High-Dose Stereotactic Re-Irradiation of Recurrent High-Grade Gliomas: Clinical Outcome and Experience with AI-Based Target Volume Simulation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Cohort
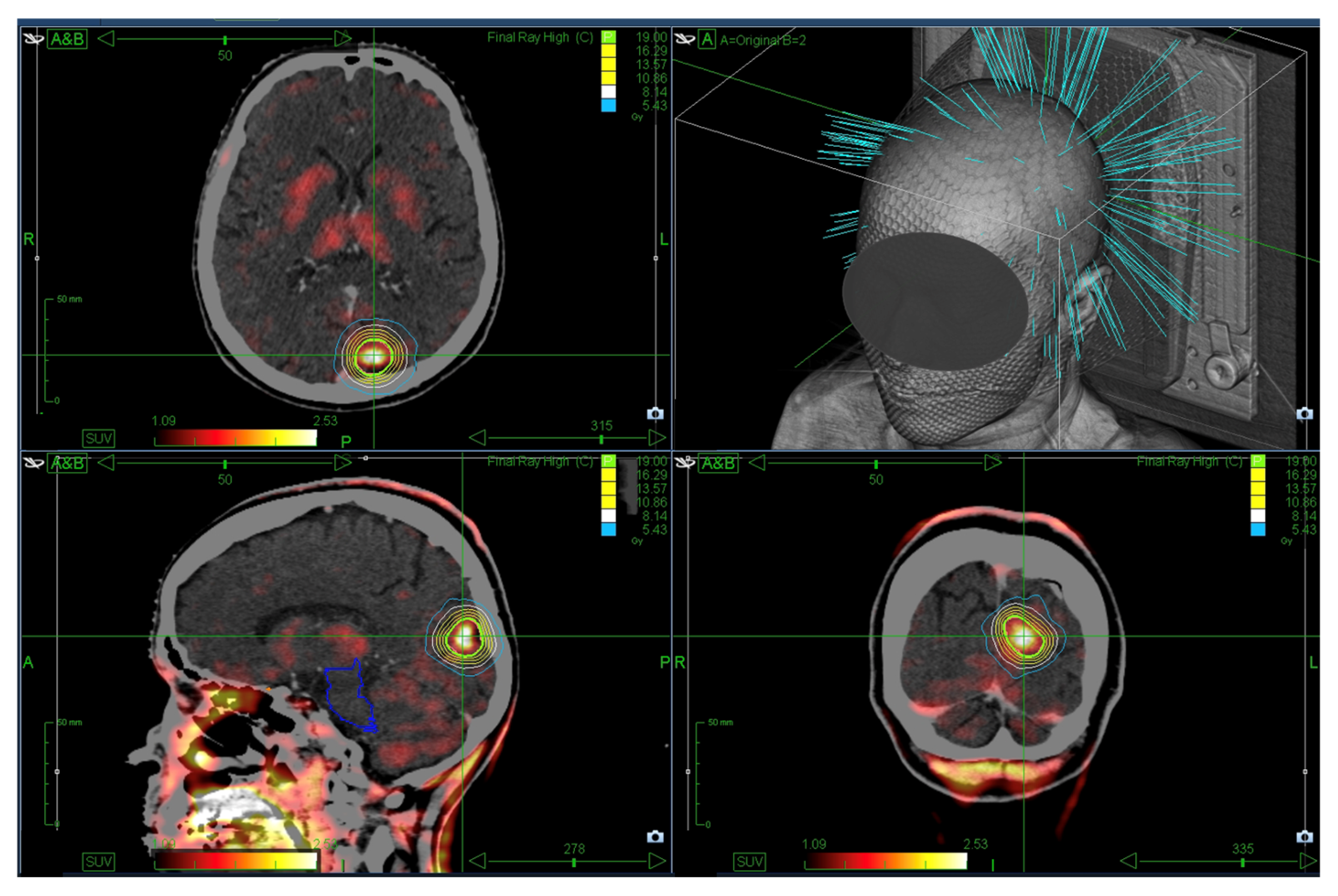
2.2. SRS Treatment
2.3. Data Collection
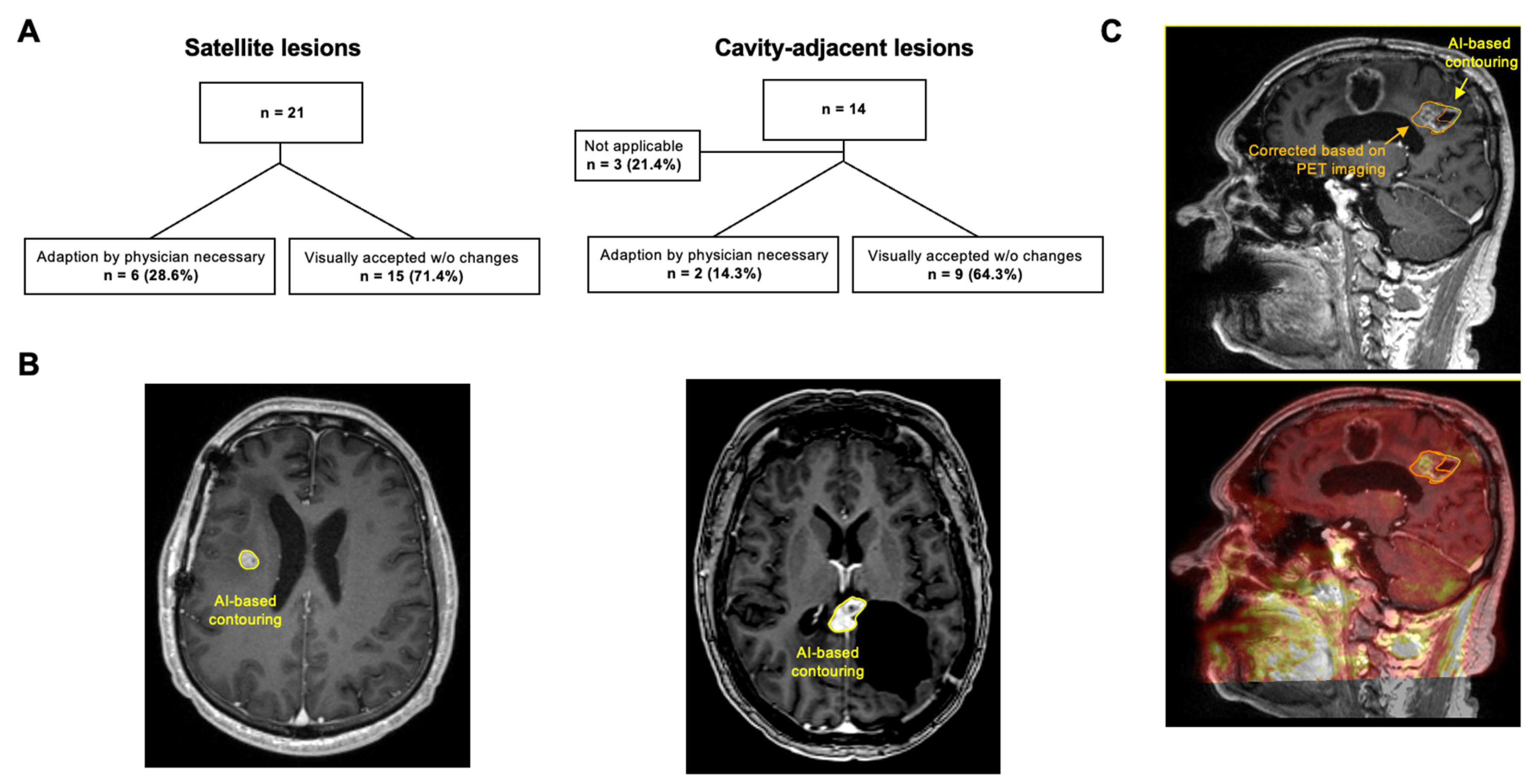
2.4. Simulation of AI-Based GTV Segmentation
2.5. Statistical Analysis
3. Results
3.1. Baseline and SRS Treatment Characteristics
3.2. Local Tumor Control and Clinical Outcome
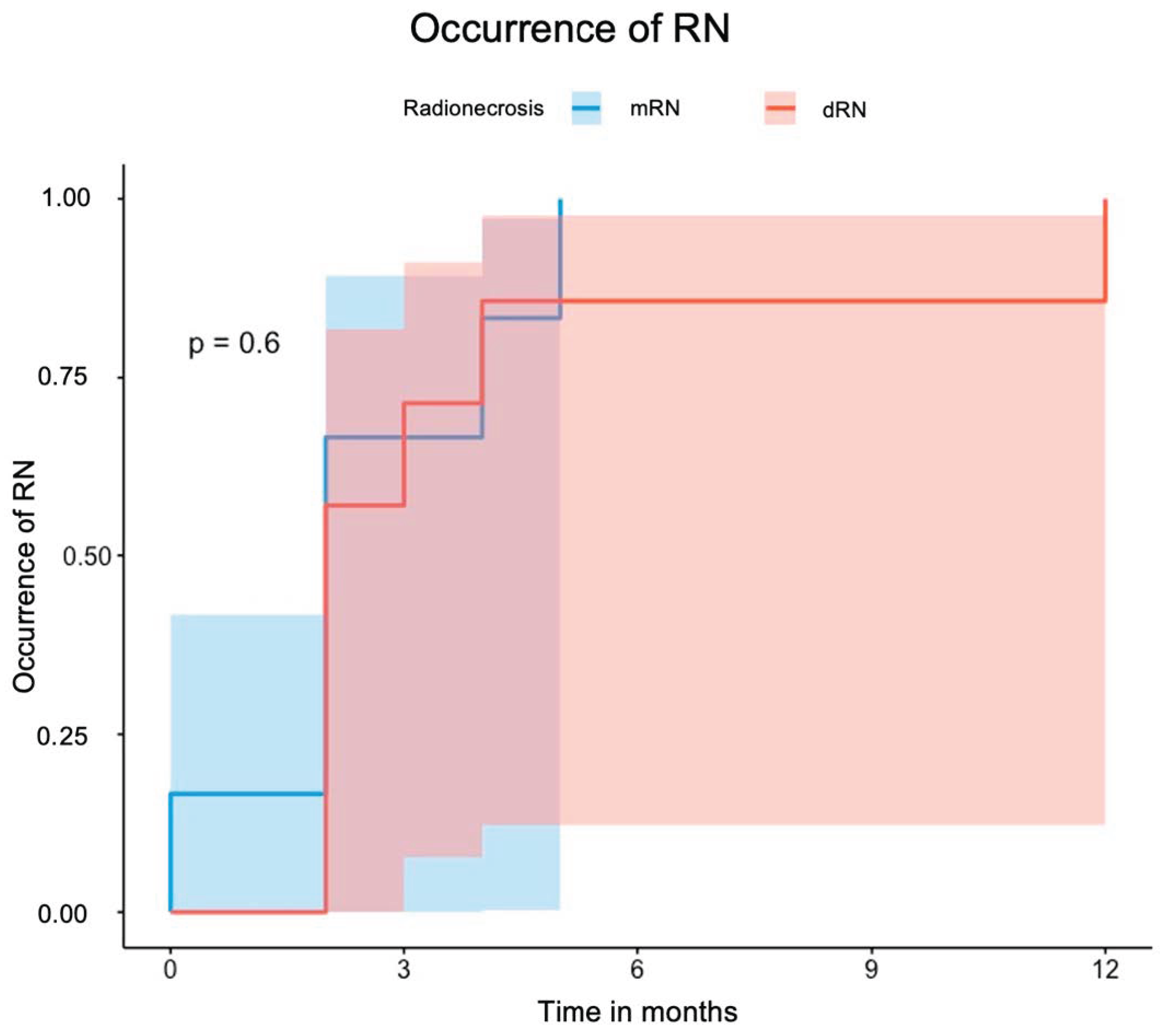
3.3. Radiation Necrosis (RN)
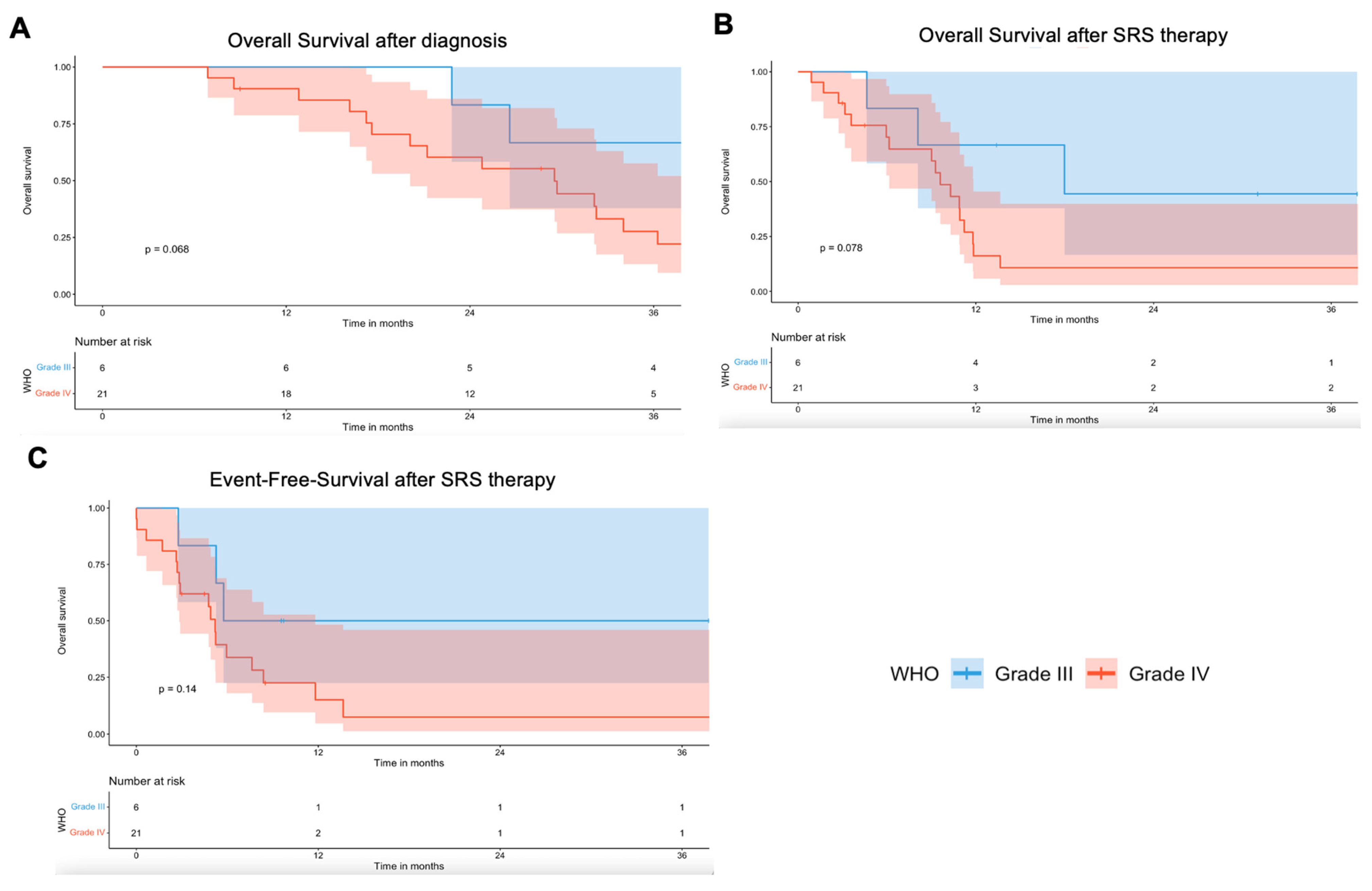
3.4. Overall and Local Event-Free-Survival
3.5. AI-Based GTV Segmentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| CR | Complete Response |
| CTCAE | Common Terminology Criteria for Adverse Events |
| dRN | Definite Radiation Necrosis |
| mRN | MRI-based Radiation Necrosis |
| FET | Fluorethyltyrosine |
| GTR | Gross Total Resection |
| GTV | Gross Total Volume |
| HGG | High Grade Glioma |
| IQR | Interquartile Range |
| KPS | Karnofsky Performance Score |
| PR | Partial Remission |
| PTV | Planning Target Volume |
| RCL | Resection Cavity-Adjacent Lesion |
| RN | Radiation Necrosis |
| SD | Stable Disease |
| SL | Satellite Lesion |
| STR | Subtotal Resection |
| TMZ | Temozolomide |
References
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22, iv1–iv96. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Wirsching, H.G.; Galanis, E.; Weller, M. Glioblastoma. Handb. Clin. Neurol. 2016, 134, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Price, S.J.; Gillard, J.H. Imaging biomarkers of brain tumour margin and tumour invasion. Br. J. Radiol. 2011, 84, S159–S167. [Google Scholar] [CrossRef] [PubMed]
- Bergman, D.; Modh, A.; Schultz, L.; Snyder, J.; Mikkelsen, T.; Shah, M.; Ryu, S.; Siddiqui, M.S.; Walbert, T. Randomized prospective trial of fractionated stereotactic radiosurgery with chemotherapy versus chemotherapy alone for bevacizumab-resistant high-grade glioma. J. Neuro-Oncol. 2020, 148, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Andratschke, N.; Heusel, A.; Albert, N.L.; Alongi, F.; Baumert, B.G.; Belka, C.; Castellano, A.; Dhermain, F.; Erridge, S.C.; Grosu, A.-L.; et al. ESTRO/EANO recommendation on reirradiation of glioblastoma. Radiother. Oncol. 2025, 204. [Google Scholar] [CrossRef]
- Kirkpatrick, J.P.; Sampson, J.H. Recurrent malignant gliomas. Semin. Radiat. Oncol. 2014, 24, 289–298. [Google Scholar] [CrossRef]
- Mallick, S.; Benson, R.; Hakim, A.; Rath, G.K. Management of glioblastoma after recurrence: A changing paradigm. J. Egypt. Natl. Cancer Inst. 2016, 28, 199–210. [Google Scholar] [CrossRef]
- Ehret, F.; Wolfgang, J.; Allwohn, L.; Onken, J.; Wasilewski, D.; Roohani, S.; Oertel, J.; Zips, D.; Kaul, D. Outcomes of Isocitrate Dehydrogenase Wild Type Glioblastoma after Re-irradiation. Clin. Transl. Radiat. Oncol. 2023, 42, 100653. [Google Scholar] [CrossRef]
- Straube, C.; Combs, S.E.; Bernhardt, D.; Gempt, J.; Meyer, B.; Zimmer, C.; Schmidt-Graf, F.; Vajkoczy, P.; Grün, A.; Ehret, F.; et al. Adjuvant re-irradiation vs. no early re-irradiation of resected recurrent glioblastoma: Pooled comparative cohort analysis from two tertiary centers. J. Neurooncol. 2024, 168, 49–56. [Google Scholar] [CrossRef]
- Muacevic, A.; Sahgal, A.; Tonn, J.-C. Spinal Robotic Radiosurgery. In Oncology of CNS Tumors; Springer: Berlin/Heidelberg, Germany, 2019; pp. 695–701. [Google Scholar]
- Bucholz, R.D.; Laycock, K.A.; Cuff, L.E. CyberKnife stereotactic radiosurgery for intracranial neoplasms, with a focus on malignant tumors. Technol. Cancer Res. Treat. 2010, 9, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.R., Jr.; Chang, S.D.; Murphy, M.J.; Doty, J.; Geis, P.; Hancock, S.L. The Cyberknife: A frameless robotic system for radiosurgery. Stereotact. Funct. Neurosurg. 1997, 69, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio, A.T.; Burneikiene, S.; Romanelli, P.; Fariselli, L.; McNeely, L.; Lipani, J.D.; Chang, S.D.; Nelson, E.L.; McIntyre, M.; Broggi, G.; et al. Survival following stereotactic radiosurgery for newly diagnosed and recurrent glioblastoma multiforme: A multicenter experience. Neurosurg. Rev. 2009, 32, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, V.; Biagioli, E.; Roberto, A.; Galli, F.; Rizzi, M.; Chiappa, F.; Brenna, G.; Fariselli, L.; Floriani, I. Radiosurgery for intracranial meningiomas: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2017, 113, 122–134. [Google Scholar] [CrossRef]
- Oermann, E.; Collins, B.T.; Erickson, K.T.; Yu, X.; Lei, S.; Suy, S.; Hanscom, H.N.; Kim, J.; Park, H.U.; Eldabh, A.; et al. CyberKnife enhanced conventionally fractionated chemoradiation for high grade glioma in close proximity to critical structures. J. Hematol. Oncol. 2010, 3, 22. [Google Scholar] [CrossRef]
- Lipani, J.D.; Jackson, P.S.; Soltys, S.G.; Sato, K.; Adler, J.R. Survival following CyberKnife radiosurgery and hypofractionated radiotherapy for newly diagnosed glioblastoma multiforme. Technol. Cancer Res. Treat. 2008, 7, 249–255. [Google Scholar] [CrossRef]
- Guan, Y.; Xiong, J.; Pan, M.; Shi, W.; Li, J.; Zhu, H.; Gong, X.; Li, C.; Mei, G.; Liu, X.; et al. Safety and efficacy of Hypofractionated stereotactic radiosurgery for high-grade Gliomas at first recurrence: A single-center experience. BMC Cancer 2021, 21, 123. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Saito, K.; Kajiwara, K.; Nomura, S.; Ishihara, H.; Suzuki, M. CyberKnife stereotactic radiotherapy for patients with malignant glioma. Minim. Invasive Neurosurg. 2006, 49, 110–115. [Google Scholar] [CrossRef]
- Madhugiri, V.S.; Prasad, D. Early experience with an artificial intelligence-based module for brain metastasis detection and segmentation. J. Neuro-Oncol. 2025, 171, 365–372. [Google Scholar] [CrossRef]
- Albert, N.L.; Galldiks, N.; Ellingson, B.M.; van den Bent, M.J.; Chang, S.M.; Cicone, F.; de Groot, J.; Koh, E.-S.; Law, I.; Le Rhun, E. PET-based response assessment criteria for diffuse gliomas (PET RANO 1.0): A report of the RANO group. Lancet Oncol. 2024, 25, e29–e41. [Google Scholar] [CrossRef]
- Holzgreve, A.; Nitschmann, A.; Maier, S.H.; Büttner, M.; Schönecker, S.; Marschner, S.N.; Fleischmann, D.F.; Corradini, S.; Belka, C.; la Fougere, C. FET PET-based target volume delineation for the radiotherapy of glioblastoma: A pictorial guide to help overcome methodological pitfalls. Radiother. Oncol. 2024, 198, 110386. [Google Scholar] [CrossRef]
- Trone, J.C.; Vallard, A.; Sotton, S.; Ben Mrad, M.; Jmour, O.; Magné, N.; Pommier, B.; Laporte, S.; Ollier, E. Survival after hypofractionation in glioblastoma: A systematic review and meta-analysis. Radiat. Oncol. 2020, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Acker, G.; Kluge, A.; Lukas, M.; Conti, A.; Pasemann, D.; Meinert, F.; Anh Nguyen, P.T.; Jelgersma, C.; Loebel, F.; Budach, V.; et al. Impact of 68Ga-DOTATOC PET/MRI on robotic radiosurgery treatment planning in meningioma patients: First experiences in a single institution. Neurosurg. Focus 2019, 46, E9. [Google Scholar] [CrossRef] [PubMed]
- Oehlke, O.; Mix, M.; Graf, E.; Schimek-Jasch, T.; Nestle, U.; Götz, I.; Schneider-Fuchs, S.; Weyerbrock, A.; Mader, I.; Baumert, B.G. Amino-acid PET versus MRI guided re-irradiation in patients with recurrent glioblastoma multiforme (GLIAA)–protocol of a randomized phase II trial (NOA 10/ARO 2013-1). BMC Cancer 2016, 16, 769. [Google Scholar] [CrossRef] [PubMed]
- Grosu, A.L.; Weber, W.A.; Franz, M.; Stärk, S.; Piert, M.; Thamm, R.; Gumprecht, H.; Schwaiger, M.; Molls, M.; Nieder, C. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. J. Radiat. Oncol. Biol. Phys. 2005, 63, 511–519. [Google Scholar] [CrossRef]
- Preusser, M.; Kazda, T.; Le Rhun, E.; Sahm, F.; Smits, M.; Gempt, J.; Koekkoek, J.A.; Monti, A.F.; Csanadi, M.; Pitter, J.G. Lomustine with or without reirradiation for first progression of glioblastoma, LEGATO, EORTC-2227-BTG: Study protocol for a randomized phase III study. Trials 2024, 25, 366. [Google Scholar] [CrossRef]
- Lévy, S.; Chapet, S.; Scher, N.; Debbi, K.; Ruffier, A.; Bernadou, G.; Pointreau, Y.; Calais, G. Reirradiation of gliomas under stereotactic conditions: Prognostic factors for survival without relapse or side effects, a retrospective study at Tours regional university hospital (France). Cancer Radiother. 2017, 21, 759–765. [Google Scholar] [CrossRef]
- Adachi, K.; Hayashi, K.; Kagawa, N.; Kinoshita, M.; Sumida, I.; Akino, Y.; Shiomi, H.; Tamari, K.; Suzuki, O.; Hirayama, R.; et al. Feasibility of Salvage Re-irradiation With Stereotactic Radiotherapy for Recurrent Glioma Using CyberKnife. Anticancer Res. 2019, 39, 2935–2940. [Google Scholar] [CrossRef]
- Dhawan, S.; Patil, C.G.; Chen, C.; Venteicher, A.S. Early versus delayed postoperative radiotherapy for treatment of low-grade gliomas. Cochrane Database Syst. Rev. 2020, 1, Cd009229. [Google Scholar] [CrossRef]
- Khan, L.; Soliman, H.; Sahgal, A.; Perry, J.; Xu, W.; Tsao, M.N. External beam radiation dose escalation for high grade glioma. Cochrane Database Syst. Rev. 2020, 5, Cd011475. [Google Scholar] [CrossRef]
- Azoulay, M.; Chang, S.D.; Gibbs, I.C.; Hancock, S.L.; Pollom, E.L.; Harsh, G.R.; Adler, J.R.; Harraher, C.; Li, G.; Hayden Gephart, M.; et al. A phase I/II trial of 5-fraction stereotactic radiosurgery with 5-mm margins with concurrent temozolomide in newly diagnosed glioblastoma: Primary outcomes. Neuro-Oncology 2020, 22, 1182–1189. [Google Scholar] [CrossRef]
- Brehmer, S.; Grimm, M.A.; Förster, A.; Seiz-Rosenhagen, M.; Welzel, G.; Stieler, F.; Wenz, F.; Groden, C.; Mai, S.; Hänggi, D.; et al. Study Protocol: Early Stereotactic Gamma Knife Radiosurgery to Residual Tumor After Surgery of Newly Diagnosed Glioblastoma (Gamma-GBM). Neurosurgery 2019, 84, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Q.; Yuan, Z.; Zhao, L.; Wang, X.; Wang, P. Clinical Efficacy of CyberKnife Radiosurgery for Adult Brainstem Glioma: 10 Years Experience at Tianjin CyberKnife Center and Review of the Literature. Front Oncol 2019, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Navarria, P.; Pessina, F.; Cozzi, L.; Tomatis, S.; Reggiori, G.; Simonelli, M.; Santoro, A.; Clerici, E.; Franzese, C.; Carta, G.; et al. Phase II study of hypofractionated radiation therapy in elderly patients with newly diagnosed glioblastoma with poor prognosis. Tumori 2019, 105, 47–54. [Google Scholar] [CrossRef]
- Song, A.; Andrews, D.W.; Werner-Wasik, M.; Kim, L.; Glass, J.; Bar-Ad, V.; Evans, J.J.; Farrell, C.J.; Judy, K.D.; Daskalakis, C.; et al. Phase I trial of alisertib with concurrent fractionated stereotactic re-irradiation for recurrent high grade gliomas. Radiother. Oncol. 2019, 132, 135–141. [Google Scholar] [CrossRef]
- Hu, Y.J.; Chen, D.; Zhang, L.F.; Chen, J. Efficacy and Safety of Hypofractionated Stereotactic Radiotherapy for Recurrent Malignant Gliomas: A Systematic Review and Meta-analysis. World Neurosurg. 2019, 127, 176–185. [Google Scholar] [CrossRef]
- Abbassy, M.; Missios, S.; Barnett, G.H.; Brewer, C.; Peereboom, D.M.; Ahluwalia, M.; Neyman, G.; Chao, S.T.; Suh, J.H.; Vogelbaum, M.A. Phase I Trial of Radiosurgery Dose Escalation Plus Bevacizumab in Patients With Recurrent/Progressive Glioblastoma. Neurosurgery 2018, 83, 385–392. [Google Scholar] [CrossRef]
- Shi, W.; Blomain, E.S.; Siglin, J.; Palmer, J.D.; Dan, T.; Wang, Y.; Werner-Wasik, M.; Glass, J.; Kim, L.; Bar Ad, V.; et al. Salvage fractionated stereotactic re-irradiation (FSRT) for patients with recurrent high grade gliomas progressed after bevacizumab treatment. J. Neurooncol. 2018, 137, 171–177. [Google Scholar] [CrossRef]
- Repka, M.C.; Lei, S.; Campbell, L.; Suy, S.; Voyadzis, J.M.; Kalhorn, C.; McGrail, K.; Jean, W.; Subramaniam, D.S.; Lischalk, J.W.; et al. Long-Term Outcomes Following Conventionally Fractionated Stereotactic Boost for High-Grade Gliomas in Close Proximity to Critical Organs at Risk. Front. Oncol. 2018, 8, 373. [Google Scholar] [CrossRef]
- Romanelli, P.; Paiano, M.; Crocamo, V.; Beltramo, G.; Bergantin, A.; Pantelis, E.; Antypas, C.; Clerico, A. Staged Image-guided Robotic Radiosurgery and Deferred Chemotherapy to Treat a Malignant Glioma During and After Pregnancy. Cureus 2018, 10, e2141. [Google Scholar] [CrossRef]
- Pinzi, V.; Orsi, C.; Marchetti, M.; Milanesi, I.M.; Bianchi, L.C.; DiMeco, F.; Cuccarini, V.; Farinotti, M.; Ferroli, P.; Finocchiaro, G.; et al. Radiosurgery reirradiation for high-grade glioma recurrence: A retrospective analysis. Neurol. Sci. 2015, 36, 1431–1440. [Google Scholar] [CrossRef]
- Kawano, H.; Hirano, H.; Yonezawa, H.; Yunoue, S.; Yatsushiro, K.; Ogita, M.; Hiraki, Y.; Uchida, H.; Habu, M.; Fujio, S.; et al. Improvement in treatment results of glioblastoma over the last three decades and beneficial factors. Br. J. Neurosurg. 2015, 29, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; Beal, K.; Gutin, P.; Karimi, S.; Correa, D.D.; Kaley, T.J.; DeAngelis, L.M.; Chan, T.A.; Gavrilovic, I.T.; Nolan, C.; et al. Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin. Cancer Res. 2014, 20, 5023–5031. [Google Scholar] [CrossRef] [PubMed]
- Dodoo, E.; Huffmann, B.; Peredo, I.; Grinaker, H.; Sinclair, G.; Machinis, T.; Enger, P.O.; Skeie, B.S.; Pedersen, P.H.; Ohlsson, M.; et al. Increased survival using delayed gamma knife radiosurgery for recurrent high-grade glioma: A feasibility study. World Neurosurg. 2014, 82, e623–e632. [Google Scholar] [CrossRef] [PubMed]
- Greto, D.; Livi, L.; Bonomo, P.; Masi, L.; Detti, B.; Meattini, I.; Mangoni, M.; Doro, R.; Favuzza, V.; Cipressi, S.; et al. Cyberknife stereotactic radiosurgery for the re-irradiation of brain lesions: A single-centre experience. Radiol. Med. 2014, 119, 721–726. [Google Scholar] [CrossRef]
- Yazici, G.; Cengiz, M.; Ozyigit, G.; Eren, G.; Yildiz, F.; Akyol, F.; Gurkaynak, M.; Zorlu, F. Hypofractionated stereotactic reirradiation for recurrent glioblastoma. J. Neurooncol. 2014, 120, 117–123. [Google Scholar] [CrossRef]
- Uslu, N.; Karakaya, E.; Dizman, A.; Yegen, D.; Guney, Y. Optic nerve glioma treatment with fractionated stereotactic radiotherapy. J. Neurosurg. Pediatr. 2013, 11, 596–599. [Google Scholar] [CrossRef]
- McKenzie, J.T.; Guarnaschelli, J.N.; Vagal, A.S.; Warnick, R.E.; Breneman, J.C. Hypofractionated stereotactic radiotherapy for unifocal and multifocal recurrence of malignant gliomas. J. Neurooncol. 2013, 113, 403–409. [Google Scholar] [CrossRef]
- Giller, C.A.; Berger, B.D.; Fink, K.; Bastian, E. A volumetric study of CyberKnife hypofractionated stereotactic radiotherapy as salvage for progressive malignant brain tumors: Initial experience. Neurol. Res. 2007, 29, 563–568. [Google Scholar] [CrossRef]
- Einstein, D.B.; Wessels, B.; Bangert, B.; Fu, P.; Nelson, A.D.; Cohen, M.; Sagar, S.; Lewin, J.; Sloan, A.; Zheng, Y.; et al. Phase II trial of radiosurgery to magnetic resonance spectroscopy-defined high-risk tumor volumes in patients with glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 668–674. [Google Scholar] [CrossRef]
- Conti, A.; Pontoriero, A.; Arpa, D.; Siragusa, C.; Tomasello, C.; Romanelli, P.; Cardali, S.; Granata, F.; De Renzis, C.; Tomasello, F. Efficacy and toxicity of CyberKnife re-irradiation and “dose dense” temozolomide for recurrent gliomas. Acta Neurochir. 2012, 154, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Malmström, A.; Grønberg, B.H.; Marosi, C.; Stupp, R.; Frappaz, D.; Schultz, H.; Abacioglu, U.; Tavelin, B.; Lhermitte, B.; Hegi, M.E.; et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012, 13, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Xu, L.; Xu, M.; Geng, M.; Tan, Y.; Li, F. 1H-MR spectroscopy guided gamma knife radiosurgery for treatment of glioma. Turk. Neurosurg. 2012, 22, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Canazza, A.; Calatozzolo, C.; Fumagalli, L.; Bergantin, A.; Ghielmetti, F.; Fariselli, L.; Croci, D.; Salmaggi, A.; Ciusani, E. Increased migration of a human glioma cell line after in vitro CyberKnife irradiation. Cancer Biol. Ther. 2011, 12, 629–633. [Google Scholar] [CrossRef]
- Maranzano, E.; Anselmo, P.; Casale, M.; Trippa, F.; Carletti, S.; Principi, M.; Loreti, F.; Italiani, M.; Caserta, C.; Giorgi, C. Treatment of recurrent glioblastoma with stereotactic radiotherapy: Long-term results of a mono-institutional trial. Tumori 2011, 97, 56–61. [Google Scholar] [CrossRef]
- Canazza, A.; De Grazia, U.; Fumagalli, L.; Brait, L.; Ghielmetti, F.; Fariselli, L.; Croci, D.; Salmaggi, A.; Ciusani, E. In vitro effects of Cyberknife-driven intermittent irradiation on glioblastoma cell lines. Neurol. Sci. 2011, 32, 579–588. [Google Scholar] [CrossRef]
- Balducci, M.; Apicella, G.; Manfrida, S.; Mangiola, A.; Fiorentino, A.; Azario, L.; D’Agostino, G.R.; Frascino, V.; Dinapoli, N.; Mantini, G.; et al. Single-arm phase II study of conformal radiation therapy and temozolomide plus fractionated stereotactic conformal boost in high-grade gliomas: Final report. Strahlenther. Onkol. 2010, 186, 558–564. [Google Scholar] [CrossRef]
- Fogh, S.E.; Andrews, D.W.; Glass, J.; Curran, W.; Glass, C.; Champ, C.; Evans, J.J.; Hyslop, T.; Pequignot, E.; Downes, B.; et al. Hypofractionated stereotactic radiation therapy: An effective therapy for recurrent high-grade gliomas. J. Clin. Oncol. 2010, 28, 3048–3053. [Google Scholar] [CrossRef]
- Wentworth, S.; Pinn, M.; Bourland, J.D.; Deguzman, A.F.; Ekstrand, K.; Ellis, T.L.; Glazier, S.S.; McMullen, K.P.; Munley, M.; Stieber, V.W.; et al. Clinical experience with radiation therapy in the management of neurofibromatosis-associated central nervous system tumors. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 208–213. [Google Scholar] [CrossRef]
- Schwer, A.L.; Kavanagh, B.D.; McCammon, R.; Gaspar, L.E.; Kleinschmidt-De Masters, B.K.; Stuhr, K.; Chen, C. Radiographic and histopathologic observations after combined EGFR inhibition and hypofractionated stereotactic radiosurgery in patients with recurrent malignant gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1352–1357. [Google Scholar] [CrossRef]
- Schwer, A.L.; Damek, D.M.; Kavanagh, B.D.; Gaspar, L.E.; Lillehei, K.; Stuhr, K.; Chen, C. A phase I dose-escalation study of fractionated stereotactic radiosurgery in combination with gefitinib in patients with recurrent malignant gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 993–1001. [Google Scholar] [CrossRef]
- Smith, K.A.; Ashby, L.S.; Gonzalez, L.F.; Brachman, D.G.; Thomas, T.; Coons, S.W.; Battaglia, M.; Scheck, A. Prospective trial of gross-total resection with Gliadel wafers followed by early postoperative Gamma Knife radiosurgery and conformal fractionated radiotherapy as the initial treatment for patients with radiographically suspected, newly diagnosed glioblastoma multiforme. J. Neurosurg. 2008, 109, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.S.; Lee, J.I.; Park, K.; Kim, J.H.; Lim, D.H.; Nam, D.H. Efficacy of stereotactic radiosurgery as a salvage treatment for recurrent malignant gliomas. Cancer 2008, 112, 2046–2051. [Google Scholar] [CrossRef] [PubMed]
- Ashamalla, H.; Zaki, B.; Mokhtar, B.; Lewis, L.; Lavaf, A.; Nasr, H.; Colella, F.; Dosik, D.; Krishnamurthy, M.; Saad, N.; et al. Fractionated stereotactic radiotherapy boost and weekly paclitaxel in malignant gliomas clinical and pharmacokinetics results. Technol. Cancer Res. Treat. 2007, 6, 169–176. [Google Scholar] [CrossRef]
- Kohshi, K.; Yamamoto, H.; Nakahara, A.; Katoh, T.; Takagi, M. Fractionated stereotactic radiotherapy using gamma unit after hyperbaric oxygenation on recurrent high-grade gliomas. J. Neurooncol. 2007, 82, 297–303. [Google Scholar] [CrossRef]
- Cardinale, R.; Won, M.; Choucair, A.; Gillin, M.; Chakravarti, A.; Schultz, C.; Souhami, L.; Chen, A.; Pham, H.; Mehta, M. A phase II trial of accelerated radiotherapy using weekly stereotactic conformal boost for supratentorial glioblastoma multiforme: RTOG 0023. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1422–1428. [Google Scholar] [CrossRef]
- Ernst-Stecken, A.; Ganslandt, O.; Lambrecht, U.; Sauer, R.; Grabenbauer, G. Survival and quality of life after hypofractionated stereotactic radiotherapy for recurrent malignant glioma. J. Neurooncol. 2007, 81, 287–294. [Google Scholar] [CrossRef]
- Combs, S.E.; Gutwein, S.; Thilmann, C.; Debus, J.; Schulz-Ertner, D. Reirradiation of recurrent WHO grade III astrocytomas using fractionated stereotactic radiotherapy (FSRT). Strahlenther. Onkol. 2005, 181, 768–773. [Google Scholar] [CrossRef]
- Ulm, A.J., 3rd; Friedman, W.A.; Bradshaw, P.; Foote, K.D.; Bova, F.J. Radiosurgery in the treatment of malignant gliomas: The University of Florida experience. Neurosurgery 2005, 57, 512–517. [Google Scholar] [CrossRef]
- Cho, K.H.; Hall, W.A.; Lo, S.S.; Dusenbery, K.E. Stereotactic radiosurgery versus fractionated stereotactic radiotherapy boost for patients with glioblastoma multiforme. Technol. Cancer Res. Treat. 2004, 3, 41–49. [Google Scholar] [CrossRef]
- Sato, K.; Baba, Y.; Inoue, M.; Omori, R. Radiation necrosis and brain edema association with CyberKnife treatment. Acta Neurochir. Suppl. 2003, 86, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.A.; Prados, M.; Lamborn, K.R.; Smith, V.; Sneed, P.K.; Chang, S.; Nicholas, K.M.; Wara, W.M.; Devriendt, D.; Kunwar, S.; et al. Phase II study of high central dose Gamma Knife radiosurgery and marimastat in patients with recurrent malignant glioma. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Boëthius, J.; Ulfarsson, E.; Rähn, T.; Lippittz, B. Gamma knife radiosurgery for pilocytic astrocytomas. J. Neurosurg. 2002, 97, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Regine, W.F.; Patchell, R.A.; Strottmann, J.M.; Meigooni, A.; Sanders, M.; Young, A.B. Preliminary report of a phase I study of combined fractionated stereotactic radiosurgery and conventional external beam radiation therapy for unfavorable gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 421–426. [Google Scholar] [CrossRef]
- Regine, W.F.; Patchell, R.A.; Strottmann, J.M.; Meigooni, A.; Sanders, M.; Young, B. Combined stereotactic split-course fractionated gamma knife radiosurgery and conventional radiation therapy for unfavorable gliomas: A phase I study. J. Neurosurg. 2000, 93 (Suppl. 3), 37–41. [Google Scholar] [CrossRef]
- Cardinale, R.M.; Schmidt-Ullrich, R.K.; Benedict, S.H.; Zwicker, R.D.; Han, D.C.; Broaddus, W.C. Accelerated radiotherapy regimen for malignant gliomas using stereotactic concomitant boosts for dose escalation. Radiat. Oncol. Investig. 1998, 6, 175–181. [Google Scholar] [CrossRef]
- Shenouda, G.; Souhami, L.; Podgorsak, E.B.; Bahary, J.P.; Villemure, J.G.; Caron, J.L.; Mohr, G. Radiosurgery and accelerated radiotherapy for patients with glioblastoma. Can. J. Neurol. Sci. 1997, 24, 110–115. [Google Scholar] [CrossRef]
- Hirato, M.; Nakamura, M.; Inoue, H.K.; Ohye, C.; Hirato, J.; Shibazaki, T.; Andou, Y. Gamma Knife radiosurgery for the treatment of brainstem tumors. Stereotact. Funct. Neurosurg. 1995, 64 (Suppl. 1), 32–41. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Barba, D.; Kormanik, P.; Shea, W.M. Stereotactic radiosurgery for recurrent gliomas. Cancer 1994, 74, 1342–1347. [Google Scholar] [CrossRef]
- Mehta, M.P.; Masciopinto, J.; Rozental, J.; Levin, A.; Chappell, R.; Bastin, K.; Miles, J.; Turski, P.; Kubsad, S.; Mackie, T.; et al. Stereotactic radiosurgery for glioblastoma multiforme: Report of a prospective study evaluating prognostic factors and analyzing long-term survival advantage. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 541–549. [Google Scholar] [CrossRef]
- Curran, W.J., Jr.; Scott, C.B.; Weinstein, A.S.; Martin, L.A.; Nelson, J.S.; Phillips, T.L.; Murray, K.; Fischbach, A.J.; Yakar, D.; Schwade, J.G.; et al. Survival comparison of radiosurgery-eligible and -ineligible malignant glioma patients treated with hyperfractionated radiation therapy and carmustine: A report of Radiation Therapy Oncology Group 83-02. J. Clin. Oncol. 1993, 11, 857–862. [Google Scholar] [CrossRef]
- Ali, F.S.; Arevalo, O.; Zorofchian, S.; Patrizz, A.; Riascos, R.; Tandon, N.; Blanco, A.; Ballester, L.Y.; Esquenazi, Y. Cerebral Radiation Necrosis: Incidence, Pathogenesis, Diagnostic Challenges, and Future Opportunities. Curr. Oncol. Rep. 2019, 21, 66. [Google Scholar] [CrossRef]
- Vordermark, D.; Kölbl, O.; Ruprecht, K.; Vince, G.H.; Bratengeier, K.; Flentje, M. Hypofractionated stereotactic re-irradiation: Treatment option in recurrent malignant glioma. BMC Cancer 2005, 5, 55. [Google Scholar] [CrossRef]
- Kossmann, M.R.P.; Ehret, F.; Roohani, S.; Winter, S.F.; Ghadjar, P.; Acker, G.; Senger, C.; Schmid, S.; Zips, D.; Kaul, D. Histopathologically confirmed radiation-induced damage of the brain—An in-depth analysis of radiation parameters and spatio-temporal occurrence. Radiat. Oncol. 2023, 18, 198. [Google Scholar] [CrossRef]
- van de Weijer, T.; Broen, M.P.G.; Moonen, R.P.M.; Hoeben, A.; Anten, M.; Hovinga, K.; Compter, I.; van der Pol, J.A.J.; Mitea, C.; Lodewick, T.M.; et al. The Use of (18)F-FET-PET-MRI in Neuro-Oncology: The Best of Both Worlds—A Narrative Review. Diagnostics 2022, 12, 1202. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, G.; Alshamy, G.; Chan, B.; Abrams, R.; Greenberg, E.; Saxena, A.; Bradbury, M.; Edgar, M.; Gutin, P.; Tabar, V. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS ONE 2007, 2, e588. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, D.; König, L.; Grosu, A.; Wiestler, B.; Rieken, S.; Wick, W.; Gempt, J.; Krieg, S.M.; Schmidt-Graf, F.; Sahm, F.; et al. DEGRO practical guideline for central nervous system radiation necrosis part 1: Classification and a multistep approach for diagnosis. Strahlenther. Onkol. 2022, 198, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, T.; Hatano, K.; Imagunbai, T.; Kodama, T.; Uchino, Y.; Tohyama, N.; Sakaida, T.; Kawasaki, K.; Hasegawa, Y. Para-ventricular radiation necrosis after radiation therapy for malignant astrocytomas. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, S274. [Google Scholar] [CrossRef]
- Chen, A.T.C.; Serante, A.R.; Ayres, A.S.; Tonaki, J.O.; Moreno, R.A.; Shih, H.; Gattás, G.S.; Lopez, R.V.M.; dos Santos de Jesus, G.R.; de Carvalho, I.T.; et al. Prospective Randomized Phase 2 Trial of Hypofractionated Stereotactic Radiation Therapy of 25 Gy in 5 Fractions Compared With 35 Gy in 5 Fractions in the Reirradiation of Recurrent Glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 1122–1132. [Google Scholar] [CrossRef]


| Patients (n = 27) | |
|---|---|
| Age, Median (IQR) | 57 (49.5–65) |
| Gender, n (%) | |
| Female | 11 (40.7) |
| Male | 16 (59.3) |
| Leading Symptom, n (%) | |
| Epilepsy of any kind | 11 (41) |
| Frontal deficiencies | 2 (7.4) |
| Headache | 2 (7.4) |
| Motoric deficiencies | 1 (3.7) |
| Sensory deficiencies | 2 (7.4) |
| Speech deficiencies | 3 (11) |
| Other | 6 (22) |
| Prior Surgeries, n (%) | |
| 1 | 14 (51.9) |
| 2 | 10 (37.0) |
| 3 | 3 (11.1) |
| KPS Score (%), n (%) | |
| 60 | 3 (11.1) |
| 70 | 6 (22.2) |
| 80 | 5 (18.5) |
| 90 | 9 (33.3) |
| 100 | 4 (14.8) |
| Amount of lesions, n (%) | |
| 1 | 17 (63.0) |
| 2 | 6 (22.2) |
| 3 | 4 (14.8) |
| Lesions (n = 41) | ||
|---|---|---|
| Diagnosis (WHO 2016), n (%) | ||
| Anaplastic Astrocytoma | 4 (9.8) | |
| Anaplastic Xanthoastrocytoma | 2 (4.9) | |
| Anaplastic Oligoastrocytoma | 2 (4.9) | |
| Glioblastoma | 33 (80) | |
| WHO grade, n (%) | ||
| III | 8 (19.5) | |
| IV | 33 (80.5) | |
| Localization, n (%) | ||
| Basal ganglia | 1 (2.4) | |
| Corpus callosum | 2 (4.9) | |
| Frontal | 15 (37) | |
| Frontoparietal | 1 (2.4) | |
| Frontotemporal | 1 (2.4) | |
| Hippocampal | 2 (4.9) | |
| Lateral ventricle | 3 (7.3) | |
| Occipital | 5 (12) | |
| Parietal | 3 (7.3) | |
| Parietooccipital | 1 (2.4) | |
| Precentral | 1 (2.4) | |
| Temporal | 6 (15) | |
| Periventricular location, n (%) | 11 (26.8) | |
| Located within previous irradiated field, n (%) | ||
| Marginal area | 10 (24) | |
| No | 3 (7.3) | |
| Yes | 28 (68 | |
| Status | ||
| WHO grade III lesions (n = 33) | All lesions (n = 41) | |
| IDH mutated, n (%) | 3 (9.1) | 8 (19.5) |
| MGMT methylated, n (%) | 20 (60.6) | 21 (55.3) |
| Unknown | 0 | 3 |
| TP53 mutation, n (%) | 9 (40.1) | 13 (39) |
| Unknown | 7 | 8 |
| ATRX loss, n (%) | 3 (9.1) | 5 (14.7) |
| Unknown | 5 | 7 |
| Lesions (n = 41) | |
|---|---|
| Treatment prior SRS therapy | |
| Number of surgeries prior to SRS, n (%) | 1: 23 (56.1); 2: 13 (31.7); 3: 5 (12.2) |
| Extent of resection of first surgery (n = 41), n (%) | Biopsy: 2 (4.9); STR: 21 (51.2); GTR: 18 (43.9) |
| Extent of resection of second surgery (n = 18), n (%) | STR: 12 (29.3); GTR: 6 (14.6) |
| Extent of resection of third surgery (n = 5), n (%) | STR: 1 (2.4); GTR: 4 (9.8) |
| Time from last surgery to SRS, months, median [IQR] | 12 [7–24] |
| Number of radiations of the patient prior to SRS, n (%) | 1: 35 (87.8); 2: 5 (12.2) |
| Dose of first radiation, Gy, median [IQR] | 60 [40.5–66] |
| Dose of second radiation, Gy, median [IQR] | 50.4 [49.4–60] |
| Time from last radiation to SRS, months, median [IQR] | 12 [5–24] |
| Other therapies prior to SRS, n (%) | |
| TMZ | 41 (100) |
| Metronomic TMZ | 13 (31.7) |
| Lomustine | 9 (22.0) |
| Bevacizumab | 0 (0.0) |
| NovoTFF | 7 (16.0) |
| SRS treatment | |
| Single Session SRS, n (%) | 31 (75.6) |
| Prescription dose, Gy, median [IQR] | 21 [20–21] |
| Isodose line, %, median [IQR] | 70 [70] |
| Mean dose, Gy, median [IQR] | 25.4 [24.3–25.8] |
| Minimum dose, Gy, median [IQR] | 19.6 [18.9–20.4] |
| Maximum dose, Gy, median [IQR] | 30.0 [28.6–30.0] |
| Gross tumor volume, cm3, median [IQR] | 0.68 [0.39–1.26] |
| Planning target volume, cm3, median [IQR] | 1.19 [0.92–1.70] |
| Coverage, %, median [IQR] | 99.2 [98.6–99.7] |
| Hypofractionation, n (%) | 10 (24.4) |
| 3 Fractions, n (%) | 9 (22.0) |
| Prescription dose, Gy, median [IQR] | 24 [24–27] |
| Mean dose, Gy, median [IQR] | 29.6 [29.2–32.8] |
| Minimum dose, Gy, median [IQR] | 23.1 [22.2–26.1] |
| Maximum dose, Gy, median [IQR] | 34.3 [34.3–38.6] |
| Gross tumor volume, cm3, median [IQR] | 4.48 [3.16–7.10] |
| Planning target volume, cm3, median [IQR] | 7.40 [5.77–11.00] |
| Coverage, %, median [IQR] | 99.0 [98.3–99.8] |
| 5 Fractions, n (%) | 1 (2.4) |
| Prescription dose, Gy | 25 |
| Isodose line, % | 70 |
| Mean dose, Gy | 29.5 |
| Minimum dose, Gy | 16.8 |
| Maximum dose, Gy | 35.7 |
| Gross tumor volume, cm3 | 4.01 |
| Planning target volume, cm3 | 19.67 |
| Coverage, % | 99.9 |
| Chemotherapy during SRS, n (%) | |
| No additional chemotherapy | 29 (70.7) |
| Metronomic TMZ | 8 (29.6) |
| Lomustine | 1 (2.4) |
| Abemaciclib | 1 (2.4) |
| Treatment post SRS therapy, n (%) | |
| Re-Surgery | 8 (19.5) |
| Re-Irradiation | 6 (14.6) |
| TMZ | 5 (12.2) |
| Metronomic TMZ | 17 (41.5) |
| PC | 5 (12.2) |
| Lomustine | 12 (29.3) |
| Bevacizumab | 5 (12.2) |
| NovoTFF | 1 (2.4) |
| HR (95% CI) | p-Value | |
|---|---|---|
| Risk for the occurrence of RN (univariate) | ||
| Male gender | 0.57 (0.14–2.21) | 0.40 |
| Age | 0.95 (0.89–1.02) | 0.20 |
| WHO grade IV | 0.83 (0.17–4.72) | 0.80 |
| IDH mutated | 0.58 (0.08–3.02) | 0.50 |
| MGMT methylated | 2.07 (0.49–9.72) | 0.30 |
| TP53 | 0.86 (0.18–3.87) | 0.80 |
| Periventricular location | 6.13 (1.40–31.1) | 0.02 |
| Time from last RT to SRS | 0.98 (0.94–1.01) | 0.30 |
| PTV | 0.98 (0.81–1.15) | 0.80 |
| GTV | 1.01 (0.72–1.38) | >0.9 |
| Risk for the occurrence of RN (multivariate) | ||
| Age | 0.95 (0.88–1.02) | 0.15 |
| Periventricular location | 6.73 (1.44–38.5) | 0.02 |
| HR (95% CI) | p-Value | |
|---|---|---|
| Risk for poor overall survival from diagnosis | ||
| Univariate | ||
| Male gender | 1.05 (0.41–2.65) | 0.90 |
| Age | 1.03 (0.99–1.07) | 0.13 |
| WHO grade IV | 3.02 (0.87–10.48) | 0.05 |
| GTV | 1.27 (1.08–1.50) | 0.01 |
| IDH mutated | 0.07 (0.01–0.57) | 0.01 |
| MGMT methylated | 0.62 (0.25–1.50) | 0.30 |
| TP53 | 0.94 (0.32–2.76) | 0.90 |
| ATRX loss | 0.17 (0.02–1.30) | 0.09 |
| Amount of lesions | 0.66 (0.33–1.32) | 0.20 |
| No surgery after SRS | 3.11 (0.71–13.7) | 0.13 |
| No radiation after SRS | 2.91 (0.66–12.8) | 0.20 |
| No TMZ after SRS | 2.41 (0.96–6.04) | 0.06 |
| No lomustine after SRS | 4.19 (1.2–14.5) | 0.02 |
| Bevacizumab after SRS | 083 (0.23–2.95) | 0.80 |
| Occurrence of RN * | 0.55 (0.21–1.44) | 0.20 |
| Lesions located within previous irradiated field | 0.66 (0.11–1.94) | 0.50 |
| Multivariate | ||
| WHO grade IV | 0.73 (0.17–3.32) | 0.70 |
| GTV | 1.20 (1.01–1.42) | 0.04 |
| IDH mutated | 0.09 (0.01–1.03) | 0.05 |
| No lomustine after SRS | 3.32 (0.89–11.02) | 0.08 |
| Risk for short event-free-survival after SRS | ||
| Univariate | ||
| Male gender | 1.69 (0.66–4.34) | 0.30 |
| Age | 1.03 (1.0–1.07) | 0.10 |
| WHO grade IV | 2.48 (0.72–8.50) | 0.15 |
| GTV | 1.21 (1.03–1.44) | 0.02 |
| IDH mutated | 0.09 (0.01–0.72) | 0.02 |
| MGMT methylated | 0.76 (0.31–1.86) | 0.50 |
| TP53 | 0.51 (0.17–1.54) | 0.20 |
| ATRX loss | 0.18 (0.02–1.42) | 0.10 |
| Amount of lesions | 0.62 (0.31–1.23) | 0.20 |
| No surgery after SRS | 4.04 (0.90–18.1) | 0.07 |
| No radiation after SRS | 3.24 (0.71–14.7) | 0.13 |
| No TMZ after SRS | 1.7 (0.67–4.29) | 0.30 |
| No lomustine after SRS | 3.73 (1.07–13.0) | 0.04 |
| No bevacizumab after SRS | 0.73 (0.21–2.52) | 0.60 |
| Multivariate | ||
| GTV | 1.20 (1.01–1.44) | 0.03 |
| IDH mutated | 0.11 (0.01–0.93) | 0.04 |
| No lomustine after SRS | 3.32 (0.93–11.0) | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Früh, A.; Loebel, F.; Bodnar, B.; Kilian, L.; Misch, M.; Kalinauskaite, G.; Kluge, A.; Eitner, C.; Onken, J.; Rubarth, K.; et al. High-Dose Stereotactic Re-Irradiation of Recurrent High-Grade Gliomas: Clinical Outcome and Experience with AI-Based Target Volume Simulation. Cancers 2025, 17, 3423. https://doi.org/10.3390/cancers17213423
Früh A, Loebel F, Bodnar B, Kilian L, Misch M, Kalinauskaite G, Kluge A, Eitner C, Onken J, Rubarth K, et al. High-Dose Stereotactic Re-Irradiation of Recurrent High-Grade Gliomas: Clinical Outcome and Experience with AI-Based Target Volume Simulation. Cancers. 2025; 17(21):3423. https://doi.org/10.3390/cancers17213423
Chicago/Turabian StyleFrüh, Anton, Franziska Loebel, Bohdan Bodnar, Larissa Kilian, Martin Misch, Goda Kalinauskaite, Anne Kluge, Chiara Eitner, Julia Onken, Kerstin Rubarth, and et al. 2025. "High-Dose Stereotactic Re-Irradiation of Recurrent High-Grade Gliomas: Clinical Outcome and Experience with AI-Based Target Volume Simulation" Cancers 17, no. 21: 3423. https://doi.org/10.3390/cancers17213423
APA StyleFrüh, A., Loebel, F., Bodnar, B., Kilian, L., Misch, M., Kalinauskaite, G., Kluge, A., Eitner, C., Onken, J., Rubarth, K., Zips, D., Vajkoczy, P., Senger, C., & Acker, G. (2025). High-Dose Stereotactic Re-Irradiation of Recurrent High-Grade Gliomas: Clinical Outcome and Experience with AI-Based Target Volume Simulation. Cancers, 17(21), 3423. https://doi.org/10.3390/cancers17213423







