Evans, J.; Cook, M.; Theaker, J.; Fallowfield, M.; O’Neill, T.; et al. Excision Margins in High-Risk Malignant Melanoma. N. Engl. J. Med. 2004, 350, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Mohs, F.E. Chemosurgery for the microscopically controlled excision of cutaneous cancer. Head Neck Surg. 1978, 1, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Dummer, R.; Flaherty, K.T.; Robert, C.; Arance, A.; de Groot, J.W.B.; Garbe, C.; Gogas, H.J.; Gutzmer, R.; Krajsová, I.; et al. COLUMBUS 7-year update: A randomized, open-label, phase III trial of encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF V600E/K-mutant melanoma. Eur. J. Cancer 2024, 204, 114073. [Google Scholar] [CrossRef] [PubMed]
- Menzies, A.M.; Lo, S.N.; Saw, R.P.M.; Gonzales, M.; Ch’ng, S.; Nieweg, O.E.; Shannon, K.F.; Ferguson, P.M.; Lee, J.; Emmett, L.; et al. Five-year analysis of neoadjuvant dabrafenib and trametinib for stage III melanoma. Ann. Oncol. 2024, 35, 739–746. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzales, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Long, G.V.; Larkin, J.; Schadendorf, D.; Grob, J.J.; Lao, C.D.; Márquez-Rodas, I.; Wagstaff, J.; Lebbé, C.; Pigozzo, J.; Robert, C.; et al. Pooled Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone in Patients with Advanced Melanoma. J. Clin. Oncol. 2025, 43, 938–948. [Google Scholar] [CrossRef]
- Blank, C.U.; Lucas, M.W.; Scolyer, R.A.; van de Wiel, B.A.; Menzies, A.M.; Lopez-Yurda, M.; Hoejimakers, L.L.; Saw, R.P.M.; Lijnsvelt, J.M.; Maher, N.G.; et al. Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma. N. Engl. J. Med. 2024, 391, 1696–1708. [Google Scholar] [CrossRef]
- Dai, Y.; Sun, J.; Zhang, X.; Zhao, J.; Yang, W.; Zhou, J.; Gao, Z.; Wang, Q.; Yu, F.; Wang, B. Supramolecular assembly boosting the phototherapy performances of BODIPYs. Coord. Chem. Rev. 2024, 517, 216024. [Google Scholar] [CrossRef]
- Yan, M.; Wu, S.; Wang, Y.; Liang, M.; Wang, M.; Hu, W.; Yu, G.; Mao, Z.; Huang, F.; Zhou, J. Recent Progress of Supramolecular Chemotherapy Based on Host-Guest Interactions. Adv. Mater. 2024, 36, e2304249. [Google Scholar] [CrossRef]
- Beaton, L.; Bandula, S.; Gaze, M.N.; Sharma, R.A. How rapid advances in imaging are defining the future of precision radiation oncology. Br. J. Cancer 2019, 120, 779–790. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Rusho, M.A.; Menon, S.V.; Kaur, M.; Jabir, M.S.; Jawad, S.F.; Hasan, T.F.; Najm, M.A.A.; Jawad, M.A.; Khelief, A.J. Advancements in radiobiology techniques and applications for personalized radiation therapy in nuclear medicine. J. Radioanal. Nucl. Chem. 2024, 333, 6121–6132. [Google Scholar] [CrossRef]
- Aujla, K.S.; Clyde, J.W.; Mudd, K.; Chen, Y. Patient Reported Factors Influencing Decisions on Radiotherapy vs. Mohs Surgery for Non-Melanoma Skin Cancer of Cosmetically Challenging Head and Neck Sites at a Rural Radiation Satellite Facility. Int. J. Radiat. Oncol. 2022, 114, E602. [Google Scholar] [CrossRef]
- Emens, L.A.; Romero, P.J.; Anderson, A.C.; Bruno, T.C.; Capitini, C.M.; Collyar, D.; Gulley, J.L.; Hwu, P.; Posey, A.D., Jr.; Silk, A.W.; et al. Challenges and opportunities in cancer immunotherapy: A Society for Immunotherapy of Cancer (SITC) strategic vision. J. Immunother. Cancer 2024, 12, e009063. [Google Scholar] [CrossRef]
- Bandara, S.; Raveendran, S. Current Landscape and Future Directions in Cancer Immunotherapy: Therapies, Trials, and Challenges. Cancers 2025, 17, 821. [Google Scholar] [CrossRef]
- QIMR Berghofer. QSkin. Available online: https://www.qimrb.edu.au/studies/qskin (accessed on 4 September 2025).
- Gallagher, R.P.; Hill, G.B.; Badjdik, C.D.; Coldman, A.J.; Fincham, S.; McLean, D.I.; Threlfall, W.J. Sunlight exposure, pigmentation factors, and risk of nonmelanocytic skin cancer. II. Squamous cell carcinoma. Arch. Dermatol. 1995, 131, 164–169. [Google Scholar] [CrossRef] [PubMed]
- English, D.R.; Armstrong, B.K.; Kricker, A.; Winter, M.G.; Heenan, P.J.; Randell, P.L. Case-control study of sun exposure and squamous cell carcinoma of the skin. Int. J. Cancer 1998, 77, 347–353. [Google Scholar] [CrossRef]
- Kricker, A.; Armstrong, B.K.; English, D.R.; Heenan, P.J. Does intermittent sun exposure cause basal cell carcinoma? a case-control study in Western Australia. Int. J. Cancer 1995, 60, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Green, A.C.; Neale, N.E.; Webb, P.M.; Cicero, R.A.; Jackman, L.M.; O’Brien, S.M.; Perry, S.L.; Ranieri, B.A.; Whiteman, D.C. Cohort profile: The QSkin Sun and Health Study. Int. J. Epidemiol. 2012, 41, 929–929i. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Thompson, B.S.; Thrift, A.P.; Hughes, M.-C.; Muranushi, C.; Neale, R.E.; Green, A.C.; Olsen, C.M. A Model to Predict the Risk of Keratinocyte Carcinomas. J. Investig. Dermatol. 2016, 136, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Pandeya, N.; Thompson, B.S.; Dusingize, J.C.; Green, A.C.; Neale, R.E.; Whiteman, D.C. Association between Phenotypic Characteristics and Melanoma in a Large Prospective Cohort Study. J. Investig. Dermatol. 2019, 139, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Tabbakh, T.; Volkov, A.; Wakefield, M.; Dobbinson, S. Implementation of the SunSmart program and population sun protection behaviour in Melbourne, Australia: Results from cross-sectional summer surveys from 1987 to 2017. PLoS Med. 2019, 16, e1002932. [Google Scholar] [CrossRef]
- Garbe, C.; Peris, K.; Hauschild, A.; Saiag, P.; Middleton, M.; Spatz, A.; Grob, J.J.; Malvehy, J.; Newton-Bishop, J.; Stratigos, A.; et al. Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. Eur. J. Cancer 2010, 46, 270–283. [Google Scholar] [CrossRef]
- Cancer Science. Limitations of Prediction Models. Available online: https://cancerscience.net/about/index/limitations-of-prediction-models (accessed on 15 October 2025).
- Australian Skin and Skin Cancer Research Centre. Position Statement: Balancing the Harms and Benefits of Sun Exposure. Available online: https://www.assc.org.au/wp-content/uploads/2023/01/Sun-Exposure-Summit-PositionStatement_V1.9.pdf (accessed on 15 October 2025).
- Robinson, J.K.; Kim, J.; Rosenbaum, S.; Ortiz, S. Indoor tanning knowledge, attitudes, and behavior among young adults from 1988-2007. Arch. Dermatol. 2008, 144, 484–488. [Google Scholar] [CrossRef]
- Makin, J.K.; Warne, C.D.; Dobbinson, S.J.; Dobbinson, M.A.; Hill, D.J. Population and age-group trends in weekend sun protection and sunburn over two decades of the SunSmart programme in Melbourne, Australia. Br. J. Dermatol. 2012, 168, 154–161. [Google Scholar] [CrossRef]
- Stapleton, J.L.; Manne, S.L.; Greene, K.; Darabos, K.; Carpenter, A.; Hudson, S.V.; Coups, E.J. Sociocultural experiences, body image, and indoor tanning among young adult women. J. Health Psychol. 2017, 22, 1582–1590. [Google Scholar] [CrossRef]
- Najmi, M.; Brown, A.E.; Harrington, S.R.; Farris, D.; Sepulveda, S.; Nelson, K.C. A systematic review and synthesis of qualitative and quantitative studies evaluating provider, patient, and health care system-related barriers to diagnostic skin cancer examinations. Arch. Dermatol. Res. 2022, 314, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Falzone, A.E.; Brindis, C.D.; Chren, M.M.; Junn, A.; Pagoto, S.; Wehner, M.; Linos, E. Teens, Tweets, and Tanning Beds: Rethinking the Use of Social Media for Skin Cancer Prevention. Am. J. Prev. Med. 2017, 53, S86–S94. [Google Scholar] [CrossRef]
- West, S.E.; Martin, K.L.; Ailor, S.K. Tanning Themselves to Death: A New Teen Fad. Mo. Med. 2012, 109, 166–170. [Google Scholar] [PubMed]
- Sinikumpu, S.P.; Jokelainen, J.; Keinänen-Kiukaanniemi, S.; Huilaja, L. Skin cancers and their risk factors in older persons: A population-based study. BMC Geriatr. 2022, 22, 269. [Google Scholar] [CrossRef] [PubMed]
- Slape, D.R.; Saunderson, R.B.; Tatian, A.H.; Forstner, D.F.; Estall, V.J. Cutaneous malignancies in Indigenous Peoples of urban Sydney. J. Med. Imaging Radiat. Oncol. 2019, 63, 244–249. [Google Scholar] [CrossRef]
- Khlat, M.; Vail, A.; Parkin, M.; Green, A. Mortality from Melanoma in Migrants to Australia: Variation by Age at Arrival and Duration of Stay. Am. J. Epidemiol. 1992, 135, 1103–1113. [Google Scholar] [CrossRef]
- Horsham, C.; Baade, P.; Kou, K.; O’Hara, M.; Sinclair, C.; Loescher, L.J.; Soyer, H.P.; Janda, M. Optimizing Texting Interventions for Melanoma Prevention and Early Detection: A Latin Square Crossover RCT. Am. J. Prev. Med. 2021, 61, 348–356. [Google Scholar] [CrossRef]
- Janda, M.; Horsham, C.; Vagenas, D.; Loescher, L.J.; Gillespie, N.; Koh, U.; Curiel-Lewandrowski, C.; Hofmann-Wellenhof, R.; Halpern, A.; Whiteman, D.C.; et al. Accuracy of mobile digital teledermoscopy for skin self-examinations in adults at high risk of skin cancer: An open-label, randomised controlled trial. Lancet Digit. Health 2020, 2, e129–e137. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Susanto, A.K.; Soyer, H.P.; Janda, M.; Camargo Catapan, S. Psychosocial wellbeing in people with melanoma in-situ: A systematic review. Melanoma Res. 2025, 35, 11–23. [Google Scholar] [CrossRef]
- Ahmad, N.; Kashyap, A.; Taylor, M.; Gonna, N.; Haff, P.; Farris, D.; McQuade, J.; Roth, M.; Nelson, K. Quality of Life Among Adolescents and Young Adults with Melanoma: A Systematic Review. J. Adolesc. Young Adult Oncol. 2025. [Google Scholar] [CrossRef]
- Collins, L.G.; Gage, R.; Sinclair, C.; Lindsay, D. The Cost-Effectiveness of Primary Prevention Interventions for Skin Cancer: An Updated Systematic Review. Appl. Health Econ. Health Policy 2024, 22, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhong, Y.; Han, L.; Xie, Y.; Wan, M. Global, regional, and national trends in the burden of melanoma and non-melanoma skin cancer: Insights from the global burden of disease study 1990–2021. Sci. Rep. 2025, 15, 5996. [Google Scholar] [CrossRef]
- Harvey, V.M.; Patel, H.; Sandhu, S.; Wallington, S.F.; Hinds, G. Social determinants of racial and ethnic disparities in cutaneous melanoma outcomes. Cancer Control 2014, 21, 343–349. [Google Scholar] [CrossRef]
- Oliveria, S.A.; Christos, P.J.; Marghoob, A.A.; Halpern, A.C. Skin cancer screening and prevention in the primary care setting: National ambulatory medical care survey 1997. J. Gen. Intern. Med. 2001, 16, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, C.; Ambrosio, L.; Cucchi, C.; Meznerics, F.A.; Kiss, N.; Bánvölgyi, A.; Rega, F.; Grignaffini, F.; Barbuto, F.; Frezza, F.; et al. Melanoma Detection by Non-Specialists: An Untapped Potential for Triage? Diagnostics 2022, 12, 2821. [Google Scholar] [CrossRef]
- Sathe, N.C.; Zito, P.M. Skin Cancer. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, CA, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441949/ (accessed on 4 September 2025).
- Gordon, L.G.; Shih, S.; Watts, C.; Goldsbury, D.; Green, A.C. The economics of skin cancer prevention with implications for Australia and New Zealand: Where are we now? Public Health Res. Pract. 2022, 32, 31502119. [Google Scholar] [CrossRef]
- von Schuckmann, L.A.; Smithers, B.M.; Khosrotehrani, K.; Beesley, V.L.; van der Pols, J.C.; Hughes, M.B.; Green, A.C. Use of support services in a sample of patients with high-risk primary melanomas in urban, regional and rural Queensland. Aust. N. Z. J. Public Health 2017, 41, 315–319. [Google Scholar] [CrossRef][Green Version]
- Beames, C.; Adelson, P.; Sharplin, G.; Eckert, M. Primary care nurse’s role and educational preparedness in skin cancer screening and early detection: A scoping review. J. Adv. Nurs. 2024, 80, 2228–2251. [Google Scholar] [CrossRef]
- Souvignier, S.T.; Mayer, J.A.; Eckhardt, L. Educating the public about skin cancer prevention: A role for pharmacists. J. Clin. Pharm. Ther. 1996, 21, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Ashrafzadeh, S.; Metlay, J.P.; Choudhry, N.K.; Emmons, K.M.; Asgari, M.M. Using Implementation Science to Optimize the Uptake of Evidence-Based Medicine into Dermatology Practice. J. Investig. Dermatol. 2020, 5, 952–958. [Google Scholar] [CrossRef]
- Papachristou, P.; Söderholm, M.; Pallon, J.; Taloyan, M.; Polesie, S.; Paoli, J.; Anderson, C.D.; Falk, M. Evaluation of an artificial intelligence-based decision support for the detection of cutaneous melanoma in primary care: A prospective real-life clinical trial. Br. J. Dermatol. 2024, 191, 125–133. [Google Scholar] [CrossRef]
- Massone, C.; Maak, D.; Hofmann-Wellenhof, R.; Soyer, H.P.; Frühauf, J. Teledermatology for skin cancer prevention: An experience on 690 Austrian patients. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1103–1108. [Google Scholar] [CrossRef]
- Dunlop, K.; Rankin, N.M.; Smit, A.K.; Salgado, Z.; Newson, A.J.; Keogh, L.; Cust, A.E. Acceptability of risk-stratified population screening across cancer types: Qualitative interviews with the Australian public. Health Expect. 2021, 24, 1326–1336. [Google Scholar] [CrossRef]
- The Australasian College of Dermatologists. Skin Cancer: A Significant Gap in the National Preventive Health Strategy. Available online: https://www.dermcoll.edu.au/wp-content/uploads/2021/04/ACD_National-Preventive-Health-Strategy-submission-September-2020.pdf (accessed on 4 September 2025).
- Cramb, S.M.; Duncan, E.W.; Aitken, J.F.; Soyer, H.P.; Mengersen, K.L.; Baade, P.D. Geographical patterns in melanoma incidence across Australia: Can thickness differentials reveal the key drivers? Ann. Cancer Epidemiol. 2020, 4, 11. [Google Scholar] [CrossRef]
- Voiculescu, V.M.; Popescu, A.I.; Costache, M. Immunohistochemistry for Skin Cancers: New Insights into Diagnosis and Treatment of Melanoma. Cancers 2025, 17, 1769. [Google Scholar] [CrossRef] [PubMed]
- Greinert, R. Skin cancer: New markers for better prevention. Pathobiology 2009, 76, 64–81. [Google Scholar] [CrossRef]
- Castellani, G.; Buccarelli, M.; Arasi, M.B.; Rossi, S.; Pisanu, M.E.; Bellenghi, M.; Lintas, C.; Tabolacci, C. BRAF Mutations in Melanoma: Biological Aspects, Therapeutic Implications, and Circulating Biomarkers. Cancers 2023, 15, 4026. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Couselo, E.; Adelantado, E.Z.; Ortiz, C.; García, J.S.; Perez-Garcia, J. NRAS-mutant melanoma: Current challenges and future prospect. Onco Targets Ther. 2017, 10, 3941–3947. [Google Scholar] [CrossRef]
- Pham, D.M.; Guhan, S.; Tsao, H. KIT and Melanoma: Biological Insights and Clinical Implications. Yonsei Med. J. 2020, 61, 562–571. [Google Scholar] [CrossRef]
- Smit, A.K.; Newson, A.J.; Morton, R.L.; Kimlin, M.; Keogh, L.; Law, M.H.; Kirk, J.; Dobbinson, S.; Kanetsky, P.A.; Fenton, G.; et al. The melanoma genomics managing your risk study: A protocol for a randomized controlled trial evaluating the impact of personal genomic risk information on skin cancer prevention behaviors. Contemp. Clin. Trials 2018, 70, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Pellegrini, C.; Cardelli, L.; Cicarelli, V.; Nardo, L.D.; Fargnoli, M.C. Familial Melanoma: Diagnostic and Management Implications. Dermatol. Prac. Concept. 2019, 9, 10–16. [Google Scholar] [CrossRef]
- Maas, E.J.; Betz-Stablein, B.; Aoude, L.G.; Soyer, H.P.; McInerney-Leo, A.M. Unusual suspects in hereditary melanoma: POT1, POLE, BAP1. Trends Genet. 2022, 38, 1204–1207. [Google Scholar] [CrossRef]
- Primiero, C.A.; Maas, E.J.; Wallingford, C.K.; Soyer, H.P.; McInerney-Leo, A.M. Genetic testing for familial melanoma. Ital. J. Dermatol. Venerol. 2024, 159, 34–42. [Google Scholar] [CrossRef]
- GenoMEL. Available online: https://genomel.org (accessed on 4 September 2025).
- Barrett, J.H.; Iles, M.M.; Harland, M.; Taylor, J.C.; Aitken, J.F.; Andresen, P.A.; Akslen, L.A.; Armstrong, B.K.; Avril, M.F.; Azizi, E.; et al. Genome-wide association study identifies three new melanoma susceptibility loci. Nat. Genet. 2011, 43, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- FORCE-Facing Our Risk of Cancer Empowered. CDKN2A: Options for Managing Cancer Risk. Available online: https://www.facingourrisk.org/info/hereditary-cancer-and-genetic-testing/hereditary-cancer-genes-and-risk/genes-by-name/cdkn2a/risk-management (accessed on 14 October 2025).
- Tsoulaki, O.; Evans, D.G.; Sinha, K.; Rajan, N.; Bakr, F.; Hatcher, H.; Napolitano, A.; Finn, E.; Iyengar, S.; Sohaib, A.; et al. UK clinical practice guidelines for the management of patients with constitutional POT1 pathogenic variants. J. Med. Genet. 2025, 62, 559–565. [Google Scholar] [CrossRef] [PubMed]
- eviQ Cancer Genetics. CDKN2A—Risk Management. Available online: https://www.eviq.org.au/cancer-genetics/adult/risk-management/1516-cdkn2a-risk-management (accessed on 16 October 2025).
- eviQ Cancer Genetics. CDKN2A—Genetic Testing. Available online: https://www.eviq.org.au/cancer-genetics/adult/genetic-testing-for-heritable-pathogenic-variants/1864-cdkn2a-genetic-testing (accessed on 14 October 2025).
- Tiller, J.; Lacaze, P. Australians need more protection against genetic discrimination: Health experts. Conversation 2021. [Google Scholar] [CrossRef]
- Fallat, M.E.; Katz, A.L.; Mercurio, M.R.; Moon, M.R.; Okun, A.L.; Webb, S.A.; Weise, K.L.; Saul, R.A.; Braddock, S.R.; Chen, E.; et al. Ethical and policy issues in genetic testing and screening of children. Pediatrics 2013, 131, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Soyer, H.P.; Jayasinghe, D.; Rodriguez-Acevedo, A.J.; Collins, L.G.; Caffery, L.J.; Whiteman, D.C.; Betz-Stablein, B.; Osborne, S.R.; Finnane, A.; Horsham, C.; et al. 3D Total-Body Photography in Patients at High Risk for Melanoma: A Randomized Clinical Trial. JAMA Dermatol. 2025, 161, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Berk-Krauss, J.; Polsky, D.; Stein, J.A. Mole Mapping for Management of Pigmented Skin Lesions. Dermatol. Clin. 2017, 35, 439–445. [Google Scholar] [CrossRef]
- The International Skin Imaging Collaboration (ISIC). Available online: https://www.isic-archive.com (accessed on 8 September 2025).
- Tschandl, P.; Rinner, C.; Apalla, Z.; Argenziano, G.; Codella, N.; Halpern, A.; Janda, M.; Lallas, A.; Longo, C.; Malvehy, J.; et al. Human–computer collaboration for skin cancer recognition. Nat. Med. 2020, 26, 1229–1234. [Google Scholar] [CrossRef]
- Winkler, J.K.; Fink, C.; Toberer, F.; Enk, A.; Deinlein, T.; Hofmann-Wellenhof, R.; Thomas, L.; Lallas, A.; Blum, A.; Stolz, W.; et al. Association Between Surgical Skin Markings in Dermoscopic Images and Diagnostic Performance of a Deep Learning Convolutional Neural Network for Melanoma Recognition. JAMA Dermatol. 2019, 155, 1135–1141. [Google Scholar] [CrossRef]
- Adamson, A.S.; Smith, A. Machine Learning and Health Care Disparities in Dermatology. JAMA Dermatol. 2018, 154, 1247–1248. [Google Scholar] [CrossRef]
- Omiye, J.A.; Gui, H.; Daneshjou, R.; Cai, Z.R.; Muralidharan, V. Principles, applications, and future of artificial intelligence in dermatology. Front. Med. 2023, 10, 1278232. [Google Scholar] [CrossRef]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; van Smeden, M.; et al. TRIPOD+AI statement: Updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef] [PubMed]
- Vasey, B.; Novak, A.; Ather, S.; Ibrahim, M.; McCulloch, P. DECIDE-AI: A new reporting guideline and its relevance to artificial intelligence studies in radiology. Clin. Radiol. 2023, 78, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.E.; Kamali, K.; Dorey, R.A.; MacIntyre, O.C.; Cleminson, K.; MacGillivry, M.L.; Green, P.J.; Langley, R.G.; Purdy, K.S.; DeCoste, R.C.; et al. Using Artificial Intelligence as a Melanoma Screening Tool in Self-Referred Patients. J. Cutan. Med. Surg. 2024, 28, 37–43. [Google Scholar] [CrossRef]
- Wei, M.L.; Tada, M.; So, A.; Torres, R. Artificial intelligence and skin cancer. Front. Med. 2024, 11, 1331895. [Google Scholar] [CrossRef]
- Behara, K.; Bhero, E.; Agee, J.T. AI in dermatology: A comprehensive review into skin cancer detection. Peer J. Comput. Sci. 2024, 10, e2530. [Google Scholar] [CrossRef]
- Abdollahimajd, F.; Abbasi, F.; Motamedi, A.; Koohi, N.; Robati, R.M.; Gorji, M. Using the power of artificial intelligence to improve the diagnosis and management of nonmelanoma skin cancer. J. Res. Med. Sci. 2025, 30, 25. [Google Scholar] [CrossRef]
- Liopyris, K.; Gregoriou, S.; Dias, J.; Stratigos, A.J. Artificial Intelligence in Dermatology: Challenges and Perspectives. Dermatol. Ther. 2022, 12, 2637–2651. [Google Scholar] [CrossRef] [PubMed]
- Caffery, L.J.; Sullivan, R.P.; McInerney-Leo, A.; Lawn, C.; Mendis, R.A.; Janda, M.; Haplern, A.C.; Soyer, H.P. Privacy in Imaging for Dermatology Research. J. Investig. Dermatol. 2025, in press. [CrossRef] [PubMed]
- The University of Queensland. ACRF ACEMID Winner of 2024 Eureka Prize for Excellence in Interdisciplinary Scientific Research. Available online: https://acemid.centre.uq.edu.au/article/2024/09/acrf-acemid-winner-2024-eureka-prize-excellence-interdisciplinary-scientific-research (accessed on 8 September 2025).
- Koh, U.; Cust, A.E.; Fernández-Peñas, P.; Mann, G.; Morton, R.; Wolfe, R.; Payne, E.; Horsham, C.; Kwaan, G.; Mahumud, R.A.; et al. ACEMID cohort study: Protocol of a prospective cohort study using 3D total body photography for melanoma imaging and diagnosis. BMJ Open 2023, 13, e072788. [Google Scholar] [CrossRef] [PubMed]
- Mahumud, R.A.; Janda, M.; Soyer, H.P.; Fernández-Peñas, P.; Mar, V.J.; Morton, R.L. Assessing the value of precision medicine health technologies to detect and manage melanoma. Med. J. Aust. 2022, 217, 275–278. [Google Scholar] [CrossRef]
- Lee, K.J.; Betz-Stablein, B.; Stark, M.S.; Janda, M.; McInerney-Leo, A.M.; Caffery, L.J.; Gillepsie, N.; Yanes, T.; Soyer, H.P. The Future of Precision Prevention for Advanced Melanoma. Front. Med. 2022, 17, 818096. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute. Available online: https://seer.cancer.gov (accessed on 14 October 2025).
- Digital Imaging and Communication in Medicine (DICOM). Available online: https://www.dicomstandard.org (accessed on 8 September 2025).
- Australian Government. Specialist Training Program. Available online: https://www.health.gov.au/our-work/specialist-training-program (accessed on 16 October 2025).
- Nair, M.; Svedberg, P.; Larrson, I.; Nygren, J.M. A comprehensive overview of barriers and strategies for AI implementation in healthcare: Mixed-method design. PLoS ONE 2024, 19, e0305949. [Google Scholar] [CrossRef]
- NSW Government. AI System Implementation Issues and Risk Mitigation: Living Evidence. Available online: https://aci.health.nsw.gov.au/statewide-programs/critical-intelligence-unit/artificial/system-implementation-issues-risks (accessed on 16 October 2025).
- Scipion, C.E.A.; Manchester, M.A.; Federman, A.; Wang, Y.; Arias, J.J. Barriers to and facilitators of clinician acceptance and use of artificial intelligence in healthcare settings: A scoping review. BMJ Open 2025, 15, e092624. [Google Scholar] [CrossRef]
- Primiero, C.A.; Janda, M.; Soyer, H.P. Skin 2.0: How Cutaneous Digital Twins Could Reshape Dermatology. J. Investig. Dermatol. 2025, 145, 18–21. [Google Scholar] [CrossRef]
- Brinker, T.J.; Klode, J.; Esser, S.; Schadendorf, D. Facial-Aging App Availability in Waiting Rooms as a Potential Opportunity for Skin Cancer Prevention. JAMA Dermatol. 2018, 154, 1085–1086. [Google Scholar] [CrossRef]
- Kahler, S.; Betz-Stablein, B.; Lee, F.; Torrano, J.; Janda, M.; Primiero, C.; Soyer, H.P.; Jayasinghe, D. Estimation of Body Mass Index from 3-Dimensional Total Body Photography. J. Investig. Dermatol. 2025, 145, 972–975. [Google Scholar] [CrossRef]
- Susanto, A.; Kahler, S.; Dulanjala Sewwandi, M.A.N.; Mothershaw, A.; Goldinger, S.M.; Mar, V.J.; Janda, M.; Soyer, H.P.; Primiero, C.A. Three-dimensional total body photography enables automated obesity-related comorbidity screening in dermatology. J. Investig. Dermatol. 2025, in press. [Google Scholar] [CrossRef]
- Susanto, A.; Nathan, V.; Janda, M.; Khosrotehrani, K.; McMeniman, E.; Soyer, H.P.; Betz-Stablein, B. Diversifying dermatology: Improving skin of colour representation. Aust. J. Dermatol. 2024, 65, e208–e210. [Google Scholar] [CrossRef]
- Liu, Y.; Primiero, C.A.; Kulkarni, V.; Soyer, H.P.; Betz-Stablein, B. Artificial Intelligence for the Classification of Pigmented Skin Lesions in Populations with Skin of Color: A Systematic Review. Dermatology 2023, 239, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Gloster, H.M., Jr.; Neal, K. Skin cancer in skin of color. J. Am. Acad. Dermatol. 2006, 55, 741–764. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare (AIHW). Australian Cancer Database. Available online: https://www.aihw.gov.au/about-our-data/our-data-collections/australian-cancer-database (accessed on 14 October 2025).
- Horsham, C.; Antrobus, J.; Olsen, C.; Ford, H.; Abernethy, D.; Hacker, E. Testing Wearable UV Sensors to Improve Sun Protection in Young Adults at an Outdoor Festival: Field Study. JMIR mHealth uHealth 2020, 8, e21243. [Google Scholar] [CrossRef]
- Singh, N.; Dunlop, K.L.A.; Woolley, N.; Vaishishtha, T.W.; Damian, D.L.; Vuong, K.; Cust, A.E.; Smit, A.K. A review of skin cancer primary prevention activities in primary care settings. Public Health Res. Pract. 2024, 34, 34012401. [Google Scholar] [CrossRef] [PubMed]
- Asdul, P.; Chambers, D.; Brandt, H.M.; Fernandez, M.E.; Ramanadhan, S.; Torres, E.; Leeman, J.; Baquero, B.; Fleischer, L.; Escoffery, C.; et al. Grounding implementation science in health equity for cancer prevention and control. Implement. Sci. Commun. 2022, 3, 56. [Google Scholar] [CrossRef] [PubMed]
- Lacson, J.C.A.; Doyle, S.H.; Qian, L.; Del Rio, J.; Forgas, S.M.; Valavanis, S.; Carvajal, R.; Gonzales-Calderon, G.; Kim, Y.; Roetzheim, R.G.; et al. A Randomized Trial of Precision Prevention Materials to Improve Primary and Secondary Melanoma Prevention Activities among Individuals with Limited Melanoma Risk Phenotypes. Cancers 2021, 13, 3143. [Google Scholar] [CrossRef]
- Green, A.; Williams, G.; Neale, R.; Hart, V.; Leslie, D.; Parsons, P.; Marks, G.C.; Gaffney, P.; Battistutta, D.; Frost, C.; et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: A randomised controlled trial. Lancet 1999, 354, 723–729. [Google Scholar] [CrossRef]
- Green, A.C.; Williams, G.M.; Logan, V.; Strutton, G.M. Reduced melanoma after regular sunscreen use: Randomized trial follow-up. J. Clin. Oncol. 2011, 29, 257–263. [Google Scholar] [CrossRef]
- Darlington, S.; Williams, G.; Neale, R.; Frost, C.; Green, A. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch. Dermatol. 2003, 139, 451–555. [Google Scholar] [CrossRef]
- Janda, M.; Neale, R.; Khosrotehrani, K.; Whiteman, D.; Julian, A.; Loughran, S. Report of the Pre-Conference Sunscreen Workshop. In Proceedings of the 6th International Conference on UV and Skin Cancer Prevention, Brisbane, Australia, 11–13 September 2024; pp. 1–13. [Google Scholar]
- Anderson, P.; Eckert, M. Skin cancer in regional, rural, and remote Australia; opportunities for service improvement through technological advances and interdisciplinary care. Aust. J. Adv. Nurs. 2020, 37, 25–30. [Google Scholar] [CrossRef]
- Ahuja, S.; Briggs, S.M.; Collier, S.M. Teledermatology in Rural, Underserved, and Isolated Environments: A Review. Curr. Dermatol. Rep. 2022, 11, 328–335. [Google Scholar] [CrossRef]
- Griffith, V.; Foy, V. Teledermatology as a Solution for Rural and Rare Dermatologic Care: A Review of Strategies and Opportunities. Dermatol. Rev. 2025, 6, e70049. [Google Scholar] [CrossRef]
- Insight+. Current Skin Cancer Biomarkers ‘Ineffective’ But Research Gives New Hope. Available online: https://insightplus.mja.com.au/2023/6/current-skin-cancer-biomarkers-ineffective-but-research-gives-new-hope (accessed on 16 October 2025).
- Felzmann, H.; Villaronga, E.F.; Lutz, C.; Tamò-Larrieux, A. Transparency you can trust: Transparency requirements for artificial intelligence between legal norms and contextual concerns. Big Data Soc. 2019, 6, 2053951719860542. [Google Scholar] [CrossRef]
- Zlatolas, L.N.; Welzer, T.; Lhotska, L. Data breaches in healthcare: Security mechanisms for attack mitigation. Clust. Comput. 2024, 27, 8639–8654. [Google Scholar] [CrossRef]
- Ratwani, R.M.; Sutton, K.; Galarraga, J.E. Addressing AI Algorithmic Bias in Healath Care. JAMA 2024, 332, 1051–1052. [Google Scholar] [CrossRef] [PubMed]
- Australian Government. Safe and Responsible Artificial Intelligence in Health Care—Legislation and Regulation Review. Available online: https://www.health.gov.au/sites/default/files/2025-07/safe-and-responsible-artificial-intelligence-in-health-care-legislation-and-regulation-review-final-report.pdf (accessed on 16 October 2025).
- Cancer Council. Climate Change: Skin Cancer Statistics and Issues Prevention Policy. Available online: https://www.cancer.org.au/about-us/policy-and-advocacy/prevention/uv-radiation/related-resources/climate-change (accessed on 14 October 2025).
- Neale, R.E.; Hamilton, A.R.; Janda, M.; Gies, P.; Green, A.C. Seasonal variation in measured solar ultraviolet radiation exposure of adults in subtropical Australia. Photochem. Photobiol. 2010, 86, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Bais, A.F.; McKenzie, R.L.; Bernhard, G.; Aucamp, P.J.; Ilyas, M.; Madronich, S.; Tourpali, K. Ozone depletion and climate change: Impacts on UV radiation. Photochem. Photobiol. Sci. 2015, 14, 19–52. [Google Scholar] [CrossRef] [PubMed]
- Mohajerani, A.; Bakaric, J.; Jeffrey-Bailey, T. The urban heat island effect, its causes, and mitigation, with reference to the thermal properties of asphalt concrete. J. Environ. Manag. 2017, 197, 522–538. [Google Scholar] [CrossRef] [PubMed]
- SunSmart. Free SunSmart Global UV App. Available online: https://www.sunsmart.com.au/resources/sunsmart-app (accessed on 16 October 2025).
- Government of South Australia: SafeWorkSA. Heat & UV. Available online: https://safework.sa.gov.au/workers/health-and-wellbeing/heat-and-uv (accessed on 14 October 2025).
- Vojvodic, A.; Vlaskovic-Jovicevic, T.; Vojvodic, P.; Vojvodic, J.; Goldust, M.; Peric-Hajzler, Z.; Matovic, D.; Sija, G.; Stepic, N.; Nguyen, V.T.; et al. Psychological Impact of Melanoma, How to Detect, Support and Help. Open Access Maced. J. Med. Sci. 2019, 7, 3043–3045. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).