The Impact of Local Ablative Therapies as Bridging Treatment on Overall Survival Following Liver Transplantation in Patients with HCC
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Neoadjuvant Therapy
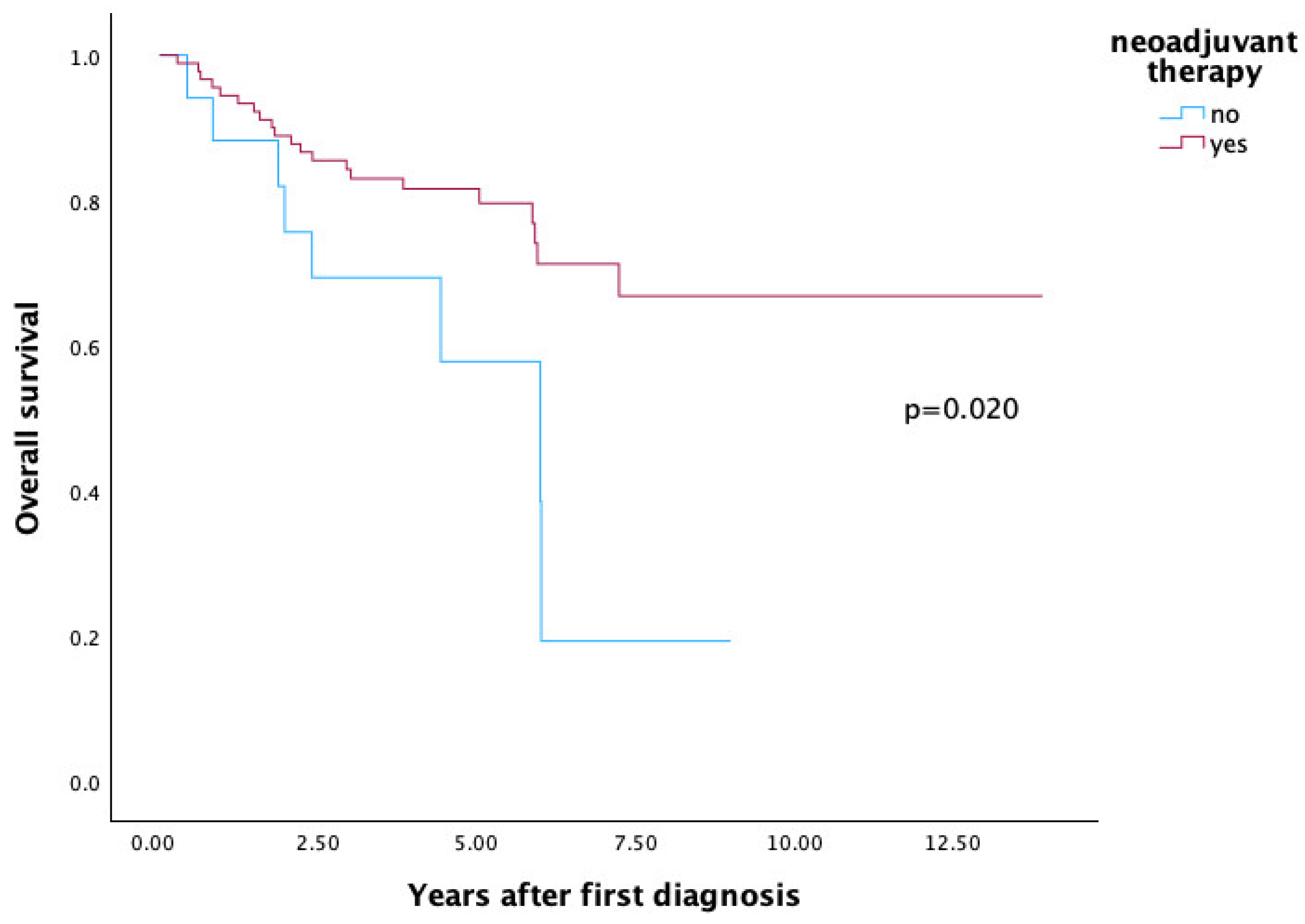
3.2. Survival
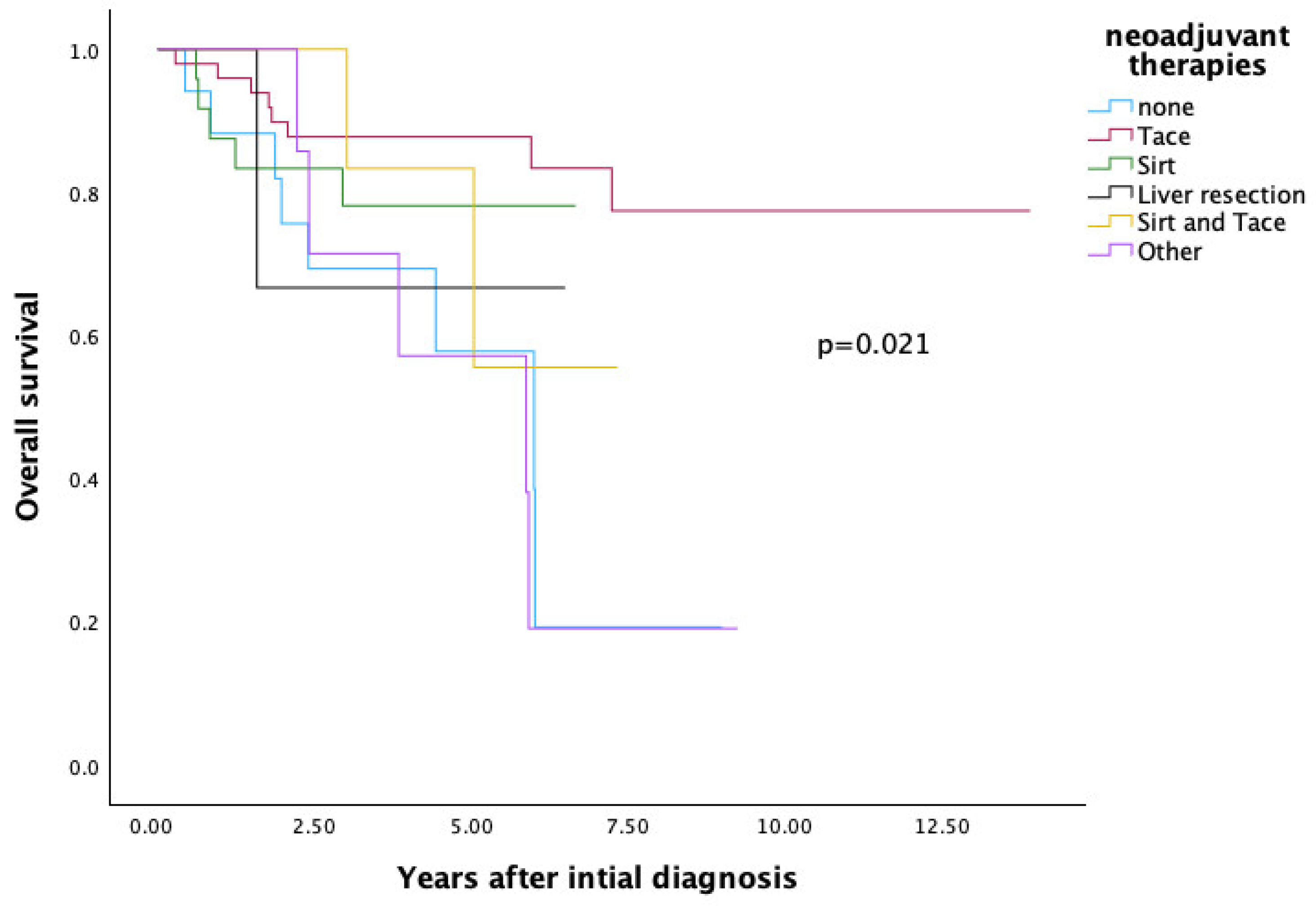
3.3. Comparison of Neoadjuvant Therapies Regarding Overall Survival
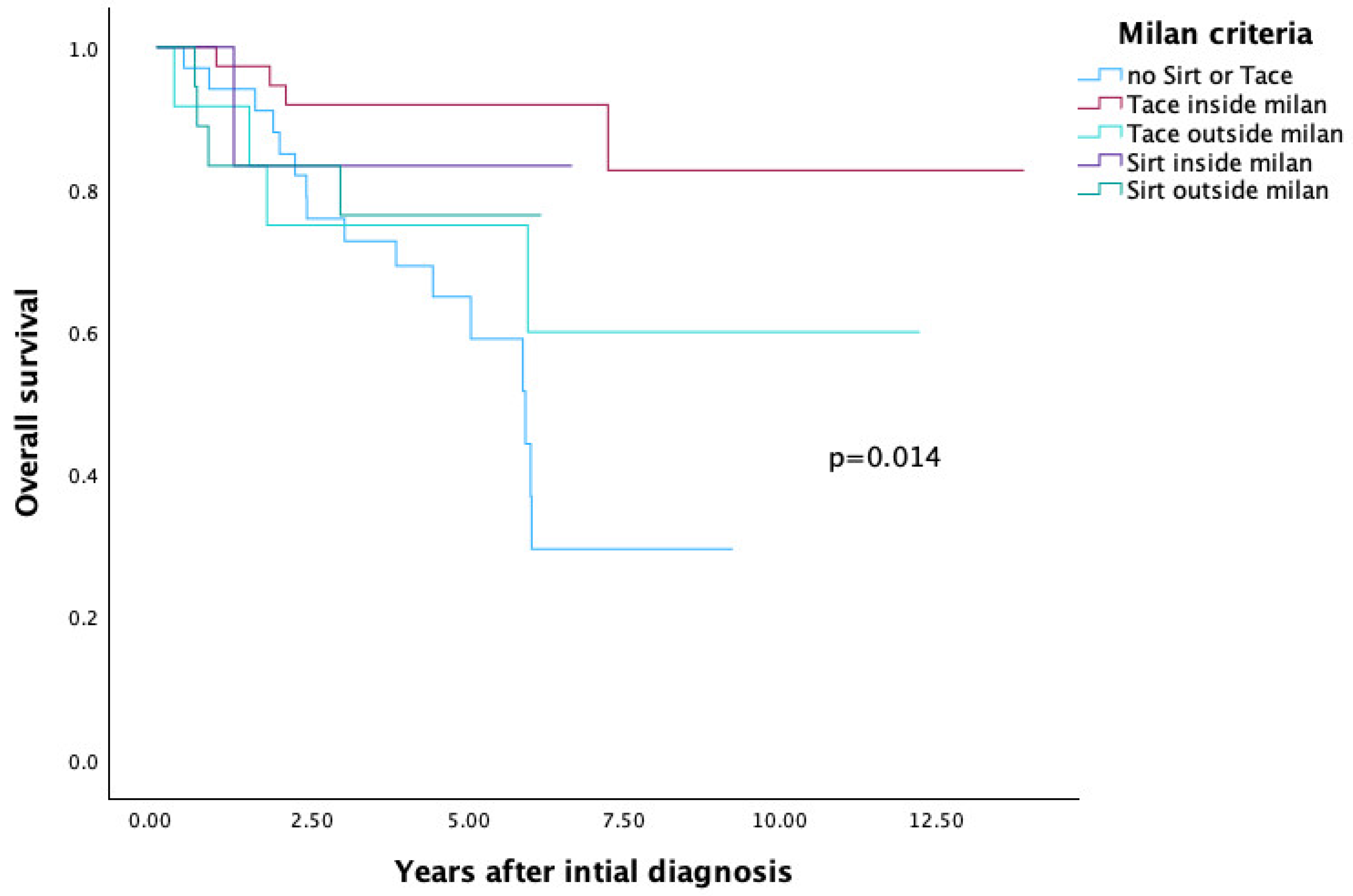
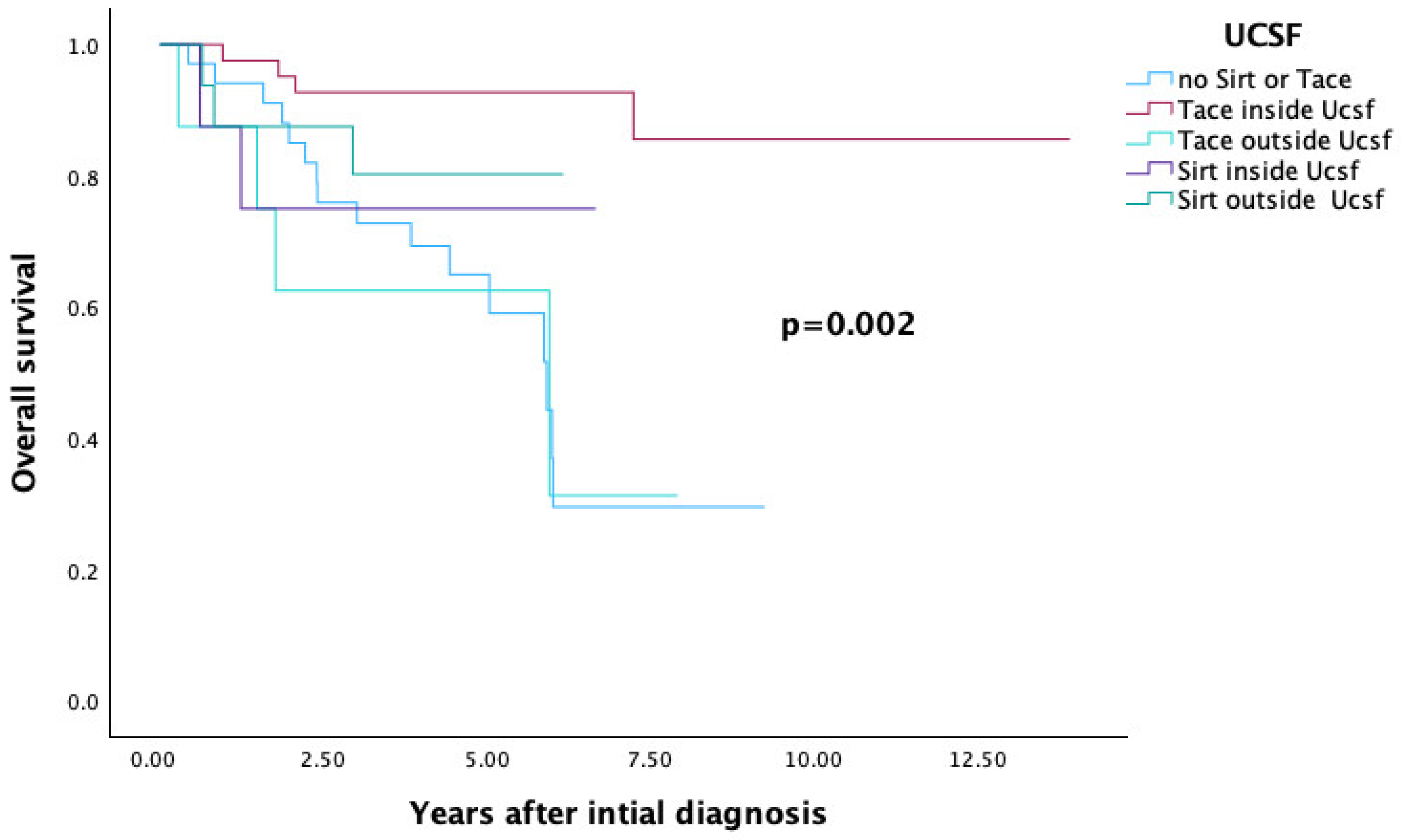
3.4. SIRT and TACE—Comparison of Overall Survival Based on Transplantation Criteria
3.4.1. Milan Criteria
3.4.2. UCSF Criteria
3.4.3. Up-to-Seven Criteria
3.5. Multivariate Analysis
3.6. Recurrence Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singal, A.G.; Kanwal, F.; Llovet, J.M. Global trends in hepatocellular carcinoma epidemiology: Implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 864–884. [Google Scholar] [CrossRef] [PubMed]
- Ringe, B.; Pichlmayr, R.; Wittekind, C.; Tusch, G. Surgical treatment of hepatocellular carcinoma: Experience with liver resection and transplantation in 198 patients. World J. Surg. 1991, 15, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.M.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Bhoori, S.; Sposito, C.; Bongini, M.; Langer, M.; Miceli, R.; Mariani, L. Milan criteria in liver transplantation for hepatocellular carcinoma: An evidence-based analysis of 15 years of experience. Liver Transplant. 2011, 17, S44–S57. [Google Scholar] [CrossRef]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver Transplantation for Hepatocellular Carcinoma: Expansion of the Tumor Size Limits Does Not Adversely Impact Survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef]
- Mazzaferro, V.M.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bruix, J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003, 37, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Berardi, G.M.; Guglielmo, N.; Cucchetti, A.; Usai, S.; Colasanti, M.; Meniconi, R.L.; Ferretti, S.; Mariano, G.; Angrisani, M.; Sciuto, R.; et al. Transarterial Radioembolization Can Downstage Intermediate and Advanced Hepatocellular Carcinoma to Liver Transplantation. Transplantation 2024, 109, e54–e63. [Google Scholar] [CrossRef]
- Livraghi, T.; Meloni, F.; Di Stasi, M.; Rolle, E.; Solbiati, L.; Tinelli, C.; Rossi, S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2007, 47, 82–89. [Google Scholar] [CrossRef]
- Baker, E.H.; Thompson, K.; McKillop, I.H.; Cochran, A.; Kirks, R.; Vrochides, D.; Martinie, J.B.; Swan, R.Z.; Iannitti, D.A. Operative microwave ablation for hepatocellular carcinoma: A single center retrospective review of 219 patients. J. Gastrointest. Oncol. 2017, 8, 337–346. [Google Scholar] [CrossRef]
- Andolino, D.L.; Johnson, C.S.; Maluccio, M.; Kwo, P.; Tector, A.J.; Zook, J.; Johnstone, P.A.; Cardenes, H.R. Stereotactic Body Radiotherapy for Primary Hepatocellular Carcinoma. Int. J. Radiat. Oncol. 2011, 81, e447–e453. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Chun, Y.S.; Poon, R.T.P.; Schwartz, M.E.; Yao, F.Y.; Marsh, J.W.; Bhoori, S.; Lee, S.-G. Liver Transplantation for Hepatocellular Carcinoma. Ann. Surg. Oncol. 2008, 15, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- McHugh, P.P.; Gilbert, J.; Vera, S.; Koch, A.; Ranjan, D.; Gedaly, R. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB 2010, 12, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Mas, X.; Aponte, J.J.; Fuster, J.; Navasa, M.; Christensen, E.; Rodés, J.; Bruix, J. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut 2002, 50, 123–128. [Google Scholar] [CrossRef]
- Andorno, E.; Bottino, G.; Morelli, N.; Casaccia, M.; Gelli, M.; Piredda, D.; Immordino, G.; Ferrante, R.; Nardi, I.; Troilo, B.; et al. Preliminary Results of Liver Transplantation for Hepatocellular Carcinoma Among Allocation Organ Policy Strategies, Neoadjuvant Treatments, and Intention-to-Treat Analysis. Transplant. Proc. 2008, 40, 1972–1973. [Google Scholar] [CrossRef]
- Frangakis, C.; Geschwind, J.-F.; Kim, D.; Chen, Y.; Koteish, A.; Hong, K.; Liapi, E.; Georgiades, C.S. Chemoembolization Decreases Drop-Off Risk of Hepatocellular Carcinoma Patients on the Liver Transplant List. Cardiovasc. Interv. Radiol. 2010, 34, 1254–1261. [Google Scholar] [CrossRef]
- Lai, Q.; Vitale, A.; Iesari, S.; Finkenstedt, A.; Mennini, G.; Onali, S.; Hoppe-Lotichius, M.; Manzia, T.M.; Nicolini, D.; Avolio, A.W.; et al. The Intention-to-Treat Effect of Bridging Treatments in the Setting of Milan Criteria–In Patients Waiting for Liver Transplantation. Liver Transplant. 2019, 25, 1023–1033. [Google Scholar] [CrossRef]
- Xing, M.; Sakaria, S.; Dhanasekaran, R.; Parekh, S.; Spivey, J.; Knechtle, S.J.; Zhang, D.; Kim, H.S. Bridging Locoregional Therapy Prolongs Survival in Patients Listed for Liver Transplant with Hepatocellular Carcinoma. Cardiovasc. Interv. Radiol. 2016, 40, 410–420. [Google Scholar] [CrossRef]
- Akateh, C.; Black, S.M.; Conteh, L.; Miller, E.D.; Noonan, A.; Elliott, E.; Pawlik, T.M.; Tsung, A.; Cloyd, J.M. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 3704–3721. [Google Scholar] [CrossRef]
- Matsumoto, M.M.; Mouli, S.; Saxena, P.; Gabr, A.; Riaz, A.; Kulik, L.; Ganger, D.; Maddur, H.; Boike, J.; Flamm, S.; et al. Comparing Real World, Personalized, Multidisciplinary Tumor Board Recommendations with BCLC Algorithm: 321-Patient Analysis. Cardiovasc. Interv. Radiol. 2021, 44, 1070–1080. [Google Scholar] [CrossRef]
- Finkenstedt, A.; Vikoler, A.; Portenkirchner, M.; Mülleder, K.; Maglione, M.; Margreiter, C.; Moser, P.; Vogel, W.; Bale, R.; Freund, M.; et al. Excellent post-transplant survival in patients with intermediate stage hepatocellular carcinoma responding to neoadjuvant therapy. Liver Int. 2015, 36, 688–695. [Google Scholar] [CrossRef]
- Jotz, R.d.F.; Horbe, A.F.; Coral, G.P.; Fontana, P.C.; de Morais, B.G.; de Mattos, A.A. Results of transarterial chemoembolization of hepatocellular carcinoma as a bridging therapy to liver transplantation. Radiol. Bras. 2023, 56, 235–241. [Google Scholar] [CrossRef]
- Cannon, R.M.; Bolus, D.N.; White, J.A. Irreversible Electroporation as a Bridge to Liver Transplantation. Am. Surg. 2019, 85, 103–110. [Google Scholar] [CrossRef]
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Lo, C.-M.; Ngan, H.; Tso, W.-K.; Liu, C.-L.; Lam, C.-M.; Poon, R.T.-P.; Fan, S.-T.; Wong, J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002, 35, 1164–1171. [Google Scholar] [CrossRef]
- Grąt, M.; Krawczyk, M.; Stypułkowski, J.; Morawski, M.; Krasnodębski, M.; Wasilewicz, M.; Lewandowski, Z.; Grąt, K.; Patkowski, W.; Zieniewicz, K. Prognostic Relevance of a Complete Pathologic Response in Liver Transplantation for Hepatocellular Carcinoma. Ann. Surg. Oncol. 2019, 26, 4556–4565. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Hwang, S.; Song, G.; Lee, Y.; Kim, K.; Ahn, C.; Moon, D.; Jung, D.; Park, G.; Lee, S. Prognostic effect of transarterial chemoembolization–induced complete pathological response in patients undergoing liver resection and transplantation for hepatocellular carcinoma. Liver Transplant. 2017, 23, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163.e2. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Kassab, I.; Massani, M.; Townsend, W.; Singal, A.G.; Soydal, C.; Moreno-Luna, L.; Roberts, L.R.; Chen, V.L.; Parikh, N.D. TACE versus TARE for patients with hepatocellular carcinoma: Overall and individual patient level meta analysis. Cancer Med. 2022, 12, 2590–2599. [Google Scholar] [CrossRef]
- de Alcântara, J.P.T.L.; Götz, G.W.X.d.R. Transarterial Radioembolization with Yttrium-90 and SIRT Versus Conventional Transarterial Chemoembolization for Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Acad. Radiol. 2025. [Google Scholar] [CrossRef]

| Variables | Entire Cohort (n = 107) |
|---|---|
| Age at the time of first diagnosis (years, mean, range) | 60 (24–72) |
| Age at the time of liver transplantation (years, mean, range) | 61 (24–73) |
| Gender (Male, Female, n (%)) | 97 (90.7), 10 (9.3) |
| BMI (kg/m2, mean, range) | 28 (19.5–37.6) |
| Transplantation (LDLT, DDLT,n (%)) | 39 (36.4), 68 (63.6) |
| Liver Cirrhosis (yes, n (%)) | 99 (92.5) |
| Child (none, A,B,C, n(%)) | 8 (7.5), 52 (48.6), 31 (29), 16 (15) |
| Nutritional toxic liver cirrhosis (yes, n (%)) | 70 (65.4) |
| Diagnosis (n (%)) | |
| HCC | 103 (96.3) |
| HCC-iCCA | 4 (3.7) |
| T Stage (n (%)) | |
| T0 | 18 (16.8) |
| T1 | 30 (28) |
| T2 | 49 (45.8) |
| T3 | 7 (6.5) |
| T4 | 2 (1.9) |
| Relapse (n (%)) | |
| Yes | 19 (17.8) |
| No | 88 (82.2) |
| Site of recurrence | |
| Adrenal gland | 1 |
| Lung | 4 |
| Lymph node | 2 |
| Liver | 7 |
| Bone | 4 |
| Brain | 1 |
| Neoadjuvant therapy (n (%)) | |
| Yes | 90 (84.1) |
| No | 17 (15.9) |
| Neoadjuvant therapy (n (%)) | |
| none | 17 (15.9) |
| SIRT | 24 (22.4) |
| TACE | 49 (45.8) |
| Liver resection | 3 (2.8) |
| SIRT and TACE | 7 (6.5) |
| Other: | 7 (6.5) |
| RFA | 2 |
| Radiatio | 3 |
| Chemotherapy | 2 |
| Response to neoadjuvant therapy (n (%)) | |
| Yes | 57 (53.3) |
| No | 7 (6.5) |
| No follow-up imaging | 26 (24.3) |
| No neoadjuvant therapy | 17 (15.9) |
| Number of lesions (n (%)) | |
| at time of first diagnosis | |
| 1 | 42 (39.3) |
| 2 | 30 (28) |
| 3 | 12 (11.2) |
| 4 | 5 (4.7) |
| ≥5 | 18 (16.8) |
| postoperative (histopathological examination) | |
| 0 | 8 (7.5) |
| 1 | 47 (43.9) |
| 2 | 17 (15.9) |
| 3 | 14 (13.1) |
| 4 | 3 (2.8) |
| ≥5 | 18 (16.8) |
| Largest tumor diameter (cm, mean, range) | |
| at time of first diagnosis | 3.7 (0.5–14) |
| postoperative histopathological examination | 3 (0–14.5) |
| AFP at initial diagnosis (n) | |
| Elevated | 66 |
| Negative | 41 |
| AFP after neoadjuvant therapy (n) | |
| Elevated | 50 |
| Negative | 49 |
| Not available | 8 |
| AFP postoperatively (n) | |
| Elevated | 17 |
| Negative | 83 |
| Not available | 7 |
| Transplantation Criteria | Entire Cohort (n = 107) |
|---|---|
| Milan at time of first diagnosis n (%) | |
| Inside | 62 (57.9) |
| Outside | 45 (42.1) |
| Milan after neoadjuvant therapy n (%) | |
| Inside | 66 (61.7) |
| Outside | 41 (38.3) |
| UCSF at time of first diagnosis n (%) | |
| Inside | 71 (66.4) |
| Outside | 36 (33.6) |
| USCSF after neoadjuvant therapy n (%) | |
| Inside | 75 (70.1) |
| Outside | 32 (29.9) |
| Up to seven at time of first diagnosis n (%) | |
| Inside | 76 (71) |
| Outside | 31 (29) |
| Up to seven after neoadjuvant therapy n (%) | |
| Inside | 82 (76.6) |
| Outside | 25 (23.4) |
| Neoadjuvanttherapy | Number of patients outside the Milan criteria (at the time of pre-transplant evaluation)/(based on postoperative histopathological findings) | Number of patients inside the Milan criteria (at the time of pre-transplant evaluation)/(based on postop-erative histopathological find-ings) |
| None | 9/11 | 8/6 |
| TACE | 12/15 | 37/34 |
| SIRT | 18/12 | 6/12 |
| Liver resection | 1/0 | 2/3 |
| SIRT and TACE | 3/1 | 4/6 |
| Other | 2/2 | 5/5 |
| Neoadjuvant therapy | Number of patients outside the UCSF criteria (at the time of pre-transplant evaluation)/(based on post-operative histopathological findings) | Number of patients inside the UCSF criteria (at the time of pre-transplant evaluation)/(based on postoperative histopathological findings) |
| None | 7/9 | 10/8 |
| TACE | 8/11 | 41/38 |
| SIRT | 16/9 | 8/15 |
| Liver resection | 1/0 | 2/3 |
| SIRT and TACE | 2/1 | 5/6 |
| Other | 2/2 | 5/5 |
| Neoadjuvant therapy | Number of patients outside the up to seven criteria (at the time of pre-transplant evaluation)/(based on postoperative histopathological findings) | Number of patients inside the up to seven criteria (at the time of pre-transplant evaluation)/(based on postoperative histopathological findings) |
| None | 6/9 | 11/8 |
| TACE | 6/6 | 43/43 |
| SIRT | 14/7 | 10/17 |
| Liver resection | 1/0 | 2/3 |
| SIRT and TACE | 2/1 | 5/6 |
| Other | 2/2 | 5/5 |
| Number of Patients | OS (%) | p | |
|---|---|---|---|
| MILAN (Figure 4) | 0.014 | ||
| No SIRT and no TACE | 34 | 29.5 | |
| TACE inside milan | 37 | 82.7 | |
| SIRT inside milan | 6 | 83.3 | |
| TACE outside milan | 12 | 60 | |
| SIRT outside milan | 18 | 76.4 | |
| UCSF (Figure 5) | 0.002 | ||
| No SIRT and no TACE | 34 | 29.5 | |
| TACE inside UCSF | 41 | 85.6 | |
| SIRT inside UCSF | 8 | 75 | |
| TACE outside UCSF | 8 | 31.3 | |
| SIRT outside UCSF | 16 | 80.2 | |
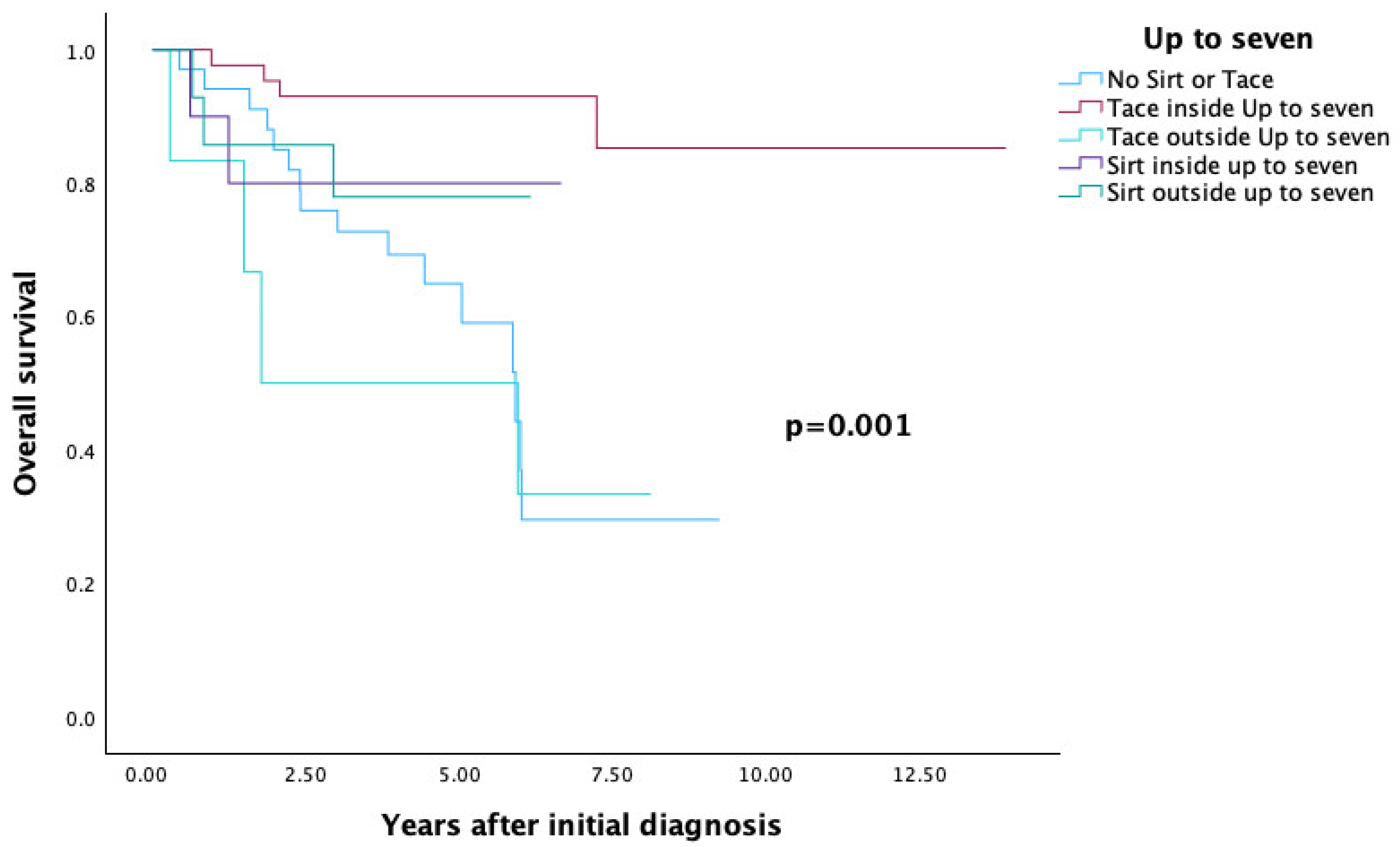
| Up to seven (Figure 6) | 0.001 | ||
| No SIRT and no TACE | 34 | 29.5 | |
| TACE inside up to seven | 43 | 85.3 | |
| SIRT inside up to seven | 10 | 80 | |
| TACE outside up to seven | 6 | 33.3 | |
| SIRT outside up to seven | 14 | 77.9 |
| Multivariate Analysis | |||
|---|---|---|---|
| Variables | HR | 95%CI | p-value |
| Transplantation (LDLT, DDLT) | 1.706 | 0.554–5.253 | 0.352 |
| Steatosis | 2.101 | 0.255–17.283 | 0.490 |
| Blood type | 0.954 | 0.605–1.504 | 0.839 |
| AFP negative (at initial diagnosis) | 0.472 | 0.161–1.387 | 0.172 |
| AFP negative after neoadjuvant therapy | 2.190 | 1.047–4.579 | 0.037 |
| AFP negative postoperatively | 5.401 | 1.596–18.276 | 0.007 |
| Liver Cirrhosis | 0.416 | 0.045–3.839 | 0.439 |
| Neoadjuvant therapy | 1.450 | 1.101–1.908 | 0.008 |
| Child score | 0.936 | 0.539–1.624 | 0.813 |
| Response to neoadjuvant therapy | 3.592 | 1.461–8.834 | 0.005 |
| T Stage | 0.959 | 0.557–1.650 | 0.880 |
| Age at the time of liver transplantation | 1.014 | 0.952–1.081 | 0.658 |
| Gender | 0.864 | 0.185–4.039 | 0.853 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwenk, L.; Dondorf, F.; Rohland, O.; Ali-Deeb, A.; Settmacher, U.; Rauchfuß, F. The Impact of Local Ablative Therapies as Bridging Treatment on Overall Survival Following Liver Transplantation in Patients with HCC. Cancers 2025, 17, 3393. https://doi.org/10.3390/cancers17203393
Schwenk L, Dondorf F, Rohland O, Ali-Deeb A, Settmacher U, Rauchfuß F. The Impact of Local Ablative Therapies as Bridging Treatment on Overall Survival Following Liver Transplantation in Patients with HCC. Cancers. 2025; 17(20):3393. https://doi.org/10.3390/cancers17203393
Chicago/Turabian StyleSchwenk, Laura, Felix Dondorf, Oliver Rohland, Aladdin Ali-Deeb, Utz Settmacher, and Falk Rauchfuß. 2025. "The Impact of Local Ablative Therapies as Bridging Treatment on Overall Survival Following Liver Transplantation in Patients with HCC" Cancers 17, no. 20: 3393. https://doi.org/10.3390/cancers17203393
APA StyleSchwenk, L., Dondorf, F., Rohland, O., Ali-Deeb, A., Settmacher, U., & Rauchfuß, F. (2025). The Impact of Local Ablative Therapies as Bridging Treatment on Overall Survival Following Liver Transplantation in Patients with HCC. Cancers, 17(20), 3393. https://doi.org/10.3390/cancers17203393





