Implications of Podoplanin Overexpression in the Malignant Transformation of Oral Potentially Malignant Disorders: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Evaluation and Risk of Bias
2.6. Statistical Analysis
3. Results
3.1. Results of the Literature Search
3.2. Study Characteristics
3.3. Qualitative Evaluation
3.4. Quantitative Evaluation (Meta-Analysis)
3.5. Quantitative Secondary Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma—An update. CA. Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- González-Ruiz, I.; Ramos-García, P.; Ruiz-Ávila, I.; González-Moles, M.Á. Early Diagnosis of Oral Cancer: A Complex Polyhedral Problem with a Difficult Solution. Cancers 2023, 15, 3270. [Google Scholar] [CrossRef]
- Monteiro, L.; Mello, F.W.; Warnakulasuriya, S. Tissue biomarkers for predicting the risk of oral cancer in patients diagnosed with oral leukoplakia: A systematic review. Oral Dis. 2021, 27, 1977–1992. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Celentano, A.; Glurich, I.; Borgnakke, W.S.; Jensen, S.B.; Peterson, D.E.; Delli, K.; Ojeda, D.; Vissink, A.; Farah, C.S. World Workshop on Oral Medicine VII: Prognostic biomarkers in oral leukoplakia: A systematic review of longitudinal studies. Oral Dis. 2019, 25, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Christofori, G. The potential role of podoplanin in tumour invasion. Br. J. Cancer 2007, 96, 1–5. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Monteiro, L.; Mariano, L.C.; Warnakulasuriya, S. Podoplanin could be a predictive biomarker of the risk of patients with oral leukoplakia to develop oral cancer: A systematic review and meta-analysis. Oral Dis. 2024, 30, 207–215. [Google Scholar] [CrossRef]
- Briggs, E.E.; Schlosser, N.; Nguyen, S.A.; Graboyes, E.; Newman, J.G.; Kejner, A.E.; Albergotti, G.; Bobian, M.; Yoon, A.; Peterson, Y.; et al. Podoplanin Expression and the Risk of Malignant Transformation of Oral Pre-Malignant Disease: A Systematic Review and Meta-Analysis. Head Neck 2025, 47, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Ridley, G.; Williams, K.; Altman, D.G.; Hayden, J.; de Vet, H.C.W. Prognosis research: Toward evidence-based results and a Cochrane methods group. J. Clin. Epidemiol. 2007, 60, 863–865; author reply 865–866. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Available online: https://www.cochrane.org/authors/handbooks-and-manuals/handbook (accessed on 5 October 2025).
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Chichester, UK, 2008; ISBN 9780470712184. [Google Scholar]
- Hayden, J.A.; Côté, P.; Bombardier, C. Evaluation of the quality of prognosis studies in systematic reviews. Ann. Intern. Med. 2006, 144, 427–437. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Ayén, Á.; González-Ruiz, I.; de Porras-Carrique, T.; González-Ruiz, L.; Ruiz-Ávila, I.; Ramos-García, P. Prognostic and Clinicopathological Significance of FADD Upregulation in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 2393. [Google Scholar] [CrossRef]
- Ramos-García, P.; González-Moles, M. Prognostic and Clinicopathological Significance of the Aberrant Expression of β-Catenin in Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 479. [Google Scholar] [CrossRef]
- Ramos-García, P.; González-Moles, M.Á.; Warnakulasuriya, S. Significance of p53 overexpression in the prediction of the malignant transformation risk of oral potentially malignant disorders: A systematic review and meta-analysis. Oral Oncol. 2022, 126, 105734. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Moya-González, E.; García-Ferrera, A.; Nieto-Casado, P.; Ramos-García, P. Prognostic and Clinicopathological Significance of Telomerase Reverse Transcriptase Upregulation in Oral Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3673. [Google Scholar] [CrossRef]
- Cívico-Ortega, J.L.; González-Ruiz, I.; Ramos-García, P.; Cruz-Granados, D.; Samayoa-Descamps, V.; González-Moles, M.Á. Prognostic and Clinicopathological Significance of Epidermal Growth Factor Receptor (EGFR) Expression in Oral Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 11888. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Higgins, J.P.T. Meta-Analysis and Subgroups. Prev. Sci. 2013, 14, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W.; Cheung, M.W.-L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Alkan, U.; Bachar, G.; Nachalon, Y.; Zlotogorsky, A.; Levin, E.G.; Kaplan, I. Proliferative verrucous leukoplakia: A clinicopathological comparative study. Int. J. Oral Maxillofac. Surg. 2022, 51, 1027–1033. [Google Scholar] [CrossRef]
- D’Souza, B.; Nayak, R.; Kotrashetti, V.S. Immunohistochemical Expression of Podoplanin in Clinical Variants of Oral Leukoplakia and Its Correlation with Epithelial Dysplasia. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 132–139. [Google Scholar] [CrossRef]
- Verma, V.; Chandrashekar, C. Evaluation of SOX2 and podoplanin expression in oral epithelial dysplasia and its correlation with malignant transformation. J. Investig. Clin. Dent. 2019, 10, e12450. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, K.Y.; Zheng, Z.; Bazarsad, S.; Kim, J. Nomogram for risk prediction of malignant transformation in oral leukoplakia patients using combined biomarkers. Oral Oncol. 2017, 72, 132–139. [Google Scholar] [CrossRef] [PubMed]
- De Vicente, J.C.; Rodrigo, J.P.; Rodriguez-Santamarta, T.; Lequerica-Fernández, P.; Allonca, E.; García-Pedrero, J.M. Podoplanin expression in oral leukoplakia: Tumorigenic role. Oral Oncol. 2013, 49, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Q.; Mi, J.G.; Wu, L.; Ma, L.W.; Shi, L.J.; Yang, X.; Liu, W.; Zhang, C.P.; Zhou, Z.T. Expression of podoplanin and ABCG2 in oral erythroplakia correlate with oral cancer development. Oral Oncol. 2012, 48, 848–852. [Google Scholar] [CrossRef]
- Gao, X.; Ma, L.; Zhou, Z.; Jian, X.; Liu, W. Podoplanin Expression Is Correlated with the Progression of Chronic Discoid Lupus Erythematosus to Lip Squamous Cell Carcinoma. Int. J. Surg. Pathol. 2016, 24, 595–599. [Google Scholar] [CrossRef]
- Habiba, U.; Hida, K.; Kitamura, T.; Matsuda, A.Y.; Higashino, F.; Ito, Y.M.; Ohiro, Y.; Totsuka, Y.; Shindoh, M. ALDH1 and podoplanin expression patterns predict the risk of malignant transformation in oral leukoplakia. Oncol. Lett. 2017, 13, 321–328. [Google Scholar] [CrossRef]
- Kawaguchi, H.; El-Naggar, A.K.; Papadimitrakopoulou, V.; Ren, H.; Fan, Y.H.; Feng, L.; Lee, J.J.; Kim, E.; Waun, K.H.; Lippman, S.M.; et al. Podoplanin: A novel marker for oral cancer risk in patients with oral premalignancy. J. Clin. Oncol. 2008, 26, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Kreppel, M.; Kreppel, B.; Drebber, U.; Wedemayer, I.; Rothamel, D.; Zöller, J.E.; Scheer, M. Podoplanin expression in oral leukoplakia: Prognostic value and clinicopathological implications. Oral Dis. 2012, 18, 692–699. [Google Scholar] [CrossRef]
- Monteiro, L.; Do Amaral, B.; Delgado, L.; Garcês, F.; Salazar, F.; Pacheco, J.J.; Lopes, C.; Warnakulasuriya, S. Podoplanin Expression Independently and Jointly with Oral Epithelial Dysplasia Grade Acts as a Potential Biomarker of Malignant Transformation in Oral Leukoplakia. Biomolecules 2022, 12, 606. [Google Scholar] [CrossRef]
- Shi, P.; Liu, W.; Zhou, Z.T.; He, Q.B.; Jiang, W.W. Podoplanin and ABCG2: Malignant transformation risk markers for oral lichen planus. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 844–849. [Google Scholar] [CrossRef]
- Mello, F.W.; Kammer, P.V.; Silva, C.A.B.; Parkinson, E.K.; Monteiro, L.; Warnakulasuriya, S.; Rivero, E.R.C. Prognostic and clinicopathological significance of podoplanin immunoexpression in oral and oropharyngeal squamous cell carcinoma: A systematic review. J. Oral Pathol. Med. 2021, 50, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Carvalho, V.; Wang, Q.; Li, T.; Wang, J.; Chen, Y.; Ni, C.; Liu, S.; Zhang, J. Prognostic Value of Podoplanin in Various Tumors. Technol. Cancer Res. Treat. 2021, 20, 15330338211038142. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, P.; Mei, Q.; Sun, W.; Zhou, L.; Yin, T. Podoplanin is a useful prognostic marker and indicates better differentiation in lung squamous cell cancer patients? A systematic review and meta-analysis. BMC Cancer 2020, 20, 424. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.; Rayes, J.; Miyashita, T.; Ishii, G.; Retzbach, E.P.; Sheehan, S.A.; Takemoto, A.; Chang, Y.; Yoneda, K.; Asai, J.; et al. Podoplanin: An emerging cancer biomarker and therapeutic target. Cancer Sci. 2018, 109, 1292–1299. [Google Scholar] [CrossRef]
- Renart, J.; Carrasco-Ramírez, P.; Fernández-Muñoz, B.; Martín-Villar, E.; Montero, L.; Yurrita, M.M.; Quintanilla, M. New Insights into the Role of Podoplanin in Epithelial-Mesenchymal Transition. Int. Rev. Cell Mol. Biol. 2015, 317, 185–239. [Google Scholar] [PubMed]
- Zhang, C.; Li, B.; Zeng, X.; Hu, X.S.; Hua, H. The global prevalence of oral leukoplakia: A systematic review and meta-analysis from 1996 to 2022. BMC Oral Health 2023, 23, 645. [Google Scholar] [CrossRef]
- Mello, F.W.; Miguel, A.F.P.; Dutra, K.L.; Porporatti, A.L.; Warnakulasuriya, S.; Guerra, E.N.S.; Rivero, E.R.C. Prevalence of oral potentially malignant disorders: A systematic review and meta-analysis. J. Oral Pathol. Med. 2018, 47, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Petti, S. Pooled estimate of world leukoplakia prevalence: A systematic review. Oral Oncol. 2003, 39, 770–780. [Google Scholar] [CrossRef]
- Pimenta-Barros, L.A.; Ramos-García, P.; González-Moles, M.Á.; Aguirre-Urizar, J.M.; Warnakulasuriya, S. Malignant transformation of oral leukoplakia: Systematic review and comprehensive meta-analysis. Oral Dis. 2025, 31, 69–80. [Google Scholar] [CrossRef]
- González-Ruiz, I.; Ramos-García, P.; Boujemaoui-Boulaghmoudi, H.; Mjouel-Boutaleb, N.; González-Moles, M.A. Cancer Hallmarks Expression in Oral Leukoplakia: Systematic Review and Meta-Analysis. Oral Dis. 2025, in press. [Google Scholar] [CrossRef]
- Lorenzo-Pouso, A.I.; Lafuente-Ibáñez de Mendoza, I.; Pérez-Sayáns, M.; Pérez-Jardón, A.; Chamorro-Petronacci, C.M.; Blanco-Carrión, A.; Aguirre-Urízar, J.M. Critical update, systematic review, and meta-analysis of oral erythroplakia as an oral potentially malignant disorder. J. Oral Pathol. Med. 2022, 51, 585–593. [Google Scholar] [CrossRef]
- Villa, A.; Villa, C.; Abati, S. Oral cancer and oral erythroplakia: An update and implication for clinicians. Aust. Dent. J. 2011, 56, 253–256. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Sultan, S.; Glasziou, P.; Akl, E.A.; Alonso-Coello, P.; Atkins, D.; Kunz, R.; Brozek, J.; Montori, V.; et al. GRADE guidelines: 9. Rating up the quality of evidence. J. Clin. Epidemiol. 2011, 64, 1311–1316. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D.; Ruiz-Ávila, I.; Ramos-García, P. Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 2021, 27, 813–828. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Ruiz-Ávila, I.; González-Ruiz, L.; Ayén, Á.; Gil-Montoya, J.A.; Ramos-García, P. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol. 2019, 96, 121–130. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Ramos-García, P.; Warnakulasuriya, S. An appraisal of highest quality studies reporting malignant transformation of oral lichen planus based on a systematic review. Oral Dis. 2021, 27, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Temam, S.; El-Naggar, A.; Zhou, X.; Liu, D.D.; Lee, J.J.; Mao, L. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer 2006, 107, 563–569. [Google Scholar] [CrossRef]


| Total | 12 studies |
| Year of publication | 2008–2022 |
| Total patients (range) | 857 (30–160) |
| Type of OPMDs | |
| Oral leukoplakia | 7 studies |
| Erythroplakia | 1 study |
| Discoid lupus erythematosus | 1 study |
| Oral lichen planus | 1 study |
| Mixed | 2 studies |
| Study design | |
| Retrospective | 10 studies |
| Prospective | 2 studies |
| Anti-podoplanin antibody | |
| Clone D2-40 | 10 studies |
| Not reported | 2 studies |
| Anti-podoplanin antibody dilution | |
| 1:100 | 6 studies |
| 1:150 | 4 studies |
| Not reported | 2 studies |
| Anti-podoplanin antibody incubation time | |
| Overnight | 5 studies |
| 60′ | 1 study |
| Not reported | 6 studies |
| Anti-podoplanin antibody incubation temperature | |
| 4 °C | 5 studies |
| Room temperature | 1 study |
| Not reported | 6 studies |
| Cut-off point | |
| 1% | 4 studies |
| Intensity | 2 studies |
| Not reported | 6 studies |
| Immunostaining pattern | |
| Membrane and cytoplasm | 6 studies |
| Membrane | 4 studies |
| Cytoplasm | 1 study |
| Not reported | 1 study |
| Geographical region | |
| Asia | 8 studies |
| Europe | 3 studies |
| North America | 1 study |
| Meta-Analyses | No. of Studies | No. of Patients | Stat. Model | Wt | Pooled Data | Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|
| ES (95% CI) | p-Value | Phet | I2 (%) | |||||
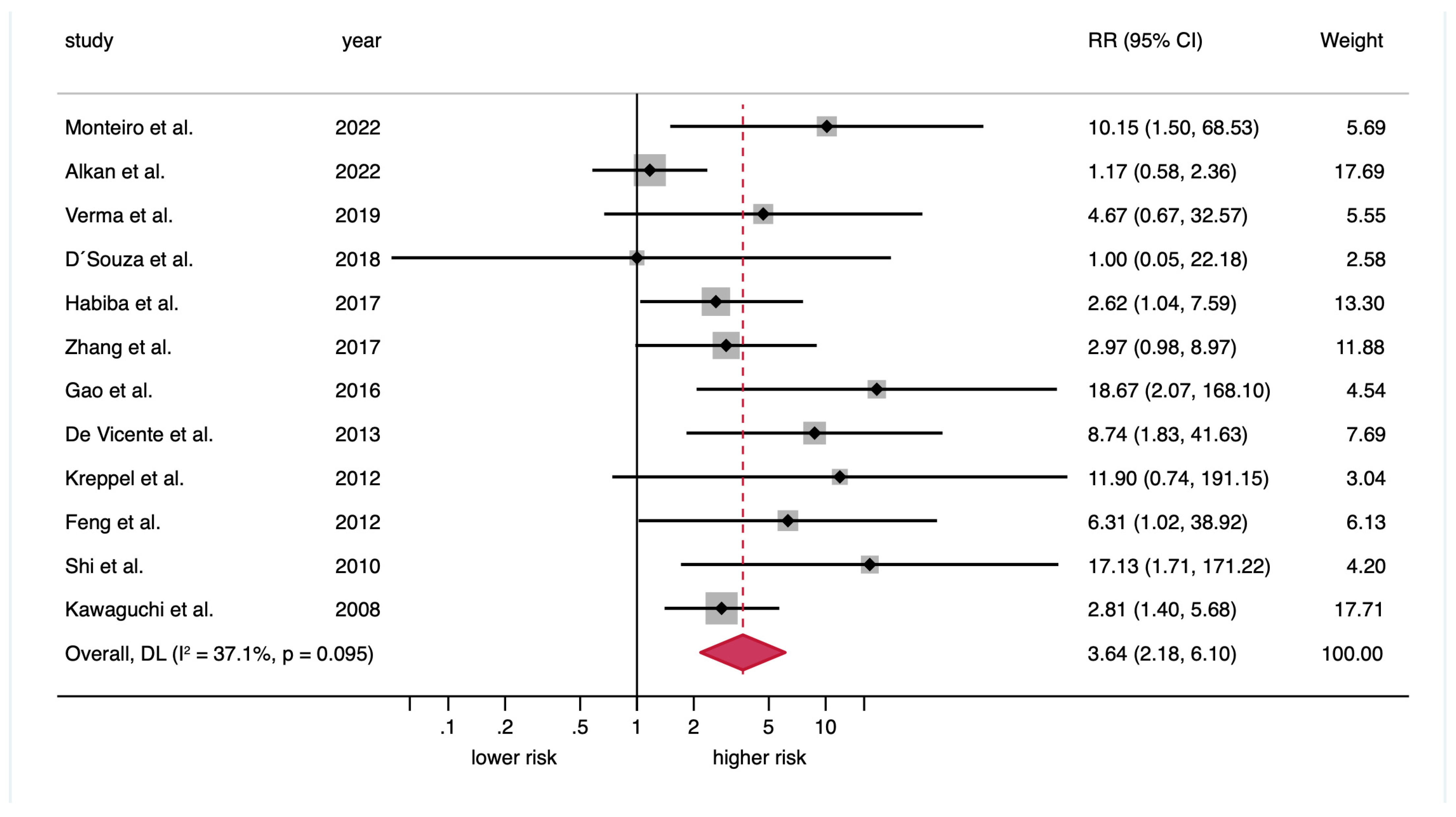
| Malignant transformation risk a | 12 | 857 | REM | D-L | RR = 3.64 (2.18–6.10) | <0.001 | 0.10 | 37.1 |
| Subgroup analysis by geographical region b | ||||||||
| Asia | 8 | 568 | REM | D-L | RR = 3.20 (1.61–6.34) | 0.001 | 0.09 | 42.6 |
| Europe | 3 | 157 | REM | D-L | RR = 9.66 (3.19–29.26) | <0.001 | 0.98 | 0.0 |
| North America | 1 | 132 | REM | D-L | RR = 2.81 (1.40–5.66) | 0.004 | — | 0.0 |
| Subgroup analysis by type of OPMD b | ||||||||
| Oral leukoplakia | 7 | 558 | REM | D-L | RR = 3.37 (2.13–5.32) | <0.001 | 0.59 | 0.0 |
| Erythroplakia | 1 | 34 | REM | D-L | RR = 6.31 (1.02–38.98) | 0.05 | — | 0.0 |
| Discoid lupus erythematosus | 1 | 52 | REM | D-L | RR = 18.67 (2.07–168.2) | 0.009 | — | 0.0 |
| Oral lichen planus | 1 | 119 | REM | D-L | RR = 17.13 (1.71–171.4) | 0.02 | — | 0.0 |
| Mixed | 2 | 94 | REM | D-L | RR = 1.72 (0.51–5.78) | 0.38 | 0.19 | 42.1 |
| Subgroup analysis by immunohistochemical pattern b | ||||||||
| Membrane and cytoplasm | 6 | 502 | REM | D-L | RR = 4.71 (2.54–8.74) | <0.001 | 0.37 | 7.5 |
| Membrane | 4 | 291 | REM | D-L | RR = 3.60 (1.96–6.62) | <0.001 | 0.50 | 0.0 |
| Cytoplasm | 1 | 30 | REM | D-L | RR = 1.00 (0.05–21.06) | 0.99 | — | 0.0 |
| Not reported | 1 | 34 | REM | D-L | RR = 1.17 (0.58–2.36) | 0.66 | — | 0.0 |
| Subgroup analysis by anti-podoplanin antibody b | ||||||||
| Clone D2-40 | 10 | 645 | REM | D-L | RR = 3.45 (1.95–6.10) | <0.001 | 0.10 | 38.4 |
| Not reported | 2 | 212 | REM | D-L | RR = 5.77 (1.02–32.55) | 0.05 | 0.14 | 53.3 |
| Subgroup analysis by anti-podoplanin antibody dilution b | 0.05 | |||||||
| 1:100 | 6 | 523 | REM | D-L | RR = 2.62 (1.51–4.56) | 0.001 | 0.14 | 39.7 |
| 1:150 | 4 | 244 | REM | D-L | RR = 11.04 (4.00–30.45) | <0.001 | 0.87 | 0.0 |
| Not reported | 2 | 90 | REM | D-L | RR = 2.99 (0.58–15.39) | 0.19 | 0.40 | 0.0 |
| Subgroup analysis by anti-podoplanin antibody incubation time b | ||||||||
| Overnight | 5 | 448 | REM | D-L | RR = 3.82 (2.11–6.92) | <0.001 | 0.33 | 12.8 |
| 60′ | 1 | 60 | REM | D-L | RR = 4.67 (0.67–32.56) | 0.12 | — | 0.0 |
| Not reported | 6 | 349 | REM | D-L | RR = 3.46 (1.36–8.78) | 0.009 | 0.05 | 54.7 |
| Subgroup analysis by anti-podoplanin antibody incubation temperature b | ||||||||
| 4 °C | 5 | 448 | REM | D-L | RR = 3.82 (2.11–6.92) | <0.001 | 0.33 | 12.8 |
| Room temperature | 1 | 60 | REM | D-L | RR = 4.67 (0.67–32.56) | 0.12 | — | 0.0 |
| Not reported | 6 | 349 | REM | D-L | RR = 3.46 (1.36–8.78) | 0.009 | 0.05 | 54.7 |
| Subgroup analysis by cut-off point for podoplanin protein overexpression b | ||||||||
| 1% | 4 | 418 | REM | D-L | RR = 3.42 (1.77–6.63) | <0.001 | 0.51 | 0.0 |
| Intensity | 2 | 73 | REM | D-L | RR = 2.85 (0.35–22.90) | 0.33 | 0.04 | 76.9 |
| Not reported | 6 | 366 | REM | D-L | RR = 4.41 (2.32–8.38) | <0.001 | 0.36 | 8.3 |
| Subgroup analysis by overall risk of bias in primary-level studies *,b | ||||||||
| Low RoB | 4 | 369 | REM | D-L | RR = 5.20 (2.16–12.51) | <0.001 | 0.26 | 26.1 |
| Moderate RoB | 6 | 420 | REM | D-L | RR = 3.70 (2.01–6.82) | <0.001 | 0.48 | 0.0 |
| High RoB | 2 | 68 | REM | D-L | RR = 2.18 (0.44–10.77) | 0.34 | 0.09 | 65.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa-Fernández, M.; Ramos-García, P.; Mjouel-Boutaleb, N.; Boujemaoui-Boulaghmoudi, H.; González-Moles, M.Á. Implications of Podoplanin Overexpression in the Malignant Transformation of Oral Potentially Malignant Disorders: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 3448. https://doi.org/10.3390/cancers17213448
Correa-Fernández M, Ramos-García P, Mjouel-Boutaleb N, Boujemaoui-Boulaghmoudi H, González-Moles MÁ. Implications of Podoplanin Overexpression in the Malignant Transformation of Oral Potentially Malignant Disorders: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(21):3448. https://doi.org/10.3390/cancers17213448
Chicago/Turabian StyleCorrea-Fernández, Marcela, Pablo Ramos-García, Noor Mjouel-Boutaleb, Hajar Boujemaoui-Boulaghmoudi, and Miguel Ángel González-Moles. 2025. "Implications of Podoplanin Overexpression in the Malignant Transformation of Oral Potentially Malignant Disorders: A Systematic Review and Meta-Analysis" Cancers 17, no. 21: 3448. https://doi.org/10.3390/cancers17213448
APA StyleCorrea-Fernández, M., Ramos-García, P., Mjouel-Boutaleb, N., Boujemaoui-Boulaghmoudi, H., & González-Moles, M. Á. (2025). Implications of Podoplanin Overexpression in the Malignant Transformation of Oral Potentially Malignant Disorders: A Systematic Review and Meta-Analysis. Cancers, 17(21), 3448. https://doi.org/10.3390/cancers17213448









