Longitudinal Bone Density During TSH Suppression in Differentiated Thyroid Cancer: A Paired PET/CT Analysis
Simple Summary
Abstract
1. Introduction
2. Methods
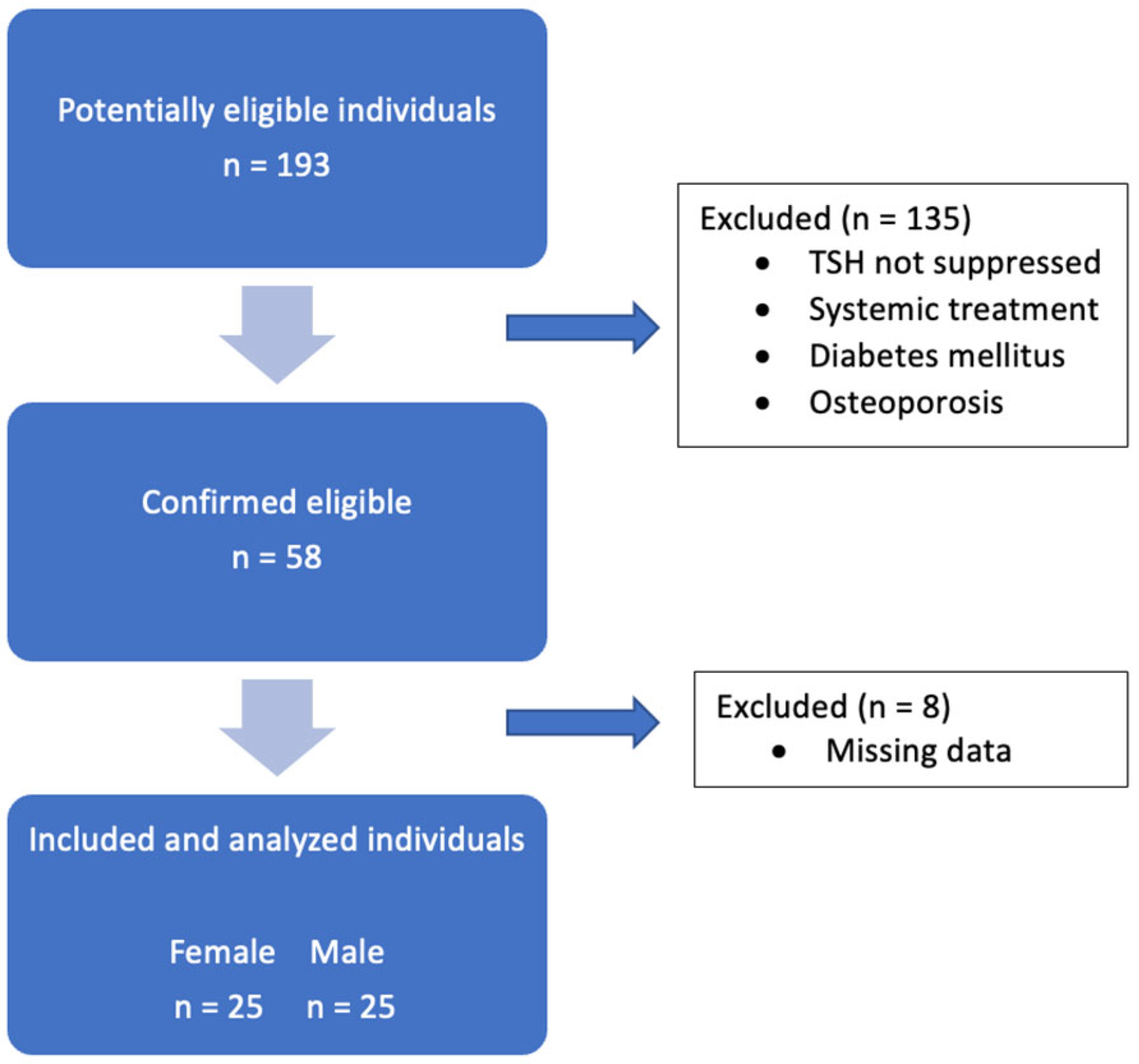
2.1. Patients
2.2. PET/CT Examinations
2.3. Image Analysis and Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patients
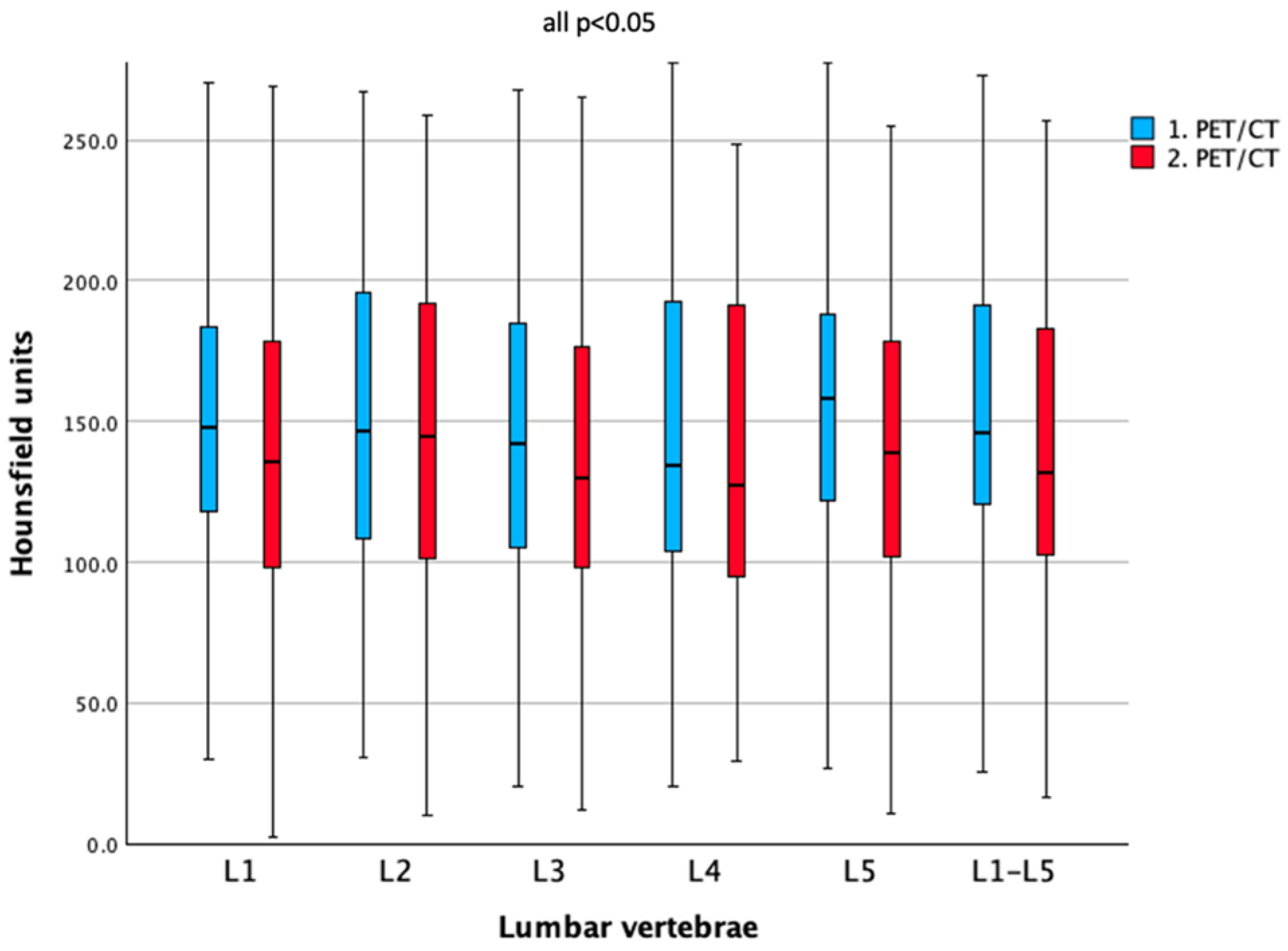
3.2. Bone Density Comparison Between Baseline and Follow-Up
3.3. Correlation Between ΔHU and Years Between PET/CT Scans
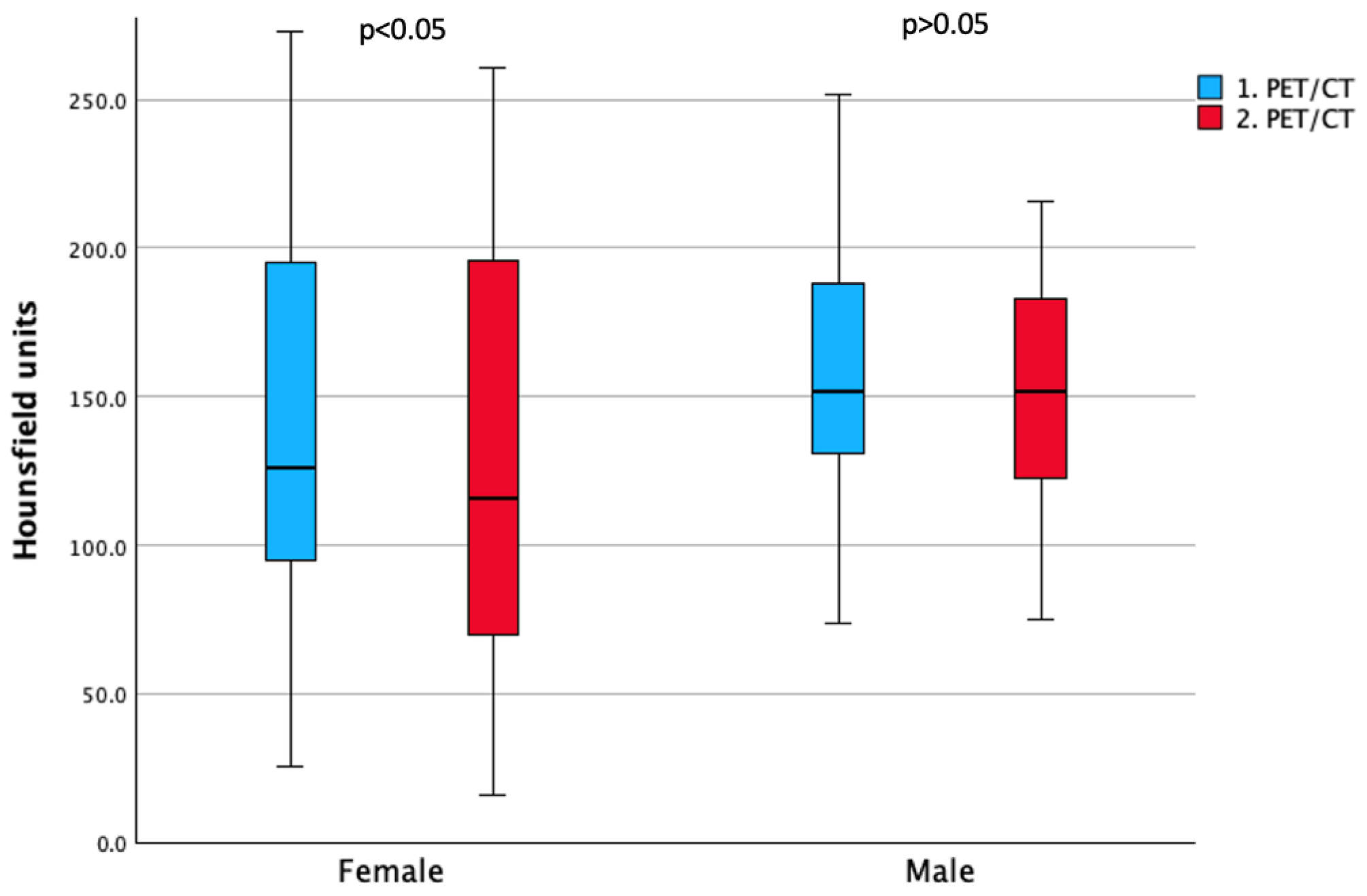
3.4. Sex Differences in Bone Density Outcomes
3.5. Impact of Age and Sex on Baseline and Longitudinal Bone Density (Two-Way ANOVA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lyu, Z.; Zhang, Y.; Sheng, C.; Huang, Y.; Zhang, Q.; Chen, K. Global burden of thyroid cancer in 2022: Incidence and mortality estimates from GLOBOCAN. Chin. Med. J. 2024, 137, 2567–2576. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Boucai, L.; Zafereo, M.; Cabanillas, M.E. Thyroid Cancer: A Review. JAMA 2024, 331, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.I. Thyroid carcinoma. Lancet 2003, 361, 501–511. [Google Scholar] [CrossRef]
- Jukić, T.; Blažeković, I.; Franceschi, M.; Ovčariček, P.P.; Butković, M.B.; Dabelić, N.; Granić, R.; Punda, M.; Sonicki, Z.; Vagić, D.; et al. Long-Term Outcome of Differentiated Thyroid Cancer Patients—Fifty Years of Croatian Thyroid Disease Referral Centre Experience. Diagnostics 2022, 12, 866. [Google Scholar] [CrossRef]
- Luis, P.-O.; Lucía, M.-J.A.; Hugo, R.-C.; Ramiro, R.-M.; Stalin, C.-Q. Differentiated Thyroid Carcinoma Long-Term Prognostic Factors. Int. J. Surg. Oncol. 2024, 2024, 1067447. [Google Scholar] [CrossRef]
- Avram, A.M.; Zukotynski, K.; Nadel, H.R.; Giovanella, L. Management of Differentiated Thyroid Cancer: The Standard of Care. J. Nucl. Med. 2022, 63, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gu, S.-Y.; Zhou, N.-M.; Jiang, J.-J. Association between thyroid stimulating hormone levels and papillary thyroid cancer risk: A meta-analysis. Open Life Sci. 2023, 18, 20220671. [Google Scholar] [CrossRef]
- Pujol, P.; Daures, J.P.; Nsakala, N.; Baldet, L.; Bringer, J.; Jaffiol, C. Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 1996, 81, 4318–4323. [Google Scholar][Green Version]
- Song, X.; Zhi, X.; Qian, L. Tailoring TSH suppression in differentiated thyroid carcinoma: Evidence, controversies, and future directions. Endocrine 2025, 89, 1–19. [Google Scholar] [CrossRef]
- Gubbi, S.; Al-Jundi, M.; Foerster, P.; Cardenas, S.; Butera, G.; Auh, S.; Wright, E.C.; Klubo-Gwiezdzinska, J. The Effect of Thyrotropin Suppression on Survival Outcomes in Patients with Differentiated Thyroid Cancer: A Systematic Review and Meta-Analysis. Thyroid 2024, 34, 674–686. [Google Scholar] [CrossRef] [PubMed]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Silverman, S.L. Quality-of-life issues in osteoporosis. Curr. Rheumatol. Rep. 2005, 7, 39–45. [Google Scholar] [CrossRef]
- Guzon-Illescas, O.; Perez Fernandez, E.; Crespí Villarias, N.; Quirós Donate, F.J.; Peña, M.; Alonso-Blas, C.; García-Vadillo, A.; Mazzucchelli, R. Mortality after osteoporotic hip fracture: Incidence, trends, and associated factors. J. Orthop. Surg. Res. 2019, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.N.; Leslie, W.D.; Schousboe, J.T. Osteoporosis: A Review. JAMA 2025, 334, 894–907. [Google Scholar] [CrossRef]
- Voulgaridou, G.; Papadopoulou, S.K.; Detopoulou, P.; Tsoumana, D.; Giaginis, C.; Kondyli, F.S.; Lymperaki, E.; Pritsa, A. Vitamin D and Calcium in Osteoporosis, and the Role of Bone Turnover Markers: A Narrative Review of Recent Data from RCTs. Diseases 2023, 11, 29. [Google Scholar] [CrossRef]
- Brancatella, A.; Marcocci, C. TSH suppressive therapy and bone. Endocr. Connect. 2020, 9, R158–R172. [Google Scholar] [CrossRef]
- Deshpande, N.; Hadi, M.S.; Lillard, J.C.; Passias, P.G.; Linzey, J.R.; Saadeh, Y.S.; LaBagnara, M.; Park, P. Alternatives to DEXA for the assessment of bone density: A systematic review of the literature and future recommendations. J. Neurosurg. Spine 2023, 38, 436–445. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Zheng, H.; Li, H.; Wang, H.; Ma, L. Hounsfield unit for assessing bone mineral density distribution within lumbar vertebrae and its clinical values. Front. Endocrinol. 2024, 15, 1398367. [Google Scholar] [CrossRef]
- Lee, S.; Chung, C.K.; Oh, S.H.; Park, S.B. Correlation between Bone Mineral Density Measured by Dual-Energy X-Ray Absorptiometry and Hounsfield Units Measured by Diagnostic CT in Lumbar Spine. J. Korean Neurosurg. Soc. 2013, 54, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, J.J.; Anderson, P.A.; Hsu, W.K. Use of computed tomography for assessing bone mineral density. Neurosurg. Focus. 2014, 37, E4. [Google Scholar] [CrossRef]
- Kim, H.J.; McLeod, D.S.A. Subclinical Hyperthyroidism and Cardiovascular Disease. Thyroid 2024, 34, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Y.; Gao, C.; Tian, L. Effect of Thyroid-Stimulating Hormone Suppression Therapy on Cardiac Structure and Function in Patients with Differentiated Thyroid Cancer After Thyroidectomy: A Systematic Review and Meta-Analysis. Endocr. Pract. 2024, 30, 177–186. [Google Scholar] [CrossRef]
- Yildiz, C.; Altay, M.; Yildiz, S.; Çağir, Y.; Akkan, T.; Ünsal, Y.A.; Beyan, E. Arterial stiffness in hyperthyroid patients is deteriorated due to thyroid hormones. Arch. Endocrinol. Metab. 2019, 63, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Einspieler, H.; Hennig, B.; Reiterits, B.; Klimpfinger, H.; Hacker, M.; Karanikas, G. A Retrospective Case-Control Study Examining the Association of Thyroid-Stimulating Hormone Suppression and Vascular Wall Inflammation on [18F]FDG-PET/CT. Thyroid 2025, 35, 357–366. [Google Scholar] [CrossRef]
- Boswijk, E.; Sanders, K.J.C.; Broeders, E.P.M.; de Ligt, M.; Vijgen, G.H.E.J.; Havekes, B.; Mingels, A.M.A.; Wierts, R.; van Marken Lichtenbelt, W.D.; Schrauwen, P.; et al. TSH suppression aggravates arterial inflammation—An (18)F-FDG PET study in thyroid carcinoma patients. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Altuntaş, S.Ç.; Hocaoğlu, Ç. Effects of Chronic Suppression or Oversuppression of Thyroid-Stimulating Hormone on Psychological Symptoms and Sleep Quality in Patients with Differentiated Thyroid Cancer. Horm. Metab. Res. 2021, 53, 683–691. [Google Scholar] [CrossRef]
- Görres, G.; Kaim, A.; Otte, A.; Götze, M.; Müller-Brand, J. Bone mineral density in patients receiving suppressive doses of thyroxine for differentiated thyroid carcinoma. Eur. J. Nucl. Med. 1996, 23, 690–692. [Google Scholar] [CrossRef]
- Guo, C.Y.; Weetman, A.P.; Eastell, R. Longitudinal changes of bone mineral density and bone turnover in postmenopausal women on thyroxine. Clin. Endocrinol. 1997, 46, 301–307. [Google Scholar] [CrossRef]
- Ku, E.J.; Yoo, W.S.; Lee, E.K.; Ahn, H.Y.; Woo, S.H.; Hong, J.H.; Chung, H.K.; Park, J.-W. Effect of TSH Suppression Therapy on Bone Mineral Density in Differentiated Thyroid Cancer: A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2021, 106, 3655–3667. [Google Scholar] [CrossRef]
- Kim, M.K.; Yun, K.-J.; Kim, M.-H.; Lim, D.-J.; Kwon, H.-S.; Song, K.-H.; Kang, M.-I.; Baek, K.H. The effects of thyrotropin-suppressing therapy on bone metabolism in patients with well-differentiated thyroid carcinoma. Bone 2015, 71, 101–105. [Google Scholar] [CrossRef]
- Lee, M.Y.; Park, J.H.; Bae, K.S.; Jee, Y.G.; Ko, A.N.; Han, Y.J.; Shin, J.Y.; Lim, J.S.; Chung, C.H.; Kang, S.J. Bone mineral density and bone turnover markers in patients on long-term suppressive levothyroxine therapy for differentiated thyroid cancer. Ann. Surg. Treat. Res. 2014, 86, 55–60. [Google Scholar] [CrossRef]
- Einspieler, H.; Walter, C.; Hacker, M.; Karanikas, G.; Tamandl, D. Effects of short- and long-term TSH suppression on lumbar bone mineral density in both genders using PET/CT. Sci. Rep. 2023, 13, 22640. [Google Scholar] [CrossRef]
- Zou, D.; Muheremu, A.; Sun, Z.; Zhong, W.; Jiang, S.; Li, W. Computed tomography Hounsfield unit–based prediction of pedicle screw loosening after surgery for degenerative lumbar spine disease. J. Neurosurg. Spine 2020, 32, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, E.; Lee, J.W. Value of computed tomography Hounsfield units in predicting pedicle screw loosening in the thoracic spine. Sci. Rep. 2022, 12, 18279. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Smith, A.W.; Palmer, F.L.; Tuttle, R.M.; Mahrous, A.; Nixon, I.J.; Patel, S.G.; Ganly, I.; Fagin, J.A.; Boucai, L. Thyrotropin Suppression Increases the Risk of Osteoporosis Without Decreasing Recurrence in ATA Low- and Intermediate-Risk Patients with Differentiated Thyroid Carcinoma. Thyroid 2015, 25, 300–307. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Han, Z.-Q.; Gong, X.-W.; Li, Q.; Ma, J. TSH-suppressive therapy can reduce bone mineral density in patients with differentiated thyroid carcinoma: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 922–929. [Google Scholar] [CrossRef]
- Reverter, J.L.; Holgado, S.; Alonso, N.; Salinas, I.; Granada, M.L.; Sanmartí, A. Lack of deleterious effect on bone mineral density of long-term thyroxine suppressive therapy for differentiated thyroid carcinoma. Endocr. Relat. Cancer 2005, 12, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.-H.; Lee, Y.; Oh, H.J.; Kim, S.H.; Lee, Y.-K. Influence of Thyroid-stimulating Hormone Suppression Therapy on Bone Mineral Density in Patients with Differentiated Thyroid Cancer: A Meta-analysis. J. Bone Metab. 2019, 26, 51–60. [Google Scholar] [CrossRef]
- Karner, I.; Hrgović, Z.; Sijanović, S.; Buković, D.; Klobucar, A.; Usadel, K.H.; Fassbender, W.J. Bone mineral density changes and bone turnover in thyroid carcinoma patients treated with supraphysiologic doses of thyroxine. Eur. J. Med. Res. 2005, 10, 480–488. [Google Scholar] [PubMed]
- Wang, X.; Teng, R.; Liu, F.; Liu, P.; Yang, Y. Effect of thyrotropin suppressive therapy on lumbar bone mineral density in patients with differentiated thyroid cancer: A retrospective cohort study. Gland. Surg. 2022, 11, 43241–43441. [Google Scholar] [CrossRef]
- Blum, M.R.; Bauer, D.C.; Collet, T.-H.; Fink, H.A.; Cappola, A.R.; da Costa, B.R.; Wirth, C.D.; Peeters, R.P.; Åsvold, B.O.; den Elzen, W.P.J.; et al. Subclinical Thyroid Dysfunction and Fracture Risk. JAMA 2015, 313, 2055–2065. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Chen, L.-R.; Chen, K.-H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.S.; Brockwell, S.E.; Mehta, V.; Greendale, G.A.; Sowers, M.R.; Ettinger, B.; Lo, J.C.; Johnston, J.M.; Cauley, J.A.; Danielson, M.E.; et al. Bone Mineral Density Changes during the Menopause Transition in a Multiethnic Cohort of Women. J. Clin. Endocrinol. Metab. 2008, 93, 861–868. [Google Scholar] [CrossRef]
- Sugitani, I.; Fujimoto, Y. Effect of postoperative thyrotropin suppressive therapy on bone mineral density in patients with papillary thyroid carcinoma: A prospective controlled study. Surgery 2011, 150, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, N.; Gao, X.; Liang, J.; Fan, X.; Zhao, Y. Meta-analysis of TSH suppression therapy and the risk of cardiovascular events after thyroid cancer surgery. Front. Endocrinol. 2022, 13, 991876. [Google Scholar] [CrossRef]
- Papaleontiou, M.; Hawley, S.T.; Haymart, M.R. Effect of Thyrotropin Suppression Therapy on Bone in Thyroid Cancer Patients. Oncologist 2016, 21, 165–171. [Google Scholar] [CrossRef] [PubMed]


| Parameters | 1. PET/CT | 2. PET/CT | p-Value |
|---|---|---|---|
| Age in years (mean ± SD) | 57.2 (±15.3) | 61.5 (±15.2) | <0.001 |
| Sex: | 1.000 | ||
| male | 25 | 25 | |
| female | 25 | 25 | |
| BMI (mean ± SD) | 27.3 (±4.6) | 26.9 (±4.9) | 0.116 |
| Blood parameters (mean ± SD): | |||
| TSH (µU/mL) | 0.08 (±0.1) | 0.09 (±0.2) | 0.421 |
| fT3 (pg/mL) | 3.2 (±0.6) | 3.1 (±0.4) | 0.089 |
| fT4 (ng/dL) | 1.81 (±0.4) | 1.72 (±0.3) | 0.256 |
| Calcium (mmol/L) | 2.29 (±0.11) | 2.25 (±0.15) | 0.093 |
| Lumbar Vertebrae | Bone Density (HU $)—1. PET/CT | Bone Density (HU $)—2. PET/CT | p-Value |
|---|---|---|---|
| L1 | 149.1 ± 53.1 | 139.8 ± 59.5 | 0.031 * |
| L2 | 149.5 ± 53.8 | 140.1 ± 57.9 | 0.017 * |
| L3 | 144.4 ± 55.3 | 136.0 ± 56.4 | 0.049 * |
| L4 | 145.5 ± 59.8 | 136.5 ± 59.8 | 0.026 * |
| L5 | 159.9 ± 61.2 | 140.3 ± 58.1 | <0.001 * |
| Mean L1–L5 | 150.4 ± 53.9 | 140.6 ± 56.4 | 0.004 * |
| Female | Male | |||
|---|---|---|---|---|
| Lumbar Vertebrae | Bone Density (HU $)—1. PET/CT | Bone Density (HU $)—2. PET/CT | Bone Density (HU $)—1. PET/CT | Bone Density (HU $)—2. PET/CT |
| L1 | 142.4 ± 62.9 * | 128.3 ± 67.7 * | 155.5 ± 42.0 | 150.9 ± 49.2 |
| L2 | 137.8 ± 63.0 * | 126.3 ± 69.7 * | 161.1 ± 40.6 | 153.9 ± 39.8 |
| L3 | 136.7 ± 65.1 | 126.5 ± 68.6 | 152.1 ± 43.5 | 145.5 ± 40.2 |
| L4 | 139.4 ± 72.0 * | 127.4 ± 71.7 * | 151.6 ± 45.2 | 145.7 ± 44.8 |
| L5 | 151.6 ± 66.8 * | 123.5 ± 66.5 * | 168.1 ± 55.3 | 157.1 ± 43.4 |
| Mean L1–L5 | 142.6 ± 64.1 * | 129.4 ± 68.9 * | 158.2 ± 41.3 | 151.8 ± 38.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Einspieler, H.; Klimpfinger, H.; Xue, S.; Debeljkovic, A.; Reiterits, B.; Hennig, B.; Hacker, M.; Karanikas, G. Longitudinal Bone Density During TSH Suppression in Differentiated Thyroid Cancer: A Paired PET/CT Analysis. Cancers 2025, 17, 3462. https://doi.org/10.3390/cancers17213462
Einspieler H, Klimpfinger H, Xue S, Debeljkovic A, Reiterits B, Hennig B, Hacker M, Karanikas G. Longitudinal Bone Density During TSH Suppression in Differentiated Thyroid Cancer: A Paired PET/CT Analysis. Cancers. 2025; 17(21):3462. https://doi.org/10.3390/cancers17213462
Chicago/Turabian StyleEinspieler, Holger, Hannah Klimpfinger, Song Xue, Aleksandar Debeljkovic, Bettina Reiterits, Bengt Hennig, Marcus Hacker, and Georgios Karanikas. 2025. "Longitudinal Bone Density During TSH Suppression in Differentiated Thyroid Cancer: A Paired PET/CT Analysis" Cancers 17, no. 21: 3462. https://doi.org/10.3390/cancers17213462
APA StyleEinspieler, H., Klimpfinger, H., Xue, S., Debeljkovic, A., Reiterits, B., Hennig, B., Hacker, M., & Karanikas, G. (2025). Longitudinal Bone Density During TSH Suppression in Differentiated Thyroid Cancer: A Paired PET/CT Analysis. Cancers, 17(21), 3462. https://doi.org/10.3390/cancers17213462







